Abstract
Pseudotumors are a complication of hip arthroplasty. The goal of this article is to review the clinical presentation, pathogenesis, histology, and the role of diagnostic imaging in clinical decision making for treatment, and surveillance of pseudotumors. We will discuss the multimodal imaging appearances, differential diagnosis, associated complications, treatment, and prognosis of pseudotumors, as an aid to the assessment of orthopedic prostheses at the hip.
Keywords: Hip, metal, pseudotumor, replacement, total hip arthroplasty

INTRODUCTION
Total hip arthroplasty (THA) has been the principle treatment of crippling hip arthropathy for several decades.[1] Nearly, 270,000 primary THA are performed each year in the United States, and may number 570,000 by 2030.[2,3] The latest generation of metal-on-metal (MOM) bearings for THA feature reduced mechanical wear, increased stability, and improved range of motion compared to traditional metal-on-polyethylene (MOP) bearings.[4,5] However, the overall 5-year revision rate for MOM bearings is 6.2%, which is actually more than double the risk of revision for non-MOM bearings.[1] Aseptic loosening is the most common reason for MOM THA failure, although infection, metal adverse reaction, periprosthetic fracture, hardware dysfunction, and malposition are also frequently encountered.[6]
Adverse reaction to metal is associated with the development of cystic or solid periprosthetic pseudotumors. The associated terminology is controversial, and these lesions also have been described as cysts, bursae, inflammatory masses, and adverse reactions to metal debris.[7,8] Classically, a pseudotumor is defined as a non-neoplastic and non-infectious mass resulting from a circumscribed fibrous exudate of inflammatory origin, fluid accumulation, or other cause. For the purposes of this article, a pseudotumor is defined as a non-neoplastic and non-infectious cystic or solid mass associated with a hip arthroplasty.
EPIDEMIOLOGY AND CLINICAL PRESENTATION
The natural history of pseudotumors is not well understood.[9] The prevalence following MOM THA is 1–39%.[10,11,12] Pseudotumors, however, are often discordant with the presence of patient symptoms or the requirement for THA revision.[10,13,14] Asymptomatic pseudotumors are incidental findings in 57–78% of cases.[10,13,15,16] Symptomatic pseudotumors, on the other hand, are less common.[10,13] Patient complaints most often include groin pain, hip discomfort, paresthesia, antalgic gait, and/or a palpable mass.[13,17] However, the actual rate of THA revision due to symptomatic pseudotumor is only 1.7–5.6%.[13,18]
ETIOPATHOGENESIS
Metal ions
Government agencies and medical organizations link metal ions to the adverse reaction to metal, including pseudotumor formation.[4,10] The Food and Drug Administration in the United States and the Medicines and Healthcare Products Regulatory Agency in the United Kingdom have recognized serum chromium (Cr) and cobalt (Co) as surrogate markers for evidence of metallic degradation of MOM THA.[4,19] Local metal concentrations in periprosthetic soft tissues also have been investigated for associations with the adverse reaction to metal.[20]
All types of MOM THA bearings have been associated with pseudotumors, including large head, small head, and hip resurfacing prostheses.[2,13,20,21] A less well-known fact, however, is that pseudotumor formation also occurs in association with MOP and ceramic-on-polyethylene (COP) THA prostheses.[17,22,23,24,25] Pseudotumor formation has been even reported in the setting of unipolar hemiarthroplasty.[26] Additional sites of MOM contact for hip prostheses are the head-neck taper junction and neck-stem junction of the femoral component of hip prostheses [Figure 1].[27,28,29,30] These sites of MOM contact are a regular feature of many MOM, MOP, COP, and other nonmetal bearing THA.[22,23,24,31] These “modular” systems were designed to bestow greater intra-operative flexibility for surgeons.[32] The modular head-neck taper junction features a male component (also known as the “trunnion”) and which is fitted to a female component.[27] Similarly, a femoral neck must be fitted to the stem in modular neck-stem systems.
Figure 1.
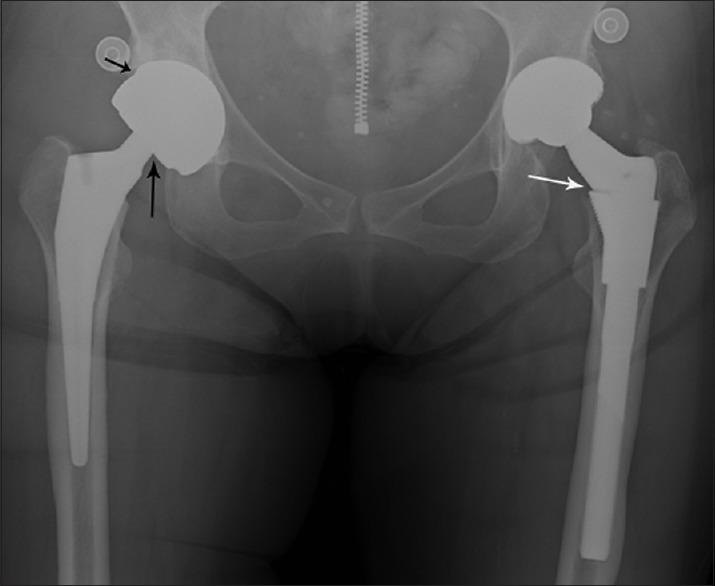
68-year-old woman with bilateral hip replacements presents with acute right hip pain attributed to her hip prosthesis. Anteroposterior view of the pelvis shows a right hip metal-on-metal total hip arthroplasty with a metal-on-metal bearing articulation (short black arrow) and a femoral head-neck taper junction (long black arrow). A modular femoral neck stem junction (long white arrow) is present at the left hip metal-on-polyethylene total hip arthroplasty. No radiographic evidence of an acute abnormality or visible metallic debris is identified.
