The S-type anion channels SLAC1 and SLAH3 inhibit stomatal opening by inhibiting the activity of the K+in channels by protein-protein interaction in response to drought stress in Arabidopsis.
Abstract
Drought stress induces stomatal closure and inhibits stomatal opening simultaneously. However, the underlying molecular mechanism is still largely unknown. Here, we show that the slow-type (S-type) anion channels SLAC1 and SLAH3 mainly inhibit the inward-rectifying K+ channel KAT1 by protein-protein interaction and consequently prevent stomatal opening in Arabidopsis thaliana. Voltage-clamp results demonstrated that SLAC1 inhibited KAT1 dramatically but did not inhibit KAT2. SLAH3 inhibited KAT1 to a lesser extent than did SLAC1. Both the N and C termini of SLAC1 inhibited KAT1, but the inhibition by the N terminus was stronger. The C terminus was essential for SLAC1-mediated inhibition of KAT1. Furthermore, drought stress strongly upregulated the expression of SLAC1 and SLAH3 in Arabidopsis guard cells, and the overexpression of wild-type and truncated SLAC1 dramatically impaired inward K+ (K+in) currents of guard cells and light-induced stomatal opening. Additionally, the inhibition of KAT1 by SLAC1 and KC1 only partially overlapped, suggesting that SLAC1 and KC1 inhibited K+in channels via different molecular mechanisms. Taken together, these findings reveal a novel regulatory mechanism for stomatal movement, in which signaling pathways for stomatal closure and opening are directly coupled by protein-protein interaction between SLAC1/SLAH3 and KAT1 in Arabidopsis.
INTRODUCTION
Stomata and their surrounding pair of guard cells are gateways in the epidermis that link the intercellular gas space to the outer environment in higher plants. The stomatal aperture is regulated by changes of turgor pressure in the guard cells, which result mainly from the flux of osmotic ions through ion channels in the vacuolar membrane and plasma membrane of guard cells (Raschke et al., 1988; Sentenac et al., 1992; Ward and Schroeder, 1994; Pandey et al., 2007; Ward et al., 2009; Kim et al., 2010; Kollist et al., 2014; Misra et al., 2015).
Light is a natural principle factor that regulates stomatal opening in plants. Red light induces stomatal opening probably by enhancing photosynthetic activity (Shimazaki et al., 2007; Inoue et al., 2010). Blue light is a specific signal for stomatal opening and is sensed by its receptors, phototropin1 (PHOT1) and PHOT2, which are consequently activated by autophosphorylation, and then bind to 14-3-3 protein (Kinoshita et al., 2001; Inoue et al., 2008, 2010). The phototropins activate H+-ATPase by phosphorylation and inhibit anion channel activity (Roelfsema and Hedrich, 2005; Roelfsema et al., 2012; Kollist et al., 2014). Both H+ extrusion through H+-ATPase and the inhibition of anion channel activity result in plasma membrane hyperpolarization, which consequently triggers the influx of external K+ (Assmann et al., 1985; Shimazaki et al., 1986; Kinoshita and Shimazaki, 1999, 2002). Abscisic acid (ABA) inhibits stomatal opening, probably through crosstalk between the blue light pathway and the ABA signaling pathway (Zhang et al., 2004, 2007).
K+ is an important macronutrient in plant cells that is involved in maintaining the ionic environment and regulating osmolality. The uptake of K+ into guard cells is the main driving force for stomatal opening (Walker et al., 1996; Lebaudy et al., 2007; Szczerba et al., 2009; Dreyer and Uozumi, 2011; Wang and Wu, 2013; Zörb et al., 2014). K+ enters guard cells mainly through inward K+ (K+in) channels, which are composed of several members of the Shaker family (Véry and Sentenac, 2003; Lebaudy et al., 2007; Ward et al., 2009; Hedrich, 2012; Roelfsema et al., 2012; Wang and Wu, 2013; Ronzier et al., 2014). Each Shaker member has six transmembrane domains, a C terminus, and an N terminus. Shaker channels are assembled as diverse homo- or heterotetramers (MacKinnon, 1991; Daram et al., 1997; Dreyer et al., 2004; Lebaudy et al., 2008). One exception is KC1, which is unable to form a homotetramer alone, but functions as an inhibitory subunit by integrating itself into the K+in channels (Reintanz et al., 2002; Geiger et al., 2009a; Jeanguenin et al., 2011). The Shaker channel family has nine members in Arabidopsis thaliana, and five of these, i.e., KAT1 (potassium channel in Arabidopsis thaliana 1), KAT2, AKT1 (Arabidopsis potassium transporter 1), AKT2, and KC1 (Arabidopsis thaliana K+ rectifying channel 1), are involved in the assembly of K+in channels in guard cells (Szyroki et al., 2001). KAT1 is the first K+ channel identified in plants (Schachtman et al., 1992; Nakamura et al., 1995; Véry et al., 1995) and represents the main K+in channel in Arabidopsis guard cells. Most K+in channels are predicted to be heterotetramers in Arabidopsis guard cells, considering the presence of the other four Shaker members in these cells. These diverse tetramers exhibit different electrophysiological characteristics (Dreyer et al., 1997; Pilot et al., 2001; Obrdlik et al., 2004; Xicluna et al., 2007). SNAREs are involved in regulating K+in currents by affecting the trafficking and subcellular localization of KAT1 (Sutter et al., 2006; Eisenach et al., 2012; Zhang et al., 2015). ABA-mediated inhibition of K+in currents was observed in Arabidopsis guard cells (Fan et al., 2008), and G protein played important roles in this process (Wang et al., 2001). K+in currents of guard cell protoplasts were strongly reduced without impairing stomatal opening in the knockout mutants kat1 and kat2 (Szyroki et al., 2001; Lebaudy et al., 2008), whereas both the K+in currents and stomatal opening were inhibited in dominant-negative KAT1 mutant plants in the wild-type background and dominant-negative KAT2 mutant plants in the kat2-1 mutant background (Kwak et al., 2001; Lebaudy et al., 2008). Interestingly, it was reported recently that K+in currents of Arabidopsis guard cells were inhibited by over 80% and stomatal opening was also impaired in slac1-1 and slac1-3 mutants (Wang et al., 2012; Laanemets et al., 2013). Changes in cytosolic Ca2+ and pH may be involved in the regulation of K+in channels in Arabidopsis guard cells (Blatt and Grabov, 1997; Wang et al., 2012; Laanemets et al., 2013). Therefore, it seems that the regulatory mechanisms underlying K+in channel activity and stomatal opening are complex and that K+in currents of guard cells may not have to be completely blocked to inhibit stomatal opening, as diverse, interconnected molecular mechanisms are involved.
ABA and drought stress induce anion efflux through anion channels and further depolarize the plasma membrane of guard cells. This membrane depolarization activates the single outward K+ (K+out) channel GORK (guard cell outward rectifying K+ channel), which allows K+ efflux and leads to stomatal closure (Véry and Sentenac, 2002; Lebaudy et al., 2007; Ward et al., 2009; Kim et al., 2010; Hedrich, 2012; Roelfsema et al., 2012; Wang and Wu, 2013). The gork knockout mutation abolishes K+out currents and impairs drought stress-induced stomatal closure (Ache et al., 2000; Hosy et al., 2003; Eisenach et al., 2014). On the other hand, plasma membrane depolarization also activates anion channels and further results in anion efflux. R-type (rapid-type) and S-type (slow-type) anion currents were first observed in Vicia faba guard cell protoplasts using a patch-clamp technique (Keller et al., 1989; Schroeder and Keller, 1992). SLAC1 (slow anion channel-associated 1) was identified as an S-type anion channel in screens for ozone-sensitive and CO2-insensitive mutants (Negi et al., 2008; Vahisalu et al., 2008). Loss-of-function mutations in SLAC1 strongly impair the S-type anion channel activity in Arabidopsis guard cells (Vahisalu et al., 2008). SLAC1 has four homologs (SLAH1 to SLAH4) in Arabidopsis (Negi et al., 2008; Vahisalu et al., 2008). SLAH3 is also involved in stomatal closure as an anion channel. Both SLAC1 and SLAH3 represent S-type anion channels in Arabidopsis guard cells that mediate stomatal closure (Geiger et al., 2011). SLAC1 is a conserved anion channel from ancient species to higher plants that is involved in stomatal closure (Kusumi et al., 2012; Lind et al., 2015). Structural analysis revealed that SLAC1 forms a trimer, but each subunit of the trimer forms a channel pore independently (Chen et al., 2010). SLAC1 and SLAH3 are activated by protein phosphorylation in a Ca2+-independent manner by OST1 (open stomata1) and CPK23 (calcium-dependent protein kinase23), respectively (Geiger et al., 2009b, 2010; Lee et al., 2009), or in a Ca2+-dependent manner by GHR1 (guard cell hydrogen peroxide-resistant 1), CPK3/6/21, and CBL1 (calcineurin B-like protein 1)/CIPK23, respectively (Geiger et al., 2010; Brandt et al., 2012; Hua et al., 2012; Scherzer et al., 2012; Demir et al., 2013; Maierhofer et al., 2014). On exposure to drought stress, plants produce ABA, which is perceived by its receptors PYR (pyrabactin resistance)/PYL (parabactin resistance 1-like)/RCAR (regulatory component of ABA receptor). These ABA receptors interact with a few phosphatases from the PP2C (protein phosphatase 2C) family, mainly including ABI1 (ABA insensitive 1), ABI2, PP2CA, and HAB1 (hypersensitive to ABA1), to release the diverse protein kinases (Ma et al., 2009; Nishimura et al., 2009; Park et al., 2009; Nishimura et al., 2010), which further activate SLAC1 and SLAH3 by phosphorylation (Geiger et al., 2009b, 2010; Lee et al., 2009; Brandt et al., 2012; Hua et al., 2012; Scherzer et al., 2012; Demir et al., 2013; Maierhofer et al., 2014). ALMT12 (aluminum-activated malate transporter 12)/QUAC (quick-activating anion channel 1) is an R-type anion channel in Arabidopsis guard cells (Meyer et al., 2010; Imes et al., 2013). The efflux of osmotic anions through R-type and S-type anion channels and the consequent K+ efflux through GORK drive stomatal closure to prevent water loss.
Stomatal opening and closure are regulated by different signaling pathways, and crosstalk between the signaling pathways is essential for a precise and efficient regulation of stomatal movement. Drought stress and ABA induce stomatal closure and simultaneously prevent light-induced stomatal opening to conserve water in the daytime under drought conditions, indicating that the ABA singling pathway is capable of inhibiting the light signaling pathway to prevent stomatal opening. However, the molecular mechanism underlying the crosstalk between the ABA signaling pathway and the light signaling pathway in guard cells is largely unknown. In this study, we discovered a novel molecular mechanism that mediates crosstalk between stomatal opening and closure. In this mechanism, the signaling pathway underlying ABA-induced stomatal closure and the signaling pathway underlying light-induced stomatal opening are directly coupled by direct protein-protein interaction between the S-type anion channels SLAC1 and SLAH3 and the K+in channels KAT1 and AKT2, for efficient inhibition of stomatal opening by drought stress and ABA in Arabidopsis.
