Abstract
BACKGROUND
Reported vaginal and seminal fluid simulants have complex compositions with multiple preparatory steps which contribute to physical instability. We report the design and characterization of stable and simplified buffers that mimic the salient physical-chemical properties of the physiological fluids.
STUDY DESIGN/METHODS
Human cervicovaginal and seminal fluid samples were collected and buffering capacity was determined. The major buffering species were identified from published compositions of reproductive tract fluids. These values were used to compute the composition of vaginal and seminal fluid simulants. Ionic strength, buffering capacities, pH and osmolalities were then calculated or experimentally determined. Finally, cytotoxicity was evaluated in HEC-1-A cells and 3D reconstructed EpiVaginal™tissue (VEC-100-FT) using naïve cells/tissue and nonoxynol-9 as controls.
RESULTS
The use of calculated amounts of conjugate acid and base for buffer development resulted in compositions that did not require end point pH adjustment and could be formulated as stable 10X concentrates. Furthermore, due to the absence of complex divalent salts, all our proposed simulants were stable at 4°C for 1 month whereas precipitation, and pH and osmolality changes were noted in reported buffers. Experimental determination of buffering capacities yielded similar values for undiluted cervicovaginal fluid (β4.2–5.2 = 35.6 ± 12.3 mM, N = 7) and human seminal fluid (β7–6 =37.5 ± 5 mM, N = 3). All neat simulants showed significant cytotoxicity in HEC-1-A cells but were well tolerated by organotypic vaginal tissue.
CONCLUSIONS
We report revised and improved compositions of buffers mimicking salient properties of vaginal and seminal fluid necessary for in vitro product evaluation.
Keywords: human cervicovaginal fluid, buffering capacity, simulant design, cytotoxicity
1. INTRODUCTION
Physiological fluid simulants are commonly used in the study and design of drug delivery systems [1]. Increasing interest in vaginal drug delivery [2, 3] requires buffers mimicking human vaginal (HVF) and human seminal fluids (HSF) for in vitro assays. Given the tradition of using simplified compositions [1], variability in compositions and assay media reported [4–9], and absence of standard pharmacoepial assay media we have reengineered the compositions proposed by Owen et al. [8, 9], that are complex, difficult to prepare, and chemically unstable.
Owen’s vaginal fluid (VFS-O) and seminal fluid (SFS-O) simulant recipes [8, 9] use two-component buffering systems of acetate and lactate in the case of HVF, and phosphate and lactate for HSF. Physiologically, both HVF and HSF have good buffering capacities over a wide pH range of 4 to 8. This is complex compared to other body fluids; for example, gastric and intestinal fluids have single buffering components and work in narrow buffering ranges [1, 10, 11]. We hypothesize the wide buffering spectrum of HVF and HSF is due to the presence of dual species, resulting in complicated buffer construction.
Since the pH and composition of vaginal fluid is variable in the population and across phases in the ovulatory cycle [12, 13], there is no single reportable buffering capacity [9]. Due to difficulty in obtaining HVF samples, previous buffering capacities were estimated from titrations of individual or pooled and diluted cervicovaginal lavages [4]. Tevi-Bennisan reported the buffering capacity of vaginal fluid based on the titration of cervicovaginal lavage with 100-fold diluted pooled seminal plasma, and found a weak buffering capacity [4]. This contradicts earlier data presented by Huggins and Preti, who used tampons to collect vaginal samples; the buffering capacity was found to be 10-fold higher than the former report [4, 13]. This conflict has led us to determine the buffering capacity of undiluted vaginal fluid using new methods of vaginal fluid collection[14]].
The Owen simulated seminal fluid (SFS-O) preparation is complex, with multiple species creating a chemical blend of unknown stability and interactions that contribute to the instability of this buffer after storage. First, the monovalent salts are dissolved, then bovine serum albumin added. Separate solutions for three divalent salts are then prepared and sequentially added to the protein-phosphate buffer solution. Finally, the pH of the buffer is adjusted to ~7.7 [8]. This complex and unstable mixture warrants the development of a stable and simplified HSF simulant recipe for in vitro studies.
Here we present our process for redesigning the simulants, informed by the work of Owen, along with the details of the reengineered solutions. We experimentally determined the buffering capacity, osmolality, and storage stability of the simplified recipes. We show titrations of HVF with HSF and the new simulants with one another. Since drug eluted in dissolution media may also be used to understand cell/tissue interactions ex vivo, we have reported the cytotoxicity of whole and diluted Owen recipes and our simplified solutions on HEC-1-A cells and reconstructed full thickness vaginal tissue (VEC-100-FT).
2. MATERIALS AND METHODS
2.1. Sample collection
Cervicovaginal secretions from 13 donors were collected as per reported method after written informed consent under protocol STU00025456 approved by the Institutional Review Board at Northwestern University [14]. HVF samples were collected using an Instead SoftCup from premenopausal donors [14]. The cup was self-placed for 3 h, followed by removal, transfer of the cup to a 50 mL centrifuge tube and centrifugation at 800 × g for 10 min to collect the contents. Pooled HSF (Lee BioSolutions, St. Louis, MO) was stored at −20°C until use.
2.2. Buffer preparation
Since reducing sugar and protein are present in high concentrations in reproductive fluids, we therefore developed two sets of buffers, one with sugars and proteins (VFS+G and SFS+F) and one without (VFS-G and SFS-F). Buffer components for proposed vaginal simulants without and with glucose (VFS-G and VFS+G) and Owen’s recipes (VFS-O) are tabulated in Table 1. To prepare 10X VFS-G buffer, 5.25 g of NaCl and 2.02 g of sodium lactate were weighed in a 100 mL volumetric flask. To this, 0.79 mL of glacial acetic acid was added followed by distilled water. The volume was made to mark and pH measured (pH ~ 4.2 ± 0.1). The stock was stored at 4°C and diluted 10X before use. Preparation of VFS+G is discussed in Appendix B.
Table 1.