The degree of metal ion release from hip prostheses is variable. In general, individual metallic particles are <50 nm in size and are shed without visible damage to the prosthesis.[33] By contrast, macroscopic metallic debris is visible to the naked eye.[22,34] Reported metal ions released from prostheses include Co, Cr, nickel, titanium, aluminum, and iron to name a few.[33] For MOM THA, mechanical edge wear at the femoral-acetabular articulation is a prominent source of metal debris.[34,35] Fretting and corrosion are additional modes of metallic degradation at the head-neck junction taper and neck-stem junction of modular hip arthroplasty prostheses.[17,23,26,27,28,31] Fretting is the process of mechanical wear by microrepetitive sliding between two metallic surfaces, whereas corrosion is the gradual degradation of metal as a result of interaction with the local surrounding environment.[17] Predisposing factors for metal ion shedding from modular interfaces include (1) poor fit from component mismatch, (2) mismatch of metal alloys, and (3) high frictional torque.[17,22,24,32,36] Complete eccentric wear of a polyethylene liner for MOP THA creates abnormal contact between metallic femoral and acetabular components and is another cause of metal ion shedding.[37]
HISTOPATHOLOGY
Periprosthetic tissues near sites of MOM contact demonstrate a distinct pattern of inflammation.[38] Two prominent histologic features include a perivascular lymphocytic infiltrate and an accumulation of plasma cells in association with macrophages containing variable amounts of metallic wear particles. The synovial linings of tissues obtained in MOM THA are more frequently ulcerated when compared to other types of implants.[38] This unique lymphocytic perivascular infiltration has been termed aseptic lymphocytic vasculitis-associated lesion.[39,40] Two different mechanisms for patient adverse tissue reaction include metal hypersensitivity and high wear debris. There is variability in the amount and distribution of metal debris, degree of necrosis, and the number, type, and arrangement of inflammatory cells. Macrophages and lymphocytes are present in all cases; however, patients with extensive infiltrates of macrophages tend to have smaller lymphocytic aggregates [Figure 2]. This is more often seen in patients with high wear, with macrophages containing high concentrations of metal particles. Contrastingly, large, dense lymphocytic aggregates are seen in association with small to moderate amounts of macrophages in patients with suspected metal hypersensitivity reactions [Figure 3]. Destruction and ulceration of the synovial lining with adherent or organized fibrin deposition and necrosis are commonly seen, although the degree of involvement of the synovial surface is often greater in patients with suspected metal sensitivity reactions.[7]
Figure 2.
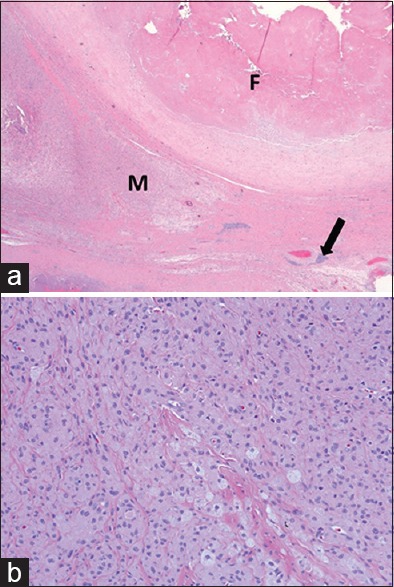
70-year-old man with increasing left hip pain diagnosed with high wear of a metal-on-metal total hip arthroplasty requiring left hip arthroplasty revision surgery. (a) Light microscopy (H and E, ×2.5) shows replacement of the synovial surface by organizing fibrin (F), prominent infiltrates of macrophages (M) with variable amounts of metallic wear debris and scattered perivascular lymphocyte aggregates (arrow). (b) Light microscopy (H and E, ×20) high power view of the perivascular lymphocyte aggregate indicated by the arrow in (a) shows prominent infiltrate of macrophages containing wear particles
Figure 3.
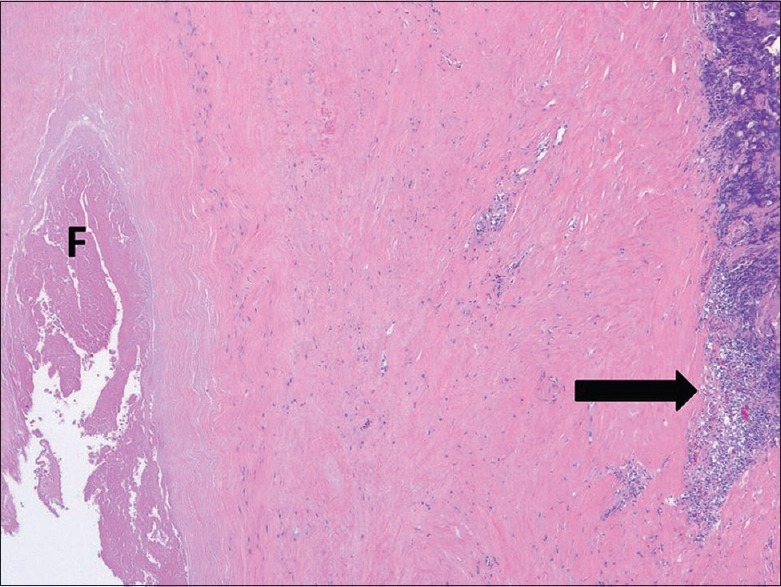
58-year-old man with complaints of worsening right hip pain diagnosed with metal hypersensitivity following surgery for revision of his right hip metal-on-metal total hip arthroplasty. Light microscopy (H and E, ×2.5) shows replacement of the synovial surface by fibrin (F) and dense lymphocyte aggregates (arrow) distal to the surface with an intervening layer of densely hyalinized, paucicellular tissue.
IMAGING
Radiographs
A common set of radiographs includes anteroposterior (AP) and lateral views of the hip and an AP view of the pelvis, which are obtained for both immediate postoperative assessment and long-term surveillance.[10,41] However, radiographs have poor sensitivity for detection of pseudotumors, and orthopedic surgeons have a low threshold to request cross-sectional imaging when a pseudotumor is suspected.[10]
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is an excellent modality for evaluation of periarticular soft-tissue complications following hip arthroplasty. Pseudotumors have a variable appearance on MRI [Figure 4] and generally show no enhancement [Figure 5]. Pseudotumors range from discrete thin-walled cystic lesions to ill-defined solid masses, often associated with synovial thickening, surrounding fluid, and/or scattered debris. Disruption of the pseudocapsule is common, allowing decompression of fluid and debris into adjacent periarticular bursae.
Figure 4.
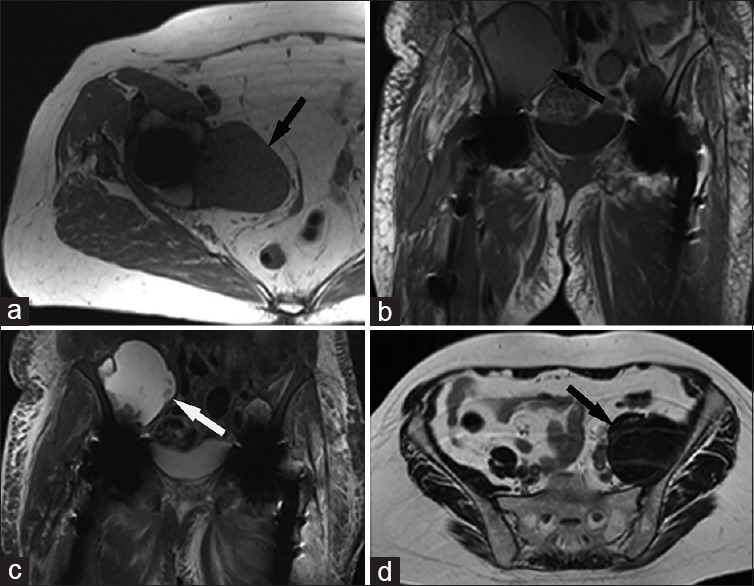
75-year-old woman with a right total hip arthroplasty presents with a pelvic mass of unknown origin. (a) Axial T1-weighted magnetic resonance image of the right hip shows a T1 intermediate pelvic mass (arrow) which is isointense to skeletal muscle. 77-year-old female with a right iliopsoas mass and bilateral total hip arthroplasty. (b) Cor T1 and (c) short tau inversion recovery-weighted magnetic resonance images of the pelvis show a right-sided mass (arrows) which is hyperintense on T1 and T2. sixty-seven-year-old female with a left total hip arthroplasty and asymptomatic retroperitoneal mass. (d) Axial T2-weighted magnetic resonance image shows a large T2 dark mass (arrow) in the expected location of the left iliopsoas muscle.
