Abstract
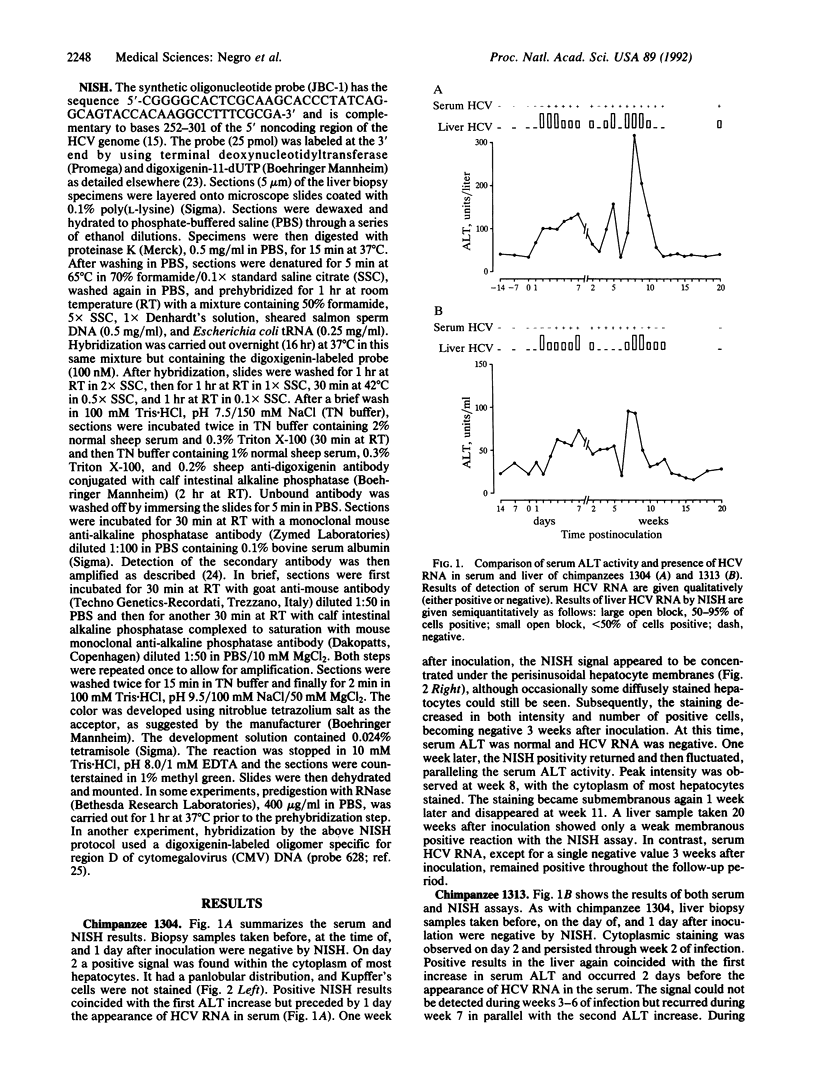
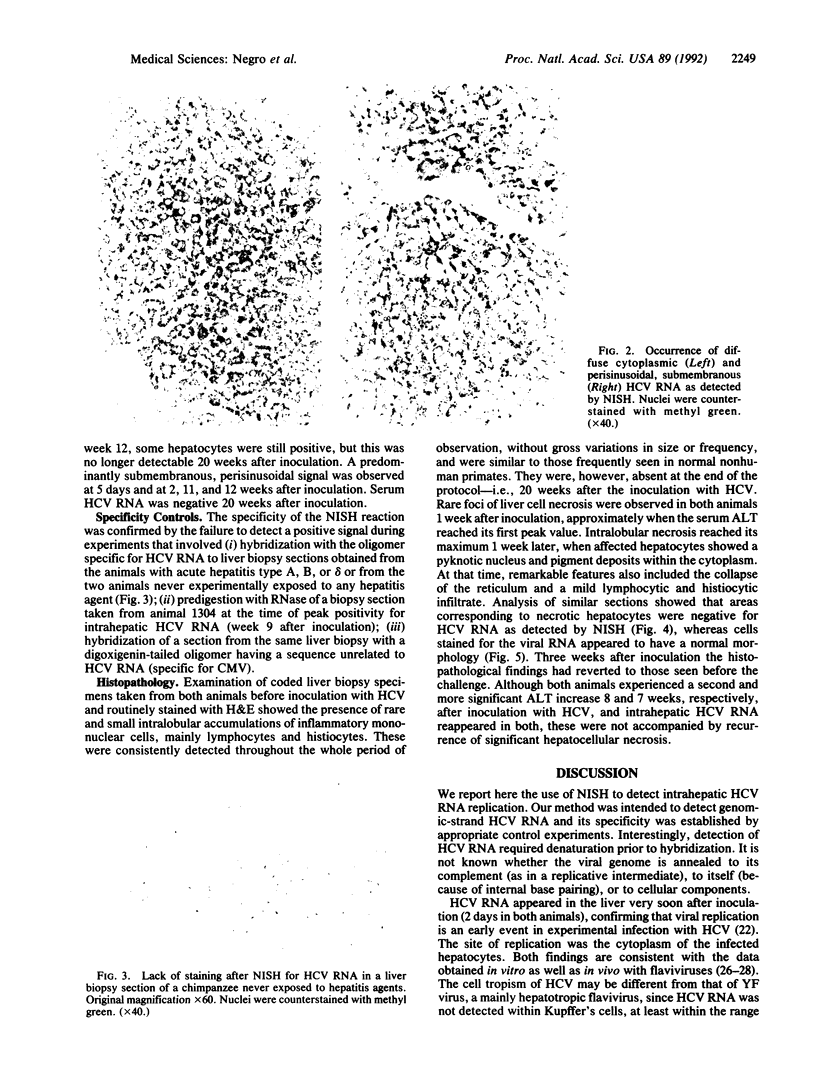
A nonisotopic in situ hybridization (NISH) assay was used to detect hepatitis C virus (HCV) RNA. A synthetic oligonucleotide complementary to bases 252-301 of the highly conserved 5' noncoding region of the HCV genome was end-labeled by terminal deoxynucleotidyltransferase using digoxigenin-conjugated dUTP. The hybridized oligomer was revealed by an immunohistochemical reaction after incubation with an alkaline phosphatase-conjugated anti-digoxigenin antibody and subsequent amplification with a complex of alkaline phosphatase and anti-alkaline phosphatase antibodies. The intracellular distribution of HCV RNA was monitored in the livers of two chimpanzees experimentally infected with the H strain of HCV and compared with the serum alanine aminotransferase activity, serum HCV RNA, and liver histopathology. Most cells were stained in the cytoplasm as early as 2 days after inoculation, 1 and 2 days, respectively, before the appearance of viral RNA in the serum. The time course of HCV RNA replication was correlated with increases in serum alanine aminotransferase. However, neither one paralleled the appearance of liver cell necrosis nor showed any correlation with the inflammatory response. The NISH signal was not found in liver biopsy specimens taken from these two animals before inoculation with HCV, from chimpanzees with acute hepatitis type A, B, or delta, or from two animals never experimentally infected with any hepatitis agent; moreover, it disappeared when the positive specimens were predigested with RNase and it was not observed after hybridization of positive controls with a labeled oligomer unrelated to HCV RNA. Thus, detection of liver HCV RNA by NISH is a sensitive and specific method for studying HCV replication at the cellular level. Intracellular replication of HCV did not appear to be associated with histopathologic changes in the liver, although the correlation with increases of liver enzyme activity in the serum suggested possible damage to the liver cell membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima T., Nagashima H., Murakami S., Kaji C., Fujita J., Shimomura H., Tsuji T. Cloning of a cDNA associated with acute and chronic hepatitis C infection generated from patients serum RNA. Gastroenterol Jpn. 1989 Oct;24(5):540–544. doi: 10.1007/BF02773882. [DOI] [PubMed] [Google Scholar]
- Arima T., Takamizawa A., Mori C., Murakami S., Kaji C., Fujita J. A lambda gt11-cDNA clone specific for chronic hepatitis C generated from pooled serum presumably infected by hepatitis C virus. Gastroenterol Jpn. 1989 Oct;24(5):545–548. doi: 10.1007/BF02773883. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Farci P., Alter H. J., Wong D., Miller R. H., Shih J. W., Jett B., Purcell R. H. A long-term study of hepatitis C virus replication in non-A, non-B hepatitis. N Engl J Med. 1991 Jul 11;325(2):98–104. doi: 10.1056/NEJM199107113250205. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Alter H. J., Dienes H. P., Shimizu Y., Popper H., Blackmore D., Sly D., London W. T., Purcell R. H. Non-A, non-B hepatitis in chimpanzees and marmosets. J Infect Dis. 1981 Dec;144(6):588–598. doi: 10.1093/infdis/144.6.588. [DOI] [PubMed] [Google Scholar]