Compositions of Owen’s recipe (VFS-O) and proposed vaginal fluid simulants with (VFS+G) and without glucose (VFS-G)
| Chemical Name |
Formula | MW (g/mol) |
CAS # | Company | Grade/ Purity |
VFS-O | VFS+G | VFS-G | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Mass(g) | Conc (mM) |
2Mass (g) |
3Conc. (mM) |
2Mass (g) |
3Conc. (mM) |
||||||
| D-glucose monohydrate |
C6H12O6.H2O | 180.1 | 14431-43-7 | Alfa Aesar |
99% | 5 | 27.8 | 5 | 25.2 | - | - |
| Sodium chloride |
NaCl | 58.44 | 7647-14-5 | Spectrum Chemicals |
USP | 3.51 | 60.5 | 4.5 | 77.6 | 5.25 | 90.6 |
| L(+)-Lactic acid |
C3H6O3 | 90.08 | 79-33-4 | Acros Organics |
90% | 42 | 26.3 | 40.61 | 8 | 40.61 | 8 |
| Potassium hydroxide |
KOH | 56.11 | 1310-58-3 | J.T.Baker | ACS | 1.4 | 25 | ||||
| Glacial acetic acid |
CH3CO2H | 60.05 | 64-19-7 | Alfa Aesar |
ACS/99.7% | 1 | 16.7 | 0.79 | 13.2 | 0.79 | 13.2 |
| Urea | NH2CONH2 | 69.06 | 57-13-6 | J.T.Baker | Ultrapure Bioreagent |
0.4 | 6.8 | - | - | - | - |
| Calcium hydroxide |
Ca(OH)2 | 74.09 | 1305-62-0 | J.T.Baker | ACS | 0.22 | 3 | - | - | - | - |
| Glycerol | C3H8O3 | 92.10 | 56-81-5 | Spectrum | USP | 0.16 | 1.7 | - | - | - | - |
| Bovine serum albumin (Fraction V) |
9048-46-8 | OmniPur | 0.018 | - | - | - | - | ||||
| DL-lactic acid sodium salt |
C3H5NaO3 | 112.06 | 72-17-3 | Fluka Analytical |
50 wt% aqueous solution |
- | - | 52.02 | 18 | 52.02 | 18 |
| Sodium acetate (anhydrous) |
C2H3NaO2 | 82.03 | 127-09-3 | Spectrum Chemicals |
USP | - | - | 0.31 | 3.8 | 0.31 | 3.8 |
(MW = molecular weight)
Mass of species to prepare 1L of 1X buffer
Mass of species to prepare 100 mL of 10X buffer
Concentration of the species in 1X buffer
Final mass of lactic acid and
sodium lactate to be added for buffer preparation.
Compositions of SFS, including Owen’s formulation (SFS-O) and proposed buffers without (SFS-F) and with fructose (SFS+F), are presented in Table 3. To make 100 mL of 10X SFS-F stock, 9 g monobasic sodium phosphate, monohydrate with 9.46 g sodium phosphate dibasic, 0.62 g lactic acid, and 0.46 g sodium chloride were weighed in a 100 mL volumetric flask followed by addition of distilled water to dissolve the solids. The mixture was brought to volume, pH measured (pH ~ 7.7 ± 0.1) and stored at 4°C until use. Preparation of SFS+F is discussed in Appendix B.
Table 3.
Summary of calculated and experimental buffering capacities and amounts of base/acid required for neutralization of VFS and SFS.
| Sample | Calculated β4.2–5.2 (mM) |
Initial pH |
Experimental β4.2–5.2 (mM; mean ± SD) |
Amount of base to reach neutralization point (mmol, mean ±SD)* |
|---|---|---|---|---|
| VFS−O | 19 | 16.0 ± 4 | 20.6 ± 2.1 | |
| VFS−G | 20 | 23.4 ± 2.1 | 28 ± 1.5 | |
| VFS+G | 22 | 19.3 ± 3 | 28.3 ± 3.2 | |
| HVF1 | - | - | ||
| HVF2 | 46.5 | 80.7 | ||
| HVF3 | 32.7 | 51.1 | ||
| HVF4 | 26.5 | 49.6 | ||
| HVF5 | 41.2 | 89.8 | ||
| HVF6 | - | - | ||
| HVF7 | 21.1 | 40.2 | ||
| HVF8 | 26.4 | 54.2 | ||
| HVF9 | - | - | ||
| HVF10 | - | - | ||
| HVF11 | 55.0 | 81.1 | ||
| HVF12 | - | - | ||
| HVF13 | - | - | ||
| Sample |
Calculated β7–6 (mM) |
Experimental β7–6 (mM; mean ± SD) |
Amount of acid to reach neutralization point (µmol, mean ±SD)* |
|
| SFS−O | 33.8 | 35 ± 3 | 132 ± 5.4 | |
| SFS−F | 32.5 | 37.5 ± 3.1 | 101 ± 7.8 | |
| SFS+F | 31.2 | 40 ± 2.9 | 117 ± 4.2 | |
| HSF−1 | 37.5 ± 5 | 372.6 ± 6.9 | ||
Data represented as mean ± SD (N = 3) except for HVF samples (N = 1). Buffering capacities not displayed for samples with initial pH above 4.2.
Amount of base or acid required to change pH of 1 mL of vaginal fluids from starting pH to pH 7 and 1 mL seminal fluids from starting pH to pH 4.2 respectively.
2.3. Buffering capacity and osmolality determination
We experimentally measured buffering capacities of undiluted HVF and HSF, Owen’s buffers [8, 9] and our simulant solutions. Buffers were titrated using a Titrando 808 automatic titrator (Metrohm, Riverview, FL) or a Machlett Automatic Burette (ThermoFischer Scientific, Waltham, MA). HVF and HSF were titrated with an analytical syringe (Hamilton, Reno, NV). The titrator was calibrated using 0.1M or 1M HCl or 0.1M or 1M NaOH standard solution (Sigma Aldrich, St Louis, MO). Buffering capacity was calculated as the moles of NaOH/HCl required to change the pH by one unit. Buffering capacity calculations and equations related to design of proposed recipes are detailed in Appendix A.
Osmolality was measured in triplicate on a pre-calibrated Wescor 5100C vapor pressure osmometer (Logan, UT).
2.4. Titration of VFS-G with SFS-F and HVF with HSF
We titrated our simplified VFS (VFS-G) with simplified SFS (SFS-F) to determine the volume of SFS-F required for one unit pH change (4.2 to 5.2) as well as neutralization of VFS-G. (Fig. 1c). Physiologically, about 0.5 mL of vaginal secretions are present [15]; we titrated an equivalent volume of VFS-G with SFS-F, and compared the results to the titration of HSF with HVF.
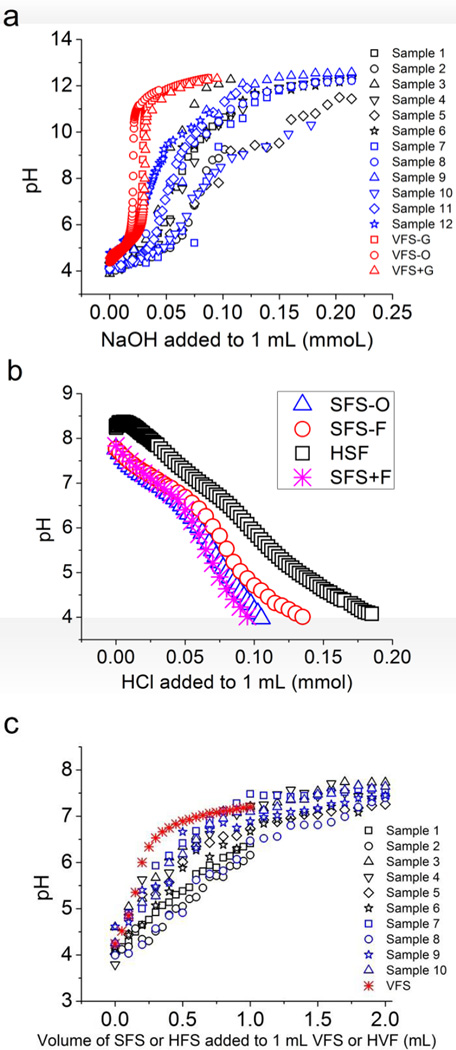
Figure 1.
