Abstract
Background:
There is a probable interaction of central angiotensin II (Ang II) and estrogen (Est) on blood pressure in deoxycorticosterone acetate (DOCA)-salt hypertensive rats. Therefore, in the present study, the interaction between Ang II and Est in ovariectomized (Ovx) and Sham rats that were treated with DOCA- salt was evaluated.
Materials and Methods:
The female rats were divided into 10 groups as follows: Sham, Ovx, Sham-DOCA, Ovx-DOCA, Sham-DOCA-estrogen (E), Ovx DOCA-E, Sham-DOCA-losartan (L), Ovx-DOCA-L, Sham–DOCA-L-E, and Ovx-DOCA-L-E. The Est groups received estradiol valerate (2 mg/kg; daily; subcutaneously (s.c)) for four weeks. Following that, several doses of Ang II (0.5, 5, 50, 500, 5000 ng/5 μl) were injected via the intracerebroventricular (i.c.v) route and the changes in systolic blood pressure (SBP) were evaluated. In the losartan groups, 200 μg losartan was injected (i.c.v) 15 minutes after the Ang II injection and the blood pressure was recorded. Treatment by DOCA was performed by removal of one kidney, injection of DOCA (45 mg/kg i.p), and adding of sodium chloride (NaCl) (1%) and potassium chloride (KCl) (0.1%) in the drinking water.
Results:
The SBP was increased by Ang II and this effect in DOCA-salt treated rat was higher than in the untreated groups. The effect of Ang II on SBP in groups that were treated with Est and L was lower than that in the DOCA-salt groups. Increase in SBP was strongly attenuated by Ang II in groups that were co-treated with both Est and L compared to the DOCA-treated rats. These results showed that Est significantly attenuated the effect of central Ang II on SBP in the DOCA-salt treated rats.
Conclusion:
We suggest that there are interactions between E and Ang II in the control of blood pressure in DOCA-salt treated rats.
Keywords: Ang II, blood pressure, DOCA-salt, estrogen, losartan, Ovx
INTRODUCTION
Hypertension is an important cause of cardiovascular disease that increases the rates of morbidity and mortality.[1] Mechanisms of hypertension are uncertain and this suggests that several factors are involved. One of the important systems that activate hypertension is the renin-angiotensin system (RAS). Angiotensin II (Ang II) is a principal effector of this system.[2] The role of RAS in cardiovascular regulation is well-known.[3,4] Both the central and peripheral effects of RAS on the cardiovascular system have been shown.[5,6] The effect of the RAS system, especially Ang II, on the central cardiovascular regulation, has been shown. All components of the RAS, especially Ang II and its receptors widely distributed in the central area involved in cardiovascular regulation such as, the rostral ventrolateral medulla (RVLM), nucleus tractus solitarius (NTS), and paraventricular nucleus (PVN).[6,7,8,9,10] The RAS also plays a critical role in the pathogenesis of several models of hypertension including DOCA-salt hypertension.[2,11] The DOCA-salt hypertension is a common model of hypertension, with several changes in the hormonal and neural activity, such as, increased Ang II activity, endothelin-1 (ET-1), vasopressin secretion, sympathetic activity, and a distorted baroreflex response.[11,12,13]
The epidemiological evidence suggests a role for sex-dependent mechanisms in the pathophysiology of hypertension. For example, the severity of hypertension has been shown to be lower in women than in men.[14] Numerous studies have reported that in blood pressure, estrogen (Est) is the main factor for sex difference.[15,16,17] Both the beneficial peripheral and central effects of Est on the cardiovascular system have been shown in several studies.[18] Its important peripheral cardiovascular effects are: Vasodilatation by increased endothelial production of nitric oxide,[19] inhibition of vascular smooth muscle cell proliferation,[20] prevention of oxidative stress, and decreased atherosclerosis.[21] The central cardiovascular effects of Est are mostly mediated by its effect on the central cardiovascular areas such as NTS and RVLM.[22,23,24]
Estrogen also has cardiovascular protective effects on DOCA-salt hypertension.[25] The mechanism of Est in DOCA-salt hypertension is not clarified, but it is suggested that it is mediated by the modulatory effect of Est on several parameters including RAS.[26] The effect of Est may also be mediated by decreased ET-1 and increased nitric oxide.[25,27] Shenoy et al. also showed that Est replacement significantly inhibits the interstitial fibrosis and cardiac hypertrophy induced by DOCA-salt hypertension.[26]
The effects of both Ang II and Est in the regulation of blood pressure are important. Several studies have shown that the effects of these agents on blood pressure are opposite and an interaction between Ang II and Est in the regulation of blood pressure in normotensive and hypertensive rats has also been reported.[28] For example, estrogen inhibits the activation of postrema neurons in the area by Ang II.[29] In addition, Est decreases blood pressure in Ang II-induced hypertension.[14] Although, the effect of Est on the pressor effect of Ang II in several animal hypertension models has been examined, the central interaction of Est and Ang II on blood pressure in DOCA-salt hypertension has not been investigated. Therefore, in the present study, the effects of central Ang II and Est on blood pressure in both Ovariectomy (Ovx) and Sham DOCA-salt treated rats have been evaluated and it has been determined whether Ang II and Est have a central interaction in this model of hypertension.