Figure 5.
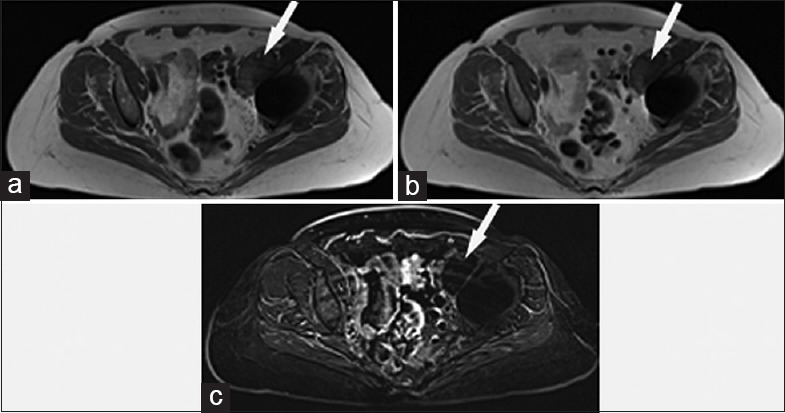
68-year-old woman with a left total hip arthroplasty and concern for a malignant left-sided periarticular mass. (a) Axial T1-weighted magnetic resonance image shows a focal left-sided periarticular mass (arrow) which hyperintense to skeletal muscle. (b) Contrast-enhanced axial T1-weighted magnetic resonance image shows similar appearance to the noncontrast sequence, although the T1 hyperintense nature of the mass (arrow) limits diagnostic sensitivity for detection of enhancement. (c) Axial subtraction sequence magnetic resonance image (produced by subtraction of the pre- and post-T1-weighted sequences) shows no enhancement of the mass (arrow).
MRI artifacts are commonly encountered when imaging hip prostheses. Local magnetic field inhomogeneity results in several imaging artifacts, including spatial misregistration, signal voids, and inhomogeneous fat suppression.[42] Common metal artifact reduction sequences (MARS) MRI techniques utilize fast-spine echo sequences in the axial, coronal, and sagittal planes. Strategies for metal artifact reduction can be achieved by increasing the amplitude of the frequency encoding gradient so that it is as large as possible compared to the susceptibility-induced gradients produced in the tissue by the metal implant.[43] Widening the receiver bandwidth and using thinner sections are also standard strategies for minimizing artifacts. Image quality also can be improved by using a high number of excitations, inversion-recovery, intermediate echo times for fluid sensitive images, and a larger matrix in the frequency direction.[44,45] Several modern MRI scanners also have vendor-specific MARS software which employs modified spin-echo sequences with view angle tilting. Multiacquisition variable-resonance image combination techniques now allow for metal reduction on 3T as well as 1.5T systems.[46]
An anatomic MRI grading system has been developed for periprosthetic pseudotumors. Pseudotumors are classified into three groups: Type I are thin-walled cystic masses (cyst wall <3 mm), Type II are thick-walled cystic masses (cyst wall >3 mm, but less than the diameter of the cystic component), and Type III are predominantly solid masses. Severity of symptoms and revision rates have been shown to increase from Type I to Type III.[47] Alternative grading schemes also have been used with similar groupings of findings.[15,48] In addition, predictive models have been recently developed for assessment of adverse tissue reactions and tissue destruction on MRI.[49]
Computed tomography
Computed tomography (CT) is a useful multiplanar imaging tool for the assessment of hip implants. CT is advantageous for evaluating bones, hardware, and bone cement, as well as heterotopic ossification, osteolysis, periprosthetic fracture, and metallosis.[40] CT allows for depiction of radiographically occult cystic and solid pseudotumors [Figures 6a and 7a], although this modality is less sensitive than MRI for evaluation of adverse local tissue reactions.[44,45] CT, however, is indicated for individuals who cannot tolerate MRI or who have extensive MRI-associated artifacts. The administration of iodinated contrast is useful for the localization and characterization of periarticular cystic pseudotumors, bursal-centered masses, and soft tissue fluid collections. Comparison of enhanced and unenhanced CT images is necessary for solid pseudotumors, since a paucity of actual enhancement is expected and internal debris within the mass may mimic enhancement when no unenhanced images are available for comparison [Figure 7].
Figure 6.
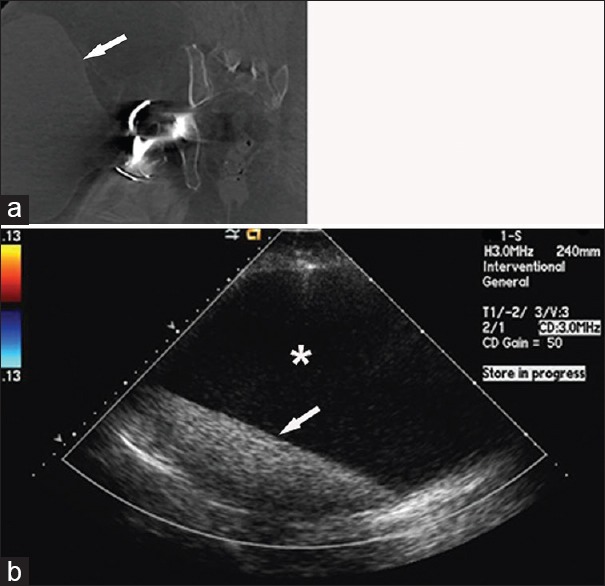
69-year-old woman with a right total hip arthroplasty presents with the right hip pain and swelling. (a) Contrast-enhanced coronal computed tomography image of the right hip shows a large cystic-appearing mass (arrow) associated with the right hip and extending into the subcutaneous soft tissues. (b) Duplex color Doppler ultrasound confirms the cystic nature of the mass. The mass is avascular, contains anechoic fluid (asterisk), and has layering internal echogenic debris (arrow).