a) Titration curves showing amount of base (NaOH) required to neutralize 1 mL of VFS-O (red), proposed VFS recipes with and without glucose (red), and thirteen human cervicovaginal fluid samples (HVF1-13) with NaOH at RT (blue and black). Since quantities of HVF samples were variable and ranged from 0.1 – 0.2 mL, data has been reported after scaling for 1 mL vaginal fluid, b) Titration curves showing amount of acid required to neutralize 1 mL of SFS-O, proposed SFS recipes with and without fructose and pooled human seminal fluid (HSF) with HCl at RT and c) Titration of VFS with SFS at RT. Data plotted as volume of SFS-F required to neutralize 0.5 mL of VFS-G. All data except for HVF titrations (N =1) represented as mean of three separate experiments.
2.5. Cytotoxicity evaluation
Cytotoxicity of the buffer solutions was evaluated on HEC-1-A cells (ATCC, Manassas, VA) and 3D engineered EpiVaginal™ tissue (VEC-100-FT, MatTek, Ashland, MA) with naive and nonoxynol-9 (N9; 0.1 mg/mL) as controls (Appendix). Pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8 and TNF-α) released in VEC-100-FT maintenance media were quantified using ELISA (R&D Systems, Minneapolis, MN)
2.6. Design of VFS
Our design of our vaginal fluid simulant was initially based on work done by Owen and Katz [9]. We identified acetate and lactate as the major contributors to buffering capacity and ionic strength. Using the experimentally determined identities and concentrations of buffering species ([lactate]+[lactic Acid] ~ 26 mM [9], Adjusted pKa = 3.84 at pH = 4.2; [acetate]+[acetic Acid] ~ 17 mM [9], Adjusted pKa = 4.74 at pH = 4.2) we obtained an estimated VFS-O buffering capacity of β4.2–5.2 ~ 20 mM by eqn A.2. Based on this buffering capacity, we calculated the necessary concentrations of buffering species for our new VFS series of equations as described by Benyon [16] (see Appendix A for details).
3. RESULTS
3.1. Buffering capacity of HVF and VFS
The buffering capacity of undiluted human vaginal fluid has not been previously studied. Figure 1a and Figure 2b show the titrations and buffering capacities of 13 HVF samples. Titration of HVF samples with 0.01 M NaOH yielded buffering capacities ranging from 21.1 to 55 mM for a pH change from 4.2 to 5.2 and an average buffering capacity of 35.6 ± 12.3 mM (N = 7, mean ± SD). Amount of base required for neutralization of these same samples (pH from 4.2 to 7) was 63.8 ± 19.5 mM.
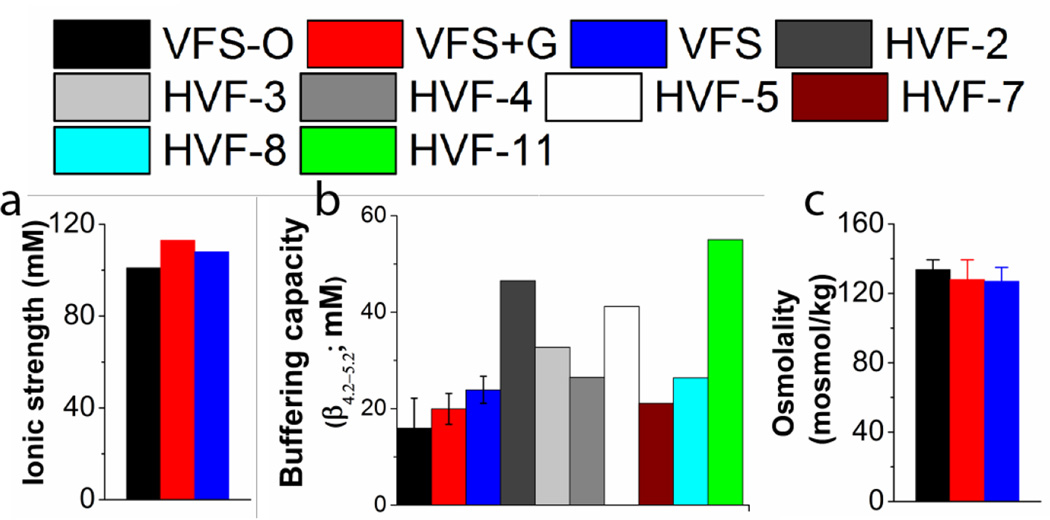
Figure 2.
a) Ionic strength, b) Buffering capacity (N = 3), and c) Osmolality (N = 3) of Owen’s and proposed recipes. Buffering capacity was calculated for for HVF samples with initial pH below 4.2 labeled as HVF 2, 3, 4, 5, 7, 8 and 11 (N = 1). Buffering capacities of all samples was determined as the slope of the curve generated by titration of samples with NaOH at room temperature.
The buffering capacity of our vaginal fluid simulants (β4.2–5.2 = 23.4 ± 2.1 mM (VFS-G) and 19.3 ± 3 mM (VFS+G) are similar to the previous simulant formulation VFS-O (β42–5.2 = 16 ± 4 mM) (Table 3). Measured buffering capacities for our VFS recipes are comparable to those of HVF and match calculated values from the concentrations of buffering species (Table 3). Our HVF samples come largely from the upper vagina and contain a higher concentration of cervical mucus than samples from lower in the vaginal tract. Thus, the titration curve of HVF samples is broader (Figure 1), reflecting the broad buffering capacities of biological macromolecules. Our fluid simulants are intended to reflect solely the non-macromolecular contribution to the buffering capacity of HVF.
Since the HVF and HSF are mixed during sex, we titrated 1 mL of our simplified VFS (VFS-G) with simplified SFS (SFS-F). A volume of 0.07 mL of SFS-F was required to change the pH of VFS-G from 4.2 to 5.2 and 0.32 mL for complete neutralization from pH 4.2 to pH 7. We additionally titrated nine HVF samples with pooled HSF. A volume of 0.32 ± .11 mL was required to change the pH of HVF from 4.2 to 5.2 and 1.3 ± 0.4 mL for neutralization from pH 4.2 to pH 7.
