Abstract
Increased vascular endothelial growth factor (VEGF) levels are associated with OA progression. Indeed, VEGF appears to be involved in OA specific pathologies including cartilage degeneration, osteophyte formation, subchondral bone cysts and sclerosis, synovitis, and pain. Moreover, a wide range of studies suggests that inhibition of VEGF signaling reduces OA progression. This review highlights both the potential significance of VEGF in OA pathology and pain as well as potential benefits of inhibition of VEGF and its receptors as an OA treatment. With the emergence of the clinical use of anti-VEGF therapy outside of OA, both as high dose systemic treatments and low dose local treatments, these particular therapies are now more widely understood. Currently, there is no established disease-modifying drug available for patients with OA, which warrants continued study of the inhibition of VEGF in OA, as standalone or adjuvant therapy.
Keywords: OSTEOARTHRITIS, VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF), VASCULAR ENDOTHELIAL GROWTH FACTOR RECEPTOR (VEGFR), ANGIOGENESIS
Introduction
In 2005, it was estimated that nearly 27 million U.S. adults had clinical osteoarthritis (OA), a prevalence that represented more than 10% of the U.S. adult population.(1,2) OA, the most common form of arthritis, is a leading cause of pain and disability of older individuals, and the incidence of OA is increasing with the increasing mean age of the population in the U.S.(3–5) Worldwide, the prevalence and incidence of OA is predicted to rise due to increased mean lifespan (aging) and obesity.(6,7)
Features of OA include cartilage degeneration, osteophyte formation, subchondral bone cysts and sclerosis, synovitis, as well as pain. There is no established clinically used disease-modifying OA drug (DMOAD). Current pharmacologic treatment options for OA, rather than inhibiting OA progression, focus on relieving pain and promoting functional improvement in patients; however, even in this regard, treatments such NSAIDS can be limited in efficacy and can pose significant adverse side effects.(8) Unfortunately, with the increasing burden of OA worldwide, there is a greater need for more effective pharmacologic treatments for OA. This review will discuss the pathophysiologic role of vascular endothelial growth factor (VEGF) in OA progression and in associated joint pain. Also, studies targeting VEGF, VEGF’s cognate receptors, or downstream effects of VEGF, such as angiogenesis, for treatment of joint degeneration and pain will be discussed.
In mammals, the VEGF family of glycoproteins is composed of VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF). Members of the VEGF family can bind to three types of receptor tyrosine kinases (RTKs), VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4), that can mediate signal transduction. VEGF-A binds to VEGFR-1 and VEGFR-2. VEGF-B and PlGF bind to VEGFR-1. VEGF-C and VEGF-D bind to VEGFR-2 and VEGFR-3.(9,10)
VEGF-A is the founding member of the VEGF family and is classically referred to as VEGF. In regard to the VEGF family of glycoproteins, VEGF-A is the most widely studied and targeted in the context of OA pathogenesis. Throughout the remainder of this text, VEGF-A will be referred to as VEGF. Major human isoforms of VEGF, which result from alternative splicing, include VEGF121, VEGF165, VEGF189, and VEGF206.(9,10) Murine counterparts of human VEGF include VEGF120, VEGF164, VEGF188, and VEGF205.(11,12) VEGF signaling can be inhibited by endogenously produced soluble VEGFR-1 (sVEGFR-1/sFlt-1), which lacks an RTK and binds to VEGF. VEGF specific isoforms also bind to neuropilin 1 (NRP-1) and neuropilin 2 (NRP-2), which act as co-receptors that can enhance VEGFR signaling.(9,10)
VEGF acts on a wide variety of cell types and has a wide-range of functions.(9,13) Well-characterized functions of VEGF include stimulation of angiogenesis, monocyte chemotaxis, vascular permeability, and vasodilation. Expression of VEGF widely occurs during embryogenesis.(9) VEGF is an important mediator of bone development.(14) In adults, well-characterized physiologic roles of VEGF include angiogenesis during the female reproductive cycle, wound healing, and bone repair.(13–15) However, there are also a number of well-known pathophysiologic effects of VEGF, including roles in tumor angiogenesis, rheumatoid arthritis (RA), psoriasis, atherosclerosis, amyloid lateral sclerosis, brain edema, age-related macular degeneration, diabetic retinopathy, retinopathy of prematurity, and sepsis.(13,15)