Figure 7.
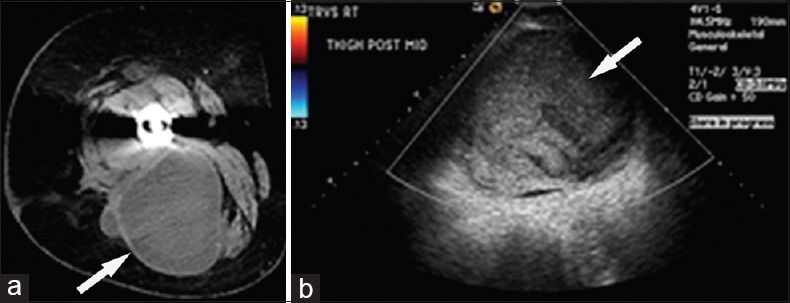
69-year-old woman complains of on-going right hip pain following placement of a right total hip arthroplasty. (a) Contrast-enhanced axial computed tomography image of the right hip shows a large mixed density mass (arrow) in the posterior compartment of the right thigh. (b) Duplex color Doppler ultrasound shows an avascular mass (arrow) with mixed internal echogenicity and posterior acoustic enhancement.
Beam-hardening, scatter, and photon starvation artifacts are the major causes of CT image degradation when imaging metallic implants.[50] There are several strategies for metal artifact reduction on conventional CT when scanning prosthetic hip implants, including increasing tube current (350–650 mAs), increasing peak kilovoltage (using 140 kVp instead of 120 kVp), using narrow collimation (0.625–0.75 mm), reducing pitch (<1), and avoiding dose modulation.[51,52] Postprocessing techniques are useful for further reduction of metal artifact. These include the use of iterative reconstruction, soft tissue reconstruction algorithms, thicker slices for multiplanar reformatted images, and vendor-specific metal reduction software.[53] Dual energy CT, where available, offers additional metal reduction capabilities compared to conventional CT.[54]
A grading scheme has been devised and validated for postoperative changes seen on CT. The grading system distinguishes between three categories of capsular changes (A, B, and C). Category A consists of those patients with a normal postoperative appearance or reactive capsular thickening without posterior bulging beyond the neck of the prosthesis or eccentric capsular enlargement. Category B includes those patients with bulging of the capsule both anteriorly and posteriorly. Category C contains those patients with eccentric capsular enlargement predominantly inferomedial to the prosthetic head as well as extensive filling of the subtrochanteric bursa posterior and/or filling of the iliopectineal bursa anterior with potential extension into the abdominal cavity.[55]
Ultrasound
Ultrasound is useful for the identification and characterization of periprosthetic masses, due to its portability and availability for patient care.[45] Examinations are conducted with a low-frequency probe using gray-scale imaging at the anterior, lateral, and posterior aspects of the hip.[16] Pseudotumors have a varied appearance on ultrasound: (1) Simple fluid-filled mass with a thin wall, (2) solid mass with no significant fluid component, or a (3) complex cystic fluid-filled mass with thick walls and/or solid internal components [Figures 6b and 7b].[56] Pseudotumors usually demonstrate no significant internal vascularity on duplex color or power Doppler imaging.[45]
The operator-dependent nature of ultrasound is a potential pitfall of this modality, as well as the familiarity of the sonographer with hip imaging. In comparison to MARS MRI, ultrasound is considered to be less practical for preoperative planning and long-term surveillance.[57] Direct communication between the radiologist and surgeon regarding the extent of pseudotumor-related sonographic findings also takes on increased importance compared to other cross-sectional imaging modalities, since nonradiologists may feel less confident about viewing ultrasound images on their own.[58]
Arthrography
Arthrography is a useful adjunct modality for problem-solving in certain cases.[59,60] The visualization of contrast filling a periarticular mass following intra-articular injection confirms communication with the hip joint and favors a pseudotumor over a malignant mass [Figure 8]. However, the lack of contrast filling a periarticular mass on arthrography does not rule out pseudotumor as the diagnosis.
Figure 8.
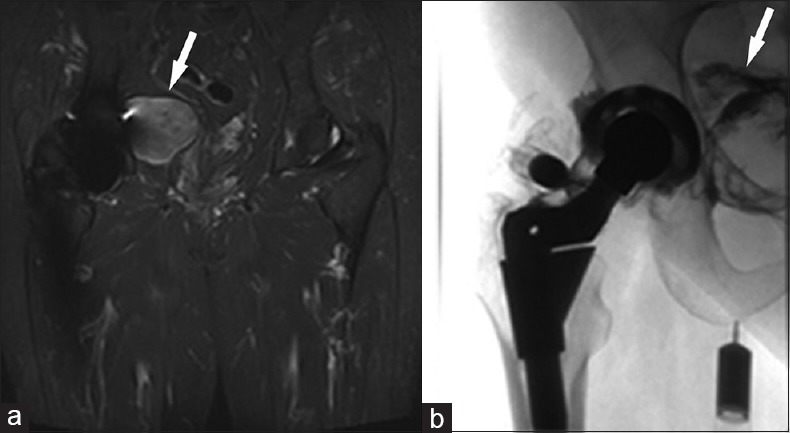
75-year-old woman with a right total hip arthroplasty and a mass of unknown origin, referred to radiology to investigate possible communication of the mass with the right hip joint. (a) Coronal short tau inversion recovery-weighted magnetic resonance image of the right hip shows a heterogeneously bright T2 mass (arrow) in the pelvis abutting the medial wall of the right acetabulum. (b) Anteroposterior fluoroscopic spot arthrogram image of the right hip show a metal-on-polyethylene total hip arthroplasty with a modular femoral neck-stem junction. A spinal needle is positioned into the right hip joint and contrast flows into the pelvic mass (arrow) after injection, confirming the mass is associated with the total hip arthroplasty.