Osmolality of human cervicovaginal fluid has not been reported previously. Using the measured value of VFS-O (133.7±5.7 mOsm), we adjusted the osmolality of VFS-G and VFS+G using NaCl (Fig. 2c) resulting in final values of 127 ± 8 mOsm and 128 ± 11.4 mOsm for VFS-G and VFS+G, respectively A detailed formula to construct a 10X buffer is provided in the appendix to this work.
3.2. Development of SFS
We prepared SFS-O as per the protocol given by Owen et al. [8]; acid-base titration of this recipe (pH 7.76) resulted in an average buffering capacity of 35 ± 3 mM as compared to 37.5 ± 5 mM for pooled HSF (N = 3; Fig. 1b and Fig. 3b).
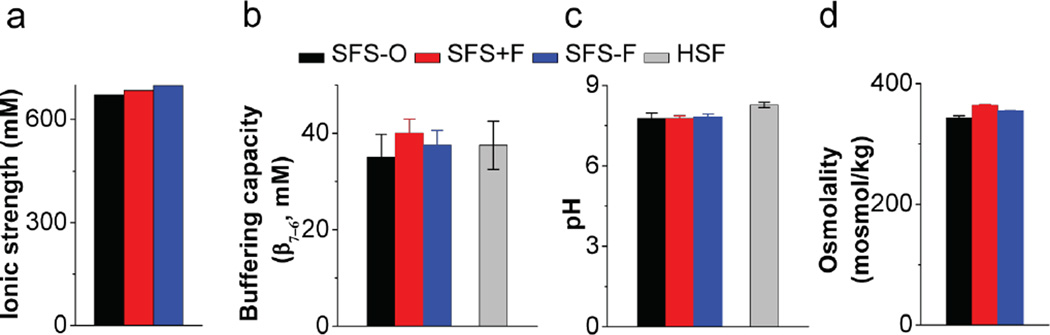
Figure 3.
a) Ionic strength, b) Buffering capacity (N = 3), c) pH (N = 3) and d) Osmolality (N = 3) of Owen’s and proposed SFS recipes. Buffering capacity and pH were measured for pooled HSF sample as obtained. Buffering capacities of all samples was determined as the slope of the curve generated by titration of samples with HCl at room temperature.
Based on prior reported recipes, phosphate was determined as the major buffering species [5, 8, 16]. To simplify buffer construction, we first composed a single buffering component system comprising of mono- and dibasic sodium phosphates with NaCl to adjust ionic strength. Acid-base titration of our phosphate buffer resulted in a buffering capacity of 30 ± 2 mM, however, the buffer showed negligible buffering activity below pH 6. Adding 8 mM of lactic acid equivalent to that reported by Owen [8] provided improved buffering capacity at pH 5 and a slight increase in the Slyke value to 35 µM. Figure 1b shows the titration of simulated seminal fluids and HSF with HCl.
Osmolality of HSF samples has been reported to be in the range of 360–380 mOsm [17]. Measured values for SFS-O were 343 ± 3.6 mOsm. We added NaCl to our buffer, resulting in an osmolality of 355 ± 0.6 mOsm while maintaining the buffering capacity (Fig. 3d). Reducing sugars (such as fructose and glucose) and proteins are present in high concentrations in HSF. This led us to formulate SFS+fructose (SFS+F), with albumin and fructose concentrations as per Owen et al. [8]. Quantity of NaCl was adjusted to account for the contribution of sugars to osmolality, the final values were 364±1.7 mOsm. A detailed formula to construct a 10X buffer is provided in the Appendix.