Aberrant VEGF Signaling in OA Tissues
During later stages of OA in affected patients, VEGF expression has been found to be increased in the articular cartilage,(16–24) synovium,(25–29) synovial fluid,(30–34) subchondral bone, (35–37) and serum.(30,31,33,38) Assessment of VEGF as a biomarker in patients with OA demonstrated that increased synovial fluid VEGF is not only correlated with grade of OA severity but also with the degree of OA pain.(34) A meta-analysis of studies assessing VEGF expression in OA compared to non-OA human joint tissue found significantly elevated levels of VEGF in OA tissue.(39) A meta-analysis of nine genome-wide association studies (GWAS), assessing 199 OA-related candidate genes, concluded that VEGF and one other candidate gene are significantly correlated with OA.(40)
Angiogenesis, which is the generation of new blood vessels from pre-existing vessels, within an affected joint has also been attributed to OA progression. With increasing OA severity, greater vascular invasion into articular cartilage has been described;(19,22,41) indices of angiogenesis, including increased vascular density and endothelial cell (EC) proliferation have been demonstrated to be increased within OA synovium,(27,41–43) while increased vascular invasion has been reported at the meniscus.(44) Table 1 summarizes clinical studies demonstrating the association between increased expression of VEGF, VEGFRs, and angiogenesis in OA progression.
Table 1.
Increased VEGF, VEGFRs, and Angiogenesis are Correlated with Osteoarthritis Progression in Human Studies
| Tissue | Change in OA Tissue | Ref. |
|---|---|---|
| Cartilage | ↑ VEGF (OA chondrocytes) | (16–24) |
| ↑ VEGFR-1 (OA chondrocytes) | (21) | |
| ↑ VEGFR-2 (OA chondrocytes) | (18,21) | |
| ↑ NRP-1 (OA chondrocytes) | (21) | |
| ↑ Vascular invasion | (19,22,41) | |
| ↑ VEGF | (23) | |
| Synovium | ↑ VEGF (synovial macrophages and fibroblasts) | (25–29) |
| ↑ Angiogenesis | (27,41–43) | |
| Synovial fluid | ↑ VEGF | (30–34) |
| Subchondral bone | ↑ VEGF (osteoblasts) | (35–37) |
| Serum | ↑ VEGF | (30,31,33,38) |
| Meniscus | ↑ 40;Vascular invasion | (44) |
Abbreviations: NRP-1: neuropilin-1; VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor.
Recent in vivo studies have examined the role of intra-articular (IA) administration of VEGF and have reported that VEGF independently causes characteristic features of OA. Ludin et al. (2013) demonstrated that local intra-articular (IA) injection of VEGF in the knee joint of mice induced OA-like changes in the joint, reflected by proteoglycan loss, calcification of the articular cartilage, cartilage degradation, bone sclerosis, osteophyte formation, and synovial hyperplasia. These pathologic changes in the knee joint were progressively more severe with increased numbers of VEGF injections. Control groups receiving IA saline did not show any signs of OA-like changes. VEGF precipitated bone sclerosis, synovial hyperplasia, and calcification of articular cartilage initially; while, VEGF-mediated cartilage degeneration occurred at later time points. Osteophytes were only identified in the VEGF-treated group. Discontinuation of IA VEGF led to resolution of both bone sclerosis and synovial hyperplasia.(45)
Shen et al. (2015) demonstrated that IA injection of VEGF into the temporomandibular joint (TMJ) in mice resulted in progressive cartilage degeneration as well as subchondral bone lesions and sclerosis. Increased expression of matrix metalloproteinase-9 (MMP-9) and MMP-13 were demonstrated at the articular chondrocytes with IA injection of VEGF. The condylar cartilage of VEGF treated joints demonstrated a greater number of apoptotic as well as VEGFR-2 positive chondrocytes. Additionally, the condylar chondrocytes demonstrated increased expression of nuclear factor-kappa-B ligand (RANKL), which suggests that chondrocytes are a potential mediator of VEGF mediated subchondral bone resorption through stimulation of RANKL. The subchondral bone of VEGF treated joints showed higher number of tartrate-resistant acid phosphatase (TRAP) positive osteoclasts.(46) In contrast to Ludin et al. (2013), discontinuation of IA VEGF treatment did not lead to resolution of subchondral bone pathology at 4 weeks post administration.