DIFFERENTIAL DIAGNOSIS
Malignant masses
Periprosthetic primary malignant tumors are rare in the setting of hip arthroplasty, with an estimated incidence of 1.43/100,000.[61] Malignant fibrous histiocytoma is the most common periprosthetic soft tissue malignant neoplasm, although liposarcoma, synovial cell sarcoma, malignant peripheral nerve sheath tumor, and non-Hodgkin lymphoma, among others, have been reported.[61,62] Periprosthetic malignant tumors of bone also have been infrequently reported, including osteosarcoma, chondrosarcoma, malignant fibrous histiocytoma, fibrosarcoma, epidermoid carcinoma, and non-Hodgkin lymphoma.[61,62,63,64,65,66] Although metastasis to bone is by far the most common form of malignant bone tumor, only a handful of case reports exist in the literature at the site of THA.[61]
Despite the relative common incidence of pseudotumors and rarity of malignant neoplasms, patients are often alarmed by the discovery of a periprosthetic “mass”. Unfamiliarity with pseudotumors also may lead radiologists and clinicians to misinterpret these masses as worrisome for malignancy. This situation not uncommonly leads clinicians to request biopsy. However, biopsy is not without risk, since pathologists unfamiliar with pseudotumors may be confused by the histology or may misinterpret tissue specimens as suspicious for malignancy.[67] The misdiagnosis of pseudotumor as a spindle cell sarcoma is a pitfall, and close communication among clinicians, radiologists, and pathologists is necessary for ensuring accurate interpretation of biopsy results.[67]
Benign masses
Seroma and hematoma formation are well-known complications of hip arthroplasty.[68] Seromas are simple fluid collections occurring along the surgical tract or periarticular soft tissues of the hip. Hematomas are caused by bleeding in the juxtaarticular soft tissues or along the surgical tract. In general, hematomas have a variable appearance on cross-sectional imaging, depending on the age of the mass.[69] Hematoma formation is also a known complication of anticoagulants.[68] Seroma and hematoma are differentiated from a pseudotumor by their development in the immediate postoperative period and subsequent resolution over time.
Soft tissue abscess is an additional periarticular mass which must be distinguished from pseudotumor. Local or systemic symptoms and signs related to pain, erythema, fever, malaise, and palpable mass prompt a clinical work up to rule out this diagnosis.[70] Peripheral enhancement of the cystic fluid collection is the typical finding on postcontrast imaging.[70,71] Ultimately, positive cultures following percutaneous aspiration or surgical debridement confirm the diagnosis.
PSEUDOTUMOR-RELATED COMPLICATIONS
Pseudotumors exert direct pressure effects on adjacent structures. Compression of intrapelvic and thigh veins are associated with the development of deep venous thrombosis.[72,73] External compression of venous structures also has been described which produce lower extremity edema and mimic the clinical symptoms and signs of deep venous thrombosis.[59,74,75] Femoral and sciatic nerve palsy are known to occur following compression or encasement by an adjacent pseudotumor.[58,76] Ureteral obstruction and vesical compression are urinary complications.[77,78]
Periarticular soft tissue and bone destruction are other complications of pseudotumor formation.[22,58,79] Dehiscence of thigh and gluteal muscular compartments following periarticular infiltration often leads to instability and recurrent dislocations.[58,79]
TREATMENT AND PROGNOSIS
Clinical management of pseudotumors is controversial since there is no clear consensus for optimal treatment or surveillance.[10] What is clear regarding pseudotumors, however, is the propensity for poor outcomes following surgical intervention.[10,16] The incidence of major complications following revision hip arthroplasty due to pseudotumor occurs in up to 50% cases compared to only 14% for all other indications.[79] Nearly, one-third of patients revised for pseudotumor will receive additional revisions, with many experiencing the same level of pain and hip dysfunction that existed before the primary surgery.[79] Coordination among several surgical subspecialties for a staged intervention is often necessary due to the pseudotumor location and extent of tissue destruction.[58] The difficult nature of revision arthroplasty contributes to poor outcomes, especially with solid pseudotumors.[58]
Although there is no validated algorithm for treatment and surveillance of pseudotumors, there is four basic elements in current clinical practice: (1) Clinical examination, (2) hip radiography, (3) cross-sectional imaging, and (4) serum metal ion evaluation.[19] Patients report subjective complaints and surgeons assess for objective abnormalities at the time of clinical examination. This step is critical for subsequent clinical decision making, since this process identifies a hip as symptomatic or asymptomatic. Hip radiographs are obtained to establish a baseline examination and providing long-term surveillance. However, radiographs are of limited usefulness for the symptomatic hip when the arthroplasty appears normal.[10] Cross-sectional imaging methods, on the other hand, are sensitive for the diagnosis of cystic and solid pseudotumors.[47,48,55,56] MRI, CT, and ultrasound studies provide information necessary for clinical decision making, treatment, and surveillance.[19,58,67]
Governmental agencies, such as the Medicines and Health Care Products Regulatory Agency in the United Kingdom, have stated that serum Cr and Co metal ion levels >7 μg/L are worrisome in the setting of unilateral MOM THA.[19] However, the use of a specific “threshold” metal ion level is considered controversial by the medical community, since asymptomatic pseudotumors not uncommonly present with levels >7 μg/L and symptomatic hips often present with levels <7 μg/L.[16,19] Some investigators have proposed a normal range for Co (0–4.0 μg/L) and Cr (0–4.6 μg/L), although this subject remains controversial.[19] Nonuniform application of metal ion levels for clinical decision making exists in current practice.[16,19]
