Abstract
OBJECTIVE
Ovarian vein thrombosis is associated with pregnancy and pelvic surgery. Postpartum ovarian vein thrombosis is associated with infection and a high morbidity rate, and is treated with anticoagulant and intravenous antibiotic therapy. The natural history of such thrombotic events after debulking surgery for ovarian cancer has not been well described. Our objective was to characterize the presentation and outcomes for patients with this condition at our institution.
STUDY DESIGN
We conducted a retrospective study of patients who underwent surgical debulking for ovarian cancer at Memorial Sloan Kettering Cancer Center between the years 2001 and 2010. Patients were included if contrast computed tomography scans of the abdomen and pelvis were performed both within 12 weeks before and 12 weeks after the surgery. The images were reviewed to assess for the presence and extent of a new post-operative ovarian vein thrombosis. When available, subsequent studies were assessed for thrombus progression. Medical records were reviewed to determine if anticoagulation was used for treatment of the thrombotic episode and to record the occurrence of any new significant venous thromboembolic event in the following year.
RESULTS
159 patients had satisfactory imaging. New ovarian vein thrombosis was a common complication of debulking surgery, as found in 41 (25.8%) of patients. Only 5 women with ovarian vein thrombosis were started on anticoagulation, of which 2 individuals had an independent venous thromboembolic event as indication for treatment. Only 2 (4.9%) of the ovarian vein thromboses progressed to the inferior vena cava or left renal vein on subsequent scan. The estimated cumulative incidence of venous thromboembolism one year after the first post-operative scan was 17.1% for patients in the new ovarian vein thrombosis group, versus 15.3% of individuals for the group without a post-operative ovarian vein thrombosis (p=0.78).
CONCLUSION
Ovarian vein thrombosis is commonly encountered after debulking surgery for ovarian cancer. Anticoagulation is usually not indicated and clinically meaningful thrombus progression rarely occurs.
Introduction
Ovarian vein thrombosis (OVT) is a rare event which is usually encountered in the postpartum period.1 It has also been found in association with malignancy, abdominopelvic surgery, inflammatory bowel disease and pelvic inflammatory disease.2, 3 Treatment of the pregnancy-associated variant has consisted of antibiotic therapy and anticoagulation, with a low rate of pulmonary embolism.4 Appropriate management in the setting of cancer has not been established, with observation and short-term anticoagulation both being considered acceptable strategies.5, 6
Debulking surgery for initial treatment of ovarian cancer usually includes bilateral oophorectomy, resulting in the formation of a stump at the level of the ovarian veins. Such anatomic change leads to absent blood flow in the affected vessels and a high rate of thrombosis. The effects of local surgical manipulation and thrombophilia associated with neoplasia may also contribute to a high rate of OVT in the immediate post-operative period of debulking surgery for ovarian cancer.7
Post-operative OVT is typically asymptomatic and identified incidentally on a follow-up, routine contrast enhanced computed tomography (CT) scan.7 However the natural history of this complication has not been well documented and optimal management remains unclear. Given the high rate of symptomatic lower extremity deep vein thrombosis associated with abdominopelvic cancer surgery, going into this project we were concerned that some women might be experiencing clinically significant ovarian vein thrombosis after debulking for ovarian cancer. We characterized the epidemiology and natural history of OVT after debulking for ovarian cancer at our institution in order to better inform therapeutic decisions in this area.
Methods
This project was deemed exempt from review by the institutional review board (IRB) based on the low risk to the privacy of individual patients. Informed consent was not obtained based on the retrospective nature of the study and in agreement with the IRB. All cases undergoing debulking surgery for ovarian cancer at Memorial Sloan Kettering Cancer Center (MSKCC) in the years 2001 to 2010 were assessed. Data captured included date of birth, comorbid conditions, tumor histology, last follow-up, and survival. This information was entered prospectively at the occasion of clinical encounters with the physicians caring for individual subjects. No cases are excluded from entry.
Records were included for this analysis if satisfactory information was available, including: adequate quality of CT scans of the abdomen and pelvis with intravenous contrast performed within 12 weeks before (baseline) and 12 weeks after debulking surgery. These were reviewed independently by two radiologists (DS and WM) for the presence of OVT with or without extension into the left renal vein or inferior vena cava (IVC). Only patients without a pre-existing OVT on pre-operative CT scans were included in this analysis.