RESULTS
SLAC1 Interacts with Several K+in Channels
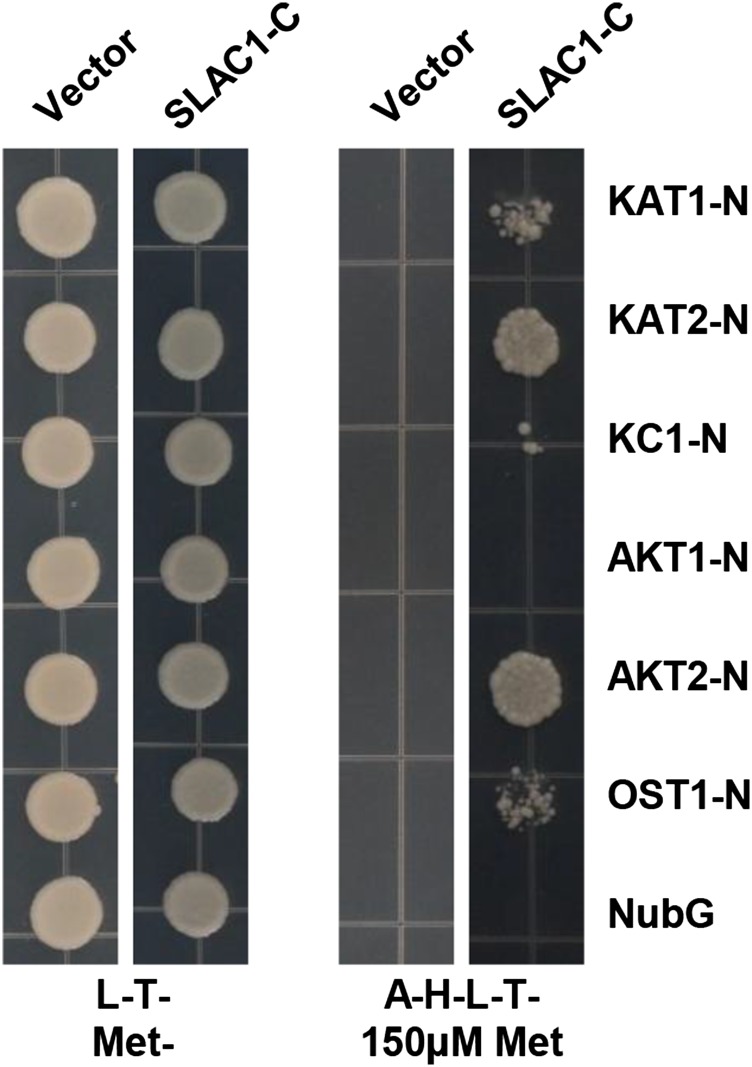
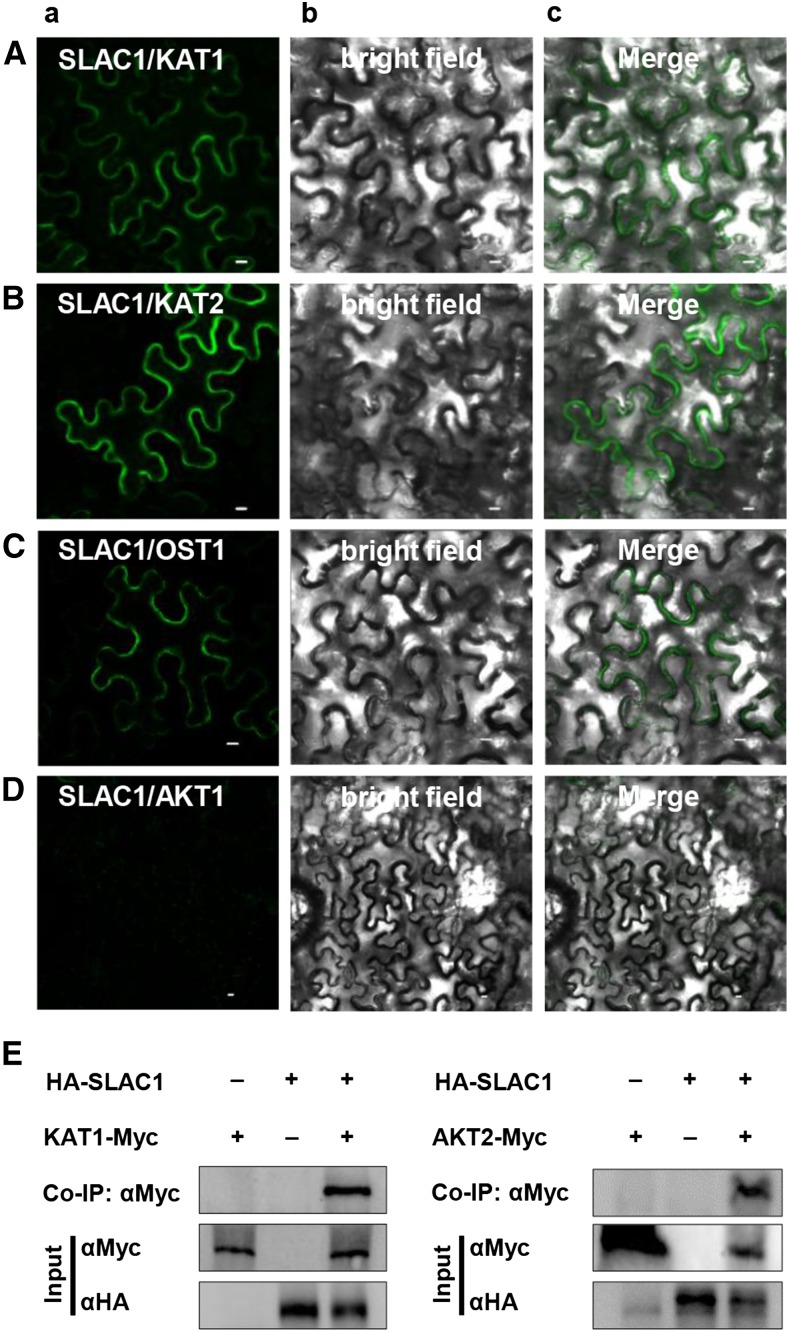
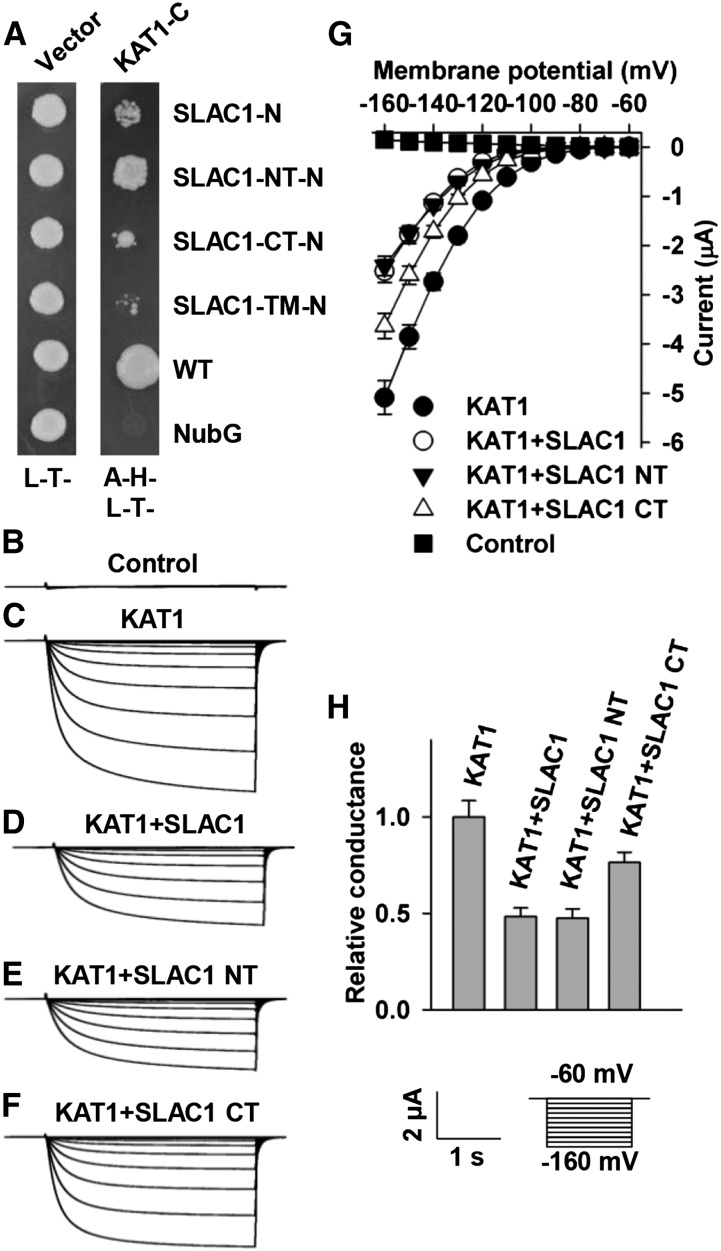
To investigate whether SLAC1 has an impact on K+in channels, we performed a yeast two-hybrid (Y2H) assay using the split-ubiquitin system (Obrdlik et al., 2004). The results demonstrated that SLAC1 interacted with most of the Shaker channels present in guard cells, including KAT1, KAT2, AKT2, and KC1, but not AKT1. For this experiment, we included the N-terminal half of ubiquitin (NubG) as a negative control and OST1, which is known to activate SLAC1 (Geiger et al., 2009b), as a positive control (Figure 1). We next performed a bimolecular fluorescence complementation (BiFC) assay in Nicotiana benthamiana leaves using the split YFP system (Waadt et al., 2008). YFP fluorescence was observed in epidermal cells coexpressing SLAC1 and KAT1 (Figure 2A), SLAC1 and KAT2 (Figure 2B), and the positive control SLAC1 and OST1 (Figure 2C), but not in the negative control SLAC1 and AKT1 (Figure 2D). We further conducted coimmunoprecipitation (co-IP) experiments and observed a clear protein-protein interaction between SLAC1 and either KAT1 and AKT2 (Figure 2E). SLAC1 and SLAH3 represent the main anion channels for anion efflux and stomatal closure, while KAT1 and KAT2 represent the main K+in channels for K+ influx and stomatal opening. We therefore focused on the regulation of K+in channels KAT1 and KAT2 by the S-type anion channels SLAC1 and SLAH3.
Figure 1.
Y2H Results Show That SLAC1 Interacts with KAT1, KAT2, and AKT2, but Not AKT1.
L-T-MET- stands for synthetic defined (SD) medium lacking Leu (L), Trp (T), and Met. A-H-L-T- plus 150 μM Met stands for SD medium lacking Leu, Trp, adenine (A), and His (H), but containing 150 μM Met.
Figure 2.
Protein-Protein Interaction Analysis.
(A) to (D) BiFC results showing that SLAC1 interacts with KAT1 (A), KAT2 (B), and the positive control OST1 (C), but not with AKT1 (D). YFP (A), bright-field (B), and merged (C) images are shown. Bars = 10 μm.
(E) Co-IP assay showing protein-protein interaction between SLAC1 and either KAT1 or AKT2 in oocytes.
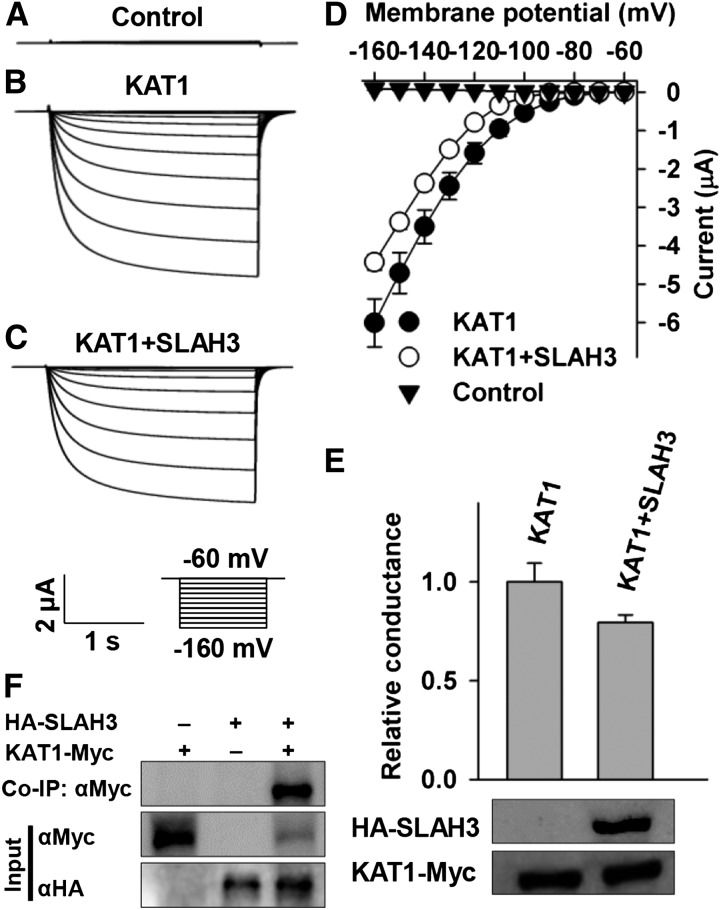
SLAC1 Strongly Inhibits KAT1, but Not KAT2, in Xenopus laevis Oocytes
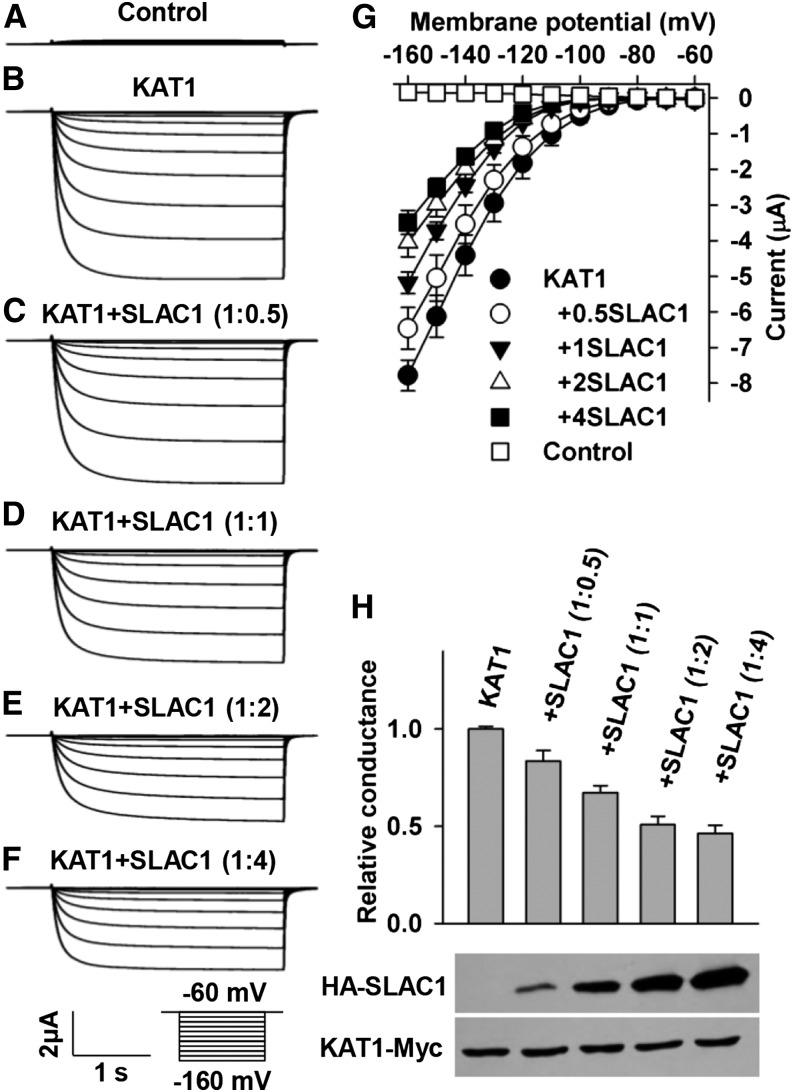
To investigate whether SLAC1 regulates K+in channels, we conducted voltage-clamp experiments in X. laevis oocytes. We observed large K+in currents in positive control oocytes expressing KAT1 alone and small background conductance in the negative control oocytes injected with water (Figures 3A, 3B, and 3G). The large KAT1-mediated K+in currents were in agreement with previous reports (Schachtman et al., 1992; Sottocornola et al., 2008; Sato et al., 2009, 2010; Lebaudy et al., 2010). However, the K+in currents were reduced significantly by the coexpression of KAT1 and SLAC1 in a [cRNA] ratio of KAT1/SLAC1-dependent manner compared with the positive control (Figures 3C to 3H). The K+in currents were inhibited significantly by ∼50% at a [cRNA] ratio of 1:2 of KAT1/SLAC1 (t test, P value < 0.005) (Figures 3E, 3G, and 3H), and the activation threshold of the KAT1 channel was negatively shifted by ∼10 mV (Figure 3G). These data indicate that SLAC1 inhibits KAT1 dramatically by both reducing the K+in current amplitude and shifting the threshold voltage negatively, which implies a decrease of voltage sensitivity of KAT1 as reported previously (Hoshi, 1995; Véry et al., 1995; Marten and Hoshi, 1997). The K+in currents were further reduced by changing the [cRNA] ratio of KAT1/SLAC1 from 1:2 to 1:4; however, this reduction was not significant relative to the K+in currents in the [cRNA] ratio of 1:2 (Figures 3E to 3G). Therefore, we used the [cRNA] ratio of KAT1/SLAC1 of 1:2 in further voltage-clamp experiments in oocytes.
Figure 3.
SLAC1 Inhibits the Activity of KAT1 in a Manner That Depends on the [cRNA] Ratio of KAT1/SLAC1 in X. laevis Oocytes.
(A) to (F) Typical whole-oocyte recordings of the negative control (A), oocytes expressing KAT1 alone (B), and oocytes coexpressing KAT1 and SLAC1 in a [cRNA] ratio of KAT1/SLAC1 of 1:0.5 (C), 1:1 (D), 1:2 (E), and 1:4 (F).