Despite the lack of general consensus, there are certain trends for the management of pseudotumors. Symptomatic hips associated with pain and dysfunction, large pseudotumors, and elevated metal ion levels are likely to receive consideration for revision arthroplasty. Pseudotumors without associated pain, dysfunction, or elevated metal ion levels are more likely to receive ongoing surveillance without surgical intervention. Pseudotumors with serum metal ion levels >20 μg/L, with or without symptoms, are likely to receive serious consideration for revision arthroplasty since these cases are associated with severe cardiac and neurological complications from metal poisoning.[19,80]
CONCLUSION
Hip arthroplasty is the principle treatment option for crippling arthropathy. Pseudotumors manifest in a varying range of clinical presentations, occurring in patients with or without symptoms. Knowledge of the imaging appearance and pathogenesis of pseudotumors will aid clinical decision making for patients with hip arthroplasty.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Jane C. Morrison, Department of Pathology, Oregon Health and Science University for her input and preparation of histologic slides.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2016/6/1/17/181493
REFERENCES
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century: Total hip replacement. Lancet. 2007;370:1508–19. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW. National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: Analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379:1199–204. doi: 10.1016/S0140-6736(12)60353-5. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Wiley KF, Ding K, Stoner JA, Teague DC, Yousuf KM. Incidence of pseudotumor and acute lymphocytic vasculitis associated lesion (ALVAL) reactions in metal-on-metal hip articulations: A meta-analysis. J Arthroplasty. 2013;28:1238–45. doi: 10.1016/j.arth.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Engh CA, Jr, Ho H, Engh CA. Metal-on-metal hip arthroplasty: Does early clinical outcome justify the chance of an adverse local tissue reaction? Clin Orthop Relat Res. 2010;468:406–12. doi: 10.1007/s11999-009-1063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabi D, Levine B, Paprosky W, Della Valle C, Sporer S, Klein G, et al. Metal-on-metal total hip arthroplasty: Causes and high incidence of early failure. Orthopedics. 2012;35:e1009–16. doi: 10.3928/01477447-20120621-12. [DOI] [PubMed] [Google Scholar]
- 7.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–7. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: Prevalence and metal ion study. J Arthroplasty. 2011;26:511–8. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Almousa SA, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. The natural history of inflammatory pseudotumors in asymptomatic patients after metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2013;471:3814–21. doi: 10.1007/s11999-013-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Weegen W, Sijbesma T, Hoekstra HJ, Brakel K, Pilot P, Nelissen RG. Treatment of pseudotumors after metal-on-metal hip resurfacing based on magnetic resonance imaging, metal ion levels and symptoms. J Arthroplasty. 2014;29:416–21. doi: 10.1016/j.arth.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Bosker BH, Ettema HB, Boomsma MF, Kollen BJ, Maas M, Verheyen CC. High incidence of pseudotumour formation after large-diameter metal-on-metal total hip replacement: A prospective cohort study. J Bone Joint Surg Br. 2012;94:755–61. doi: 10.1302/0301-620X.94B6.28373. [DOI] [PubMed] [Google Scholar]
- 12.Murray DW, Grammatopoulos G, Gundle R, Gibbons CL, Whitwell D, Taylor A, et al. Hip resurfacing and pseudotumour. Hip Int. 2011;21:279–83. doi: 10.5301/HIP.2011.8405. [DOI] [PubMed] [Google Scholar]
- 13.Bisschop R, Boomsma MF, Van Raay JJ, Tiebosch AT, Maas M, Gerritsma CL. High prevalence of pseudotumors in patients with a Birmingham Hip Resurfacing prosthesis: A prospective cohort study of one hundred and twenty-nine patients. J Bone Joint Surg Am. 2013;95:1554–60. doi: 10.2106/JBJS.L.00716. [DOI] [PubMed] [Google Scholar]
- 14.Munro JT, Masri BA, Duncan CP, Garbuz DS. High complication rate after revision of large-head metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472:523–8. doi: 10.1007/s11999-013-2979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: A case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94:317–25. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 16.Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011;93:2164–71. doi: 10.2106/JBJS.J.01884. [DOI] [PubMed] [Google Scholar]
- 17.Scully WF, Teeny SM. Pseudotumor associated with metal-on-polyethylene total hip arthroplasty. Orthopedics. 2013;36:e666–70. doi: 10.3928/01477447-20130426-33. [DOI] [PubMed] [Google Scholar]
- 18.Berend KR, Morris MJ, Adams JB, Lombardi AV., Jr Metal-on-metal hip arthroplasty: Going, going, gone… – affirms. J Bone Joint Surg Br. 2012;94(11 Suppl A):75–7. doi: 10.1302/0301-620X.94B11.30745. [DOI] [PubMed] [Google Scholar]
- 19.Van Der Straeten C, Grammatopoulos G, Gill HS, Calistri A, Campbell P, De Smet KA. The 2012 Otto Aufranc Award: The interpretation of metal ion levels in unilateral and bilateral hip resurfacing. Clin Orthop Relat Res. 2013;471:377–85. doi: 10.1007/s11999-012-2526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohmann CH, Meyer H, Nuechtern JV, Singh G, Junk-Jantsch S, Schmotzer H, et al. Periprosthetic tissue metal content but not serum metal content predicts the type of tissue response in failed small-diameter metal-on-metal total hip arthroplasties. J Bone Joint Surg Am. 2013;95:1561–8. doi: 10.2106/JBJS.L.01273. [DOI] [PubMed] [Google Scholar]
- 21.Beaulé PE, Kim PR, Hamdi A, Fazekas A. A prospective metal ion study of large-head metal-on-metal bearing: A matched-pair analysis of hip resurfacing versus total hip replacement. Orthop Clin North Am. 2011;42:251–7. doi: 10.1016/j.ocl.2011.01.005. ix. [DOI] [PubMed] [Google Scholar]
- 22.Bisseling P, Tan T, Lu Z, Campbell PA, Susante JL. The absence of a metal-on-metal bearing does not preclude the formation of a destructive pseudotumor in the hip – A case report. Acta Orthop. 2013;84:437–41. doi: 10.3109/17453674.2013.823590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu AR, Gross CE, Levine BR. Pseudotumor from modular neck corrosion after ceramic-on-polyethylene total hip arthroplasty. Am J Orthop (Belle Mead NJ) 2012;41:422–6. [PubMed] [Google Scholar]
