Abstract
Background:
Mycobacterium tuberculosis (MTB) is the causative agent of tuberculosis (TB), which remains one of the major public health problems in the world. The increasing incidence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) worldwide highlights the urgent need to search for alternative antimycobacterial agents. More and more people in developing countries utilize traditional medicine for their major primary health care needs. It has been determined that the medicinal plants Pulicaria gnaphalodes and Perovskia abrotanoides possess strong antibacterial effect.
Materials and Methods:
In this study, the antimycobacterial effects of P. gnaphalodes and P. abrotanoides essential oil on MTB were examined. Essential oil was prepared from P. gnaphalodes aerial parts and P. abrotanoides flower. The effects of six different concentrations (20 μg/ml, 40 μg/ml, 80 μg/ml, 160 μg/ml, 320 μg/ml, and 640 μg/ml) were examined against sensitive isolates of MTB and MTB H37Rv (ATCC 27294).
Results:
The results showed that P. gnaphalodes and P. abrotanoides essential oil extracts have strong inhibitory effects on MTB. This activity for P. gnaphalodes was observed from very low (4%) to good (70.9%) effect; meanwhile, this activity for P. abrotanoides was observed from very low (4%) to strong (86%) effect.
Conclusion:
The mean of inhibition percentage for P. gnaphalodes and P. abrotanoides in 640 μg/ml was 58.1% and 76.2%, respectively. So, P. abrotanoides plant is more effective against MTB than P. gnaphalodes. Identification of the effective fraction against MTB is a further step to be studied.
Keywords: Antimycobacterial effect, essential oil, Perovskia abrotanoides, Pulicaria gnaphalodes
INTRODUCTION
Mycobacterium tuberculosis (MTB), the causative agent of tuberculosis (TB), is one of the top killer diseases in the world. In 2011, 8.7 million people were overtaken with TB, including 1.1 million cases among people with HIV. It was estimated that 1.4 million people died from TB in this year.[1] Today, the emergence of resistance to antimycobacterial agents has become an important public health issue in many developing countries. The increasing incidence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant TB (XDR-TB) worldwide highlights the urgent need to search for newer antituberculosis compounds.[2] More and more people in developing countries utilize traditional medicine for their major primary health care needs.[3] Medicinal plants offer a great hope to fulfill these needs and have been used for curing diseases for many centuries.[4] The scientific experiments that have been carried out on antimicrobial properties of plant components were first documented in the late nineteenth century.[5] Plants have been a source of effective chemotherapeutic agents for various infectious diseases, and there is a growing interest in the development of drugs of plant origin. A number of plants have shown significant in vitro antimycobacterial activities. These findings have therefore stimulated further research toward the isolation of new antimycobacterial agents from natural products.[6]
The Pulicaria genus belongs to the family Compositae (Asteraceae), tribe Inuleae, which contains more than 77 species that are widely distributed across Asia, Europe, and Africa. P. gnaphalodes is effective on B. anthracis, S. aureus, K. pneumoniae, E. coli, P. aeruginosa, P. vulgaris, Sh. sonnei, S. paratyphi A, and V. cholerae.[7] This species completely inhibited lettuce seed germination, thus it has allelopathic potential.[8] Perovskia is a genus that belongs to the Lamiaceae family, which is allied to Salvia, Nepeta, and Rosmarinus. Perovskia is represented in the Flora Iranica by only three species. Perovskis abrotanoides is an aromatic erect herb used in Iranian folk medicine as an analgesic for rheumatic pains. Previous investigations of this plant resulted in the isolation of bioactive tanshinones with leishmanicidal, antiplasmodial, and cytotoxic activities.[9] There are also other reports of the isolation and identification of two triterpenes with a novel carbon skeleton from P. abrotanoides.[10] Essential oil from the flowers of P. abrotanoides showed in vitro antibacterial activity on B. cereus, S. aureus, K. pneumoniae, and E. coli.[11] This plant is often used as a fortifier, as an antiseptic, and for rheumatic pains, and is known to be antiinflammatory, leishmanicidal, and anthelmintic.[9] However, what has not yet been reported is the antimycobacterial effect of P. gnaphalodes and P. abrotanoides plants essential oils toward MTB that has been recognized as the major organism involved in deaths worldwide. In this study, the effect of the essential oils of these plantson MTB isolates and MTB H37Rv (ATCC 27294)were evaluated.