If a second, post-operative contrast-enhanced CT of the abdomen and pelvis was available, it was reviewed for the progression of an existing OVT into the IVC or left renal vein, for right and left ovarian vein thromboses, respectively. Differences in assessment for the presence or absence of OVT for a given study were resolved by a discussion between the radiologists.
The survival status up to one year after debulking surgery was assessed from the MSK clinical data system. The latter was also the source of information for diagnosis of any concomitant or new VTE episode during the observation period, as well as anticoagulant administered if any.
The data was analyzed with the R for Windows statistical platform, version 3.0. The clinical outcomes of the patients who developed an OVT in the post-operative period were compared to those of the patients who did not. The cumulative incidence of new VTE episodes was assessed with the cmprsk package, treating mortality as a competing event. The difference in cumulative incidence of new VTE episodes for patients with and without post-operative new OVT was assessed using Gray's test. Overall survival was estimated using Kaplan-Meier methods, and the difference in survival was assessed using the log-rank test. Fisher's exact tests and Wilcoxon rank sum tests were used to compare the characteristics of the two groups.
Results
575 patients underwent surgical debulking for ovarian cancer between 2001 and 2010. Study analysis was performed on 159 subjects (161 with adequate pre-operative and post-operative contrast enhanced CT scans, removing 2 with pre-existing OVT on the baseline CT scan). Cohen's Kappa for initial inter-observer agreement on the presence of a new OVT was 0.63. All differences were resolved between the two radiologists. All records were reviewed for events occurring up to one year after the index post-operative CT. The baseline characteristics of patients are shown in Table 1; median age was 60 years (range=37-74 years) in the group of patients with a new OVT, compared to 59.5 years (range=23-85) in the group without an event (p=0.17). 75.6% of patients had stage IIIC ovarian cancer and 22.0% had stage IV disease in the subset of patients with a new OVT, compared to 72.9% and 22.9% respectively for those without thrombosis (p=0.99).
Table 1.
Characteristics of Patients (n=159)
| Characteristic | New OVT (n=41) | No New OVT (n=8 | p * |
|---|---|---|---|
| Age in years, median (range) | 60 (37-74) | 59.5 (23-85) | 0.17 |
| Stage, number (%) | 0.99 | ||
| IIIB | 1 (2.4) | 5 (4.2) | |
| IIIC | 31 (75.6) | 86 (72.9) | |
| IV | 9 (22.0) | 27 (22.9) | |
| Hypertension, number (%) | 11 (26.8) | 26 (22.0) | 0.64 |
| Diabetes, number (%) | 3 (7.3) | 2 (1.7) | 0.11 |
| Pulmonary disease, number (%) | 0 | 4 (3.4) | 0.57 |
| Coronary artery disease, number (%) | 1 (2.4) | 3 (2.5) | 0.99 |
| Renal disease, number (%) | 1 (2.4) | 2 (1.7) | 0.99 |
| Congestive heart failure, number | 0 | 0 | NA |
| Dementia, number | 0 | 0 | NA |
| Stroke, number (%) | 0 (0) | 1 (0.8) | 0.99 |
| Hepatic disease, number (%) | 2 (4.9) | 3 (2.5) | 0.60 |
| Peripheral vascular disease, number (%) | 1 (2.4) | 1 (0.8) | 0.45 |
| Weight in kg, median (range) | 70.2 (44.5-112) | 64.0 (40.7-134.5) | 0.063 |
| Height in cm, median (range) | 163.0 (150-179) | 160.0 (131-176.5) | 0.81 |
Abbreviation: OVT, ovarian vein thrombosis
Fisher's exact test or Wilcoxon rank sum test
41 of 159 subjects (25.8%) developed an OVT in the post-operative period. Only 5 (12.2%) were anticoagulated with a low-molecular weight heparin and none of these cases were treated for uncomplicated OVT alone. All of those individuals had a second post-operative CT available for review. One had initial involvement of the IVC, 1 progression of the OVT into the left renal vein on the second post-operative CT, 1 had presented with abdominal pain along with worsening bilateral OVT on the third postoperative CT and 2 had other concomitant VTE at time of OVT diagnosis. Only 2 patients did not undergo oophorectomy due to the intra-operative decision to cancel debulking. Neither of these were found to have a post-operative OVT event. Additionally, 5 individuals only had a unilateral oophorectomy performed. In 4 cases this was because the other ovary had already been removed at a prior time, including one patient who had had a prior right oophorectomy and was found to have a left ovarian vein thrombosis after debulking. Finally, one individual had already undergone bilateral oophorectomy about 4 years prior. Interestingly, this patient was found to have a 3 cm left ovarian vein thrombus situated 8.3 cm from the left renal vein, while the pathology report mentioned the presence of “residual left ovarian vessel” in the resected material.