IA injection of VEGF-expressing muscle-derived stem cells (MDSCs) in an osteochondral defect (OCD) rat model resulted in cartilage degradation, synovial hypertrophy, pannus invasion into cartilage and bone, osteolysis, as well as subchondral cyst and osteophyte formation.(47) This study assessed treatment of a cartilage defect with MDSCs alone or MDSCs expressing bone morphogenetic protein 4 (BMP-4), VEGF, or sFlt-1. Only treatment groups receiving VEGF-expressing MDSCs resulted in global OA-like changes in the joint, and the VEGF treatment groups were terminated early due to severe joint deterioration.(47) Table 2 summarizes studies that assessed the effects of introduction of VEGF into a joint.
Table 2.
Administration of VEGF at the Joint Induces Osteoarthritic Changes in Animal Models
| Model | Treatment | Results | Ref. |
|---|---|---|---|
| Mouse | IA VEGF at knee | ↑ Cartilage degradation, calcification of articular cartilage, subchondral bone sclerosis, synovial hyperplasia, and osteophyte formation |
(45) |
| Mouse | IA VEGF at TMJ |
↑ Cartilage degradation, subchondral bone lesions and sclerosis, chondrocyte apoptosis, MMP-9, MMP-13, and RANKL by articular chondrocytes, TRAP positive osteoclasts in the subchondral bone |
(46) |
| Rat OCD | IA VEGF-MDSCs at knee |
↑ Cartilage degradation, pannus invasion into cartilage, and subchondral bone osteolysis |
(47) |
Abbreviations: IA: intra-articular; MMP: matrix metalloproteinase; OCD: osteochondral defect; RANKL: nuclear factor-kappa-B ligand; TMJ; temporomandibular joint; TRAP: tartrate-resistant acid phosphatase; VEGF: vascular endothelial growth factor; VEGF-MDSCs: VEGF expressing muscle derived stem cells.
A wide number of studies have discovered that VEGF signaling at chondrocytes influences pro-catabolic mediators. Enomoto et al. (2003) compared OA with non-OA human chondrocytes and found that VEGFR-1, VEGFR-2, and NRP-1 are expressed only in OA chondrocytes but not in non-OA chondrocytes. OA chondrocytes, which were stimulated with VEGF, induced MMP-1 and MMP-3 expression; however stimulation of non-OA chondrocytes with VEGF did not lead to increased MMP expression.(21) These results suggest that VEGF is acting directly through VEGFRs expressed by OA chondrocytes to mediate a catabolic response.