MATERIALS AND METHODS
Plant material
P. gnaphalodes and P. abrotanoides were collected from Khorasan province on May 2012 and identified by botanist Dr. Rahiminejad, and the voucher specimen has been deposited in the herbarium of the School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran.
Preparation of essential oils
The plant's aerial part including leaves, stem, and flower were air-dried for 3 days in the shade at temperatures 23°C-27°C, then powdered mechanically using a blender; 550 gm of plant aerial part powder was mixed with water; 1:10 plant material/water volume ratio was maintained, and the mixture was distillated for hours. Essential oils were obtained from the plant samples using a Clevenger-type apparatus where the plant powder is subjected to hydro-distillation. The oils were collected in a screwed tube glass and stored in at 4°C. The essential oils of these plants were prepared for polar and semipolar compound extraction.
MTB strains
Fifty isolates of MTB species were collected from tuberculosis centers of Isfahan. Isolates subcultured on Löwenstein-Jensen (LJ) medium and incubated for 21 days at 37°C, and characterized by conventional methods including staining, colony characteristics, pigmentation, growth temperature, and time of growth.[12]
Susceptibility testing of MTB
The susceptibility of isolates to isonicotinylhydrazine (INH) (0.2 μg/ml), rifampin (RIF) (40 μg/ml), streptomycin (STR) (4 μg/ml), and ethambutol (EMB) (2 μg/ml) was tested using the proportion method.[13,14] Briefly, the isolates were subcultured on LJ medium and incubated for 21 days. A standard suspension of 107 colony-forming units (CFU)/ml (equivalent to a 1 McFarland standard) of MTB isolates was prepared by dissolving of 3-5 colonies of MTB in distilled water.[15] Subsequently, LJ media without and with subjected antibiotics were prepared and inoculated with 0.2 ml of 10-4 dilutions of MTB strains. The inoculated plates were then incubated for 42 days at 37°C (1st reading after 28 days, 2nd after 42 days), and the percentage of inhibition was determined.[16] Resistance was defined as growth on drug-containing tubes greater than 1% of the growth of drug free control medium for INH, RIF, EMB, and 10% for STR.[17,18] MTB H37Rv (ATCC 27294) was set as the control in all culturing and sensitivity testing processes.
Determination of antimycobacterial activity
The antimycobacterial effects of six concentrations (20 μg/ml, 40 μg/ml, 80 μg/ml, 160 μg/ml, 320 μg/ml, and 640 μg/ml) of P. gnaphalodes and P. abrotanoides essential oil on MTB isolates and MTB H37Rv (ATCC 27294) weredetermined according to the standard procedures.[14] LJ medium containing and free of subjected plants were prepared and inoculated with 0.2 ml of 10-4 dilutions of a 1 McFarland standard of each isolate. RIF and INH were used as the positive control and water as the negative control. The percentage of inhibition was determined after 3-6 weeks of inoculation by the following formula:[19,20]

The P value was used to quantify the idea of statistical significance of evidence.
RESULTS AND DISCUSSION
All isolates of were confirmed as MTB species by conventional biochemical and phenotypic methods (staining, colonial morphology, pigmentation, biochemical profiles, and growth rate). Drug susceptibility testing was done using the proportion method with INH (0.2 μg/ml), RIF (40 μg/ml), STR (4 μg/ml), and EMB (2 μg/ml), and two sensitive isolates that were susceptible to all drugs and MTB H37Rv (ATCC 27294) were selected for the study. The antimycobacterial effect of P. gnaphalodes and P. abrotanoides against sensitive isolates of MTB were determined using the proportion method. The results showed that the essential oils of the plants had a strong inhibitory effect against MTB strains [Tables 1 and 2, Figure 1]. The maximum percentage inhibition for these plants (P. gnaphalodes and P. abrotanoides) was related to the highest concentration. So the essential oils of these plants were more effective in the 640 μg/ml concentration.