(G) Average current-voltage curves of steady state currents recorded in oocytes injected with water (n = 6), KAT1 cRNA (n = 7), and a cRNA mixture of KAT1 and SLAC1 in a [cRNA] ratio of KAT1/SLAC1 of 1:0.5 (n = 10), 1:1 (n = 11), 1:2 (n = 6), and 1:4 (n = 9).
(H) Normalized macroscopic conductance and immunoblot analysis. The oocyte numbers for K+in recordings in (H) were the same as described for (G). Error bars depict means ± se.
To test whether the SLAC1-mediated inhibition of KAT1 resulted from a mistargeting of KAT1 in oocytes, we analyzed the subcellular localization of KAT1. We observed a clear plasma membrane localization of KAT1 in oocytes either expressing KAT1-eGFP alone or coexpressing KAT1-eGFP and SLAC1 in a [cRNA] ratio of KAT1-eGFP/SLAC1 of either 1:1 or 1:2 (Supplemental Figure 1), suggesting that the presence of SLAC1 did not lead to any mistargeting of KAT1 in oocytes. We then conducted an immunoblot assay. The experimental results showed a clear increase of SLAC1 protein level and a stable KAT1 protein level upon changes of the [cRNA] ratio of KAT1/SLAC1 from 1:0.5 to 1:4 (Figure 3H), demonstrating that the expression of KAT1 in oocytes was not repressed by the expression of SLAC1. These results together demonstrate that SLAC1 inhibits KAT1 neither by repressing the expression of KAT1 nor by leading to any mistargeting of KAT1 in oocytes, leaving the protein-protein interaction the main cause for the inhibition of KAT1 by SLAC1.
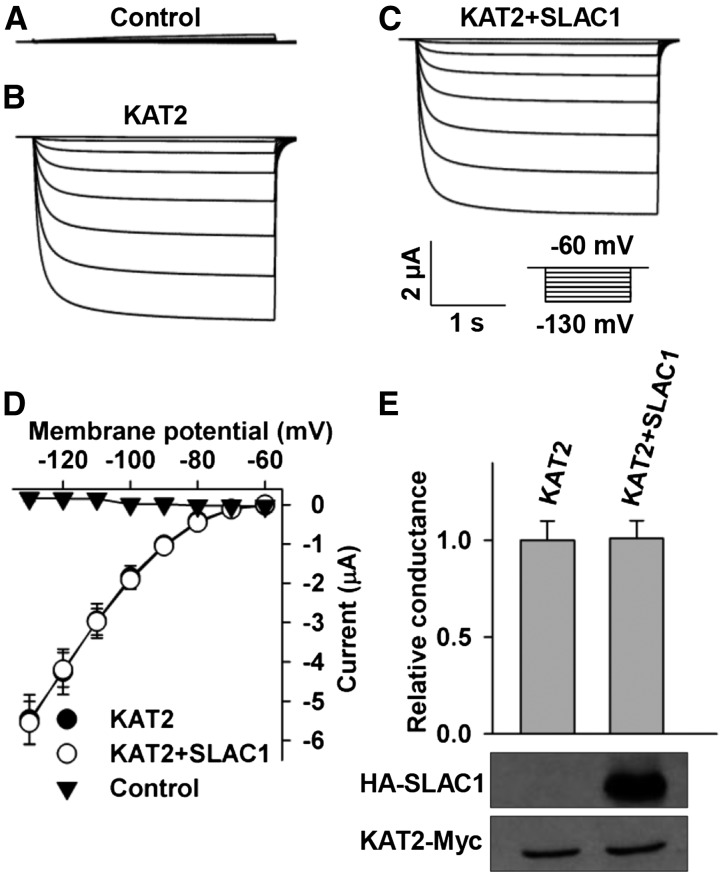
KAT2 is the closest homolog of KAT1 in the Shaker family with the highest level of amino acid sequence similarity. We therefore tested whether SLAC1 inhibits KAT2. We observed large K+in currents in positive control oocytes expressing KAT2 alone (Figures 4B, 4D, and 4E), which was in agreement with previous reports (Pilot et al., 2001; Lebaudy et al., 2010; Ronzier et al., 2014). We also observed large K+in currents in oocytes coexpressing KAT2 and SLAC1 in a [cRNA] ratio of KAT2/SLAC1 of 1:2 (Figure 4C), and the K+in currents of KAT2 were not significantly altered compared with the positive control (Figures 4B to 4E). Further analysis showed that neither the current amplitude nor the voltage threshold of KAT2 was markedly altered by SLAC1 (Figures 4D and 4E). We then conducted an immunoblot assay and found that the expression of KAT2 was not altered by SLAC1 (Figure 4E), demonstrating that the voltage-clamp results were reliable.
Figure 4.
SLAC1 Does Not Obviously Inhibit KAT2 in X. laevis Oocytes.
(A) to (C) Typical whole-oocyte recordings of negative control oocytes (A), positive control oocytes expressing KAT2 alone (B), and oocytes coexpressing KAT2 and SLAC1 with a [cRNA] ratio of KAT2/SLAC1 of 1:2.
(D) Average current-voltage curves of steady state whole-oocyte currents recorded in oocytes injected with water (n = 4), KAT2 cRNA (n = 6), and a cRNA mixture of KAT2 + SLAC1 (n = 6).
(E) Normalized macroscopic conductance and immunoblot analysis of the oocytes expressing KAT2 with and without SLAC1. The oocyte numbers for K+in recordings in (E) were the same as described for (D). Error bars depict means ± se.
Considering that we observed a strong interaction between SLAC1 and AKT2 (Figures 1 and 2), we also tested whether SLAC1 was capable of inhibiting AKT2. The voltage-clamp results showed that AKT2-mediated K+in currents were significantly inhibited, whereas the K+out currents were not obviously altered by SLAC1 (Supplemental Figure 2). We also analyzed whether KAT1 could alter the activity of SLAC1. We added 10 mM BaCl2 to the bath solution to block K+in currents and avoid any disturbance of anion currents by K+in currents. We observed similar S-type anion channel currents both in the presence and absence of KAT1 (Supplemental Figure 3), indicating that KAT1 had no obvious effect on the channel activity of SLAC1.
SLAH3 Inhibits KAT1 to a Lesser Extent Than Does SLAC1
SLAH3 is a homolog of SLAC1 and functions in guard cells as an anion channel (Geiger et al., 2011). We tested whether SLAH3 could inhibit KAT1. KAT1-mediated K+in currents were reduced significantly by ∼25% in oocytes coexpressing KAT1 and SLAH3 in a [cRNA] ratio of KAT1/SLAC1 of 1:2 compared with the positive control oocytes expressing KAT1 alone (Figures 5A to 5E), demonstrating a clear inhibition of KAT1 by SLAH3, but to a much smaller extent than in SLAC1 (Figure 3). We then conducted co-IP and immunoblot assays. The co-IP data showed a clear protein-protein interaction between KAT1 and SLAH3 (Figure 5F), and the immunoblot assay showed that the expression of KAT1 was not repressed by SLAH3 (Figure 5E). Thus, the co-IP and immunoblot data supported the notion that SLAH3 inhibited KAT1-mediated K+in currents by protein-protein interaction and not by repressing the expression of KAT1.
Figure 5.
SLAH3 Inhibits KAT1 Significantly, but to a Lesser Extent than Does SLAC1.
(A) to (C) Typical whole-oocyte recordings of negative control oocytes injected with water (A), positive control oocytes expressing KAT1 alone (B), and oocytes coexpressing KAT1 and SLAH3 in a [cRNA] ratio of KAT1/SLAH3 of 1:2 (C).
(D) Average current-voltage curves of steady state whole-oocyte currents recorded in the negative control (n = 5), positive control (n = 10), and oocytes coexpressing KAT1 and SLAH3 (n = 7).
(E) Normalized macroscopic conductance and immunoblot analysis. The oocyte numbers in (E) were the same as described for (D).
(F) Co-IP data showing protein-protein interaction between SLAH3 and KAT1. Error bars depict means ± se.
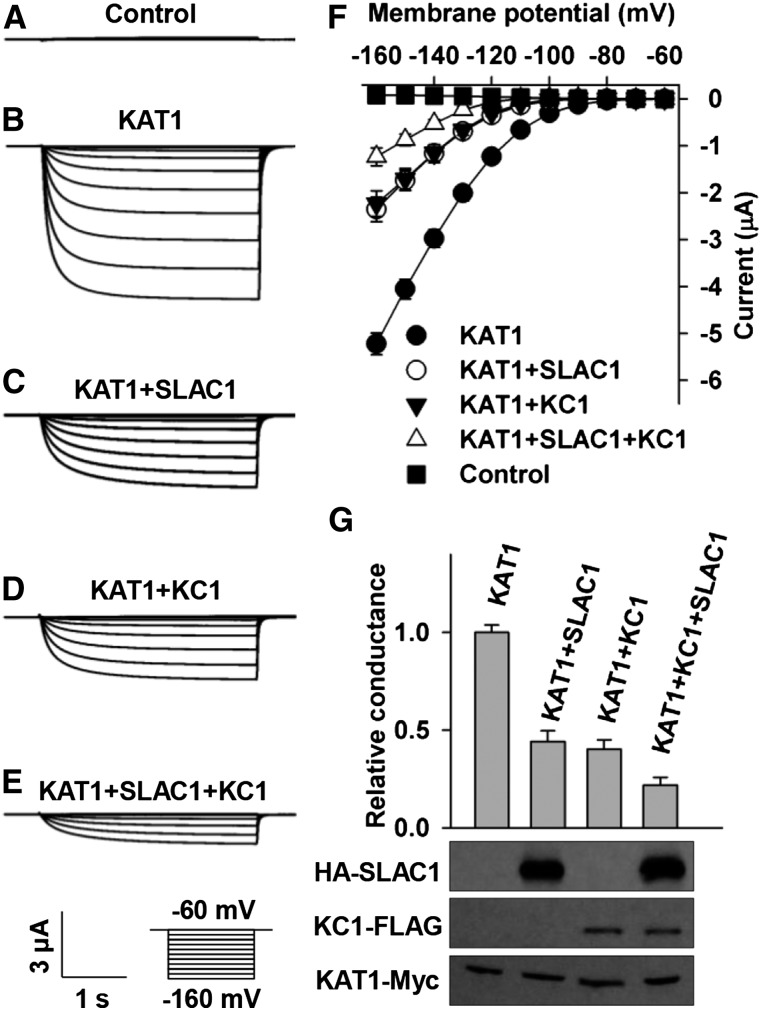
KC1 and SLAC1 Inhibit KAT1 through Different Mechanisms
KC1 has been reported as a general inhibitory regulating subunit of Shaker channels in plants (Geiger et al., 2009a; Jeanguenin et al., 2011). We tested whether SLAC1 and KC1 inhibited KAT1 independently considering the fact that KC1 also directly interacts with and inhibits KAT1. We observed KAT1-mediated large K+in currents in positive control oocytes expressing KAT1 alone (Figures 6B, 6F, and 6G). The inhibition of K+in currents by SLAC1 and KC1 was ∼55 and 60%, respectively, compared with the positive control (Figures 6C, 6D, 6F, and 6G). Interestingly, KAT1-mediated K+in currents were diminished by ∼80% in the presence of both SLAC1 and KC1 compared with the positive control (Figures 6B and 6E to 6G). This inhibition of KAT1 in the presence of both SLAC1 and KC1 was significantly stronger than the inhibition of KAT1 by either SLAC1 or KC1 alone (t test, P value = 0.010 for SLAC1 and 0.017 for KC1), indicating that the inhibition of KAT1 by SLAC1 and KC1 partially overlapped. To confirm these voltage-clamp results, we conducted an immunoblot assay and observed a stable protein level of KAT1 in either the presence or absence of SLAC1 and/or KC1 (Figure 6G), demonstrating that KC1 and SLAC1 inhibited KAT1-mediated K+in currents not by repressing the expression of KAT1. Together, these results suggest that SLAC1 and KC1 may use different mechanisms to inhibit KAT1.
Figure 6.
SLAC1 and KC1 Inhibit KAT1 in X. laevis Oocytes Independently.
(A) to (E) Typical whole-oocyte recordings of negative control oocytes injected with water (A), positive control oocytes expressing KAT1 alone (B), and oocytes injected with a mixture of cRNA of KAT1 and SLAC1 at a ratio of 1:2 (C), a mixture of cRNA of KAT1 and KC1 at a ratio of 1:1 (D), and a mixture of cRNA of KAT1, SLAC1, and KC1 at a ratio of 1:2:1 (E).
(F) Average current-voltage curves of steady state currents recorded in the negative control (n = 5), the positive control (n = 7), and oocytes injected with a cRNA mixture of KAT1 + SLAC1 (n = 5), KAT1 + KC1 (n = 5), and KAT1 + SLAC1 + KC1 (n = 5).
(G) Normalized macroscopic Shaker conductance and immunoblot analysis. The oocyte numbers for K+in recordings in (G) were the same as described for (F). Error bars depict means ± se.