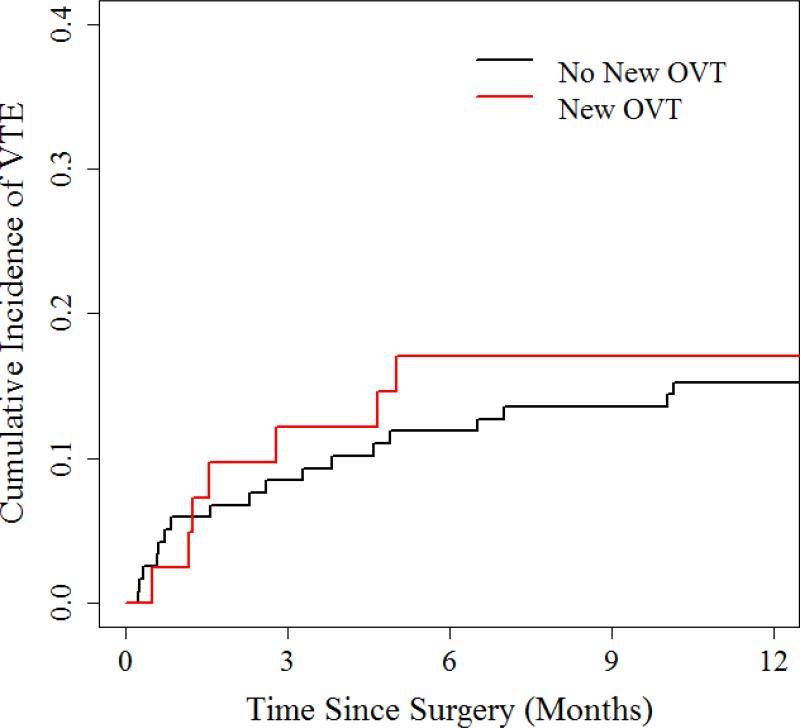
Rates of overall survival and development of a future thrombosis were comparable in patients with or without an OVT (Table 2, Figures 1 and 2). Within one year of follow-up, the cumulative incidence of a new VTE episode was 17.1% in women with a post-operative OVT and 15.3% in the group without new OVT (p=0.78). There was also no significant difference in survival; estimated 1-year values of 95.1% of patients with a new OVT and 93.2% of patients without a new OVT.
Table 2.
Summary of Clinical Outcomes
| Outcome | New OVT (n=41) | No New OVT (n=118) | p |
|---|---|---|---|
| Treated with anticoagulation*, number (%) | 5 (12.2) | 2 (1.7) | 0.012† |
| Concomitant VTE, number (%) | 3 (7.3) | 4 (3.4) | 0.38† |
| Progression of OVT into IVC or left renal vein, number (%) | 2 (4.9) | NA | NA |
| Cumulative incidence of VTE at 1 year, % | 17.1 | 15.3 | 0.78‡ |
| Overall survival at 1 year (%) | 95.1 | 93.2 | 0.84§ |
Abbreviations: OVT, ovarian vein thrombosis; VTE, venous thromboembolic event
New starts who were not already anticoagulated for VTE before the index CT
Fisher's exact test
Gray's test
Log-rank test
Figure 1.
Cumulative Incidence of New Venous Thromboembolic Event
Figure 2.
Overall Survival
Discussion
Ovarian vein thrombosis has long been recognized as being associated with debulking surgery for ovarian cancer. The loss of blood flow following ligation of the vessel likely contributes to the high rate of OVT in this setting. In theory such a thrombus can progress into the IVC (on the right) or the renal vein and eventually IVC (on the left). However, the general impression in the field has been that symptoms or complications from such post-operative OVT events are uncommon, leading to few of these patients being treated with anticoagulants.