Pufe et al. (2004) tested chondrocytes expressing VEGFR-2 but not VEGFR-1. Stimulation of these chondrocytes with VEGF led to increased production of MMP-1, MMP-3, MMP-13, with concomitant reduction of tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2.(48) This VEGF-mediated pro-catabolic response by chondrocytes was reversed with a VEGFR-2 inhibitor in in vitro and ex vivo conditions.(48,49) VEGF treatment of chondrocytes also increased production of nitric oxide (NO).(48) NO is a direct and specific inducer of chondrocyte apoptosis.(50) Stimulation of chondrocytes with exogenous VEGF also increased IL-1β, IL-6, and TNF-α.(48)
The dual effects of VEGF and inflammatory cytokines on chondrocytes may potentiate cartilage degeneration. For example, an in vitro study of rat articular chondrocytes performed by Chen et al. (2012) showed that treatment of chondrocytes with VEGF decreased expression of key extracellular matrix components, including aggrecan and type II collagen. When VEGF was combined with IL-1β, there appeared to be further synergistic reduction of aggrecan and type II collagen production.(51) Moreover, in an OA animal model, treatment with an anti-VEGF antibody increased aggrecan and type II collagen expression in the articular chondrocytes.(52)
The biological impact of anti-VEGF antibody on chondrocytes was also assessed in an in vitro/ex vivo culture system. Cartilage explants placed in a VEGF-rich medium increased chondrocyte expression of MMP-3, MMP-13, and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5). Addition of an anti-VEGF antibody to this VEGF-rich medium reduced chondrocyte metalloproteinase expression.(53)
Aberrant VEGF signaling at the joint in OA may be influencing both abnormal endochondral ossification at the articular surface and subchondral bone remodeling. These processes mediated by aberrant VEGF signaling may be leading to cartilage degeneration, osteophyte formation, as well as subchondral bone cysts and sclerosis. Therefore, this warrants further investigation into the specific roles of VEGF on specific cells types involved in influencing both endochondral ossification and bone remodeling.
VEGF signaling has a fundamental role in angiogenesis, stimulating EC proliferation, migration, maturation, and survival.(9,54) Stimulation of angiogenesis by VEGF occurs principally through stimulation of EC VEGFR-2. VEGFR-1 on ECs is classically described as serving as a decoy receptor, decreasing VEGF/VEGFR-2 activity; however, activation of EC VEGFR-1 under certain conditions may promote VEGFR-2 activity.(9,10) Further, recruitment and activation of macrophages mediated by VEGFR-1 may further stimulate angiogenesis by macrophage secretion of pro-angiogenic cytokines, including VEGF.(27,55–57)
VEGF is associated with survival, differentiation, maturation, migration, and increased resorptive activity of osteo(chondro)clasts.(58–63) Osteoclasts and chondroclasts share similar phenotypes, and both appear capable of cartilage matrix resorption.(64) In regard to VEGFR signaling, it has been reported that VEGFR-1 plays a more important role in stimulating osteoclastogenesis, while VEGFR-2 appears to more greatly influence stimulation of mature osteoclast function.(58,61,63,65) VEGF has also been suggested to indirectly stimulate osteoclastogenesis by stimulating production of RANKL at articular chondrocytes, osteoblasts, and synovial fibroblasts.(46,65,66)
VEGF stimulates osteoblast migration,(67) activity,(68) and differentiation.(59,69) Activation of VEGFR-1 by VEGF induces osteoblast migration, and VEGFR-1 is the primary receptor that mediates the biological effects of VEGF signaling on mature osteoblasts.(67). Both VEGFR-1 and VEGFR-2 are involved in osteoblast differentiation from mescenchymal stem cells (MSCs) through VEGF-mediated Runx2 expression.(59)
The process of endochondral ossification is classically described during fracture healing and long bone development. During this process, hypertrophic chondrocytes produce VEGF, and vascular invasion, which is stimulated by VEGF occurs into the previously avascular cartilage; further, osteo(chondro)clasts, are recruited to the cartilage at sites of vascular invasion, under stimulation by VEGF, to degrade the cartilage matrix, and osteoblasts, which are recruited and stimulated by VEGF, actively lay down new bone matrix.(14)
The process of endochondral ossification can be disrupted by the inhibition of VEGF. Inhibition of VEGF with a soluble chimeric protein (Flt-IgG) reduced chondrocyte apoptosis, reduced recruitment and/or differentiation of chondroclasts expressing MMP-9, decreasing cartilage resorption during endochondral ossification at the growth plate.(70) Similarly, it was discussed that inhibition of VEGF with Flt-IgG during secondary fracture healing inhibited cartilage resorption and ossification at the fracture site.(68) It has been suggested that endochondral ossification, under the stimulation of aberrant VEGF signaling, is facilitating osteophyte formation and cartilage degeneration in OA, which will be discussed further below.