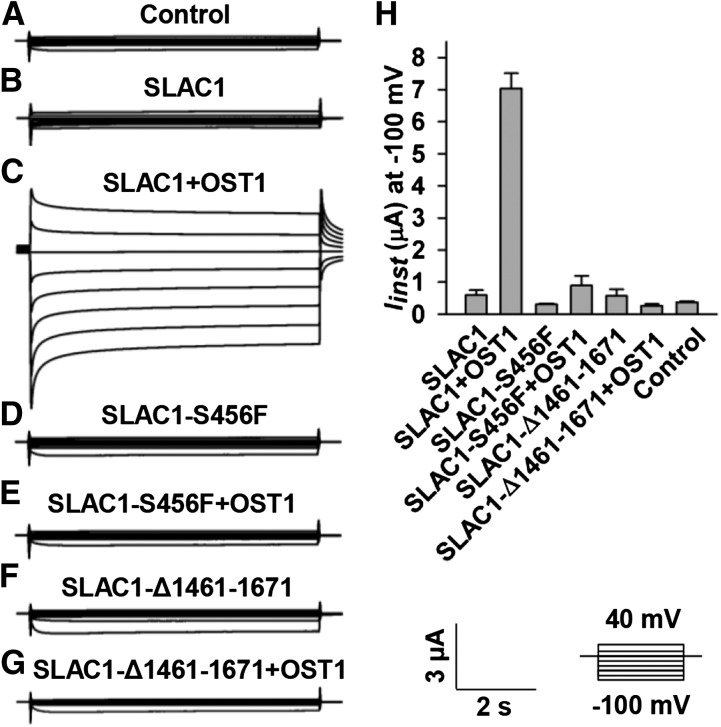
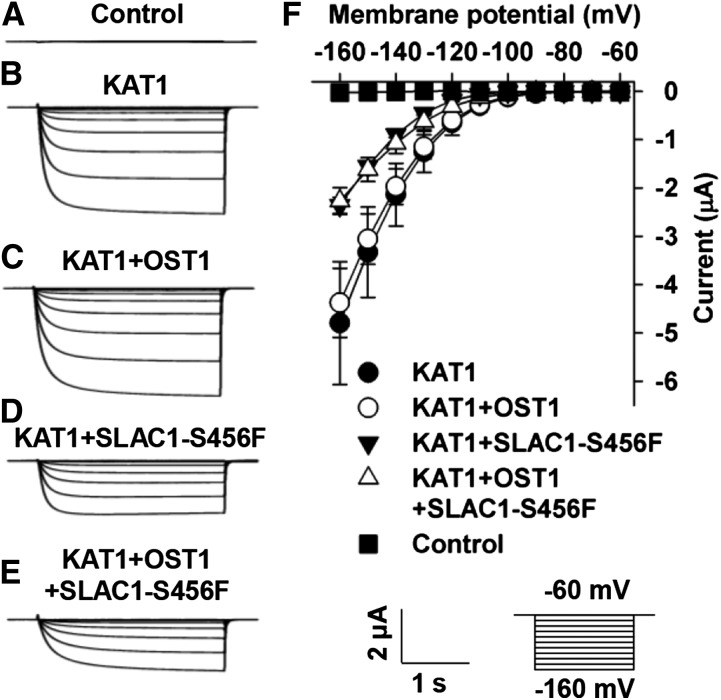
SLAC1-Mediated Inhibition of KAT1 Does Not Depend on the Function of SLAC1 as an Anion Channel
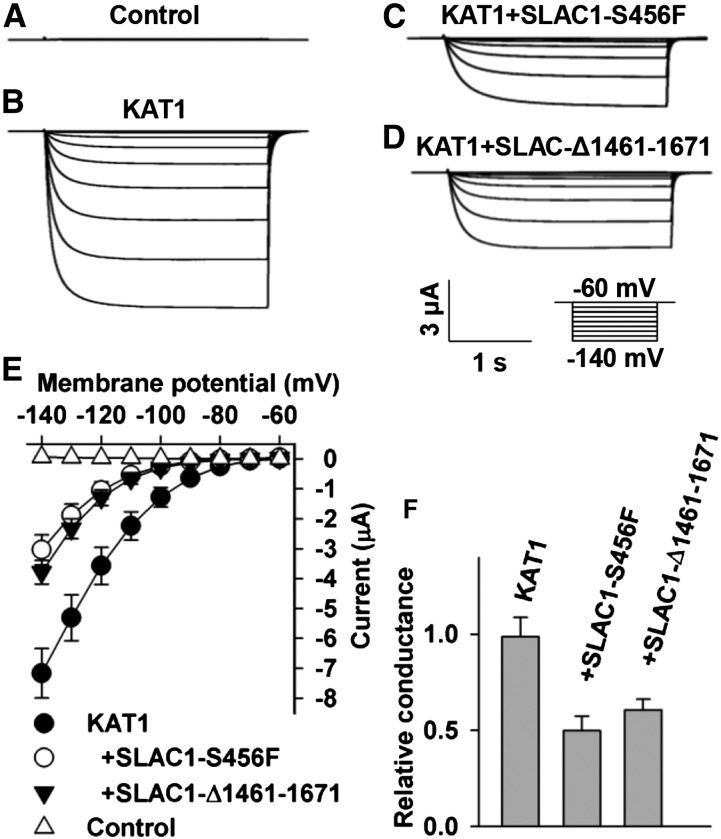
SLAC1 is an S-type anion channel (Negi et al., 2008; Vahisalu et al., 2008). We therefore tested whether the inhibition of KAT1 by SLAC1 depends on SLAC1’s function as an S-type anion channel. It has been reported that both a point-mutated version SLAC1-S456F in the slac1-1 mutant background and a truncated version SLAC1-Δ1461-1671 in the slac1-3 mutant background lost their function as an anion channel (Negi et al., 2008; Vahisalu et al., 2008). We therefore constructed a point-mutated SLAC1, SLAC1-S456F, and a truncated SLAC1, SLAC1-Δ1461-1671, in the pGEMHE vector and tested whether the two loss-of-function versions of SLAC1 could inhibit KAT1 in oocytes. We first tested the S-type anion channel activity of the two mutated versions of SLAC1. Large OST1-activated S-type anion currents of wild-type SLAC1 were observed (Figures 7C and 7H), but neither SLAC1-S456F nor SLAC-Δ1461-1671 exhibited any OST1-activated anion channel activity (Figures 7D to 7H). Thus, we confirmed the loss of function of the two mutated SLAC1 versions. We next coexpressed KAT1 with either SLAC1-S456F or SLAC1-Δ1461-1671 in oocytes and observed a significant reduction of KAT1-mediated K+in currents by ∼50% compared with the positive control oocytes expressing KAT1 alone (Figure 8). These data indicate that the loss-of-function SLAC1-S456F and SLAC1-Δ1461-1671 inhibited KAT1 to a similar extent as did wild-type SLAC1 (Figure 3). Thus, the inhibition of KAT1 by SLAC1 is independent of SLAC1’s function as an anion channel, leaving structural interaction as the main cause of the inhibition.
Figure 7.
SLAC1-S456F and SLAC1-Δ1461-1671 Show No S-Type Anion Channel Activity.
(A) to (G) Typical whole-oocyte recordings of control oocytes injected with water (A) and oocytes injected with the cRNA of SLAC1 alone (B), SLAC1 + OST1 (C), SLAC1-S456F alone (D), SLAC1-S456F + OST1 (E), SLAC1-Δ1461-1671 alone (F), and SLAC1-Δ1461-1671 + OST1 (G). The ratios of cRNA mixture in (C) and (E) to (G) were 1:1.
(H) Average instantaneous SLAC1 currents recorded at −100 mV. The number of oocytes tested was 5 for the control, SLAC1, SLAC1 + OST1, SLAC1-S456F, SLAC1-S456F + OST1, and SLAC1-Δ1461-1671, and four for SLAC1-Δ1461-1671 + OST1. Error bars depict means ± se.
Figure 8.
Loss-of-Function SLAC1-S456F and SLAC1-Δ1461-1671 Inhibit KAT1 Dramatically in X. laevis Oocytes.
(A) to (D) Typical whole-oocyte recordings of control oocytes injected with water (A) and oocytes expressing KAT1 alone (B), KAT1 + SLAC1-S456F (C), and KAT1 + SLAC1-Δ1461-1671 (D). The [cRNA] ratios of KAT1/SLAC1-S456F in (C) and KAT1/SLAC1-Δ1461-1671 in (D) were 1:2.
(E) Average current-voltage curves of whole-oocyte currents recorded in oocytes injected with water (n = 5), KAT1 cRNA (n = 7), a cRNA mixture of KAT1 + SLAC1-S456F (n = 6), and a cRNA mixture of KAT1 + SLAC1-Δ1461-1671 (n = 10).
(F) Relative macroscopic Shaker conductance and immunoblot analysis. The oocyte numbers for K+in recordings in (F) were the same as described for (E). Error bars depict means ± se.
To close stomata, SLAC1 needs to be phosphorylated by OST1 in the guard cells. We therefore tested whether the phosphorylation states of SLAC1 and KAT1 were involved in the SLAC1-mediated inhibition of KAT1. We used SLAC1-S456F in the experiments to avoid any disturbance of K+in currents by anion channel currents. The amplitudes of the K+in currents recorded in oocytes coexpressing KAT1+SLAC1-S456F or KAT1+SLAC1-S456F+OST1 were similar to each other (Figures 9D to 9F) and were significantly reduced to a similar extent compared with the positive control oocytes expressing KAT1 alone (Figures 9B and 9D to 9F). We also observed similar KAT1-mediated K+in currents in oocytes in the presence or absence of OST1 (Figures 9B, 9C, and 9F). These data demonstrate that the phosphorylation states of SLAC1 and KAT1 are not involved in the inhibition of KAT1 by SLAC1.
Figure 9.
SLAC1-Mediated Inhibition of KAT1 Was Independent of the Phosphorylation States of SLAC1, and OST1 Had No Obvious Effects on the Channel Activity of KAT1.
(A) to (E) Typical whole-oocyte recordings of oocytes injected with water (A) and the cRNA of KAT1 (B), KAT1 + OST1 (C), KAT1 + SLAC1-S456F (D), and KAT1 + OST1 + SLAC1-S456F (E).
(F) The average current-voltage curves of whole-oocyte currents and the oocyte numbers were 4 for the oocytes injected with water and the oocytes injected with the cRNA of KAT1, KAT1 + OST1, KAT1 + SLAC1-S456F, and KAT1 + OST1 + SLAC1-456F. Error bars depict means ± se.
Both N and C Termini of SLAC1 Are Capable of Inhibiting KAT1
To investigate which domain(s) of SLAC1 interacted with and inhibited KAT1, we conducted Y2H experiments. The experimental results showed that both the N and C termini of SLAC1 interacted with KAT1, and the N terminus was stronger than the C terminus for the inhibition of KAT1 (Figure 10A). We then pursued voltage-clamp experiments. The experimental results showed that the N and C termini of SLAC1 inhibited KAT1-mediated K+in currents by ∼50 and 25%, respectively, compared with positive control oocytes expressing KAT1 alone (Figures 10C to 10H). Moreover, the N terminus of SLAC1 inhibited KAT1-mediated K+in currents in a percentage similar to that of full-length SLAC1 and was stronger than the C terminus of SLAC1 (Figures 10C, 10D, and 10F to 10H). The Y2H data and voltage-clamp results were consistent with each other. These results demonstrate that both the N and C termini of SLAC1 are involved in the inhibition of KAT1.
Figure 10.
Both the N and C Termini of SLAC1 Are Capable of Interacting with and Inhibiting KAT1.
(A) Y2H data show protein-protein interaction between KAT1 and either SLAC1’s N terminus or SLAC1’s C terminus.
(B) to (F) Typical whole-oocyte recordings of oocytes injected with water (B) and the cRNA of KAT1 (C), KAT1 + full-length SLAC1 (D), KAT1+ SLAC1 N terminus (E), and KAT1 + SLAC1 C terminus (F).
(G) The average current-voltage curves of whole-oocyte currents of the oocytes injected with water (n = 5), the cRNA of KAT1 (n = 9), KAT1 + full-length SLAC1 (n = 7), KAT1 + SLAC1 N terminus (n = 9), and KAT1 + SLAC1 C terminus (n = 8).
(H) Normalized macroscopic Shaker conductance of the oocytes. Normalization was performed as described in Methods, and the numbers of oocytes tested were the same as described for (G). Error bars depict means ± se.
The C Terminus of KAT1 Is Essential for Its Inhibition by SLAC1
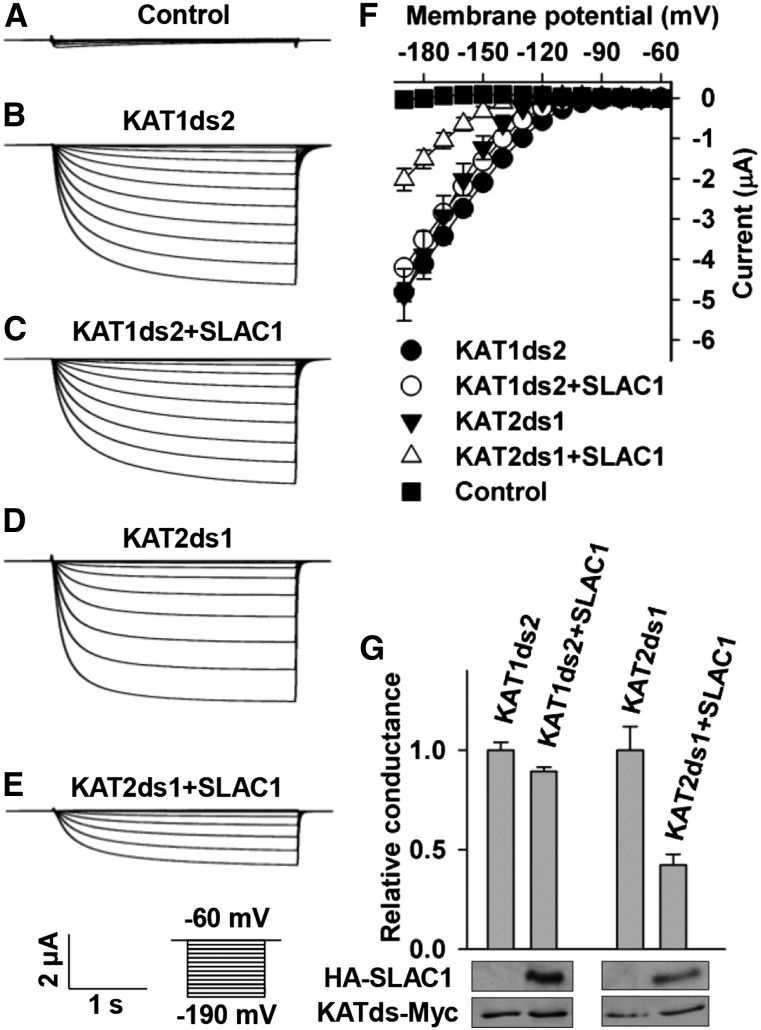
To find out which domain(s) of KAT1 was responsible for its inhibition by SLAC1, we aligned the amino acid sequences of KAT1 and KAT2. The alignment results showed that the major differences between KAT1 and KAT2 amino acid sequences were in the C termini (Supplemental Figure 4). We therefore hypothesized that the C terminus of KAT1 was the main domain to interact with SLAC1 for the SLAC1-mediated inhibition of KAT1. We then conducted domain swapping experiments by exchanging the C-terminal domain between KAT1 and KAT2. The chimeric KAT1 with a C terminus of KAT2 was termed KAT1ds2, while the chimeric KAT2 with a C terminus of KAT1 was termed KAT2ds1. We then pursued voltage-clamp experiments and observed large K+in currents in oocytes expressing either KAT1ds2 or KAT2ds1 alone (Figures 11B, 11D, 11F, and 11G), demonstrating that the two chimeric K+in channels have normal K+in channel activity as for wild-type KAT1 and KAT2 (Figures 3 and 4). We then coexpressed SLAC1 with either KAT1ds2 or KAT2ds1 in oocytes and found that SLAC1 reduced the K+in currents of KAT2ds1 significantly by ∼60% compared with the K+in currents recorded in oocytes expressing KAT2ds1 alone (Figures 11D to 11G) but failed to inhibit KAT1ds2-mediated K+in currents compared with the K+in currents recorded in oocytes expressing KAT1ds2 alone (Figures 11B, 11C, 11F, and 11G). We then performed an immunoblot assay and observed stable protein levels of KAT1ds2 and KAT2ds1 in the presence and absence of SLAC1 (Figure 11G), demonstrating that SLAC1 inhibited the K+in currents of KAT2ds1 not by repressing the expression of KAT12ds1. Together, these results demonstrate that the C terminus of KAT1 is essential for the inhibition of KAT1 by SLAC1.