Table 1.
Efficiency of essential oil extracted from P. gnaphalodes against M. tuberculosis strains

Table 2.
Efficiency of essential oil extracted from P. abrotanoides against M. tuberculosis strains
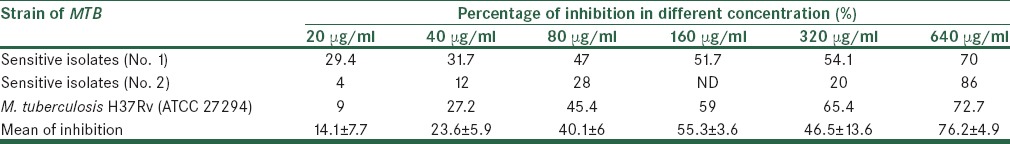
Figure 1.
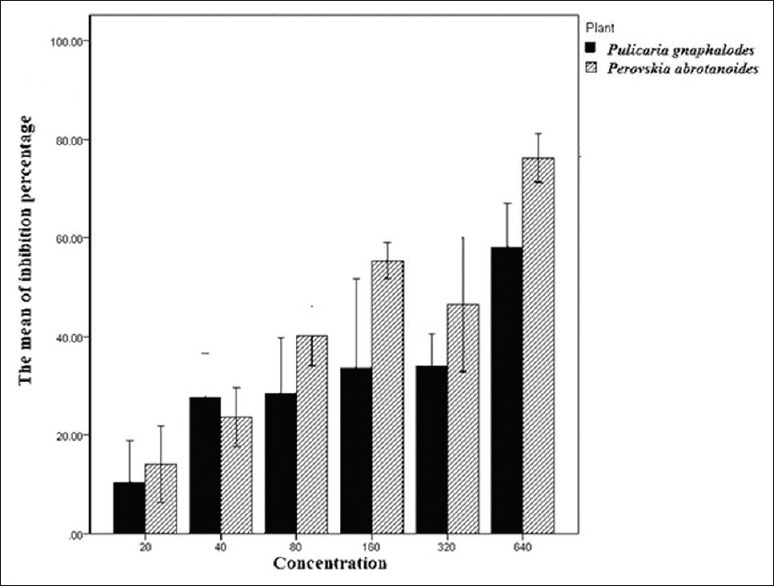
The mean of M. tuberculosis inhibition percentage according to the kind of plant and concentration
The prevalence of MDR strains of M. tuberculosis is an important reason for the resurgence of TB as a major disease in many parts of the world. There is, therefore, an urgent need for new, inexpensive TB drugs that are effective and have fewer side effects.[21] In many countries, medicinal plants are used by traditional medical practitioners to combat TB. Medicinal plants are an important natural source of novel leads in the field of antimycobacterial therapeutics.[22,23] Various biological activities have been reported for some species of Pulicaria, such as antibacterial, antifungal, and insecticidal properties.[24] Khani et al. showed that the essential oil of this plant has good insecticidal activity against Callosobruchus maculates.[25] Mahboubi reported that P. abrotanoides flower oil shows antifungal activity against Aspergillus flavus, Candida albicans, and Trichophyton mentagrophytes.[26]
In this study, the antimycobacterial activity of the P. gnaphalodes and P. abrotanoides essential oils in concentrations of 20 μg/ml, 40 μg/ml, 80 μg/ml, 160 μg/ml, 320 μg/ml, and 640 μg/ml on sensitive isolates of MTB was determined by the proportion method according to standard procedures.[13,27] These plants showed a noticeable inhibitory activity against MTB strains [Tables 1 and 2]. This activity for P. gnaphalodes was observed from very low (4%) to good (70.9%) effect. Meanwhile, this activity for P. abrotanoides was observed from very low (4%) to strong (86%) effect. These plants showed similar activity on MTB H37Rv (ATCC 27294) strain of MTB in 640 μg/ml (70.9% for P. gnaphalodes plant and 72.7% for P. abrotanoides plant). Maximum percentage inhibition for these plants was related to 640 μg/ml concentration, and for P. gnaphalodes and P. abrotanoides was 70.9% and 86%, respectively. So, the essential oils of these plants were more effective in the 640 μg/ml concentration. The mean of inhibition percentage for P. gnaphalodes and P. abrotanoides in 640 μg/ml was observed to be 58.1% and 76.2%, respectively. So the P. abrotanoides plant is more effective on MTB than P. gnaphalodes. Renu Gupta et al. evaluated the antituberculosis activity of Allium sativum and Adhatoda vasica by the proportion method. Allium sativum and Adhatoda vasica showed 63% and 70% inhibition on the MTB H37Rv (ATCC 27294) strain, respectively. They showed that P. gnaphalodes and P. abrotanoides in comparison with Adhatoda vasica have similar activity on MTB; meanwhile they showed stronger antituberculosis activity than Allium sativum.[16] The identification of the potent constituents of these essential oils against MTB is a problem for further study.