Osteophyte formation has been well described as a process of endochondral ossification that is stimulated by VEGF. Hashimito et al. (2002) showed that during osteophyte formation in an OA animal model hypertrophic chondrocytes produce VEGF, leading to vascular invasion into the cartilage matrix, and bone formation. Osteoclast-like cells and osteoblasts were found at the leading front of vascular invasion into the cartilage at the osteophyte. Additionally, it was shown that NO is produced in ECs and in hypertrophic chondrocytes at osteophyte formation sites, leading to chondrocyte apoptosis.(71) As discussed previously, VEGF induces NO in chondrocytes.(48) A major, and well-characterized, stimulator of NO production in ECs is VEGF.(72,73)
Components of endochondral ossification under stimulation by VEGF have also been described along the articular surface leading to cartilage degeneration. Healthy hyaline cartilage is an avascular tissue. However, increased vascular invasion into hyaline cartilage at the articular surface occurs during OA progression. Previous work has demonstrated that OA cartilage loses its ability to remain avascular, and areas of neovascularization in OA cartilage is associated with increased loss of cartilage matrix.(74) Human OA chondrocytes (compared to non-OA chondrocytes) at osteochondral junctions showed increased VEGF expression, which is correlated with increased osteochondral vascular density and chondropathy.(22) Further work demonstrated a direct correlation of osteochondral vascular density with chondropathy and clinical disease severity of OA.(19,41) Aside from increased VEGF expression at OA articular chondrocytes, osteochondral vascularity in OA has also been associated with subchondral bone marrow replacement by fibrovascular tissue expressing VEGF.(19) While chondrocyte hypertrophy and resulting VEGF secretion is well described in initiating vascular invasion of cartilage, it has also been suggested that chondrocytes may be influenced to undergo a hypertrophic phenotype in regions of neovascularization.(74,75)
It has been suggested that during OA progression, similar to endochondral ossification, hyaline cartilage becomes increasingly calcified, which may be a result of chondrocyte hypetrophy.(76) Increased VEGF expression in the articular cartilage is associated with osteoclast recruitment into the calcified cartilage at sites of OA-like lesions.(77) VEGF-mediated osteoclast invasion at the articular cartilage can result in resorption of the calcified matrix, allowing endochondral ossification to occur by deposition of bone by osteoblasts within the degraded cartilaginous matrix. Imaging at the articular cartilage showed that blood vessels invading into calcified cartilage are surrounded by osteoblasts actively laying down bone.(78) Evidence for endochondral ossification resulting in cartilage thinning can be further inferred by assessment of the calcified cartilage. With aging, tidemark duplication occurs, which is a result of progressive calcification of deep hyaline cartilage. Tidemark duplication would suggest that the calcified cartilage zone would expand. However, with aging, the calcified cartilage zone becomes thinner, suggesting that ossification is occurring in the calcified cartilage.(79)
VEGF signaling is also critical for bone remodeling. As described previously, VEGF regulates osteoblast and osteoclast activity, influencing both bone resorption and bone formation. (46,58–63,65–69) Aberrant VEGF signaling in regard to bone remodeling may have an important contribution to subchondral bone cysts and sclerosis. In arthritic joints, VEGF signaling has been suggested to play a role in mediating bone destruction at the joint, through stimulating osteoclast recruitment and activity.(80) While VEGF is involved in bone resorption, this process is also coupled to bone formation, which is also stimulated by VEGF, and may be leading to sclerosis.