Figure 11.
The C Terminus of KAT1 Plays an Essential Role in the SLAC1-Mediated Inhibition of KAT1.
(A) to (E) Typical whole-oocyte recordings of oocytes injected with water (A), the cRNA of KAT1ds2 (B), a cRNA mixture of KAT1ds2 + SLAC1 (C), the cRNA of KAT2ds1 (D), and a cRNA mixture of KAT2ds1 + SLAC1 (E).
(F) Average current-voltage curves of whole-oocyte currents recorded in the oocytes injected with water (n = 6) and the cRNA of KAT1ds2 (n = 6), KAT1ds2 + SLAC1 (n = 5), KAT2ds1 (n = 6), and KAT2ds1 + SLAC1 (n = 6).
(G) Normalized macroscopic Shaker conductance and immunoblot analysis. The oocyte numbers for K+in recordings in (G) were the same as described for (F). Error bars depict means ± se.
The Overexpression of Wild-Type SLAC1 and Truncated SLAC1 Dramatically Reduces K+in Currents of Arabidopsis Guard Cells
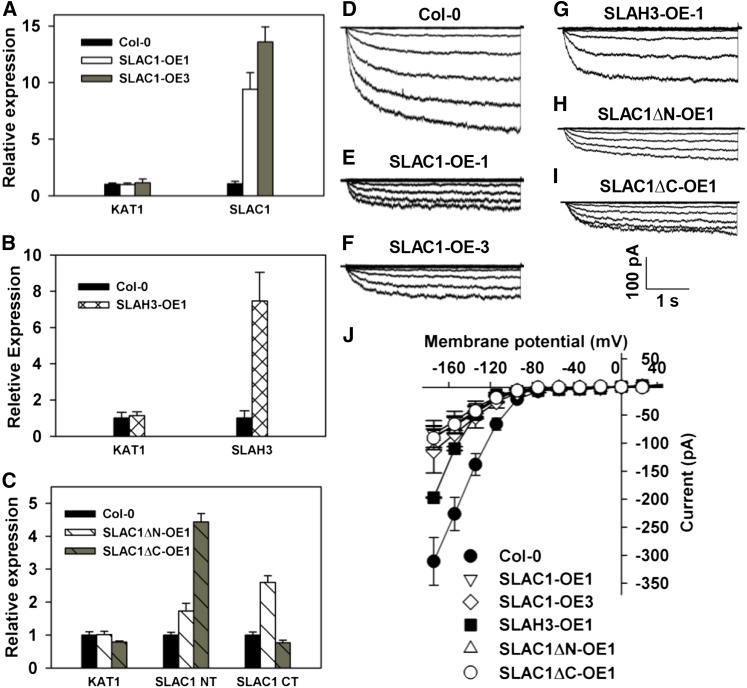
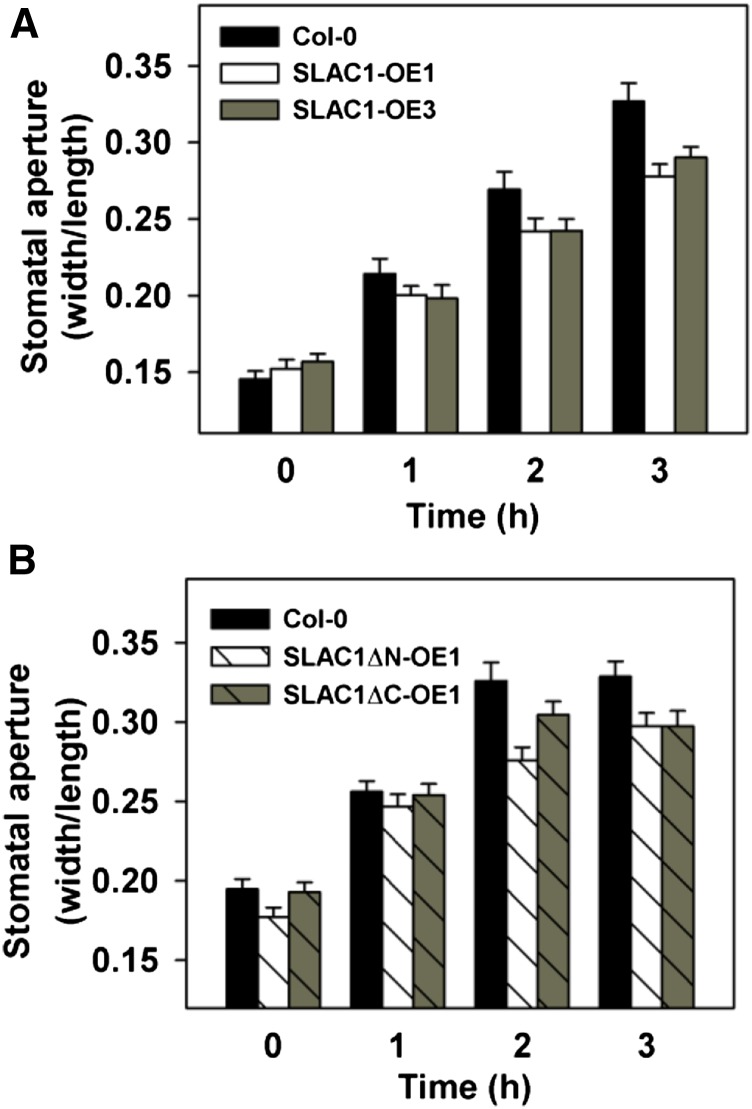
To test whether the overexpression of SLAC1 and SLAH3 leads to the inhibition of KAT1 in Arabidopsis guard cells, we generated transgenic Arabidopsis lines by overexpressing SLAC1 driven by a 35S promoter in the wild-type Columbia background. Two lines showing significant increases in SLAC1 expression were selected and were termed SLAC1-OE1 and SLAC1-OE3 (Figure 12A). We also generated transgenic lines by expressing SLAH3 in the Columbia wild-type background. One transgenic line was selected and was termed SLAH3-OE1 (Figure 12B). We performed patch-clamp experiments in the guard cell protoplasts of the three transgenic lines and Columbia wild type. Large whole-cell K+in currents were readily observed in the wild-type guard cell protoplasts (Figures 12D and 12J), while only much smaller K+in currents with a reduction of ∼70% compared with the wild type were observed in SLAC1-OE1 and SLAC1-OE3 (Figures 12D to 12F and 12J). The K+in currents of SLAH3-OE1 guard cells were significantly reduced by ∼35% compared with the Columbia wild type (Figures 12D, 12G, and 12J). The inhibition of K+in currents by SLAH3 was ∼50% less than the inhibition of K+in currents by SLAC1 (Figure 12). These data are consistent with the voltage-clamp results in X. laevis oocytes (Figures 3 and 5).
Figure 12.
K+in Currents of Arabidopsis Guard Cells Are Dramatically Reduced by the Overexpression of SLAC1 and SLAH3.
(A) to (C) qRT-PCR data show the overexpression of SLAC1 in transgenic lines SLAC1-OE1 and SLAC1-OE3 (A), SLAH3 in transgenic line SLAH3-OE1 (B), and SLAC1∆N in transgenic line SLAC1∆N-OE1 and SLAC1∆C in transgenic line SLAC1∆C-OE1 (C). Three replicates were conducted for qRT-PCR.
(D) to (I) Typical whole-cell K+in current recordings in Arabidopsis guard cell protoplasts of Columbia wild type (D) and transgenic lines SLAC1-OE1 (E), SLAC1-OE3 (F), SLAH3-OE1 (G), SLAC1∆N-OE1 (H), and SLAC1∆C-OE1 (I).
(J) Average current-voltage curves of steady state K+in currents recorded in the guard cell protoplasts of Columbia wild type (n = 11), SLAC1-OE1 (n = 9), SLAC1-OE3 (n = 8), SLAH3-OE1 (n = 5), SLAC1∆N-OE1 (n = 8), and SLAC1∆C-OE1 (n = 7). SLAC1-NT and SLAC1-CT denote the N terminus and the C terminus of SLAC1, respectively. Error bars depict means ± se.
We next generated transgenic Arabidopsis lines by overexpressing truncated SLAC1 with the N terminus deleted, but with the 10 transmembrane domains retained (termed SLAC1ΔN hereafter), or by expressing truncated SLAC1 with the C terminus deleted, but with the 10 transmembrane domains retained (termed SLAC1ΔC hereafter), under a 35S promoter. The 10 transmembrane domains could ensure a plasma membrane localization of the truncated SLAC1 in guard cells. We isolated four lines overexpressing SLAC1ΔN, which were termed SLAC1ΔN-OE1 to SLAC1ΔN-OE4. We also isolated five lines overexpressing SLAC1ΔC, which were termed SLAC1ΔC-OE1 to SLAC1ΔC-OE5. SLAC1ΔN-OE1 and SLAC1ΔC-OE1 were selected for whole-cell patch-clamp experiments in guard cell protoplasts. The patch-clamp results showed that the K+in currents of the guard cells of both SLAC1ΔN-OE1 and SLAC1ΔC-OE1 were significantly inhibited by ∼70% compared with Columbia wild type (Figures 12D and 12H to 12J). These results demonstrate that both the N and C terminuses of SLAC1 are capable of inhibiting K+in channel currents in vivo.
Together, these results strongly suggest that SLAC1 functions as an essential inhibitory regulator of K+in currents in Arabidopsis guard cells, while SLAH3 is involved in the inhibitory regulation of K+in currents of the guard cells, but to a lesser extent than is SLAC1.
Light-Induced Stomatal Opening Is Significantly Impaired by the Overexpression of Either Full-Length SLAC1 or Truncated SLAC1 in Arabidopsis
We next performed a light-induced stomatal opening assay using epidermal strips. The results showed that light-induced stomatal opening was impaired significantly by the overexpression of full-length SLAC1 in the transgenic Arabidopsis lines SLAC1-OE1 and SLAC1-OE3 (Figure 13A) and by the overexpression of the truncated SLAC1 in transgenic lines SLAC1ΔN-OE1 and SLAC1ΔC-OE1, compared with Columbia wild type (Figure 13B). These results demonstrate that the upregulation of the expression level of SLAC1 is essential for the inhibition of stomatal opening in Arabidopsis.
Figure 13.
The Upregulation of SLAC1 Inhibits Light-Induced Stomatal Opening.
(A) Light-induced stomatal opening was impaired in the transgenic lines SLAC1-OE1 and SLAC1-OE3 compared with the Columbia wild type.
(B) Light-induced stomatal opening was impaired in the transgenic lines SLAC1∆N-OE1 and SLAC1∆C-OE1 compared with the Columbia wild type. Error bars depict means ± se.
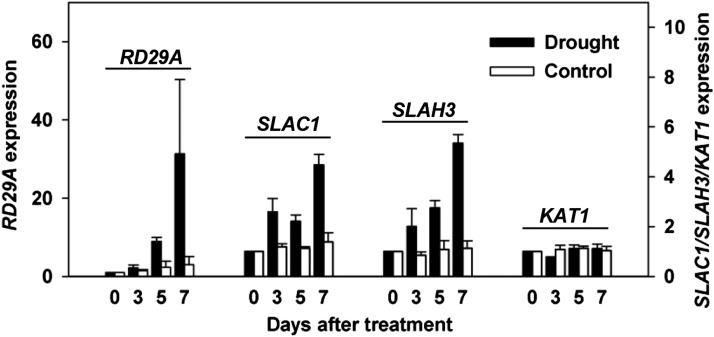
Drought Stress Induces a Significant Upregulation of the Expression of SLAC1 and SLAH3 in Arabidopsis Guard Cells
To test whether drought stress actually upregulates the expression of S-type anion channel genes, we tested the expression of a number of ion channel genes, including SLAC1, SLAH3, and KAT1, in guard cell-enriched isolates derived from blended leaves using qRT-PCR. Well-watered plants were used as a negative control, while RD29A was used as a reporter for drought stress. The experimental results showed a significant drought stress-induced upregulation of the expression of SLAC1 and SLAH3 by ∼2- to 4-fold in wild-type Arabidopsis guard cells (Figure 14), whereas the expression of KAT1 was not obviously upregulated (Figure 14). The method of blending leaves could greatly enrich the guard cells in the materials for qRT-PCR; however, this method cannot guarantee the removal of all other cells, including mesophyll cells, epidermal cells, and vasculature cells. In addition, the 35S promoter is not guard cell specific. We therefore could not absolutely exclude the effects of those cells on the qRT-PCR results. However, considering the guard cell-specific expression of SLAC1 (Negi et al., 2008) and the strong upregulation of the expression of SLAC1 and SLAH3 (∼4-fold), we are confident that the qRT-PCR data reflect the expression levels of the ion channels in guard cells.
Figure 14.
Drought Stress Induces the Upregulation of the Expression of SLAC1 and SLAH3, but Not KAT1, in Arabidopsis Guard Cells.
Three biological replicates were conducted.