MATERIALS AND METHODS
Animals and drugs
Female Wistar rats, eight weeks old (240 ± 10 g) were used. The animals were housed in numbers of four to five per standard cage, at room temperature (24 ± 1°C) on a 12 hour light/dark cycle. Food and water were available ad libitum. Animal handling and all related procedures were approved by the Mashhad Medical University Committee on Animal Research. Ang II (Sigma, USA), Ketamine (Daru-Pakhsh Pharmaceutical Mfg Co, Iran), and Estrogen (Temad Ltd., Tehran, Iran) were used in this experiment.
Groups and desoxycorticostron acetate-salt model
The animal groups were: (Groups 1 and 2) Sham and Ovariectomy (Ovx); (Groups 3 and 4) Sham-Desoxycorticostron (DOCA), Ovx-DOCA; Groups (5 and 6) Sham-DOCA-Est (E), Ovx-DOCA-E; (Groups 7 and 8) Sham-DOCA losartan (L), Ovx-DOCA-L; (Groups 9 and 10) Sham-DOCA-L-E and Ovx-DOCA-L-E.
The animals were ovariectomized under ketamine anesthesia (150 mg/kg and xylazine 0.1 mg/kg i.p).[30,31] The abdomens were exposed and the ovaries and ovarian fats were removed. The same procedure was performed on Sham rats except that the wound was closed without removing the ovaries.[25] After surgery, the rats were given i.p 300,000 units of procaine penicillin G, to prevent infection. Treatment by DOCA was performed by removal of one kidney, injection of DOCA (45 mg/kg i.p), and adding of NaCl (1%) and KCl (0.1%) in drinking tap water. The animals were allowed seven days to recover from surgery.[32] The Est groups received estradiol valerate (2 mg/kg; s.c/four weeks).
Blood pressure recording and intracerebroventricular injection
After four weeks, for systolic blood pressure (SBP) recording, the animals were anesthetized with urethane (1.2 g/kg, i.p),[33] the left femoral artery was cannulated by a polyethylene tube, and an arterial catheter was connected to a blood pressure transducer and power laboratory system (AD instrument), after which the SBP was recorded.[34,35] For the intracerebroventricular (i.c.v) injection of Ang II, the animals were placed in a stereotaxic instrument (Stolting Instruments, USA). Stainless steel, 23-gauge guide cannulas were implanted 0.6 mm above the left lateral cerebral ventricle. Sterotaxic coordinates were selected according to the rat brain atlas of Paxinos and Watson (0.9 mm posterior to the bregma, lateral + 1.6 mm lateral to the sagittal suture, and 3 mm from the top of skull).[36,37] The cannulas were fixed with dental acrylic cement and anchored by two screws placed in the skull. A stylet (26-gauge stainless steel) was placed into the guide cannula to allow the guide cannula to maintain patency.[36,37]
After stabilization of SBP, for i.c.v injections of Ang II, an injection needle (26-gauge) was inserted into the guide cannula. The tip of the injection needle was 1 mm longer than guide cannula. The injection needle was attached to a 10-µl Hamilton syringe by a polyethylene tube. After recording of the baseline systolic blood pressure (SBP), saline and various doses of Ang II (0.5, 5, 50, 500, 5000 ng/5µl) were injected and the SBP measured 15 minutes after each injection.[38] In the losartan groups, 200 µg losartan was injected 15 minutes after the Ang II injection and the blood pressure was followed 45 minutes after each injection. The volume of the i.c.v injection was 5 µl in all groups.