- 24.Mao X, Tay GH, Godbolt DB, Crawford RW. Pseudotumor in a well-fixed metal-on-polyethylene uncemented hip arthroplasty. J Arthroplasty. 2012;27:493.e13–7. doi: 10.1016/j.arth.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Walsh AJ, Nikolaou VS, Antoniou J. Inflammatory pseudotumor complicating metal-on-highly cross-linked polyethylene total hip arthroplasty. J Arthroplasty. 2012;27:324.e5–8. doi: 10.1016/j.arth.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Whitehouse MR, Endo M, Masri BA. Adverse local tissue reaction associated with a modular hip hemiarthroplasty. Clin Orthop Relat Res. 2013;471:4082–6. doi: 10.1007/s11999-013-3133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthies AK, Racasan R, Bills P, Blunt L, Cro S, Panagiotidou A, et al. Material loss at the taper junction of retrieved large head metal-on-metal total hip replacements. J Orthop Res. 2013;31:1677–85. doi: 10.1002/jor.22431. [DOI] [PubMed] [Google Scholar]
- 28.Gill IP, Webb J, Sloan K, Beaver RJ. Corrosion at the neck-stem junction as a cause of metal ion release and pseudotumour formation. J Bone Joint Surg Br. 2012;94:895–900. doi: 10.1302/0301-620X.94B7.29122. [DOI] [PubMed] [Google Scholar]
- 29.Langton DJ, Jameson SS, Joyce TJ, Gandhi JN, Sidaginamale R, Mereddy P, et al. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br. 2011;93:1011–6. doi: 10.1302/0301-620X.93B8.26040. [DOI] [PubMed] [Google Scholar]
- 30.Burge AJ, Gold SL, Lurie B, Nawabi DH, Fields KG, Koff MF, et al. MR imaging of adverse local tissue reactions around rejuvenate modular dual-taper stems. Radiology. 2015;277:142–50. doi: 10.1148/radiol.2015141967. [DOI] [PubMed] [Google Scholar]
- 31.Cooper HJ, Della Valle CJ, Berger RA, Tetreault M, Paprosky WG, Sporer SM, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94:1655–61. doi: 10.2106/JBJS.K.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blakey CM, Eswaramoorthy VK, Hamilton LC, Biant LC, Field RE. Mid-term results of the modular ANCA-fit femoral component in total hip replacement. J Bone Joint Surg Br. 2009;91:1561–5. doi: 10.1302/0301-620X.91B12.22638. [DOI] [PubMed] [Google Scholar]
- 33.Keegan GM, Learmonth ID, Case CP. Orthopaedic metals and their potential toxicity in the arthroplasty patient: A review of current knowledge and future strategies. J Bone Joint Surg Br. 2007;89:567–73. doi: 10.1302/0301-620X.89B5.18903. [DOI] [PubMed] [Google Scholar]
- 34.Glyn-Jones S, Roques A, Taylor A, Kwon YM, McLardy-Smith P, Gill HS, et al. The in vivo linear and volumetric wear of hip resurfacing implants revised for pseudotumor. J Bone Joint Surg Am. 2011;93:2180–8. doi: 10.2106/JBJS.J.01206. [DOI] [PubMed] [Google Scholar]
- 35.Grammatopoulos G, Pandit H, Kamali A, Maggiani F, Glyn-Jones S, Gill HS, et al. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013;95:e81. doi: 10.2106/JBJS.L.00775. [DOI] [PubMed] [Google Scholar]
- 36.Chana R, Esposito C, Campbell PA, Walter WK, Walter WL. Mixing and matching causing taper wear: Corrosion associated with pseudotumour formation. J Bone Joint Surg Br. 2012;94:281–6. doi: 10.1302/0301-620X.94B2.27247. [DOI] [PubMed] [Google Scholar]
- 37.Murgatroyd SE. Pseudotumor presenting as a pelvic mass: A complication of eccentric wear of a metal on polyethylene hip arthroplasty. J Arthroplasty. 2012;27:820.e1–4. doi: 10.1016/j.arth.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 39.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 40.Cahir JG, Toms AP, Marshall TJ, Wimhurst J, Nolan J. CT and MRI of hip arthroplasty. Clin Radiol. 2007;62:1163–71. doi: 10.1016/j.crad.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Mulcahy H, Chew FS. Current concepts of hip arthroplasty for radiologists: Part 1, features and radiographic assessment. AJR Am J Roentgenol. 2012;199:559–69. doi: 10.2214/AJR.12.8843. [DOI] [PubMed] [Google Scholar]
- 42.Olsen RV, Munk PL, Lee MJ, Janzen DL, MacKay AL, Xiang QS, et al. Metal artifact reduction sequence: Early clinical applications. Radiographics. 2000;20:699–712. doi: 10.1148/radiographics.20.3.g00ma10699. [DOI] [PubMed] [Google Scholar]
- 43.Toms AP, Smith-Bateman C, Malcolm PN, Cahir J, Graves M. Optimization of metal artefact reduction (MAR) sequences for MRI of total hip prostheses. Clin Radiol. 2010;65:447–52. doi: 10.1016/j.crad.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Fritz J, Lurie B, Miller TT, Potter HG. MR imaging of hip arthroplasty implants. Radiographics. 2014;34:E106–32. doi: 10.1148/rg.344140010. [DOI] [PubMed] [Google Scholar]
- 45.Ostlere S. How to image metal-on-metal prostheses and their complications. AJR Am J Roentgenol. 2011;197:558–67. doi: 10.2214/AJR.11.6840. [DOI] [PubMed] [Google Scholar]
- 46.Nawabi DH, Hayter CL, Su EP, Koff MF, Perino G, Gold SL, et al. Magnetic resonance imaging findings in symptomatic versus asymptomatic subjects following metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013;95:895–902. doi: 10.2106/JBJS.K.01476. [DOI] [PubMed] [Google Scholar]
- 47.Hauptfleisch J, Pandit H, Grammatopoulos G, Gill HS, Murray DW, Ostlere S. A MRI classification of periprosthetic soft tissue masses (pseudotumours) associated with metal-on-metal resurfacing hip arthroplasty. Skeletal Radiol. 2012;41:149–55. doi: 10.1007/s00256-011-1329-6. [DOI] [PubMed] [Google Scholar]
- 48.Anderson H, Toms AP, Cahir JG, Goodwin RW, Wimhurst J, Nolan JF. Grading the severity of soft tissue changes associated with metal-on-metal hip replacements: Reliability of an MR grading system. Skeletal Radiol. 2011;40:303–7. doi: 10.1007/s00256-010-1000-7. [DOI] [PubMed] [Google Scholar]
- 49.Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2014;472:471–81. doi: 10.1007/s11999-013-2788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boas FE, Fleischmann D. CT artifacts: Causes and reduction techniques. Imaging Med. 2012;4:229–40. [Google Scholar]
- 51.Buckwalter KA, Lin C, Ford JM. Managing postoperative artifacts on computed tomography and magnetic resonance imaging. Semin Musculoskelet Radiol. 2011;15:309–19. doi: 10.1055/s-0031-1286013. [DOI] [PubMed] [Google Scholar]
- 52.Roth TD, Maertz NA, Parr JA, Buckwalter KA, Choplin RH. CT of the hip prosthesis: Appearance of components, fixation, and complications. Radiographics. 2012;32:1089–107. doi: 10.1148/rg.324115183. [DOI] [PubMed] [Google Scholar]
- 53.Boas FE, Fleischmann D. Evaluation of two iterative techniques for reducing metal artifacts in computed tomography. Radiology. 2011;259:894–902. doi: 10.1148/radiol.11101782. [DOI] [PubMed] [Google Scholar]
- 54.Nicolaou S, Liang T, Murphy DT, Korzan JR, Ouellette H, Munk P. Dual-energy CT: A promising new technique for assessment of the musculoskeletal system. AJR Am J Roentgenol. 2012;199(5 Suppl):S78–86. doi: 10.2214/AJR.12.9117. [DOI] [PubMed] [Google Scholar]