DISCUSSION
A Novel and “Smart” Molecular Mechanism for the Inhibition of KAT1 and Stomatal Opening in Arabidopsis
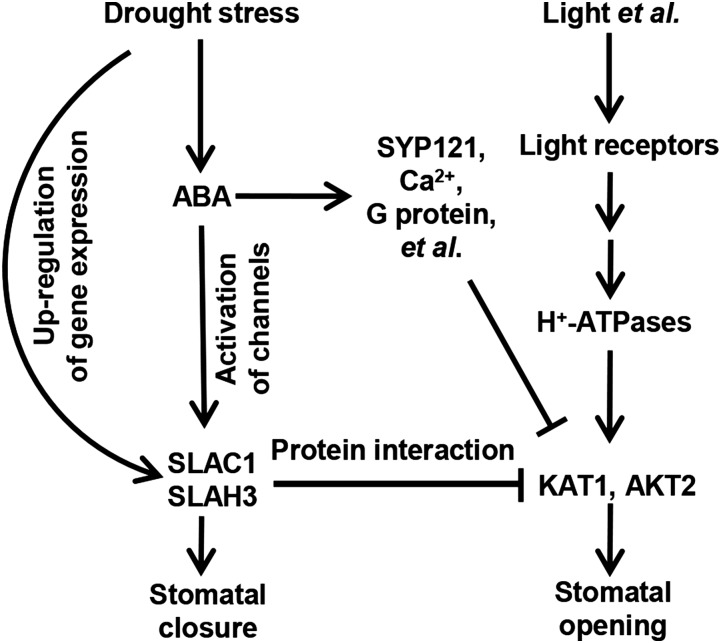
KAT1 is regulated by multiple regulators. ABA triggers the endocytosis of KAT1, which further leads to the inhibition of K+in channel currents in Arabidopsis guard cells, and SNAREs play essential roles in the endocytosis of KAT1 (Mikosch et al., 2006; Sutter et al., 2007; Eisenach et al., 2012; Zhang et al., 2015). High cytosolic Ca2+ has been reported to inhibit K+in channels (Schroeder and Hagiwara, 1989). ABA needs to first trigger the production of reactive oxygen species to activate Ca2+ channels, which mediate the influx of external Ca2+ to increase the cytosolic Ca2+ concentration (Pei et al., 2000; Allen et al., 2001, 2002). It has also been reported that KAT1 can be inhibited through phosphorylation by kinases and that the C terminus of KAT1 is the main targeting site for this phosphorylation event (Mori et al., 2000; Sato et al., 2009, 2010; Acharya et al., 2013). All these diverse regulators are involved in KAT1 regulation, which may be slow and consume energy. In this study, we discovered a novel molecular mechanism for the inhibition of stomatal opening by ABA (Figure 15). In this mechanism, the signaling pathways for stomatal opening and closure are directly coupled by the protein-protein interaction between S-type anion channels and K+in channels (Figure 15). When plants are stressed by drought, ABA triggers several signaling branches. One branch is the activation of SLAC1 and SLAH3 through the well-known signaling pathway, ABA-ABA receptors-phosphatases-protein kinases-anion channels, to close stomata (Figure 15). In this branch, ABA may use the SLAC1 and SLAH3 proteins synthesized before the onset of drought stress. The second branch is the upregulation of the expression of SLAC1 and SLAH3, and the third branch is the inhibition of KAT1 by SLAC1 and SLAH3 (Figure 15). The de novo-synthesized anion channels through the second branch can join the first branch to be activated by protein kinases to accelerate and facilitate the efflux of anions and hence lead to stomatal closure. The accumulated SLAC1 and SLAH3 simultaneously inhibit K+in channels to prevent stomatal opening by protein-protein interaction (Figure 15). This mechanism seems quite “smart” because the signaling pathways for stomatal closure and opening are coupled directly, which makes both stomatal closure and the inhibition of stomatal opening more efficient. Furthermore, the inhibition of KAT1 by SLAC1 and SLAH3 is independent of the functions of SLAC1 and SLAH3 as anion channels. Therefore, this direct coupling mechanism allows ABA and drought stress to close stomata and prevent stomatal opening more efficiently and in a more energy-efficient way relative to the multistep mechanisms. Nevertheless, guard cells may integrate different mechanisms for a precise regulation of stomatal movement. There are still remaining questions to be addressed. For example, it remains to be determined how SLAC1 and SLAH3 find and inhibit KAT1 in the plasma membrane of Arabidopsis guard cells in a situation where the protein-protein interaction is required. One possibility is that SLAC1 and SLAH3 may diffuse around to catch KAT1, probably with the help of unknown partners. SLAC1 can interact with KC1, KAT2, and AKT2. These three K+in channels could be the partners of SLAC1 and could function as mediators to recruit SLAC1 and KAT1 by protein-protein interaction with both sides, so that SLAC1 and KAT1 have a greater chance of coming in contact. In addition, the accumulation of SLAC1 and SLAH3 could also increase the protein density in the plasma membrane of guard cells to make it easier for SLAC1 and SLAH3 to come in contact with KAT1.
Figure 15.
Schematic Model of the S-Type Anion Channel- and K+in Channel-Mediated Regulatory Network for Stomatal Movement.
Drought stress triggers the production of ABA, which in turn triggers three signaling branches, including the upregulation of SLAC1, the activation of anion channels SLAC1 and SLAH3, and the inhibition of K+in channels KAT1 and AKT2 and stomatal opening. SLAC1 and SLAH3 play dual roles of mediating anion efflux and inhibiting K+in currents. Arrows and T-bars represent positive and negative regulation, respectively.
SLAC1 and SLAH3 Inhibit KAT1 by Affecting the Regulatory Nature of the C Terminus of KAT1, a Mechanism Different from That of KC1
KC1 functions in plant cells as a general inhibitory regulator of Shaker K+ channels by integrating itself into tetramers as a subunit of the K+in channels (Jeanguenin et al., 2011). Shaker channels have six transmembrane domains for each subunit (Anderson et al., 1992) and assemble as homo- or heterotetramers (Ward et al., 2009; Kim et al., 2010; Dreyer and Uozumi, 2011; Hedrich, 2012; Kollist et al., 2014). On the other hand, SLAC1 has 10 transmembrane domains for each subunit (Negi et al., 2008; Vahisalu et al., 2008) and assembles as a trimer (Chen et al., 2010). Since the topological structures of SLAC1 and Shaker channels are quite different, it seems unlikely that SLAC1 integrates itself, like KC1, into K+in channels as a regulatory subunit. Indeed, only partial overlap of the inhibition of KAT1 by SLAC1 and KC1 (Figure 6) indicates that SLAC1 and KC1 regulate the activity of KAT1 probably through different mechanisms. Furthermore, the partial overlap in the inhibitory effects of SLAC1 and KC1 on KAT1 (Figure 6) also implies that SLAC1 and KC1 may affect each other in the inhibition of KAT1. In other words, KC1 may be involved in and amplify the inhibitory effects of SLAC1 on KAT1 (see below for more discussion).
Shaker channels are K+ selective, and their ion selectivity is conferred by the pore-surrounding helixes and P loops of each subunit in the tetramers (Lebaudy et al., 2007; Szczerba et al., 2009; Dreyer and Uozumi, 2011). On the other hand, the SLAC1/SLAH family is known as the anion channel family, and its members show different anion selectivity. SLAC1 and SLAH3 are permeable to both nitrate and chloride, whereas SLAH2 is nitrate specific without obvious permeability to chloride. Structural analysis revealed that the ion selectivity of SLAC1 may be conferred by its own topological structure (Chen et al., 2010). Recent research showed that the selectivity of SLAC/SLAH is determined by the polarity of pore-lining residues, which are localized in the alpha helix 3 (Maierhofer et al., 2014). Nevertheless, considering the structural difference between the two types of channels, it seems that S-type anion channels may not be able to alter the topological structures of Shaker members and vice versa. This may account for the observation that the inhibition of KAT1 by SLAC1 is independent of SLAC1’s function as an anion channel (Figures 8, 9, and 12); similarly, KAT1 has no effect on the anion channel activity of SLAC1 (Supplemental Figure 3).
The C terminus of KAT1 and the N terminus of SLAC1 are regulatory domains (Marten and Hoshi, 1997, 1998; Vahisalu et al., 2010; Brandt et al., 2012; Maierhofer et al., 2014). The interaction between SLAC1 and the C terminus of KAT1 may affect the regulatory functions of the C terminus of KAT1, including open probability (Supplemental Figure 6) and voltage sensitivity, and may further lead to the inhibition of KAT1. Therefore, the N terminus of SLAC1 may simultaneously play dual roles for the regulation of SLAC1 itself and KAT1. In addition, the C terminus of SLAC1 is also capable of inhibiting KAT1, but to a weaker extent (Figure 10). Both N and C termini of SLAC1 may coordinate to strengthen the protein interaction with KAT1 for the inhibition of K+in currents.
The C-terminal amino acid sequences of AKT2 are similar to KAT1 (Supplemental Figure 4). This similarity may account for the inhibition of AKT2-mediated K+in currents by SLAC1 (Supplemental Figure 2). However, the K+out currents may be from AKT2 and/or native channels of the oocytes. Thus, it is unclear why the K+out currents were not altered by SLAC1 in oocytes.
Different Molecular Mechanisms Underlie the SLAC1-Mediated Inhibition of K+in Channels in Arabidopsis Guard Cells
K+in currents were reduced by SLAC1 by ∼50% in oocytes and over 70% in guard cells. The stronger inhibition of K+in currents in guard cells relative to the inhibition of K+in currents in oocytes by SLAC1 might result from higher protein ratio between SLAC1/SLAH3 and KAT1 than in oocytes because our qRT-PCR data showed that the expression of SLAC1 and SLAH3 was upregulated strongly by drought stress. Second, K+in channels must be homotetramers in oocytes expressing either KAT1 or AKT2 alone because of the absence of other Shaker members. However, most K+in channels in guard cells are expected to be heterotetramers because of the presence of diverse Shaker members. The heterotetramers can be inhibited by SLAC1 and SLAH3 if they contain one or more KAT1 subunits. The heterotetramers also have a greater chance of being inhibited by SLAC1 if they contain KAT1 and other Shaker members, such as AKT2, KC1, and KAT2, which could interact with SLAC1, so that SLAC1 could be recruited to further interact with KAT1 and inhibit the tetramers. Thus, the inhibitory effects of SLAC1 on K+in currents could be amplified. In this situation, the formation of K+in channels as heterotetramers is an important regulating mechanism for K+in channels, and SLAC1 could function more efficiently as negative regulators of the Shaker K+in channels based on this formation of the tetramers.
We reported previously that the mutations in SLAC1 led to a strong inhibition of K+in currents by ∼80% in Arabidopsis guard cells and that stomatal opening was also impaired in slac1-1 and slac1-3 mutants under well-watered conditions (Laanemets et al., 2013). Another group reported a similar phenotype in slac1-1 mutant (Wang et al., 2012). In this research, a novel regulating mechanism for K+in channels is revealed. It seems surprising that both loss-of-function mutations and overexpression of SLAC1 would inhibit K+in channels and stomatal opening in Arabidopsis. Considering that the strong inhibition of K+in currents is independent of SLAC1’s function as an anion channel in both oocytes and Arabidopsis guard cells (Figures 8, 9, and 12), we propose that the mutations in SLAC1 could induce the overexpression of loss-of-function SLAC1, including the point-mutated version and the truncated SLAC1 as a feedback. This is because the stomata do not close in the presence of drought stress and ABA in slac1-1 and slac1-3 mutants, and plants may perceive the expression level of SLAC1 as being low. Therefore, plants may try to restore anion efflux by upregulating the expression of anion channel genes. The accumulation of the loss-of-function SLAC1 could further result in the inhibition of K+in currents. To test the hypothesis, we pursued qRT-PCR experiments repeatedly but failed to observe any obvious upregulation of the mutated SLAC1 in the Arabidopsis mutants slac1-1 and slac1-3 (Supplemental Figure 5). Therefore, it seems that different molecular mechanisms in the Arabidopsis slac1-1 and slac1-3 mutants mediate the inhibition of K+in currents of guard cells. Cytosolic Ca2+ is known as a negative regulator of K+in channels in guard cells (Schroeder and Hagiwara, 1989). In fact, we reported previously that the decrease of cytosolic Ca2+ concentration partially released the inhibition of K+in currents in slac1-1 and slac1-3 mutants (Laanemets et al., 2013), indicating a signaling connection between Ca2+ signaling and K+in channels through SLAC1. In addition, it has been reported that the elevation of cytosolic pH as a result of the mutation in SLAC1 was a cause of the inhibition of K+in channels in Arabidopsis guard cells (Blatt and Grabov, 1997; Wang et al., 2012). Considering the involvement of other factors, such as G proteins and SNAREs, it seems that the mechanisms that regulate K+in channels in guard cells are quite complex, and diverse regulators and molecular mechanisms may be involved in this process.
It has been proposed that K+in currents of guard cells need to be completely inhibited to impair stomatal opening in Arabidopsis (Szyroki et al., 2001). K+in currents were strongly inhibited in the knockout mutant kat1, while light-induced stomatal opening was not impaired (Szyroki et al., 2001). On the other hand, a similar reduction in K+in currents of Arabidopsis guard cells has been observed in dominant-negative KAT1 mutants in the wild-type background and dominant-negative KAT1 mutants in the knockout mutant kat2 background, and stomatal opening was impaired in these mutants (Kwak et al., 2001; Lebaudy et al., 2008). The strong inhibition of K+in currents by the loss-of-function mutations (Wang et al., 2012; Laanemets et al., 2013) and the overexpression of SLAC1 in this research revealed two more mechanisms that regulate K+in channels and stomatal opening. Thus, it seems that the regulation of K+in channels in guard cells is a complex process and that K+in currents need not be completely blocked to impair stomatal opening, as different molecular mechanisms are involved. Further research is needed to reveal the signaling network underlying K+in channel regulation and stomatal movements.
METHODS
In Vitro Transcription and Gene Expression in Xenopus laevis Oocytes
For K+in current recordings in oocytes, the coding sequences of KAT1, KAT2, KC1, KAT1ds2, KAT2ds1, SLAC1, SLAH3, SLAC1-S456F, SLAC1-Δ1461-1671, SLAC1-NT, and SLAC1-CT were cloned into pGEMHE as described previously (Liman et al., 1992; Xu et al., 2006). SLAC1-NT and SLAC1-CT denote the N terminus and the C terminus of SLAC1, respectively. For anion current recordings in oocytes, the coding sequences of SLAC1, SLAC1-S456F, and SLAC1-Δ1461-1671 were cloned into the pGEMHE-YFPC vector, and OST1 was cloned into the pGEMHE-YFPN vector, as described previously (Geiger et al., 2009b). The N terminus, transmembrane domains, and C terminus of SLAC1 refer to the Met1-Phe188, Pro189-Phe498, and Val499-His556 peptides, respectively (Chen et al., 2010). The C terminus of KAT1 refers to the His301-Asn677 peptide, whereas the C terminus of KAT2 refers to the His301-Ser697 peptide (Uozumi et al., 1998). The cRNAs were transcribed in vitro using the T7 RiboMAX large-scale RNA production system (Promega). Oocytes were isolated from X. laevis, and each oocyte was injected with 20 ng cRNA. The oocytes were incubated in ND96 solution at 16°C for 2 to 3 d after injection (Xu et al., 2006), and ion currents were recorded after incubation in a bath solution as described below.