Histology
Immediately after the tests, 2 µl of methylene blue was injected into the lateral ventricle, and then the rats were anesthetized with a high dose of urethane and the brains transcardially perfuse with 100 ml of saline, followed by 100 ml of formalin (10%). Then the brains were removed and placed in formalin (10%). After three days, the brains were sliced into 60 µm thin slices. Data from the rats with incorrect placement were excluded from the analysis.[30,31,36,37]
Data analyses
All data were expressed as means ± SEM. The systolic blood pressure was recorded and compared using two-way analysis of variance (ANOVA), followed by a post hoc test. The criterion for statistical significance was P < 0.05.
RESULTS
Baseline systolic blood pressure before injection of Ang II in the experimental groups
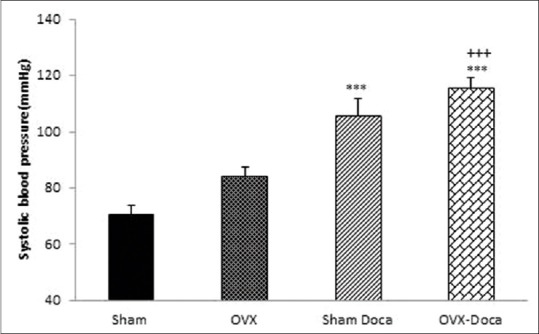
Baseline systolic blood pressure in Sham, Sham-DOCA, Ovx, Ovx-DOCA, Sham-DOCA-E, and Ovx DOCA E groups is shown in Table 1. As shown, although the basal SBP in the Ovx group was higher than in the Sham group, it was not significant compared to the Sham group. The SBP in both the Sham and Ovx groups, treated by DOCA, was significantly higher than in the untreated groups (P < 0.001 and P < 0.05, respectively). Administration of Est significantly decreased SBP in the DOCA-treated groups (P < 0.01). In addition, SBP in the Sham DOCA-E was lower than in the Ovx-DOCA-E group (P < 0.05) [Figures 1 and 2].
Table 1.
Comparison of systolic blood pressure induced by i.c.v injection of various doses of Ang II in Sham, Ovx, Sham and Ovx treated by DOCA, Sham and Ovx groups that treated by DOCA and estrogen + losartan
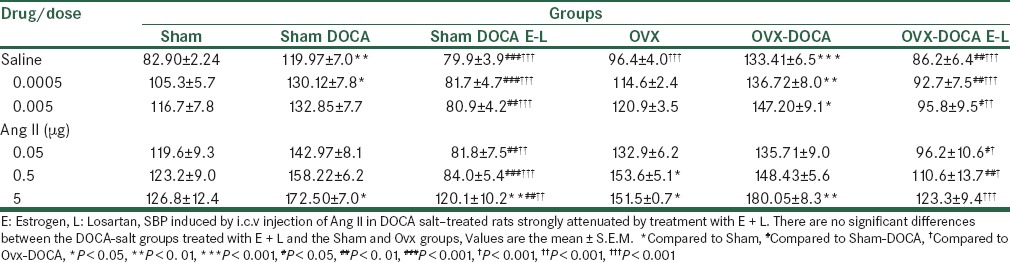
Figure 1.
Comparison of systolic blood pressure (SBP) in Sham, Ovx, Sham-DOCA, and Ovx-DOCA groups Values are the mean ± S.E.M, n = 8, *Compared to Sham, +Compared to Ovx, ***P < 0.001, +++P < 0.001
Figure 2.
Comparison of systolic blood pressure (SBP) in Sham- DOCA, Ovx-DOCA, Sham-DOCA-estrogen, and Ovx-DOCA-estrogen groups Values are the mean ± S.E.M. n=8, *Compared to Sham-DOCA, +Compared to Ovx-DOCA, #Sham-DOCA-estrogen, *P<0.05, +P<0.05, ##P<0.01
Effect of i.c.v injection of Ang II on systolic blood pressure in Sham DOCA rats treated with Estrogen and losartan
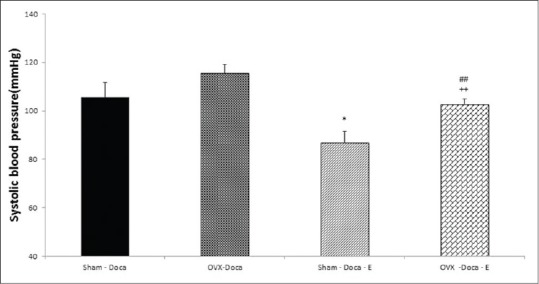
As shown in Figure 3, i.c.v injection of Ang II in various doses increased blood pressure in the Sham-DOCA group (P < 0.05 to P < 0.01). The effect of Ang II in Sham-DOCA-E and Sham-DOCA-L groups was lower than in the Sham-DOCA group (P < 0.05 to P < 0.01). In addition, the effect of Ang II on SBP in the Sham-DOCA E-L group was strongly attenuated, so it did not significantly compare to the Sham group.