3.3. Stability of vaginal and seminal fluid simulants
Stability of our solutions (VFS-G/+G and SFS-F/+F) at 4°C was compared with Owen’s recipes after 1-month of storage. Osmolality, pH, and buffering capacity of the simplified simulants matched initial values (p > 0.05; paired t-test). Owen’s formulations showed significant changes in osmolality (339 ± 5 mosmol/kg; t = 0 and 311 ± 4 mosmol/kg; t = 24 h at 4°C, p = 0.002) and pH (7.7 ± 0.1; t = 0 and 8.0 ± 0.1; t = 24 h at 4°C, p = 0.02; unpaired t-tests assuming unequal variance with two-tailed distribution), as well as extensive precipitation due to the incorporation of divalent cations in the buffer formulation. Approximately 5.9% solids were recovered as precipitate from SFS-O within 24 h of preparation and storage at 4°C.
3.4 Cytotoxicity
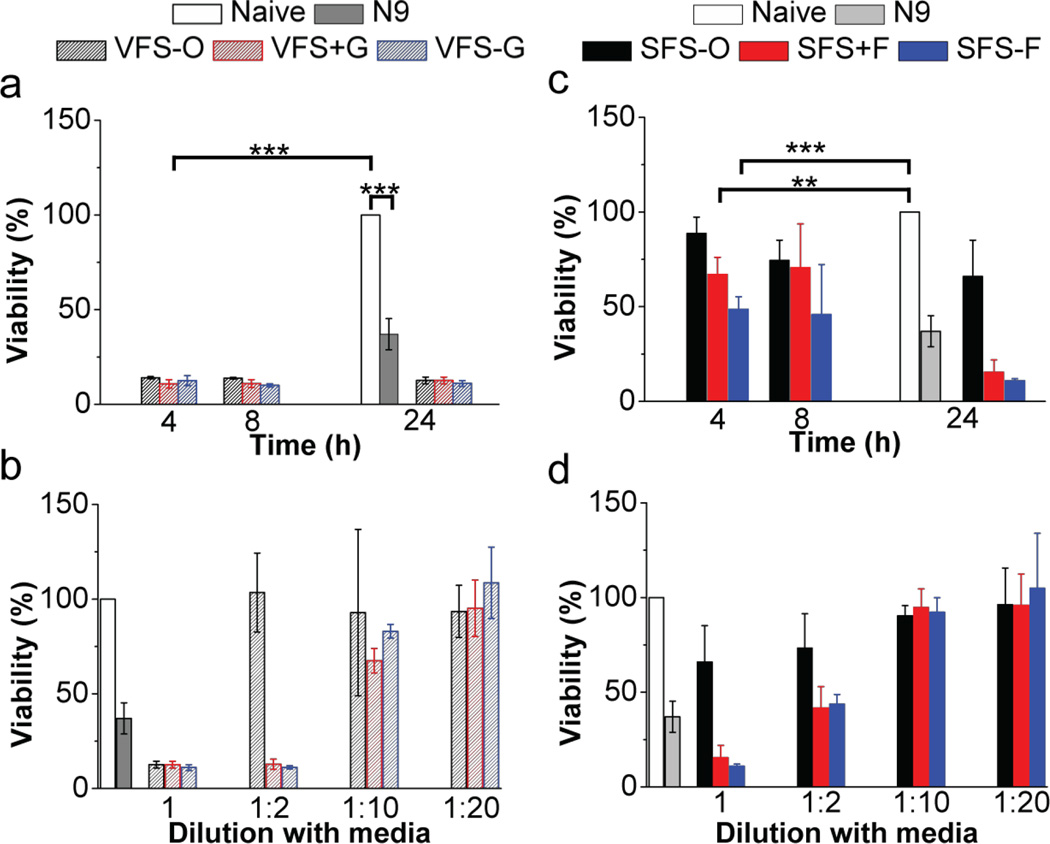
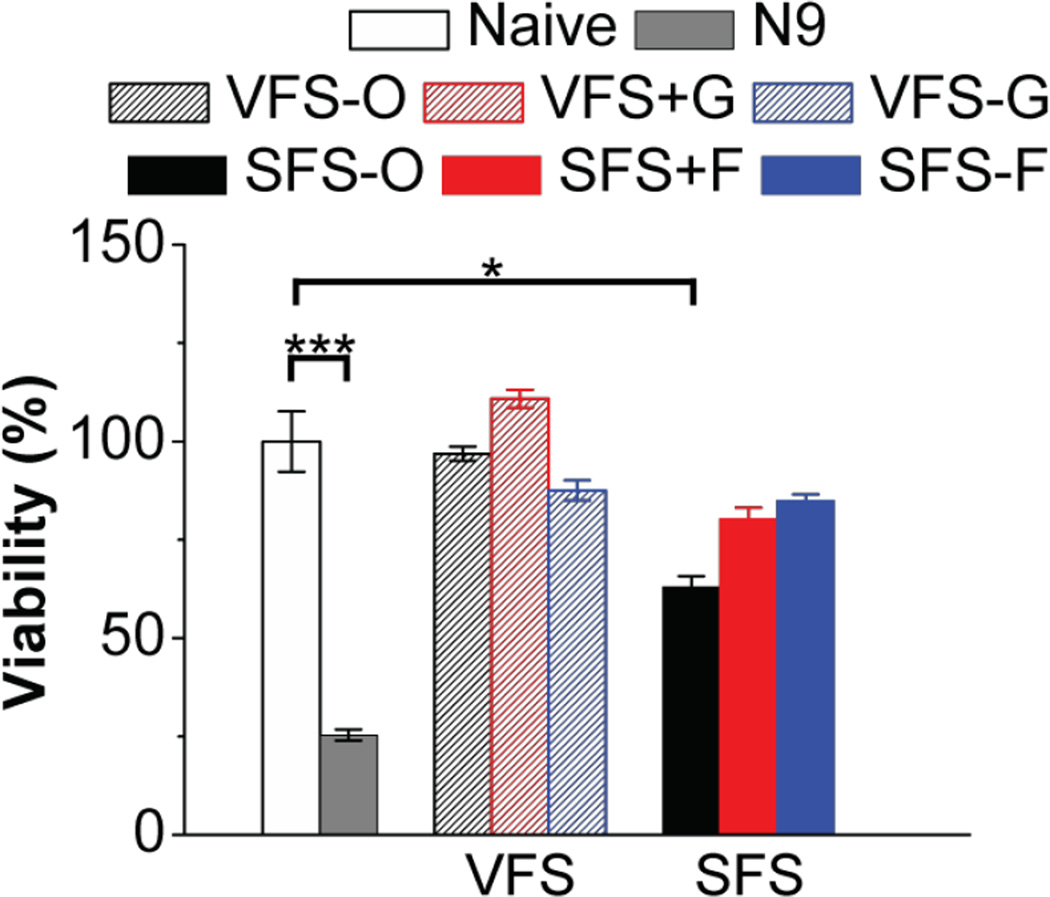
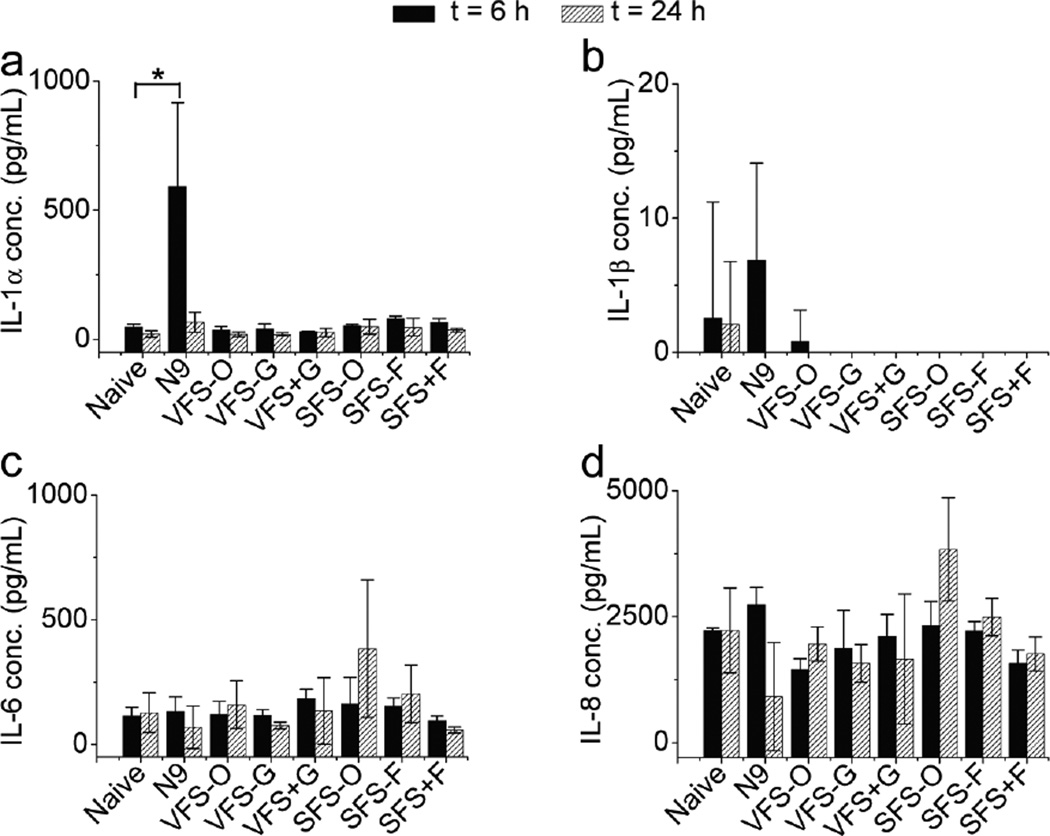
Cytotoxicity of all buffers was estimated both in epithelial cell layers and VEC-100-FT engineered tissue samples. Details are found in Appendix B. Although it is unlikely that these fluids would be used neat in cell based assays we compared their cytoxicity. We tested the toxicity of neat and diluted buffers on HEC-1A cells and VEC-100-FT tissues (Fig. 4 and Fig. 5). Neat solutions, except SFS-O showed significant loss in cell viability at all time-points, however dilutions were well tolerated. The decreased cell viability in the presence of VFS may be more pronounced due to acidic pH at 4 and 8 h time-points. At 24 h, our SFS buffers showed a great loss in cell viability possibly due to absence of nutrients in comparison to the more complex SFS-O. We then evaluated the cytotoxicity of the buffers on 3D–reconstructs derived from non-transformed, human ectocervical epithelial cells (VEC-100FT). These tissues can be used in multi-day experiments to study the effect of repeated exposures on cell/tissue viability and inflammatory response [18]. All solutions, except N9 and SFS-O were well tolerated with 3-once-daily apical application. Further basal solutions were analyzed for secreted cytokine concentration to determine any inflammatory response. Only a transient increase in IL-1α level was noted with N9 treatment at 6 h.