- Han J. H., Shyamala V., Richman K. H., Brauer M. J., Irvine B., Urdea M. S., Tekamp-Olson P., Kuo G., Choo Q. L., Houghton M. Characterization of the terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5' untranslated region and poly(A) tails at the 3' end. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1711–1715. doi: 10.1073/pnas.88.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia K., Spector D. H., Lawrie J., Spector S. A. Enzymatic amplification of human cytomegalovirus sequences by polymerase chain reaction. J Clin Microbiol. 1989 Aug;27(8):1802–1809. doi: 10.1128/jcm.27.8.1802-1809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantolino D., Bonino F., Zanetti A. R., Lesniewski R. R., Barbazza R., Chiaramonte M. Localization of hepatitis C virus (HCV) antigen by immunohistochemistry on fixed-embedded liver tissue. Ital J Gastroenterol. 1990 Aug;22(4):198–199. [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Takeuchi K., Boonmar S., Katayama T., Choo Q. L., Kuo G., Weiner A. J., Bradley D. W., Houghton M., Saito I. A cDNA fragment of hepatitis C virus isolated from an implicated donor of post-transfusion non-A, non-B hepatitis in Japan. Nucleic Acids Res. 1989 Dec 25;17(24):10367–10372. doi: 10.1093/nar/17.24.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Maéno M., Kaminaka K., Sugimoto H., Esumi M., Hayashi N., Komatsu K., Abe K., Sekiguchi S., Yano M., Mizuno K. A cDNA clone closely associated with non-A, non-B hepatitis. Nucleic Acids Res. 1990 May 11;18(9):2685–2689. doi: 10.1093/nar/18.9.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Alter H. J., Miller R. H., Purcell R. H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Tanaka T., Sugai Y., Akahane Y., Machida A., Mishiro S., Yoshizawa H., Miyakawa Y. Detection of hepatitis C virus RNA by a two-stage polymerase chain reaction with two pairs of primers deduced from the 5'-noncoding region. Jpn J Exp Med. 1990 Aug;60(4):215–222. [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Yotsumoto S., Tanaka T., Yoshizawa H., Tsuda F., Miyakawa Y., Mayumi M. The 5'-terminal sequence of the hepatitis C virus genome. Jpn J Exp Med. 1990 Jun;60(3):167–177. [PubMed] [Google Scholar]
- Salzberg S., Bakhanashvili M., Aboud M. Effect of interferon on mouse cells chronically infected with murine leukaemia virus: kinetic studies on virus production and virus RNA synthesis. J Gen Virol. 1978 Jul;40(1):121–130. doi: 10.1099/0022-1317-40-1-121. [DOI] [PubMed] [Google Scholar]