- 55.Boomsma MF, Edens MA, Van Lingen CP, Warringa N, Ettema HB, Verheyen CC, et al. Development and first validation of a simplified CT-based classification system of soft tissue changes in large-head metal-on-metal total hip replacement: Intra- and interrater reliability and association with revision rates in a uniform cohort of 664 arthroplasties. Skeletal Radiol. 2015;44:1141–9. doi: 10.1007/s00256-015-2146-0. [DOI] [PubMed] [Google Scholar]
- 56.Lainiala O, Elo P, Reito A, Pajamäki J, Puolakka T, Eskelinen A. Good sensitivity and specificity of ultrasound for detecting pseudotumors in 83 failed metal-on-metal hip replacements. Acta Orthop. 2015;86:339–44. doi: 10.3109/17453674.2014.1001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siddiqui IA, Sabah SA, Satchithananda K, Lim AK, Cro S, Henckel J, et al. A comparison of the diagnostic accuracy of MARS MRI and ultrasound of the painful metal-on-metal hip arthroplasty. Acta Orthop. 2014;85:375–82. doi: 10.3109/17453674.2014.908345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liddle AD, Satchithananda K, Henckel J, Sabah SA, Vipulendran KV, Lewis A, et al. Revision of metal-on-metal hip arthroplasty in a tertiary center: A prospective study of 39 hips with between 1 and 4 years of follow-up. Acta Orthop. 2013;84:237–45. doi: 10.3109/17453674.2013.797313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butler RA, Barrack RL. Total hip wear debris presenting as lower extremity swelling. A report of two cases. J Bone Joint Surg Am. 2004;86-A:142–5. doi: 10.2106/00004623-200401000-00022. [DOI] [PubMed] [Google Scholar]
- 60.Gruber FW, Böck A, Trattnig S, Lintner F, Ritschl P. Cystic lesion of the groin due to metallosis: A rare long-term complication of metal-on-metal total hip arthroplasty. J Arthroplasty. 2007;22:923–7. doi: 10.1016/j.arth.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Visuri T, Pulkkinen P, Paavolainen P. Malignant tumors at the site of total hip prosthesis. Analytic review of 46 cases. J Arthroplasty. 2006;21:311–23. doi: 10.1016/j.arth.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 62.Keel SB, Jaffe KA, Petur Nielsen G, Rosenberg AE. Orthopaedic implant-related sarcoma: A study of twelve cases. Mod Pathol. 2001;14:969–77. doi: 10.1038/modpathol.3880420. [DOI] [PubMed] [Google Scholar]
- 63.Martin A, Bauer TW, Manley MT, Marks KE. Osteosarcoma at the site of total hip replacement. A case report. J Bone Joint Surg Am. 1988;70:1561–7. [PubMed] [Google Scholar]
- 64.Bagó-Granell J, Aguirre-Canyadell M, Nardi J, Tallada N. Malignant fibrous histiocytoma of bone at the site of a total hip arthroplasty. A case report. J Bone Joint Surg Br. 1984;66:38–40. doi: 10.1302/0301-620X.66B1.6319423. [DOI] [PubMed] [Google Scholar]
- 65.Harris WR. Chondrosarcoma complicating total hip arthroplasty in Maffucci's syndrome. Clin Orthop Relat Res. 1990;260:212–4. [PubMed] [Google Scholar]
- 66.Ganapathi M, Lake DN, Griffiths AP. Periprosthetic high-grade B-cell lymphoma complicating an infected revision total hip arthroplasty. J Arthroplasty. 2001;16:229–32. doi: 10.1054/arth.2001.9827. [DOI] [PubMed] [Google Scholar]
- 67.Singh C, Kaplan A, Pambuccian SE. Necrotic granulomatous pseudotumor following metal-on-metal hip arthroplasty: A potential mimic of sarcoma on fine needle aspiration cytology. Diagn Cytopathol. 2012;40(Suppl 2):E104–8. doi: 10.1002/dc.21605. [DOI] [PubMed] [Google Scholar]
- 68.Mortazavi SM, Hansen P, Zmistowski B, Kane PW, Restrepo C, Parvizi J. Hematoma following primary total hip arthroplasty: A grave complication. J Arthroplasty. 2013;28:498–503. doi: 10.1016/j.arth.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 69.Wu JS, Hochman MG. Soft-tissue tumors and tumorlike lesions: A systematic imaging approach. Radiology. 2009;253:297–316. doi: 10.1148/radiol.2532081199. [DOI] [PubMed] [Google Scholar]
- 70.Turecki MB, Taljanovic MS, Stubbs AY, Graham AR, Holden DA, Hunter TB, et al. Imaging of musculoskeletal soft tissue infections. Skeletal Radiol. 2010;39:957–71. doi: 10.1007/s00256-009-0780-0. [DOI] [PubMed] [Google Scholar]
- 71.Fayad LM, Carrino JA, Fishman EK. Musculoskeletal infection: Role of CT in the emergency department. Radiographics. 2007;27:1723–36. doi: 10.1148/rg.276075033. [DOI] [PubMed] [Google Scholar]
- 72.Parfitt DJ, Wood SN, Chick CM, Lewis P, Rashid MH, Evans AR. Common femoral vein thrombosis caused by a metal-on-metal hip arthroplasty-related pseudotumor. J Arthroplasty. 2012;27:1581.e9–11. doi: 10.1016/j.arth.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 73.Regis D, Sandri A, Costa A, Bartolozzi P, Mazzilli G. Recurrent femoral deep vein thrombosis: Rare complication of a pelvic mass induced by polyethylene wear debris following total hip arthroplasty. A case report. Thromb Res. 2008;121:593–5. doi: 10.1016/j.thromres.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 74.Kawakita K, Shibanuma N, Tei K, Nishiyama T, Kuroda R, Kurosaka M. Leg edema due to a mass in the pelvis after a large-diameter metal-on-metal total hip arthroplasty. J Arthroplasty. 2013;28:197.e1–4. doi: 10.1016/j.arth.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 75.Maurer-Ertl W, Friesenbichler J, Liegl-Atzwanger B, Kuerzl G, Windhager R, Leithner A. Noninflammatory pseudotumor simulating venous thrombosis after metal-on-metal hip resurfacing. Orthopedics. 2011;34:e678–81. doi: 10.3928/01477447-20110826-32. [DOI] [PubMed] [Google Scholar]
- 76.Clayton RA, Beggs I, Salter DM, Grant MH, Patton JT, Porter DE. Inflammatory pseudotumor associated with femoral nerve palsy following metal-on-metal resurfacing of the hip. A case report. J Bone Joint Surg Am. 2008;90:1988–93. doi: 10.2106/JBJS.G.00879. [DOI] [PubMed] [Google Scholar]
- 77.Hananouchi T, Saito M, Nakamura N, Yamamoto T, Yonenobu K. Huge pelvic mass secondary to wear debris causing ureteral obstruction. J Arthroplasty. 2005;20:946–9. doi: 10.1016/j.arth.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Hattrup SJ, Bryan RS, Gaffey TA, Stanhope CR. Pelvic mass causing vesical compression after total hip arthroplasty. Case report. Clin Orthop Relat Res. 1988;227:184–9. [PubMed] [Google Scholar]
- 79.Grammatopoulos G, Pandit H, Kwon YM, Gundle R, McLardy-Smith P, Beard DJ, et al. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg Br. 2009;91:1019–24. doi: 10.1302/0301-620X.91B8.22562. [DOI] [PubMed] [Google Scholar]
- 80.Tower SS. Arthroprosthetic cobaltism: Neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: A case report. J Bone Joint Surg Am. 2010;92:2847–51. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]