Two-Electrode Voltage-Clamp Recordings in X. laevis Oocytes
Whole-oocyte voltage-clamp experiments were performed 2 to 3 d after cRNA injection using two-electrode voltage-clamp equipment with an Axoclamp 900A amplifier connected to a 1440A interface (Axon Instruments) at room temperature (∼20°C). Glass pipettes were prepared using a glass capillary puller (model PC-10; Narishige). The glass microelectrodes were filled with 3 M KCl as pipette solution. Anion currents were recorded using Kulori-based solutions containing 1 mM CaCl2, 1 mM MgCl2, 2 mM KCl, 24 mM NaCl, 70 mM Na-gluconate, and 10 mM MES-Tris (pH 5.6) as described previously (Geiger et al., 2009b). For K+in current recordings, bath solution contained 96 mM KCl, 1.8 mM MgCl2, 1.8 mM CaCl2, and 10 mM HEPES-NaOH (pH 7.2) as described previously (Xu et al., 2006). Whole-oocyte currents were filtered using a 20-MHz low-pass filter, digitized through a Digidata 1440A, and saved on a hard drive of a personal computer. Clampex10.2 software (Axon Instruments) was used for data acquisition and analysis. For normalization of K+in currents, the conductance of each oocyte was divided by the mean conductance of oocytes expressing KAT1 or KAT2 alone as described previously (Jeanguenin et al., 2011).
Yeast Two-Hybrid Assay
The coding sequences of SLAC1 were amplified using gene-specific primers (forward, 3′-ACAAGTTTGTACAAAAAAGCAGGCTCTCCAACCACCATGGAGAGGAAACAGTCAAA-5′, and reverse, 3′-TCCGCCACCACCAACCACTTTGTACAAGAAAGCTGGGTAGTGATGCGACTCTTCCT-5′). For CubPLV fusions, pMetYC gate was cleaved with PstI/HindIII and used together with PCR products to cotransform THY.AP4. Transformants were selected on SC (Selenite Cystine Broth) lacking Leu. Several clones from THY.AP4 transformants were transferred and incubated in liquid SC media (-Leu), and yeast plasmids were extracted. The yeast plasmids were then transformed into Escherichia coli, and DNA sequences of the constructs (SLAC1-Cub) were confirmed by sequencing. KAT2 and OST1 were cloned into the vector pNXgate32-3HA using EcoRI/SmaI (Nub-KAT2 and Nub-OST1). N-KAT1-3HA (Nub-KAT1) was prepared using the mbSUS system (Obrdlik et al., 2004). For Y2H assays, SLAC1-Cub was cotransformed with Nub-KAT1, Nub-KAT2, Nub-OST1, or empty Nub vector into the yeast strain THY.AP4 using the lithium acetate transformation method (Obrdlik et al., 2004). To select positive clones, the transformants were selected on SD/-Leu-Trp medium and then streaked on selective medium (SD/-Leu-Trp-His-Ade).
BiFC Assay in Nicotiana benthamiana Epidermal Cells
The coding regions of OST1 and KAT1 were cloned into pSPYNE(R)173 using BamHI/SalI sites, and the coding region of SLAC1 was cloned into pSPYCE(MR) using the same sites (Waadt et al., 2008). KAT2 and AKT1 were cloned into pSPYNE(MR) using BamHI/XhoI and XbaI(SpeI)/SalI sites, respectively. All the plasmids were introduced into Agrobacterium tumefaciens GV3101 and coinfiltrated at OD600 = 0.6 to ∼0.8. The YFP fluorescence of the N. benthamiana leaves was observed 2 to 4 d after the infiltration. The images were acquired using a Zeiss LSM510 META confocal microscope (Carl Zeiss).
Immunoblot Analysis and Co-IP Experiments
For immunoblots in oocytes, the coding sequences of KAT1, KAT2, KAT1ds2, KAT2ds1, and KC1 were fused upstream of Myc or FLAG as indicated, while SLAC1 and SLAH3 were fused downstream of HA in the pGEMHE vector. Total oocyte membrane protein was extracted using extraction buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.5 mM EDTA, 1% SDS, 0.5 mM PMSF, and protein inhibitor [Roche]) (Liu et al., 2008). Oocyte homogenate was centrifuged at 12,000g for 3 min at 4°C, and the supernatant was used for immunoblot assay. To determine the protein levels, antibodies for Myc (Millipore), HA (Sigma-Aldrich), and FLAG (Sigma-Aldrich) were used in immunoblots.
For co-IP experiments, total membrane proteins were extracted with lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5 mM PMSF, and protein inhibitor [Roche]). Oocyte homogenates were incubated at 4°C for 1 h and were centrifuged at 12,000g for 3 min at 4°C. Anti-HA agarose (Sigma-Aldrich) was incubated with the supernatant for 5 h at 4°C. The co-IP products were washed gently five times using washing buffer, which contained 150 mM NaCl, 1 mM EDTA, 0.1% Triton X-100, 0.5 mM PMSF, protein inhibitor (Roche), and 20 mM Tris-HCl (pH 7.5). The chemiluminescence signal was detected using autoradiography.
Plant Growth and Isolation of Arabidopsis thaliana Guard Cell Protoplasts
Arabidopsis plants were grown in a controlled growth room with a cycle of 16 h light and 8 h dark at 22°C. Leaves of 3- to 4-week-old plants were used to isolate guard cell protoplasts. Epidermal strips were manually peeled from the leaves using a pair of tweezers and were enzymatically digested for ∼16 h at 23°C in a shaker with a shaking speed of 60 rpm, and guard cell protoplasts were collected by centrifugation as described (Pei et al., 1997; Vahisalu et al., 2008).
Whole-Cell Patch-Clamp Experiments and Data Analysis
For whole-cell K+in current recordings using the patch-clamp technique in Arabidopsis guard cell protoplasts, the bath solution contained 30 mM KCl, 1 mM CaCl2, 2 mM MgCl2, and 10 mM MES-Tris, pH 5.8, and the pipette solution contained 30 mM KCl, 70 mM K-glutamate, 2 mM MgCl2, 6.7 mM EGTA, 3.35 mM CaCl2, 5 mM ATP, and 10 mM HEPES-Tris, pH 7.1. Glass pipettes were prepared using a glass capillary puller (model PC-10; Narishige) and a microforge (model MF-830; Narishige). Whole-cell patch-clamp experiments were performed using an Axopatch 200B amplifier (Axon Instruments) equipped with an inverted microscope (model A1; Carl Zeiss) and connected to a computer through a 1440A interface (Axon Instruments). Whole-cell K+in currents were recorded 3 min after accessing to a whole-cell configuration, and the membrane voltage with a 4-s duration for each voltage was stepped from −180 to +40 mV in a +20-mV increment as indicated. Seals were >10 GΩ, and a liquid junction potential of −5.7 mV was measured and corrected as described previously (Ward and Schroeder, 1994). No leak subtractions were applied.
Stomatal Opening Assay
Epidermal strips were peeled manually from the leaves of 2- to 3-week-old plants and incubated for 3 h in opening buffer in darkness as described previously (Vahisalu et al., 2008). Epidermal strips were then exposed to light for a certain period of time as indicated. The stomatal apertures were then measured using an inverted microscope (model D1; Carl Zeiss) equipped with a neo CCD camera (Andor Technology). Image J was used to measure stomatal apertures.
qRT-PCR
Rosette leaves of 3-week-old plants were blended using a commercial blender in deionized water three times for 5 s each to remove most of the other types of cells, including mesophyll cells, vasculature cells, and some epidermal cells. The materials derived from blended leaves and greatly enriched in guard cells were collected using a nylon mesh (pore size, 100 μm). Total RNA was extracted from the guard cell-enriched isolates using Trizol reagent (Invitrogen), and cDNA was synthesized from RNA with TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix. qRT-PCR experiments using SYBRGreen were performed using TransStart Top Green qPCR SuperMix (Transgen) on a Bio-Rad CFX Connect real-time PCR system according to the manufacturer’s protocols as described previously (Wang et al., 2013). Quantification of the relative gene expression was achieved by normalization to ACT2. Primers are listed in Supplemental Table 1.
Accession Numbers
Sequence data from this article can be found in GenBank database under accession numbers AT1G12480 (SLAC1), AT5G24030 (SLAH3), AT5G46240 (KAT1), AT4G18290 (KAT2), AT2G26650 (AKT1), AT4G22200 (AKT2), AT4G32650 (KC1), AT4G33950 (OST1), AT5G52310 (RD29A), and AT3G18780 (ACTIN2).
Supplemental Data
Supplemental Figure 1. KAT1 localizes in the plasma membrane of oocytes in either presence or absence of SLAC1.
Supplemental Figure 2. AKT2-mediated K+in currents were inhibited by SLAC1 in oocytes.
Supplemental Figure 3. KAT1 had no effects on SLAC1-mediated anion channel currents.
Supplemental Figure 4. Amino acid sequence alignment among KAT1, KAT2, and AKT2.
Supplemental Figure 5. Expression levels of full-length, point-mutated, and truncated SLAC1 were not altered in slac1-1 and slac1-3 mutants compared with the wild type.
Supplemental Figure 6. The relative open probability of KAT1 was inhibited by the presence of SLAC1 in oocytes.
Supplemental Table 1. Primers for qRT-PCR experiments.
Acknowledgments
We thank Hongtao Liu (SIPPE, CAS, China) and Peng Zhang (SIPPE, CAS, China) for suggestions and assistance with immunoblotting and the co-IP assay. The mbSUS system for Y2H was obtained from the ABRC. pSPYCE(MR) and pSPYNE(R)173 were provided by Wei-Hua Wu’s group (China Agricultural University, Beijing, China). This work was supported by the National Basic Research Program of China (973 program; Grant 2012CB114300), by the National Natural Science Foundation of China (31170227), and by the Hundred Talents Program of the Chinese Academy of Sciences (2010OHTP06).
AUTHOR CONTRIBUTIONS
G.-N.Q. observed the inhibition of KAT1 by SLAC1 in X. laevis oocytes, which was confirmed by A.Z. and F.-Y.Y. A.Z. and S.-J.S. performed the immunoblot and co-IP experiments. H.-M.R. conducted the Y2H and BiFC experiments. Y.-Q.T. conducted the stomatal assay. G.-L.W. and L.-W.Y. provided assistance with the plant growth, vector construction, and project management. Y.-F.W. proposed this project and designed the experiments. Y.-F.W., A.Z., and J.H. wrote the manuscript.
Glossary
- ABA
abscisic acid
- Y2H
yeast two-hybrid
- BiFC
bimolecular fluorescence complementation
- co-IP
coimmunoprecipitation
References
- Acharya B.R., Jeon B.W., Zhang W., Assmann S.M. (2013). Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol. 200: 1049–1063. [DOI] [PubMed] [Google Scholar]
- Ache P., Becker D., Ivashikina N., Dietrich P., Roelfsema M.R., Hedrich R. (2000). GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K(+)-selective, K(+)-sensing ion channel. FEBS Lett. 486: 93–98. [DOI] [PubMed] [Google Scholar]
- Allen G.J., Chu S.P., Harrington C.L., Schumacher K., Hoffmann T., Tang Y.Y., Grill E., Schroeder J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057. [DOI] [PubMed] [Google Scholar]
- Allen G.J., Murata Y., Chu S.P., Nafisi M., Schroeder J.I. (2002). Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell 14: 1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.A., Huprikar S.S., Kochian L.V., Lucas W.J., Gaber R.F. (1992). Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 89: 3736–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann S.M., Simoncini L., Schroeder J.I. (1985). Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature 318: 285–287. [Google Scholar]
- Blatt M.R., Grabov A. (1997). Signal redundancy, gates and integration in the control of ion channels for stomatal movement. J. Exp. Bot. 48: 529–537. [DOI] [PubMed] [Google Scholar]
- Brandt B., Brodsky D.E., Xue S., Negi J., Iba K., Kangasjärvi J., Ghassemian M., Stephan A.B., Hu H., Schroeder J.I. (2012). Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA 109: 10593–10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.H., Hu L., Punta M., Bruni R., Hillerich B., Kloss B., Rost B., Love J., Siegelbaum S.A., Hendrickson W.A. (2010). Homologue structure of the SLAC1 anion channel for closing stomata in leaves. Nature 467: 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daram P., Urbach S., Gaymard F., Sentenac H., Chérel I. (1997). Tetramerization of the AKT1 plant potassium channel involves its C-terminal cytoplasmic domain. EMBO J. 16: 3455–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir F., Horntrich C., Blachutzik J.O., Scherzer S., Reinders Y., Kierszniowska S., Schulze W.X., Harms G.S., Hedrich R., Geiger D., Kreuzer I. (2013). Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc. Natl. Acad. Sci. USA 110: 8296–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer I., Antunes S., Hoshi T., Müller-Röber B., Palme K., Pongs O., Reintanz B., Hedrich R. (1997). Plant K+ channel α-subunits assemble indiscriminately. Biophys. J. 72: 2143–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer I., Porée F., Schneider A., Mittelstädt J., Bertl A., Sentenac H., Thibaud J.B., Mueller-Roeber B. (2004). Assembly of plant Shaker-like K(out) channels requires two distinct sites of the channel α-subunit. Biophys. J. 87: 858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer I., Uozumi N. (2011). Potassium channels in plant cells. FEBS J. 278: 4293–4303. [DOI] [PubMed] [Google Scholar]
- Eisenach C., Chen Z.H., Grefen C., Blatt M.R. (2012). The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K+ channel activity with vegetative growth. Plant J. 69: 241–251. [DOI] [PubMed] [Google Scholar]
- Eisenach C., Papanatsiou M., Hillert E.K., Blatt M.R. (2014). Clustering of the K+ channel GORK of Arabidopsis parallels its gating by extracellular K+. Plant J. 78: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L.M., Zhang W., Chen J.G., Taylor J.P., Jones A.M., Assmann S.M. (2008). Abscisic acid regulation of guard-cell K+ and anion channels in Gβ- and RGS-deficient Arabidopsis lines. Proc. Natl. Acad. Sci. USA 105: 8476–8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Becker D., Vosloh D., Gambale F., Palme K., Rehers M., Anschuetz U., Dreyer I., Kudla J., Hedrich R. (2009a). Heteromeric AtKC1middle dotAKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J. Biol. Chem. 284: 21288–21295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Maierhofer T., Al-Rasheid K.A., Scherzer S., Mumm P., Liese A., Ache P., Wellmann C., Marten I., Grill E., Romeis T., Hedrich R. (2011). Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci. Signal. 4: ra32. [DOI] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., Liese A., Wellmann C., Al-Rasheid K.A., Grill E., Romeis T., Hedrich R. (2010). Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA 107: 8023–8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., Ache P., Matschi S., Liese A., Al-Rasheid K.A., Romeis T., Hedrich R. (2009b). Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA 106: 21425–21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R. (2012). Ion channels in plants. Physiol. Rev. 92: 1777–1811. [DOI] [PubMed] [Google Scholar]
- Hoshi T. (1995). Regulation of voltage dependence of the KAT1 channel by intracellular factors. J. Gen. Physiol. 105: 309–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E., et al. (2003). The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 100: 5549–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D., Wang C., He J., Liao H., Duan Y., Zhu Z., Guo Y., Chen Z., Gong Z. (2012). A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imes D., Mumm P., Böhm J., Al-Rasheid K.A.S., Marten I., Geiger D., Hedrich R. (2013). Open stomata 1 (OST1) kinase controls R-type anion channel QUAC1 in Arabidopsis guard cells. Plant J. 74: 372–382. [DOI] [PubMed] [Google Scholar]