Figure 3.
Effects of estrogen (Est, n=10), losartan (los, n=7) and Est + los (n=8) on systolic blood pressure (SBP) induced by i.c.v injection of various doses of Ang II in Sham DOCA salt-treated rats SBP significantly attenuated by estrogen and losartan Values are the mean ± S.E.M.*Compared to Sham DOCA, #Compared to Sham DOCA-Est, *P<0.05, **P<0.01, ***P<0.001, #P<0.05, ##P<0.01, ###P<0.001
Effect of i.c.v injection of Ang II on the systolic blood pressure of Ovx-DOCA rats treated with estrogen and losartan
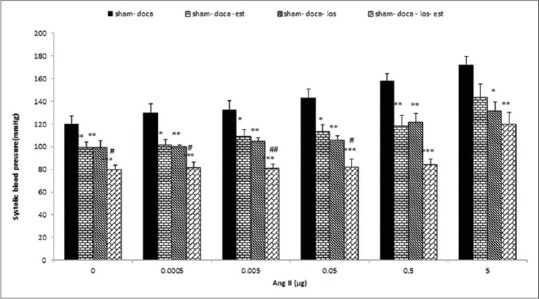
In this experiment injection of Ang II increased SBP in the Ovx-DOCA group, but in the Ovx-DOCA group treated with Est, the SBP was attenuated. Administration of losartan (Ovx-DOCA-L group) decreased the effect of Ang II on SBP in all doses (P < 0.05 to P < 0. 01). The effect of all doses of Ang II on the SBP in the Ovx- DOCA-E-L group that received both Est and losartan was also significantly lower than in the Ovx-DOCA (P < 0.01 to P < 0.001) and Ovx-DOCA-E groups (P < 0.05 to P < 0.01) [Figure 4].
Figure 4.
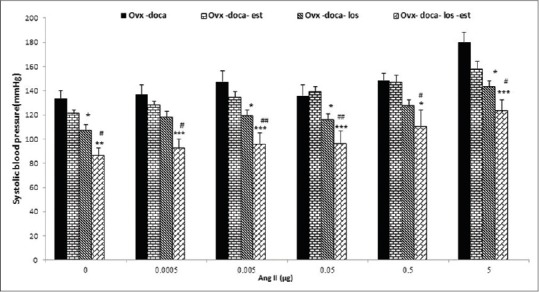
Effects of estrogen (Est, n=10), losartan (los, n=8) and Est +los (n=11) on systolic blood pressure (SBP) induced by i.c.v injection of various doses of Ang II in Ovx DOCA salt-treated rats. SBP significantly attenuated by estrogen and losartan Values are the mean ± S.E.M.*Compared to Ovx- DOCA, #Compared to Ovx DOCA-Est, *P<0.05, **P<0.01, ***P<0.001, #P<0.05, ##P<0.01, ###P < 0.001
Comparison effect of i.c.v injection of Ang II on systolic blood pressure in the Ovx and Sham, Sham-DOCAand Ovx -DOCA groups that were treated with estrogen and losartan
The SBP in the Sham-DOCA and Ovx-DOCA groups was higher than in the Sham and Ovx groups. Injection of various doses of Ang II increased SBP in the Sham-DOCA and Ovx-DOCA groups more than in the Sham and Ovx groups (P < 0.05 to P < 0.01). In groups that received both Est and losartan (Sham-DOCA-E-L and Ovx-DOCA-E-L groups), the effect of various doses of Ang II on SBP was decreased compared to the Sham-DOCA and Ovx-DOCA groups (P < 0.01 to P < 0.001). In addition, the effect of Ang II on SBP in the Sham-DOCA-E-L and Ovx-DOCA–E_L groups was not significant compared to the Sham and Ovx groups [Table 1].