This retrospective study confirms that OVT in this clinical setting are quite common. However, in our cohort, few patients were treated and progression of the OVT into the IVC or renal vein was uncommon. Importantly, OVT was not a risk marker for the subsequent development of VTE at other sites and did not predict all-cause mortality at one year, justifying the major practice of not treating with anticoagulation.
A major strength of this study is the large number of individuals included, more than any other published so far. The quality of the data was also very good, largely due to the fact that the data was collected in a prospective manner. We believe our approach was very sensitive for detecting OVT cases, since every CT evaluation was reviewed independently by two radiologists, with the explicit purpose of eliciting the presence of this outcome.
One limitation of this analysis is the lack of systematic approach to anticoagulation in individuals with an OVT event. Anticoagulation therapy was given at the discretion of the individual surgeon, without consensus guidelines. An additional potential issue is the relatively small fraction (28%) of subjects being included in the final analysis due to the fact that early post-operative CT scans are not part of routine practice. Those studies are performed as part of research protocols or when a complication was suspected, such as infectious processes or hemorrhagic events. This could lead to a selection bias, whereas the final cohort is enriched with more ill patients. In this case, one would expect an increased rate of ovarian vein thrombosis, even though the natural history and response to treatment should not be materially affected.
Conclusion
Our findings confirm that OVT is a common complication of debulking surgery for ovarian cancer. Our data also suggest that anticoagulation is not necessary for asymptomatic OVT incidentally diagnosed on routine CT scan done in the 12 weeks following debulking surgery for ovarian cancer, provided the thrombus does not extend into the IVC or left renal vein. Repeat imaging to assess progression is also not indicated, unless symptoms suggestive of VTE emerge.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding information: not applicable.
The authors report no conflict of interest.
Disclosure Statements:
Simon Mantha; no relevant conflict of interest.
Deborah Sarasohn; no relevant conflict of interest.
Weining Ma; no relevant conflict of interest.
Sean M. Devlin; no relevant conflict of interest.
Dennis S. Chi; no relevant conflict of interest.
Kara Long Roche; no relevant conflict of interest.
Rudy S. Suidan; no relevant conflict of interest.
Kaitlin Woo; no relevant conflict of interest.
Gerald A. Soff; no relevant conflict of interest.
References
- 1.Sharma P, Abdi S. Ovarian vein thrombosis. Clin Radiol. 2012;67:893–8. doi: 10.1016/j.crad.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Wysokinska EM, Hodge D, McBane RD. Ovarian vein thrombosis: Incidence of recurrent venous thromboembolism and survival. Thromb Haemost. 2006 [PubMed] [Google Scholar]
- 3.Gakhal MS, Levy HM, Spina M, Wrigley C. Ovarian vein thrombosis: analysis of patient age, etiology, and side of involvement. Del Med J. 2013;85:45–50. quiz 59. [PubMed] [Google Scholar]
- 4.De Stefano V, Martinelli I. Abdominal thromboses of splanchnic, renal and ovarian veins. Best Pract Res Clin Haematol. 2012;25:253–64. doi: 10.1016/j.beha.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Jacoby WT, Cohan RH, Baker ME, Leder RA, Nadel SN, Dunnick NR. Ovarian vein thrombosis in oncology patients: CT detection and clinical significance. AJR Am J Roentgenol. 1990;155:291–4. doi: 10.2214/ajr.155.2.2115254. [DOI] [PubMed] [Google Scholar]
- 6.Harris K, Mehta S, Iskhakov E, et al. Ovarian vein thrombosis in the nonpregnant woman: an overlooked diagnosis. Ther Adv Hematol. 2012;3:325–8. doi: 10.1177/2040620712450887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yassa NA, Ryst E. Ovarian vein thrombosis: a common incidental finding in patients who have undergone total abdominal hysterectomy and bilateral salpingo-oophorectomy with retroperitoneal lymph node dissection. AJR Am J Roentgenol. 1999;172:45–7. doi: 10.2214/ajr.172.1.9888736. [DOI] [PubMed] [Google Scholar]