- Schmitz G. G., Walter T., Seibl R., Kessler C. Nonradioactive labeling of oligonucleotides in vitro with the hapten digoxigenin by tailing with terminal transferase. Anal Biochem. 1991 Jan;192(1):222–231. doi: 10.1016/0003-2697(91)90212-c. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Sarkar N. H. Effects of interferon on the production of murine mammary tumor virus by mammary tumor cells in culture. Virology. 1980 Apr 30;102(2):431–443. doi: 10.1016/0042-6822(80)90110-5. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. K., Oomura M., Abe K., Uno M., Yamada E., Ono Y., Shikata T. Production of antibody associated with non-A, non-B hepatitis in a chimpanzee lymphoblastoid cell line established by in vitro transformation with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2138–2142. doi: 10.1073/pnas.82.7.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y. K., Purcell R. H. Cytoplasmic antigen in hepatocytes of chimpanzees infected with non-A, non-B hepatitis virus or hepatitis delta virus: relationship to interferon. Hepatology. 1989 Nov;10(5):764–768. doi: 10.1002/hep.1840100503. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. K., Weiner A. J., Rosenblatt J., Wong D. C., Shapiro M., Popkin T., Houghton M., Alter H. J., Purcell R. H. Early events in hepatitis C virus infection of chimpanzees. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6441–6444. doi: 10.1073/pnas.87.16.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Zhang L. Q., Watson H. G., Rebus S., Ferguson E. D., Balfe P., Leadbetter G. H., Yap P. L., Peutherer J. F., Ludlam C. A. Hepatitis C quantification and sequencing in blood products, haemophiliacs, and drug users. Lancet. 1990 Dec 15;336(8729):1469–1472. doi: 10.1016/0140-6736(90)93179-s. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich P. P., Romeo J. M., Lane P. K., Kelly I., Daniel L. J., Vyas G. N. Detection, semiquantitation, and genetic variation in hepatitis C virus sequences amplified from the plasma of blood donors with elevated alanine aminotransferase. J Clin Invest. 1990 Nov;86(5):1609–1614. doi: 10.1172/JCI114882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. J., Kuo G., Bradley D. W., Bonino F., Saracco G., Lee C., Rosenblatt J., Choo Q. L., Houghton M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet. 1990 Jan 6;335(8680):1–3. doi: 10.1016/0140-6736(90)90134-q. [DOI] [PubMed] [Google Scholar]
- Westaway E. G., Brinton M. A., Gaidamovich SYa, Horzinek M. C., Igarashi A., Käriäinen L., Lvov D. K., Porterfield J. S., Russell P. K., Trent D. W. Flaviviridae. Intervirology. 1985;24(4):183–192. doi: 10.1159/000149642. [DOI] [PubMed] [Google Scholar]
- van der Poel C. L., Reesink H. W., Schaasberg W., Leentvaar-Kuypers A., Bakker E., Exel-Oehlers P. J., Lelie P. N. Infectivity of blood seropositive for hepatitis C virus antibodies. Lancet. 1990 Mar 10;335(8689):558–560. doi: 10.1016/0140-6736(90)90347-8. [DOI] [PubMed] [Google Scholar]