Tibial subchondral bone harvested from OA patients showed a phenotypic change in osteoblasts with increased VEGF expression in regions of sclerosis as compared to non-sclerotic regions in the same patients.(81) Given the significant roles VEGF signaling plays in both osteoclast and osteoblast activity,(46,58–63,65–69) evidence suggesting aberrant VEGF signaling is correlated with subchondral bone resorption and sclerosis,(80,81) in additional to studies that demonstrated exogenous IA administration of VEGF is correlated with significant subchondral bone pathology, including cysts and sclerosis,(45–47) we suggest aberrant VEGF signaling during OA pathogenesis may have a strong contribution to OA pathologies evident at the subchondral bone. It is known that pathologic changes in the bone, as well as cartilage damage, during OA progression creates malalignment of the joint, leading to areas of increased focal stress and further joint damage.(82) Therefore, understanding mediators of subchondral bone changes in OA is of critical importance.
Inflammatory pathways are tied to OA progression. One characteristic feature of inflammation at any joint is synovitis. Increased angiogenesis and VEGF production in the synovium is associated with a resultant synovitis.(27,41) Angiogenesis stimulates inflammation and inflammation promotes angiogenesis.(83,84) Importantly, a key mediator of both inflammation and the angiogenic process is VEGF, which will be more completely discussed below. The process of angiogenesis itself is suggested to maintain chronic inflammation by allowing transport of inflammatory cells to the site of inflammation, as well as supplying nutrients and oxygen to the inflamed tissue; further, increased endothelial surface area allows a greater capacity for the production of cytokines, adhesion molecules, and other inflammatory stimuli.(84) Assessment of perivascular cells in OA synovium demonstrates a high amount of inflammatory cell infiltrate.(27,85) VEGF, also known as vascular permeability factor (VPF), promotes vascular permeability through EC VEGFR-2,(86,87) supporting inflammation.(13) A histologic phenomenon known as synovial hyperplasia can be a direct result of chronic inflammation.(88) Injection of VEGF into healthy mice knee joints results in synovial hyperplasia.(45)
VEGF expression is increased in synovial macrophages and fibroblast-like synovial cells in OA.(25–29) Stimulation of monocytes and macrophages and fibroblast-like synovial cells with VEGF increased the expression of TNF-α and IL-6; while, production of these inflammatory cytokines were substantially inhibited by the addition of an anti-VEGF peptide.(89) A study performed by Luttun et al. (2002) demonstrated that an anti-VEGFR-1 antibody impairs production of monocyte chemoattractant protein (MCP-1/CCL-2) and TNF-α by macrophages.(90)
VEGF is a chemoattractant and activator of monocytes/macrophages, and this biological action is mediated by activation of VEGFR-1.(90–92) Use of an anti-VEGFR-1 antibody reduced monocyte and granulocyte mobilization from the bone marrow, with reduction of leukocyte accumulation in the inflamed tissue.(90) Additionally, VEGF signaling activates NF-κB in endothelial cells, which increases production of MCP-1 and IL-8 from endothelial cells and promotes monocyte/macrophage and neutrophil recruitment.(93,94) Neutrophils are a significant source of VEGF.(95) T-cells can both secrete and respond to VEGF to promote inflammation and angiogenesis. VEGF stimulates T-cells to increase INF-γ and decrease IL-10, an anti-inflammatory cytokine.(96) T-cells in the synovial membrane participate in OA inflammation and pathogenesis.(97,98)