CONCLUSION
The two-way analysis of variance (ANOVA) showed that P. gnaphalodes and P. abrotanoides (P < 0.04) in different concentrations (P < 0.001) are effective against M. tuberculosis. The mean of inhibition percentage of the P. gnaphalodes plant is more than that of the P. abrotanoides plant.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.WHO Library Cataloguing-in-Publication Data Global tuberculosis controls. WHO report. 2011 [Google Scholar]
- 2.Serkani JE, Isfahani BN, Safaei HG, Kermanshahi RK, Asghari G. Evaluation of the effect of Humulus lupulus alcoholic extract on rifampin-sensitive and resistant isolates of Mycobacterium tuberculosis. Res Pharm Sci. 2012;7:235–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Houghton PJ. The role of plants in traditional medicine and current therapy. J Altern Complement Med. 1995;1:131–43. doi: 10.1089/acm.1995.1.131. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich M, Gibbons S. Ethnopharmacology in drug discovery: An analysis of its role and potential contribution. J Pharm Pharmacol. 2001;53:425–32. doi: 10.1211/0022357011775712. [DOI] [PubMed] [Google Scholar]
- 5.Zaika LL. Spices and herbs: Their antimicrobial activity and its determination. J Food Safety. 1975;9:97–118. [Google Scholar]
- 6.Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J Ethnopharmacol. 2002;79:57–67. doi: 10.1016/s0378-8741(01)00350-6. [DOI] [PubMed] [Google Scholar]
- 7.Surmaghi MS, Aynehchi Y, Amin GH, Mahmoodi Z. Survey of Iranian Plants for Saponins Alkaloids Flavonoids and Tannins. IV. DARU J Pharm Sci. 1992;2:281–290. [Google Scholar]
- 8.Azizi M, Amini S, Joharchi MR, Oroojalian F, Baghestani Z. Genetic resources for allelopathic and medicinal plants from traditional persian experience. 4th International Symposium on Biology, September, iran. 2014 W3-05. [Google Scholar]
- 9.Beikmohammadi M. The evaluation of medicinal properties of Perovskia abrotanoides karel. Middle-East J Sci Res. 2012;11:189–93. [Google Scholar]
- 10.Parvez A, Choudhary MI, Akhter F, Noorwala M, Mohammod FV, Hasan NM, et al. Perovskone: A triterpene with a novel carbon skeleton from Perovskia abrotanoides. J Org Chem. 1992;57:4339–40. [Google Scholar]
- 11.Nezhadali A, Masrorniab M, Solatib A, Akbarpour M, Moghaddamb MN. Analysis of the flower essential oil at different stages of plant growth and in vitro antibacterial activity of Perovskia abrotanoides Karal, in Iran. Der Pharma Chemica. 2009;1:146–50. [Google Scholar]
- 12.Oliveira DG, Prince KA, Higuchi CT, Santos AC, Lopes LM, Simoes MJ, Leite CQ. Antimycobacterial activity of some Brazilian indigenous medicinal drinks. J Basic Appl Pharm Sci. 2007;28:165–9. [Google Scholar]
- 13.Charley W, Mitchell M, Kidd K. Antibiotics laboratory medicine. In The Antimycobacterial Susceptibility Tests. New York: Williams and Wilkins Press; 1999. pp. 145–278. [Google Scholar]