Figure 4.
Cytotoxicity of VFS and SFS buffers in HEC-1-A cells. Naïve cells and N9 (0.1 mg/mL; N = 3) were used as controls and effect of exposure time (4, 8 and 24 h) and dilution with cell culture media followed by 24 h dosing was studied (N = 5, Mean ±SD). N9 (p < 0.001; unpaired t-test with two-tailed distribution and unequal variance) and undiluted VF compositions (p < 0.001; single factor ANOVA) showed significant toxicity with respect to no treatment. An increase in viability with VFS compositions diluted with cell culture media in the ratio 1:2, 1:10 and 1:20 was noted. Whole SFS-O was not toxic and diluted SFS−/+F (1:10 and 1:20) showed enhanced cell viability with no significant difference from untreated cells (p > 0.05; single factor ANOVA).
Figure 5.
Effect of whole buffers on viability of VEC-100-FT tissue after 3 days of daily apical dosing (N = 3, Mean ± SD). Naïve tissue and N9 (0.1 mg/mL) were used as negative and positive controls. Treatment with N9 (p = 0.004) and SFS-O (p = 0.02; unpaired t-test with two-tailed distribution assuming equal variance) showed significant loss in tissue viability with respect to untreated tissue. No significant differences were noted with any of the proposed simulants and VFS-O.
4. DISCUSSION
Physico-chemical properties such as pH, ionic strength, buffering capacity and osmolarity, of reproductive tract fluids impact performance of vaginal products. Thus, in vitro assay media that mimic these salient properties to evaluate products prior to clinical studies is necessary. Furthermore, assay media should be simple compositions devoid of complex divalent ions that have a tendency to interact with drugs and other test articles. In this report, we present a rational approach towards design of simulated reproductive tract fluids.
Following the literature, we identified the major buffering species in HVF and HSF [8, 9] and reconstructed buffers that contain the major species, demonstrating similar pH, osmolality and ionic strength. We then calculated molar ratios of the conjugate acid and base required to obtain the target pH.
Osmolarity of our proposed buffers was adjusted by addition of NaCl or glucose in VFS+G and fructose in SFS+F to match with Owen’s recipes (Fig. 2c and Fig. 3d). Our proposed vaginal and seminal simulants were found to be stable at 4°C for 1 month. In contrast, osmolality of VFS-O was reduced within 24 h of storage at 4°C and extensive precipitation was observed in SFS-O.
Another significant advancement is the measurement of buffering capacity of undiluted HVF samples presented in this report (Fig. 1a, Fig. 2b). Experimental determination of buffering capacities yielded similar values for HVF (β4.2–5.2 = 35.6 ± 12.3 mM) and pooled HSF (β7–6 = 37.5 ± 5 mM, N = 3); similar to reported HSF buffering capacities (β7–6 = 15–65 mM) [19, 20] thus calling into question a common assumption that the buffering capacity of HVF is significantly lower than that of HSF [4, 21].
We have presented a rational approach for formulating simplified buffers that mimic essential properties of human vaginal and seminal fluid. Our buffers, while functionally similar to the Owen simulant, are simpler and more stable, representing a significant and useful contribution to the reproductive drug delivery field.
Implications.
To support research in reproductive health and in particular drug delivery, we have designed and characterized stable new media to mimic these important fluids that can be used in a variety of in vitro studies.
Acknowledgments
This work was supported by the National Institutes of Health (grant no. U19AI076980).
APPENDIX A
A.1 Calculations
The ionic strength (I) was calculated using the standard definition:
| [A.1] |
The buffering capacity (β) of vaginal fluid is the slope of the titration curve in the basic direction and is defined as the gradient of the concentration of strong base with one unit change in pH and can be calculated as
| [A.2] |
where is the sum of the buffering capacity for each buffering species in solution. As noted from eqn. A.2, β depends on the pKa, initial pH and concentration of the buffering species (Cj). The solution for multiple buffering species buffers is more complex and beyond the scope of this paper. We, therefore, used a first order approximation of the buffering capacity for two buffering species with similar pKa (like lactic and acetic acid) where the buffering capacity reduces to the sum of the calculated β values when near their pKa [22]. If the desired pH value was more than 2 units away, we used the pKa value closest to the pH in eqn. A.2 (for example for SFS pH 7.7, only phosphate pKa (pKa = 7.21) was used in the calculation). Starting pKa for acetate (pKa = 4.74) and lactate (pKa = 3.86) were used for calculation [23, 24]. We then computed an effective pKa (pKa,e) of each of the buffering components for temperature changes and ionic strength. To correct for salt effects (pKa,I) and temperature changes (37°C use versus 22°C construction; we utilized eqn A.3 to calculate the effective pKa (pKa,e)
| [A.3] |
The pKa gradient with temperature for each buffering species was obtained from the literature [24, 25]. The pKa adjustment for ionic strength, pKa,I and the corresponding impact of salt screening effects on pKa determined using the Debye-Hückel equation is also described in literature [24].
Ionic strength and effective pKa mutually affect each other, ionic strength influences the effective pKa of a buffering species by charge screening effects, while the effective pKa at a given pH determines the concentration of charged buffering species thereby influencing the ionic strength. Thus, an iterative approach was used to obtain a convergent solution for the effective pKa (pKa,e, eqn.A.3) where the ionic strength was determined. From an initial set of concentrations of NaCl and buffering species modified from those proposed by Owen et al. [8, 9], the ionic strength was calculated and eqn. A.3 was used to compute pKa,e. This value was then used to calculate the buffering capacity at pH 4.2 for vaginal and 7.7 for seminal fluid simulants. Based on the result we altered the concentration of the buffering species to attain the target buffering capacity and adjusted the NaCl concentration to set the ionic strength. The composition of the buffering species and the [NaCl] would thus asymptote toward a single value. The calculation was repeated until the effective pKa changed less than 0.1 unit from the previous iteration. Buffers were then constructed with calculated amounts of the components using free acids and the number of equivalents of NaOH required to achieve the calculated ratios of the conjugate acid and bases. The number of moles of NaOH was used to compute how much conjugate base component (sodium lactate) would be added to the buffer. Since the buffering capacity is not a unitary value that depends on pH, and the concentration of the buffering species and their effective pKa we will use the notation, β4.2–5.2 to represent the VFS buffering capacity between pH 4.2 and 5.2 and β7–6 for buffering capacity of SFS between pH 7 and 6.