It is important to understand how VEGF signaling interactions among OA tissues may be occurring, since multiple tissues are affected at the joint during OA progression. As discussed previously, VEGF expression is increased in the cartilage,(16–23) synovium,(25–29) and subchondral bone(35–37) in OA. Also vascular invasion into articular cartilage is associated with replacement of bone marrow with fibrovascular tissue expressing VEGF.(19) Aberrant VEGF signaling at all these tissues may contribute to characteristic features of OA pathogenesis. Communication of cytokines, such as VEGF, between the cartilage, subchondral bone, and bone marrow can be potentially mediated by fissures, channels, and vascular connections between these tissues.(99–102) Additionally, the synovial fluid allows potential communication of VEGF between the articular surface and synovium. The implications associated with VEGF crosstalk from the subchondral bone to articular cartilage have recently been explored. For example, in an experimental OA model, it has been speculated that increased VEGF produced by osteoblasts and osteocytes present in subchondral bone directly stimulates metalloproteinase expression in articular chondrocytes, leading to cartilage degeneration, while reduction of VEGF secretion from the subchondral bone reduces cartilage degeneration.(53)
An important question is how does VEGF expression become elevated at various tissues within a joint? VEGF expression in chondrocytes is increased by inflammatory cytokines, NO, and reactive oxygen species (ROS).(20,103–105) At chondrocytes, increased mechanical stress and strain,(49,77,106) chondrocyte hypertrophy and increased Runx2 expression,(107) and aging(108) have also been suggested to increase VEGF expression. Mechanical overload in bovine cartilage disks increases MMP and decreases TIMP expression, and this can be reversed with a VEGFR-2 inhibitor.(49) Mechanical loads are thought to stimulate both hypoxia inducible factor-1α- and 2α-(HIF-1 α and HIF-2α)-mediated upregulation of VEGF in chondrocytes,(49,109,110) and hypoxia, itself, is a prime regulator of increased HIFs.(103,108,111,112) Mechanical loading at osteocytes also increases VEGF expression.(113) In synovial cells, hypoxia, IL-1, IL-6, IL-17, IL-18 prostaglandin E2 (PGE2), TGF-β, TNFα, and CD40 ligation are important mediators of increased VEGF expression.(28,65,114–118) Aside from inflammatory cytokines, it has been known that VEGF expression is stimulated by a wide variety of growth factors, including transforming growth factor beta (TGF-β), insulin-like growth factor 1 (IGF-1), and fibroblast growth factor (FGF).(54)
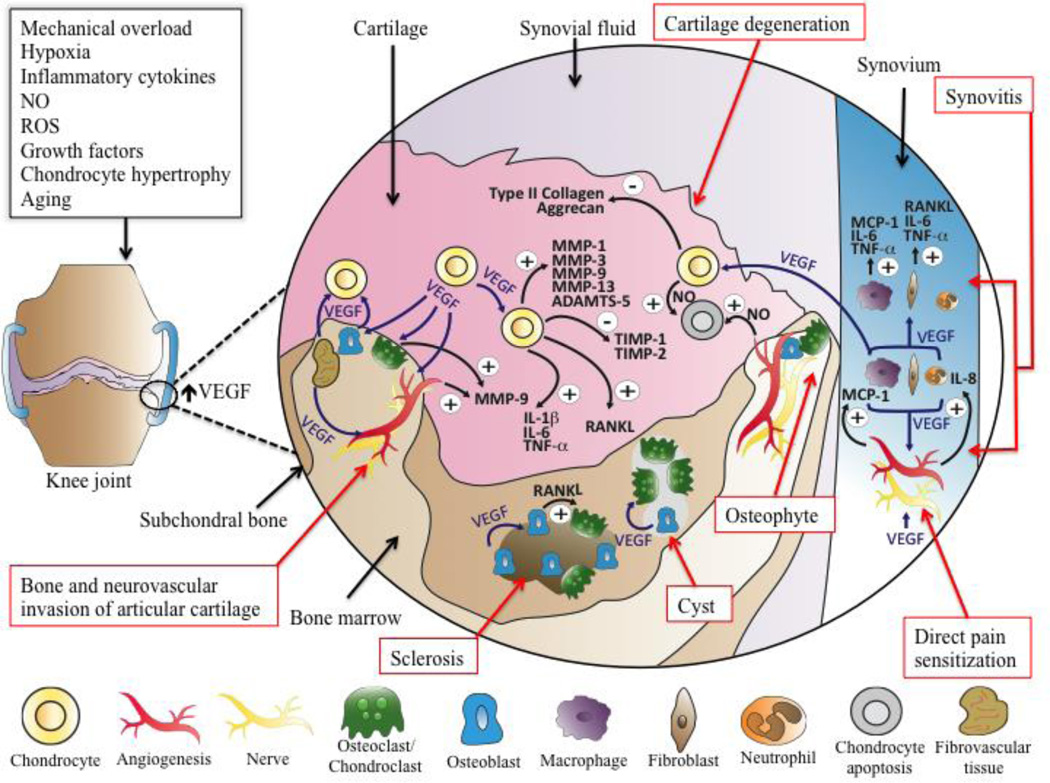
In summary, aberrant VEGF signaling at the joint, which is influenced by a wide range of mediators, has been attributed cartilage degeneration, osteophyte formation, subchondral bone cysts and sclerosis, and inflammation. Figure 1 illustrates potential pathways of VEGF mediated OA disease progression. Figure 2 overviews characterized roles of individuals VEGFRs involved with influencing cell types that may be involved in OA disease progression.