DISCUSSION
The results of the present study showed that the SBP in both Sham and Ovx DOCA salt–treated rats was significantly higher than in the Sham and Ovx groups. The pressor effect induced by DOCA treatment was attenuated by Est treatment. In addition, the pressor effect of the i.c.v injection of Ang II in the DOCA salt treatment groups was significantly attenuated in the Est-treated groups. The pressor effect of Ang II was also completely blocked when the DOCA salt–treated rats were co-treated with both losartan and Est.
Our findings are in agreement with the previous studies showing the cardiovascular protective effects of Est.[17,18,39] It has been frequently reported that the removal of ovaries, the main source of circulating Est in females, facilitates the development of blood pressure.[21,40,41,42] The effects of Est on the cardiovascular system are complicated and are not completely elucidated, but suggest that they are mediated by the direct and indirect effects of this hormone on the peripheral vascular system and brain nuclei.[43,44]
Experimental evidence indicates that Est increases the transcription of nitric oxide synthase (NOS), the key enzyme regulating the production of nitric oxide (NO).[45,46] In addition, a further possible explanation is suggested by the antioxidant properties of Est, and therefore, endothelium-dependent vasodilatation through the negative effect of superoxide anions that the latter strongly inactivate in NO.[47] Est also has an effect on blood pressure by modulating the central autonomic networks in the nuclei, such as, the nucleus solitary tract (NTS) and the rostroventrolateral medulla (RVLM) the important central areas in central cardiovascular regulation.[48,49]
As RAS activity can increase in DOCA salt hypertension,[50,51,52] in this experiment, we have evaluated the role of Ang II on blood pressure in DOCA salt–treated rats. Our results show that i.c.v injection of Ang II in various doses increases SBP in Ovx and Sham DOCA salt–treated rats. However, this effect in the Ovx groups is higher. This result is consistent with a previous study that indicated increased sensitivity to Ang II in DOCA salt–treated rats.[11] This pressor effect of Ang II is not completely blocked by losartan. Therefore, it is suggested that the central effect of Ang II in DOCA salt-treated rats is not merely mediated by the AT1 receptor. These results are partially opposite to the previous study, which shows that a pressor effect of Ang II is mediated by AT1. One possible reason for this effect is that in the previous studies the localized action of losartan has been evaluated. However, in this study, losartan has been injected by i.c.v and it is likely that losartan has not been completely distributed. Therefore, all discrete AT1 receptors in several nuclei have not been blocked.
We also examined the effect of Est on the pressor effect of the i.c.v injection of Ang II in DOCA salt–treated rat. Our result showed that Est in both the Ovx-DOCA and Sham-DOCA attenuated the pressor effect of Ang II. However, the effect of Est in the Sham DOCA was higher than in the Ovx-DOCA. This result was consistent with the previous study and showed the important protective role of Est on hypertension. The effect of Est on blood pressure and its interaction with RAS in the control of blood pressure has been reported in numerous studies.[28,53,54]
On the basis of these results Est has an important protective effect on the pressor effect of Ang II. It has been reported that Ovx female rats treated with estradiol show 30–40% reduction in the AT1 receptor of the pituitary than the Ovx and control animals.[53] The effect of Est on the pressor induced by central Ang II has not been clarified, however, it has been suggested that it is mediated by several mechanism(s). There is evidence that the reactive oxygen species (ROS) increases in the DOCA salt–treated hypertension model.[55] As ROS production is increased by Ang II and decreased by Est,[56] therefore, it is suggested that an interaction effect of Est and Ang II mediate via an interaction in ROS production.
In chronic Ang II-infusion hypertension model, Ang II causes increased sympathetic activity[57] and this effect of Ang II has been decreased by Est treatment.[29,30] Because in DOCA salt hypertension also sympathetic activity increased by Ang II, another possible effect of Est is interacting with Ang II in modulation of sympathetic activity evoked by DOCA- salt hypertension.
There is also evidence that baroreflex sensitivity (BRS) in DOCA-salt hypertension is suppressed.[58] Because BRS reduced by Ang II and facilitate by estrogen,[24] we suggest that effect of Est on the pressor of Ang II in DOCA-salt is mediated by the interaction of these agents on BRS. However, further study is needed to clarify this effect.