A.2 Statistical analysis
Paired and unpaired t-tests were performed to compare physicochemical properties of Owen’s and simplified recipes. Single factor ANOVA was used for cell and tissue viability studies to compare multiple data points.]
APPENDIX B
Preparation of VFS+G
The buffer components for VFS+G preparation are tabulated in Table 1. To make 100 mL of 10X stock, 5 g of glucose, 4.5 g of sodium chloride and 4.04 g of sodium lactate are weighed in a 100 mL volumetric flask. Since sodium lactate used in this experiment was a 50% aqueous solution, therefore, 4.04g of the solution was weighed. To this 0.79 mL of glacial acetic acid was added followed by 18 mΩ distilled water and stirred until all solids completely dissolved. The volume was made up to mark and the pH was measured. The final concentrated VFS+G should read pH ~ 4.2 ± 0.1. The stock was stored at 4°C and diluted 10X before use.
Preparation of SFS+F
Buffer components are outlined in Table 2. To make 100 mL of 10X stock, 0.97 g monobasic sodium phosphate, monohydrate with 9.46 g sodium phosphate dibasic and 0.62 g lactic acid were weighed in a clean 100 mL volumetric flask. 3.64 g of fructose and 50.4 g of bovine serum albumin were added to the flask followed by 18 mΩ distilled water and stirred until all solids completely dissolved. The volume was made up to mark and the pH was measured. The final concentrated SFS+F should read pH ~ 7.7 ± 0.1. The stock was stored at 4°C and diluted 10X before use.
Table 2.
Compositions of Owen’s recipe (SFS-O) and proposed seminal fluid simulants with (SFS+F) and without fructose and protein (SFS-F)
| Chemical Name |
Formula | MW (gmol) |
CAS # | Company | Grade | SFS-O | SFS+F | SFS-F | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Mass (g) |
3Conc. (mM) |
2Mass (g) | Conc. (mM) |
2Mass (g) |
3Conc. (mM) |
||||||
| Sodium phosphate monobasic monohydrate |
NaH2PO4.H2O | 137.99 | 10049-21-5 | Mallinckrodt Chemicals |
USP-FCC | 0.92 | 6.72 | 0.97 | 7.08 | 0.97 | 7.08 |
| Sodium phosphate dibasic |
Na2HPO4 | 141.96 | 7558-79-4 | J.T. Baker | USP-FCC | 8.58 | 60.44 | 9.46 | 66.64 | 9.46 | 66.64 |
| Sodium citrate dihydrate |
C6H5Na3O7 | 294.1 | 6132-04-3 | J.T. Baker | USP-FCC | 8.13 | 27.64 | - | - | - | - |
| Potassium chloride |
KCl | 74.55 | 7447-40-7 | BDH | ACS | 0.908 | 12.18 | - | - | - | - |
| Potassium hydroxide |
KOH | 56.11 | 1310-58-3 | J.T. Baker | ACS | 0.88 | 15.74 | - | - | - | - |
| D-(-)- Fructose |
C6H12O6 | 180.16 | 57-48-7 | J.T. Baker | 2.72 | 15.1 | 3.64 | 20.24 | - | - | |
| D-Glucose monohydrate |
C6H12O6.H2O | 180.1 | 14431-43-7 | Alfa Aesar | 99% | 1.02 | 5.15 | - | - | - | - |
| L(+)-Lactic acid |
C3H6O3 | 90.08 | 79-33-4 | Acros Organics |
90% | 40.62 | 8.15 | 40.62 | 8.15 | 40.62 | 8.15 |
| Urea | NH2CONH2 | 69.06 | 57-13-6 | J.T.Baker | Ultrapure Bioreagent |
0.45 | 7.49 | - | - | - | - |
| Bovine serum albumin (Fraction V) |
9048-46-8 | OmniPur | 50.4 | 0.76 | 50.4 | 0.76 | - | - | |||
| Calcium chloride dehydrate |
CaCl2.2H2O | 147.02 | 10035-04-8 | Mallinckrodt Chemicals |
1.01 | 9.1 | - | - | - | - | |
| Magnesium chloride hexahydrate |
MgCl2.6H2O | 203.3 | 7791-18-6 | Mallinckrodt Chemicals |
0.92 | 4.53 | - | - | - | - | |
| Zinc chloride | ZnCl2 | 136.29 | 7646-85-7 | Acros Organics |
98+% | 0.34 | 2.5 | - | - | - | - |
| Sodium chloride |
NaCl | 58.44 | 7647-14-5 | Spectrum Chemicals |
USP | - | - | - | - | 0.46 | 8 |
(MW = molecular weight)
Mass of species to prepare 1L of 1X buffer
Mass of species to prepare 100 mL of 10X buffer
Concentration of the species in 1X buffer
Final mass of lactic acid to be added for buffer preparation
Cell and tissue toxicity and cytokine analysis
Toxicity of whole and diluted buffers was tested on HEC-1-A cell layers and VEC-100-FT tissues. Briefly, 50 µL of whole or diluted buffer solutions (1:2, 1:10 and 1:20) were dosed for varying time intervals (4, 8 and 24 h) on HEC-1-A cells. Reconstructed EpiVaginal™ tissue was treated with 50 µL of test solutions and controls apically daily for 3 days. Cell viability was determined using CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI) with optical density readout at 490 nm using a Synergy HT Multi-Mode Microplate Reader (BioTek, Winooski, VT). To monitor the tissue viability following exposure, the tissues were rinsed with PBS and loaded with (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) at 37 °C, 5% CO2 (incubation time = 3 h) [18]. In case of vaginal tissues, the inserts were transferred on to 24-well plates containing 2.0 mL of isopropyl alcohol; and left undisturbed overnight in dark at RT for dye extraction. The resulting solution was quantified by measuring the optical density at 570 nm. Viability was determined by normalizing the optical density of the samples to the naïve.
Vaginal tissue maintenance media from the basal side (N = 3/sample) collected 6 and 24 h after dosing were analyzed for cytokine release. The sample concentrations were quantified using an 8-point standard calibration by fitting the standard plot to four parameter logistic curves based on logarithmically transformed optical densities.