- 14.Williams DL, Spring L, Collins L, Miller LP, Heifets LB, Gangadharam PR, et al. Contribution of rpoB mutations to development of rifamycin cross-resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:1853–7. doi: 10.1128/aac.42.7.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasr Esfahani B, Hozoorbakhsh F, Rashed Kh, Havaei SA, Heidari K, Moghim Sh. Effect of Lagerstroemia tomentosa and Diospyros virginiana methanolic extracts on different drug-resistant strains of Mycobacterium tuberculosis. Res Pharm Sci. 2014;9:193–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta R, Thakur B, Singh P, Singh HB, Sharma VD, Katoch VM, et al. Anti-tuberculosis activity of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Indian J Med Res. 2010;131:809–13. [PubMed] [Google Scholar]
- 17.Leite CQ, Beretta AL, Anno IS, Telles MA. Standardization of broth microdilution method for Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz. 2000;95:127–9. doi: 10.1590/s0074-02762000000100021. [DOI] [PubMed] [Google Scholar]
- 18.NCCLS-National Committee for Clinical Laboratory Standards: Antimycobacterial susceptibility testing for MTB. Villanova; Tentative standard M24-T, NCCLS. 2002 [Google Scholar]
- 19.Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen JO, Dooley SW. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328:521–6. doi: 10.1056/NEJM199302253280801. [DOI] [PubMed] [Google Scholar]
- 20.Isfahani BN, Tavakoli A, Salehi M, Tazhibi M. Detection of rifampin resistance patterns in Mycobacterium tuberculosis strains isolated in Iran by polymerase chain reaction-single-strand conformation polymorphism and direct sequencing methods. Mem Inst Oswaldo Cruz. 2006;101:597–602. doi: 10.1590/s0074-02762006000600004. [DOI] [PubMed] [Google Scholar]
- 21.Kato-Maeda M, Sifuentes-Osornio J, Bobadilla-del-Valle M, Ruiz-Palacios GM, Ponce-de-León A. Drug resistance among acid-fast bacilli. Lancet. 1999;353:1709. doi: 10.1016/s0140-6736(05)77019-7. [DOI] [PubMed] [Google Scholar]
- 22.Mann A, Amupitan JO, Oyewale AO, Okogun JI, Ibrahim K. An ethnobotanical survey of indigenous flora for treating tuberculosis and other respiratory diseases in Niger State, Nigeria. J Phytomed and Therap. 2007;12:1–12. [Google Scholar]
- 23.Cantrell CL, Franzblau SG, Fischer NH. Antimycobacterial plants terpenoids. Planta Med. 2001;67:685–94. doi: 10.1055/s-2001-18365. [DOI] [PubMed] [Google Scholar]
- 24.Liu LL, Yang JL, Shi YP. Phytochemicals and biological activities of Pulicaria species. Chem Biodivers. 2010;7:327–49. doi: 10.1002/cbdv.200900014. [DOI] [PubMed] [Google Scholar]
- 25.Khani A, Asghari J. Insecticide activity of essential oils of Mentha longifolia, Pulicaria gnaphalodes and Achillea wilhelmsii against two stored product pests, the flour beetle, Tribolium castaneum, and the cowpea weevil, Callosobruchus maculatus. J Insect Sci. 2012;12:73. doi: 10.1673/031.012.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahboubi M. Iranian medicinal plants as antimicrobial agents. J Microbiology, Biotech Food Sci. 2013;2:2388–405.27. [Google Scholar]
- 27.Nasr Isfahani B, Bahadorane bagh baderani M, Esmi Zh, Hoseini N, Tavakoli dastjerdi A, Javadi A, et al. Tuberculosis. Iran: Isfahan university publisher; 2009. p. 164. [Google Scholar]