In conclusion, our results show an important inhibitory effect of Est on the pressor effect of central Ang II in DOCA salt–treated rats. We suggest that there are probably interaction(s) between Est and Ang II in the control of blood pressure in DOCA salt–treated rats.
ACKNOWLEDGEMENT
This study was derived from an MSc student thesis. We would like to thank the Research Council of Mashhad University of Medical Sciences for their financial support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Whelton PK. Declining mortality from hypertension and stroke. South Med J. 1982;75:33–8. doi: 10.1097/00007611-198201000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Marc Y, Llorens-Cortes C. The role of the brain renin-angiotensin system in hypertension: Implications for new treatment. Prog Neurobiol. 2011;95:89–103. doi: 10.1016/j.pneurobio.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Skeggs LT, Dorer FE, Kahn JR, Lentz KE, Levine M. The biochemistry of the renin-angiotensin system and its role in hypertension. Am J Med. 1976;60:737–48. doi: 10.1016/0002-9343(76)90888-3. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system-focusing on the vascular system. Peptides. 2011;32:2141–50. doi: 10.1016/j.peptides.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: Recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen AM, Moeller I, Jenkins TA, Zhuo J, Aldred GP, Chai SY, et al. Angiotensin receptors in the nervous system. Brain Res Bull. 1998;47:17–28. doi: 10.1016/s0361-9230(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 7.Allen AM, Zhuo J, Mendelsohn FA. Localization and function of angiotensin AT1 receptors. Am J Hypertens. 2000;13:31–8S. doi: 10.1016/s0895-7061(99)00249-6. [DOI] [PubMed] [Google Scholar]
- 8.McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ. Interaction of circulating hormones with the brain: The roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol Suppl. 1998;25:S61–7. doi: 10.1111/j.1440-1681.1998.tb02303.x. [DOI] [PubMed] [Google Scholar]
- 9.Casto R, Phillips MI. Angiotensin II attenuates baroreflexes at nucleus tractus solitarius of rats. Am J Physiol. 1986;250:R193–8. doi: 10.1152/ajpregu.1986.250.2.R193. [DOI] [PubMed] [Google Scholar]
- 10.Becker LK, Santos RA, Campagnole-Santos MJ. Cardiovascular effects of angiotensin II and angiotensin-(1-7) at the RVLM of trained normotensive rats. Brain Res. 2005;1040:121–8. doi: 10.1016/j.brainres.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 11.Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am J Physiol. 1986;251:H261–8. doi: 10.1152/ajpheart.1986.251.2.H261. [DOI] [PubMed] [Google Scholar]
- 12.Ammarguellat F, Larouche I, Schiffrin EL. Myocardial fibrosis in DOCA-salt hypertensive rats: Effect of endothelin ET(A) receptor antagonism. Circulation. 2001;103:319–24. doi: 10.1161/01.cir.103.2.319. [DOI] [PubMed] [Google Scholar]
- 13.Yu M, Gopalakrishnan V, Robert McNeill JR. Role of endothelin and vasopressin in DOCA-salt hypertension. Br J Pharmacol. 2001;132:1447–54. doi: 10.1038/sj.bjp.0703958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–84. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 15.Shan J, Resnick LM, Liu QY, Wu XC, Barbagallo M, Pang PK. Vascular effects of 17 beta-estradiol in male Sprague-Dawley rats. Am J Physiol. 1994;266:H967–73. doi: 10.1152/ajpheart.1994.266.3.H967. [DOI] [PubMed] [Google Scholar]
- 16.Lehrer S, Rabin J, Kalir T, Schachter BS. Estrogen receptor variant and hypertension in women. Hypertension. 1993;21:439–41. doi: 10.1161/01.hyp.21.4.439. [DOI] [PubMed] [Google Scholar]
- 17.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 18.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 19.Rogers J, Sheriff DD. Role of estrogen in nitric oxide- and prostaglandin-dependent modulation of vascular conductance during treadmill locomotion in rats. J Appl Physiol (1985) 2004;97:756–63. doi: 10.1152/japplphysiol.00115.2004. [DOI] [PubMed] [Google Scholar]
- 20.Morey AK, Pedram A, Razandi M, Prins BA, Hu RM, Biesiada E, et al. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology. 1997;138:3330–9. doi: 10.1210/endo.138.8.5354. [DOI] [PubMed] [Google Scholar]