- Inoue S., Kinoshita T., Matsumoto M., Nakayama K.I., Doi M., Shimazaki K. (2008). Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc. Natl. Acad. Sci. USA 105: 5626–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Takemiya A., Shimazaki K. (2010). Phototropin signaling and stomatal opening as a model case. Curr. Opin. Plant Biol. 13: 587–593. [DOI] [PubMed] [Google Scholar]
- Jeanguenin L., Alcon C., Duby G., Boeglin M., Chérel I., Gaillard I., Zimmermann S., Sentenac H., Véry A.A. (2011). AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J. 67: 570–582. [DOI] [PubMed] [Google Scholar]
- Keller B.U., Hedrich R., Raschke K. (1989). Voltage-dependent anion channels in the plasma membrane of guard cells. Nature 341: 450–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61: 561–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Doi M., Suetsugu N., Kagawa T., Wada M., Shimazaki K. (2001). Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Shimazaki Ki. (1999). Blue light activates the plasma membrane H(+)-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 18: 5548–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Shimazaki K. (2002). Biochemical evidence for the requirement of 14-3-3 protein binding in activation of the guard-cell plasma membrane H+-ATPase by blue light. Plant Cell Physiol. 43: 1359–1365. [DOI] [PubMed] [Google Scholar]
- Kollist H., Nuhkat M., Roelfsema M.R. (2014). Closing gaps: linking elements that control stomatal movement. New Phytol. 203: 44–62. [DOI] [PubMed] [Google Scholar]
- Kusumi K., Hirotsuka S., Kumamaru T., Iba K. (2012). Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J. Exp. Bot. 63: 5635–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.M., Murata Y., Baizabal-Aguirre V.M., Merrill J., Wang M., Kemper A., Hawke S.D., Tallman G., Schroeder J.I. (2001). Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in arabidopsis. Plant Physiol. 127: 473–485. [PMC free article] [PubMed] [Google Scholar]
- Laanemets K., et al. (2013). Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via enhanced cytosolic [Ca2+] and increased Ca2+ sensitivity of K+ uptake channels. New Phytol. 197: 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaudy A., Hosy E., Simonneau T., Sentenac H., Thibaud J.-B., Dreyer I. (2008). Heteromeric K+ channels in plants. Plant J. 54: 1076–1082. [DOI] [PubMed] [Google Scholar]
- Lebaudy A., Pascaud F., Véry A.A., Alcon C., Dreyer I., Thibaud J.B., Lacombe B. (2010). Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J. Biol. Chem. 285: 6265–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaudy A., Véry A.A., Sentenac H. (2007). K+ channel activity in plants: genes, regulations and functions. FEBS Lett. 581: 2357–2366. [DOI] [PubMed] [Google Scholar]
- Lee S.C., Lan W., Buchanan B.B., Luan S. (2009). A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. USA 106: 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E.R., Tytgat J., Hess P. (1992). Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9: 861–871. [DOI] [PubMed] [Google Scholar]
- Lind C., et al. (2015). Stomatal guard cells co-opted an ancient ABA-dependent desiccation survival system to regulate stomatal closure. Curr. Biol. 25: 928–935. [DOI] [PubMed] [Google Scholar]
- Liu H., Yu X., Li K., Klejnot J., Yang H., Lisiero D., Lin C. (2008). Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322: 1535–1539. [DOI] [PubMed] [Google Scholar]
- Ma Y., Szostkiewicz I., Korte A., Moes D., Yang Y., Christmann A., Grill E. (2009). Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. (1991). Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature 350: 232–235. [DOI] [PubMed] [Google Scholar]
- Maierhofer T., Diekmann M., Offenborn J.N., Lind C., Bauer H., Hashimoto K., S Al-Rasheid K.A., Luan S., Kudla J., Geiger D., Hedrich R. (2014). Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Sci. Signal. 7: ra86. [DOI] [PubMed] [Google Scholar]
- Marten I., Hoshi T. (1997). Voltage-dependent gating characteristics of the K+ channel KAT1 depend on the N and C termini. Proc. Natl. Acad. Sci. USA 94: 3448–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten I., Hoshi T. (1998). The N-terminus of the K channel KAT1 controls its voltage-dependent gating by altering the membrane electric field. Biophys. J. 74: 2953–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S., Mumm P., Imes D., Endler A., Weder B., Al-Rasheid K.A., Geiger D., Marten I., Martinoia E., Hedrich R. (2010). AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 63: 1054–1062. [DOI] [PubMed] [Google Scholar]
- Mikosch M., Hurst A.C., Hertel B., Homann U. (2006). Diacidic motif is required for efficient transport of the K+ channel KAT1 to the plasma membrane. Plant Physiol. 142: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra B.B., Acharya B.R., Granot D., Assmann S.M., Chen S. (2015). The guard cell metabolome: functions in stomatal movement and global food security. Front. Plant Sci. 6: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I.C., Uozumi N., Muto S. (2000). Phosphorylation of the inward-rectifying potassium channel KAT1 by ABR kinase in Vicia guard cells. Plant Cell Physiol. 41: 850–856. [DOI] [PubMed] [Google Scholar]
- Nakamura R.L., McKendree W.L. Jr., Hirsch R.E., Sedbrook J.C., Gaber R.F., Sussman M.R. (1995). Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 109: 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., Uchimiya H., Hashimoto M., Iba K. (2008). CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486. [DOI] [PubMed] [Google Scholar]
- Nishimura N., Hitomi K., Arvai A.S., Rambo R.P., Hitomi C., Cutler S.R., Schroeder J.I., Getzoff E.D. (2009). Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science 326: 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N., Sarkeshik A., Nito K., Park S.-Y., Wang A., Carvalho P.C., Lee S., Caddell D.F., Cutler S.R., Chory J., Yates J.R., Schroeder J.I. (2010). PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J. 61: 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrdlik P., et al. (2004). K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. USA 101: 12242–12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S., Zhang W., Assmann S.M. (2007). Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 581: 2325–2336. [DOI] [PubMed] [Google Scholar]
- Park S.Y., et al. (2009). Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z.M., Kuchitsu K., Ward J.M., Schwarz M., Schroeder J.I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9: 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z.M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G.J., Grill E., Schroeder J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. [DOI] [PubMed] [Google Scholar]
- Pilot G., Lacombe B., Gaymard F., Cherel I., Boucherez J., Thibaud J.B., Sentenac H. (2001). Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 276: 3215–3221. [DOI] [PubMed] [Google Scholar]
- Raschke K., Hedrich R., Reckmann U., Schroeder J.I. (1988). Exploring biophysical and biochemical components of the osmotic motor that drives stomatal movement. Bot. Acta 101: 283–294. [Google Scholar]
- Reintanz B., Szyroki A., Ivashikina N., Ache P., Godde M., Becker D., Palme K., Hedrich R. (2002). AtKC1, a silent Arabidopsis potassium channel α -subunit modulates root hair K+ influx. Proc. Natl. Acad. Sci. USA 99: 4079–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema M.R., Hedrich R. (2005). In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol. 167: 665–691. [DOI] [PubMed] [Google Scholar]
- Roelfsema M.R., Hedrich R., Geiger D. (2012). Anion channels: master switches of stress responses. Trends Plant Sci. 17: 221–229. [DOI] [PubMed] [Google Scholar]
- Ronzier E., Corratgé-Faillie C., Sanchez F., Prado K., Brière C., Leonhardt N., Thibaud J.B., Xiong T.C. (2014). CPK13, a noncanonical Ca2+-dependent protein kinase, specifically inhibits KAT2 and KAT1 shaker K+ channels and reduces stomatal opening. Plant Physiol. 166: 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Gambale F., Dreyer I., Uozumi N. (2010). Modulation of the Arabidopsis KAT1 channel by an activator of protein kinase C in Xenopus laevis oocytes. FEBS J. 277: 2318–2328. [DOI] [PubMed] [Google Scholar]
- Sato A., Sato Y., Fukao Y., Fujiwara M., Umezawa T., Shinozaki K., Hibi T., Taniguchi M., Miyake H., Goto D.B., Uozumi N. (2009). Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 424: 439–448. [DOI] [PubMed] [Google Scholar]
- Schachtman D.P., Schroeder J.I., Lucas W.J., Anderson J.A., Gaber R.F. (1992). Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science 258: 1654–1658. [DOI] [PubMed] [Google Scholar]
- Scherzer S., Maierhofer T., Al-Rasheid K.A., Geiger D., Hedrich R. (2012). Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol. Plant 5: 1409–1412. [DOI] [PubMed] [Google Scholar]
- Schroeder J.I., Hagiwara S. (1989). Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338: 427–430. [Google Scholar]
- Schroeder J.I., Keller B.U. (1992). Two types of anion channel currents in guard cells with distinct voltage regulation. Proc. Natl. Acad. Sci. USA 89: 5025–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac H., Bonneaud N., Minet M., Lacroute F., Salmon J.M., Gaymard F., Grignon C. (1992). Cloning and expression in yeast of a plant potassium ion transport system. Science 256: 663–665. [DOI] [PubMed] [Google Scholar]
- Shimazaki K., Doi M., Assmann S.M., Kinoshita T. (2007). Light regulation of stomatal movement. Annu. Rev. Plant Biol. 58: 219–247. [DOI] [PubMed] [Google Scholar]
- Shimazaki K., Iino M., Zeiger E. (1986). Blue light-dependent proton extrusion by guard-cell protoplasts of Vicia faba. Nature 319: 324–326. [Google Scholar]
- Sottocornola B., Gazzarrini S., Olivari C., Romani G., Valbuzzi P., Thiel G., Moroni A. (2008). 14-3-3 proteins regulate the potassium channel KAT1 by dual modes. Plant Biol (Stuttg) 10: 231–236. [DOI] [PubMed] [Google Scholar]
- Sutter J.U., Campanoni P., Tyrrell M., Blatt M.R. (2006). Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 18: 935–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter J.U., Sieben C., Hartel A., Eisenach C., Thiel G., Blatt M.R. (2007). Abscisic acid triggers the endocytosis of the arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 17: 1396–1402. [DOI] [PubMed] [Google Scholar]
- Szczerba M.W., Britto D.T., Kronzucker H.J. (2009). K+ transport in plants: physiology and molecular biology. J. Plant Physiol. 166: 447–466. [DOI] [PubMed] [Google Scholar]
- Szyroki A., Ivashikina N., Dietrich P., Roelfsema M.R., Ache P., Reintanz B., Deeken R., Godde M., Felle H., Steinmeyer R., Palme K., Hedrich R. (2001). KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. USA 98: 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N., Nakamura T., Schroeder J.I., Muto S. (1998). Determination of transmembrane topology of an inward-rectifying potassium channel from Arabidopsis thaliana based on functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 95: 9773–9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T., Kollist H., Wang Y.F., Nishimura N., Chan W.Y., Valerio G., Lamminmäki A., Brosché M., Moldau H., Desikan R., Schroeder J.I., Kangasjärvi J. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T., et al. (2010). Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 62: 442–453. [DOI] [PubMed] [Google Scholar]
- Véry A.A., Gaymard F., Bosseux C., Sentenac H., Thibaud J.B. (1995). Expression of a cloned plant K+ channel in Xenopus oocytes: analysis of macroscopic currents. Plant J. 7: 321–332. [DOI] [PubMed] [Google Scholar]
- Véry A.A., Sentenac H. (2002). Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci. 7: 168–175. [DOI] [PubMed] [Google Scholar]
- Véry A.A., Sentenac H. (2003). Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 54: 575–603. [DOI] [PubMed] [Google Scholar]
- Waadt R., Schmidt L.K., Lohse M., Hashimoto K., Bock R., Kudla J. (2008). Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 56: 505–516. [DOI] [PubMed] [Google Scholar]
- Walker D.J., Leigh R.A., Miller A.J. (1996). Potassium homeostasis in vacuolate plant cells. Proc. Natl. Acad. Sci. USA 93: 10510–10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.Q., Ullah H., Jones A.M., Assmann S.M. (2001). G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292: 2070–2072. [DOI] [PubMed] [Google Scholar]
- Wang Y., Papanatsiou M., Eisenach C., Karnik R., Williams M., Hills A., Lew V.L., Blatt M.R. (2012). Systems dynamic modeling of a guard cell Cl- channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol. 160: 1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu W.H. (2013). Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 64: 451–476. [DOI] [PubMed] [Google Scholar]
- Wang Y.F., Munemasa S., Nishimura N., Ren H.M., Robert N., Han M., Puzõrjova I., Kollist H., Lee S., Mori I., Schroeder J.I. (2013). Identification of cyclic GMP-activated nonselective Ca2+-permeable cation channels and associated CNGC5 and CNGC6 genes in Arabidopsis guard cells. Plant Physiol. 163: 578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J.M., Mäser P., Schroeder J.I. (2009). Plant ion channels: gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 71: 59–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J.M., Schroeder J.I. (1994). Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6: 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xicluna J., Lacombe B., Dreyer I., Alcon C., Jeanguenin L., Sentenac H., Thibaud J.B., Chérel I. (2007). Increased functional diversity of plant K+ channels by preferential heteromerization of the shaker-like subunits AKT2 and KAT2. J. Biol. Chem. 282: 486–494. [DOI] [PubMed] [Google Scholar]
- Xu J., Li H.D., Chen L.Q., Wang Y., Liu L.L., He L., Wu W.H. (2006). A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360. [DOI] [PubMed] [Google Scholar]
- Zhang B., Karnik R., Wang Y., Wallmeroth N., Blatt M.R., Grefen C. (2015). The Arabidopsis R-SNARE VAMP721 interacts with KAT1 and KC1 K+ channels to moderate K+ current at the plasma membrane. Plant Cell 27: 1697–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Takemiya A., Kinoshita T., Shimazaki K. (2007). Nitric oxide inhibits blue light-specific stomatal opening via abscisic acid signaling pathways in Vicia guard cells. Plant Cell Physiol. 48: 715–723. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang H., Takemiya A., Song C.P., Kinoshita T., Shimazaki K. (2004). Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol. 136: 4150–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zörb C., Senbayram M., Peiter E. (2014). Potassium in agriculture--status and perspectives. J. Plant Physiol. 171: 656–669. [DOI] [PubMed] [Google Scholar]