Cytotoxicity in HEC-1-A epithelial cell layers
Cytotoxicity was estimated in epithelial cell layers (HEC-1-A) with media and N9 as negative and positive controls (N = 3 – 6; Fig. 4). Significant toxicity was noted for N9 (0.1 mg/mL, p < 0.001; unpaired t-test with two-tailed distribution and unequal variance) as well as for all undiluted VFS for all time-points tested (p < 0.001; single factor ANOVA) with respect to no treatment. The low pH (~4.2) of whole VFS led to significant alteration of the pH of cell culture media resulting in loss of cell viability. Thus dilutions with maintenance media were tested. We observed increased viability with VFS compositions diluted with cell culture media in the ratio 1:2, 1:10 and 1:20. Nonetheless, all SFS compositions showed higher cell viability in comparison to VFS compositions after 4 and 8 hours of exposure. Undiluted SFS-O was not toxic for any of the time-points in the study. Whole SFS−/+F showed significant loss in cell viability at 4 h (SFS-F; p = 0.002 and SFS+F; p = 0.0002 [unpaired t-test with two-tailed distribution and unequal variance]). Similar to VFS, dilution of SFS with cell culture media (1:10 and 1:20) resulted in enhanced cell viability with no significant difference from naïve cells (p > 0.05; single factor ANOVA). Overall, the more complex buffer SFS-O was well tolerated in the study.
Cytotoxicity in 3D–engineered vaginal tissue
We tested whole simulant compositions for 3 days with once-daily apical dosing to the VEC-100-FT engineered tissue samples. Complete basal media was changed each day for tissue maintenance. Significant loss in tissue viability was noted in case of N9 (p = 0.004) and SFS-O (p = 0.02; unpaired t-test with two-tailed distribution assuming equal variance) with respect to naïve tissue. No significant differences were noted with proposed simulants and VFS-O (Fig. 5).
Cytokine expression
Induction of pro-inflammatory cytokines, IL-1α, IL-1β, IL-6, IL-8 and TNF-α was studied in VEC-100-FT engineered tissue 6 and 24h post dosing with whole VFS and SFS solutions (Fig. 6). Significant increase in IL-1α was noted with N9 (positive control) at 6 h after application (p = 0.04; unpaired t-test with two-tailed distribution assuming equal variance). However, no differences were noted in IL-1α 24 h post N9 dosing. No significant increase in any of the tested cytokines was noted with the simulant compositions and TNF- α was not detected with any test article.
Figure 6.
Pro-inflammatory cytokine induction in VEC-100-FT tissue after exposure to whole buffers for 6 and 24 h. Cytokines tested include IL-1α, IL-1β, IL-6, IL-8 and TNF-α (N = 3, Mean ± SD). Cytokine levels in tissue maintenance media were compared to untreated (tissue only) and N9 (0.1 mg/mL) treatment. Significant increase in IL-1α concentration was noted 6 h post treatment with N9 (p = 0.04; unpaired t-test with two-tailed distribution assuming equal variance).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.United States Pharmacopeia. Committee of Revision., e.a. The Pharmacopoeia of the United States of America; [Google Scholar]
- 2.Kiser PF, Johnson TJ, Clark JT. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 2012;14(1):62–77. [PubMed] [Google Scholar]
- 3.Garg S, et al. Advances in development, scale-up and manufacturing of microbicide gels, films, and tablets. Antiviral Res. 2010;88(Suppl 1):S19–S29. doi: 10.1016/j.antiviral.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Tevi-Benissan C, et al. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin Diagn Lab Immunol. 1997;4(3):367–374. doi: 10.1128/cdli.4.3.367-374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker JR. The chemical control of contraception. Chapman & Hall; 1935. [Google Scholar]
- 6.Dorr RT, et al. In vitro retinoid binding and release from a collagen sponge material in a simulated intravaginal environment. J Biomed Mater Res. 1982;16(6):839–850. doi: 10.1002/jbm.820160609. [DOI] [PubMed] [Google Scholar]
- 7.Geshnizgani AM, Onderdonk AB. Defined medium simulating genital tract secretions for growth of vaginal microflora. J Clin Microbiol. 1992;30(5):1323–1326. doi: 10.1128/jcm.30.5.1323-1326.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26(4):459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 9.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 10.Dressman JB, et al. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15(1):11–22. doi: 10.1023/a:1011984216775. [DOI] [PubMed] [Google Scholar]
- 11.Galia E, et al. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705. doi: 10.1023/a:1011910801212. [DOI] [PubMed] [Google Scholar]
- 12.Wagner G, Levin R. Human vaginal pH and sexual arousal. Fertil Steril. 1984;41(3):389–394. [PubMed] [Google Scholar]
- 13.Huggins GR, Preti G. Volatile constituents of human vaginal secretions. Am J Obstet Gynecol. 1976;126(1):129–136. doi: 10.1016/0002-9378(76)90477-4. [DOI] [PubMed] [Google Scholar]
- 14.Shukair SA, et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunology. 2013;6(2):427–434. doi: 10.1038/mi.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell C, et al. Estimating Volume of Cervicovaginal Secretions in Cervicovaginal Lavage Fluid Collected for Measurement of Genital HIV-1 RNA Levels in Women. Journal of Clinical Microbiology. 2011;49(2):735–736. doi: 10.1128/JCM.00991-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shedlovsky L, Belcher D, Levenstein I. Titrations of human seminal fluid with acids and alkalis and their effects on the survival of sperm motility. American Journal of Physiology. 1942;136(3):535–541. [Google Scholar]
- 17.Gopalkrishman K, Hinduja IN, Kumar TC. Determining the osmolality of seminal fluid aids in the rapid diagnosis of the fertilizing potential of spermatozoa. J In Vitro Fert Embryo Transf. 1989;6(2):119–121. doi: 10.1007/BF01130740. [DOI] [PubMed] [Google Scholar]
- 18.Ayehunie S, et al. Development of an in vitro alternative assay method for vaginal irritation. Toxicology. 2011;279(1–3):130–138. doi: 10.1016/j.tox.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolters-Everhardt E, et al. Buffering capacity of human semen. Fertil Steril. 1986;46(1):114–119. doi: 10.1016/s0015-0282(16)49468-9. [DOI] [PubMed] [Google Scholar]
- 20.Searcy RL, Simms NM. A practical approach for acid-base characterization of human semen. Int J Fertil. 1967;12(3):329–334. [PubMed] [Google Scholar]
- 21.Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radio-telemetry of vaginal pH during human coitus. Journal of Reproduction and Fertility. 1973;33(1):69–75. doi: 10.1530/jrf.0.0330069. [DOI] [PubMed] [Google Scholar]
- 22.Asuero AG. Buffer capacity of a polyprotic acid: first derivative of the buffer capacity and pKa values of single and overlapping equilibria. Crit. Rev. Anal. Chem. 2007;37(4):269–301. [Google Scholar]
- 23.Martin A, Tartar H. The Ionization Constant of Lactic Acid, 0–50° from Conductance Measurements. J. Amer. Chem. Soc. 1937;59(12):2672–2675. [Google Scholar]
- 24.Beynon RJ, Easterby JS. Buffer Solutions. Oxford University Press; 1996. [Google Scholar]
- 25.Dawson RMC, et al. Data for Biochemical Research. 3rd. Clarendon Press; 1989. p. 592. [Google Scholar]