- 21.Hernández I, Delgado JL, Diaz J, Quesada T, Teruel MJ, Llanos MC, et al. 17beta-estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1599–605. doi: 10.1152/ajpregu.2000.279.5.R1599. [DOI] [PubMed] [Google Scholar]
- 22.Shih CD. Activation of estrogen receptor beta-dependent nitric oxide signaling mediates the hypotensive effects of estrogen in the rostral ventrolateral medulla of anesthetized rats. J Biomed Sci. 2009;16:60. doi: 10.1186/1423-0127-16-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, et al. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res. 2006;1094:163–78. doi: 10.1016/j.brainres.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 24.Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat. 2009;38:185–96. doi: 10.1016/j.jchemneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Nematbakhsh M, Khazaei M. The effect of estrogen on serum nitric oxide concentrations in normotensive and DOCA Salt hypertensive ovariectomized rats. Clin Chim Acta. 2004;344:53–7. doi: 10.1016/j.cccn.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Shenoy V, Grobe JL, Qi Y, Ferreira AJ, Fraga-Silva RA, Collamat G, et al. 17beta-Estradiol modulates local cardiac renin-angiotensin system to prevent cardiac remodeling in the DOCA-salt model of hypertension in rats. Peptides. 2009;30:2309–15. doi: 10.1016/j.peptides.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 27.David FL, Carvalho MH, Cobra AL, Nigro D, Fortes ZB, Rebouças NA, et al. Ovarian hormones modulate endothelin-1 vascular reactivity and mRNA expression in DOCA-salt hypertensive rats. Hypertension. 2001;38:692–6. doi: 10.1161/01.hyp.38.3.692. [DOI] [PubMed] [Google Scholar]
- 28.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol. 2007;292:H1770–6. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 29.Pamidimukkala J, Hay M. 17 beta-Estradiol inhibits angiotensin II activation of area postrema neurons. Am J Physiol Heart Circ Physiol. 2003;285:H1515–20. doi: 10.1152/ajpheart.00174.2003. [DOI] [PubMed] [Google Scholar]
- 30.Hosseini M, Alaei HA, Havakhah S, Neemati Karimooy HA, Gholamnezhad Z. Effects of microinjection of angiotensin II and captopril to VTA on morphine self-administration in rats. Acta Biol Hung. 2009;60:241–52. doi: 10.1556/ABiol.60.2009.3.1. [DOI] [PubMed] [Google Scholar]
- 31.Hosseini M, Alaei HA, Headari R, Eslamizadeh MJ. Effects of microinjection of angiotensin II and captopril into nucleus accumbens on morphine self-administration in rats. Indian J Exp Biol. 2009;47:361–7. [PubMed] [Google Scholar]
- 32.Rahimian R, Laher I, Dube G, van Breemen C. Estrogen and selective estrogen receptor modulator LY117018 enhance release of nitric oxide in rat aorta. J Pharmacol Exp Ther. 1997;283:116–22. [PubMed] [Google Scholar]
- 33.Stringer JL. Ethosuximide specifically antagonizes the effect of pentylenetetrazol in the rat entorhinal cortex. Epilepsy Res. 1996;25:69–77. doi: 10.1016/0920-1211(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 34.Shafei MN, Nasimi A. Effect of glutamate stimulation of the cuneiform nucleus on cardiovascular regulation in anesthetized rats: Role of the pontine Kolliker-Fuse nucleus. Brain Res. 2011;1385:135–43. doi: 10.1016/j.brainres.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 35.Nasimi A, Shafei MN, Alaei H. Glutamate injection into the cuneiform nucleus in rat, produces correlated single unit activities in the Kolliker-Fuse nucleus and cardiovascular responses. Neuroscience. 2012;223:439–46. doi: 10.1016/j.neuroscience.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 36.Hosseini M, Sharifi MR, Alaei H, Shafei MN, Karimooy HA. Effects of angiotensin II and captopril on rewarding properties of morphine. Indian J Exp Biol. 2007;45:770–7. [PubMed] [Google Scholar]
- 37.Alaei H, Hosseini M. Angiotensin converting enzyme inhibitor captopril modifies conditioned place preference induced by morphine and morphine withdrawal signs in rats. Pathophysiology. 2007;14:55–60. doi: 10.1016/j.pathophys.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Rabkin SW. Endogenous kappa opioids mediate the action of brain angiotensin II to increase blood pressure. Neuropeptides. 2007;41:411–9. doi: 10.1016/j.npep.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, et al. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovascular Res. 1999;43:985–91. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- 40.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–85. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito K, Hirooka Y, Kimura Y, Sagara Y, Sunagawa K. Ovariectomy augments hypertension through rho-kinase activation in the brain stem in female spontaneously hypertensive rats. Hypertension. 2006;48:651–7. doi: 10.1161/01.HYP.0000238125.21656.9e. [DOI] [PubMed] [Google Scholar]
- 42.Xu X, Xiao JC, Luo LF, Wang S, Zhang JP, Huang JJ, et al. Effects of ovariectomy and 17 beta-estradiol treatment on the renin-angiotensin system, blood pressure, and endothelial ultrastructure. Int J Cardiol. 2008;130:196–204. doi: 10.1016/j.ijcard.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Ceylan-Isik AF, Erdogan-Tulmac OB, Ari N, Ozansoy G, Ren J. Effect of 17beta-oestradiol replacement on vascular responsiveness in ovariectomized diabetic rats. Clin Exp Pharmacol Physiol. 2009;36:e65–71. doi: 10.1111/j.1440-1681.2009.05255.x. [DOI] [PubMed] [Google Scholar]
- 44.Freay AD, Curtis SW, Korach KS, Rubanyi GM. Mechanism of vascular smooth muscle relaxation by estrogen in depolarized rat and mouse aorta: Role of nuclear estrogen receptor and Ca2+ uptake. Circ Res. 1997;81:242–8. doi: 10.1161/01.res.81.2.242. [DOI] [PubMed] [Google Scholar]
- 45.Kleinert H, Wallerath T, Euchenhofer C, Ihrig-Biedert I, Li H, Förstermann U. Estrogens increase transcription of the human endothelial NO synthase gene: Analysis of the transcription factors involved. Hypertension. 1998;31:582–8. doi: 10.1161/01.hyp.31.2.582. [DOI] [PubMed] [Google Scholar]
- 46.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 47.Pinto S, Virdis A, Ghiadoni L, Bernini G, Lombardo M, Petraglia F, et al. Endogenous estrogen and acetylcholine-induced vasodilation in normotensive women. Hypertension. 1997;29:268–73. doi: 10.1161/01.hyp.29.1.268. [DOI] [PubMed] [Google Scholar]
- 48.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 49.Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand. 2003;177:209–18. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- 50.Shan Z, Shi P, Cuadra AE, Dong Y, Lamont GJ, Li Q, et al. Involvement of the brain (pro) renin receptor in cardiovascular homeostasis. Circ Res. 2010;107:934–8. doi: 10.1161/CIRCRESAHA.110.226977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng JF, Kimura B, Fregly MJ, Phillips MI. Reduction of cold-induced hypertension by antisense oligodeoxynucleotides to angiotensinogen mRNA and AT1-receptor mRNA in brain and blood. Hypertension. 1998;31:1317–23. doi: 10.1161/01.hyp.31.6.1317. [DOI] [PubMed] [Google Scholar]
- 52.Chan YS, Wong TM. Relationship of rostral ventrolateral medullary neurons and angiotensin in the central control of blood pressure. Biol Signals. 1995;4:133–41. doi: 10.1159/000109433. [DOI] [PubMed] [Google Scholar]
- 53.Kisley LR, Sakai RR, Fluharty SJ. Estrogen decreases hypothalamic angiotensin II AT1 receptor binding and mRNA in the female rat. Brain Res. 1999;844:34–42. doi: 10.1016/s0006-8993(99)01815-6. [DOI] [PubMed] [Google Scholar]
- 54.Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;295:H1025–32. doi: 10.1152/ajpheart.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Callera G, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1- induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond) 2006;110:243–53. doi: 10.1042/CS20050307. [DOI] [PubMed] [Google Scholar]
- 56.Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. doi: 10.1016/j.brainres.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schenk J, McNeill JH. The pathogenesis of DOCA-salt hypertension. J Pharmacol Toxicol Methods. 1992;27:161–70. doi: 10.1016/1056-8719(92)90036-z. [DOI] [PubMed] [Google Scholar]
- 58.Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP. Sex difference in pressor responsiveness to vasopressin and baroreflex function in DOC-salt hypertensive rats. J Hypertens. 1988;6:381–7. [PubMed] [Google Scholar]