Figure 1.
Potential pathways of VEGF-mediated OA progression and pain. Suggested mediators of increased VEGF expression at the joint tissue include mechanical overload, hypoxia, inflammatory cytokines, NO, ROS, growth factors, chondrocyte hypertrophy, and aging. Cartilage degeneration: Chondrocyte mediated pathways of cartilage degeneration include VEGF-mediated chondrocyte upregulation of MMP-1, MMP-3, MMP-9, MMP-13, and ADAMTS-5. VEGF can also decrease chondrocyte production of TIMP-1, TIMP-2, type II collagen, and aggrecan. VEGF can increase expression of NO in both chondrocytes and endothelial cells, leading to chondrocyte apoptosis. Increased levels of VEGF lead to a minor increase in expression of inflammatory cytokines from chondrocytes, including IL-1β, IL-6, TNF-α. Bone and neurovascular invasion of articular cartilage and osteophyte formation: VEGF stimulates vascular invasion of articular cartilage, and sensory nerve ingrowth can occur upon the tracks of vascular invasion. Increased sensory nerve ingrowth is a potential source of increased pain sensitivity in the joint. VEGF stimulates the recruitment and activity of osteo(chondro)clasts, which can resorb cartilage. Chondroclasts can express MMP-9, leading to matrix degradation of cartilage. Additionally, osteochondral angiogenesis is associated with increased fibrovascular tissue in the bone marrow expressing VEGF. Vascular invasion facilitates the migration of osteoblastic precursor cells towards the articular surface, which are further stimulated by VEGF to produce new bone. Synovitis: VEGF production is increased in synovial macrophages and fibroblasts. VEGF increases migration and/or activity of macrophages, fibroblasts, and neutrophils. Neutrophils may be an additional source of increased VEGF. VEGF stimulates macrophages to secrete MCP-1, TNF-α, and IL-6, and VEGF stimulates fibroblasts to express TNF-α and IL-6. VEGF stimulates endothelial cells to increase expression of IL-8 and MCP-1. IL-8 stimulates neutrophil recruitment, and MCP-1 stimulates macrophage recruitment. VEGF mediated angiogenesis facilitates inflammatory cell infiltration at the synovium. Subchondral bone sclerosis and resorption: VEGF stimulates osteoclast and osteoblast differentiation, migration, and ultimately activity, leading to areas of increased bone resorption and bone formation. Additionally, VEGF can potentially stimulate RANKL expression at chondrocytes, synovial fibroblasts, and osteoblasts, further stimulating osteoclastogenesis. Direct pain sensitization: VEGF can directly stimulate nociceptive sensory neurons to increase pain sensitivity and potentially nerve ingrowth; this may occur in the synovium, osteochondral junction, and meniscus (not shown).
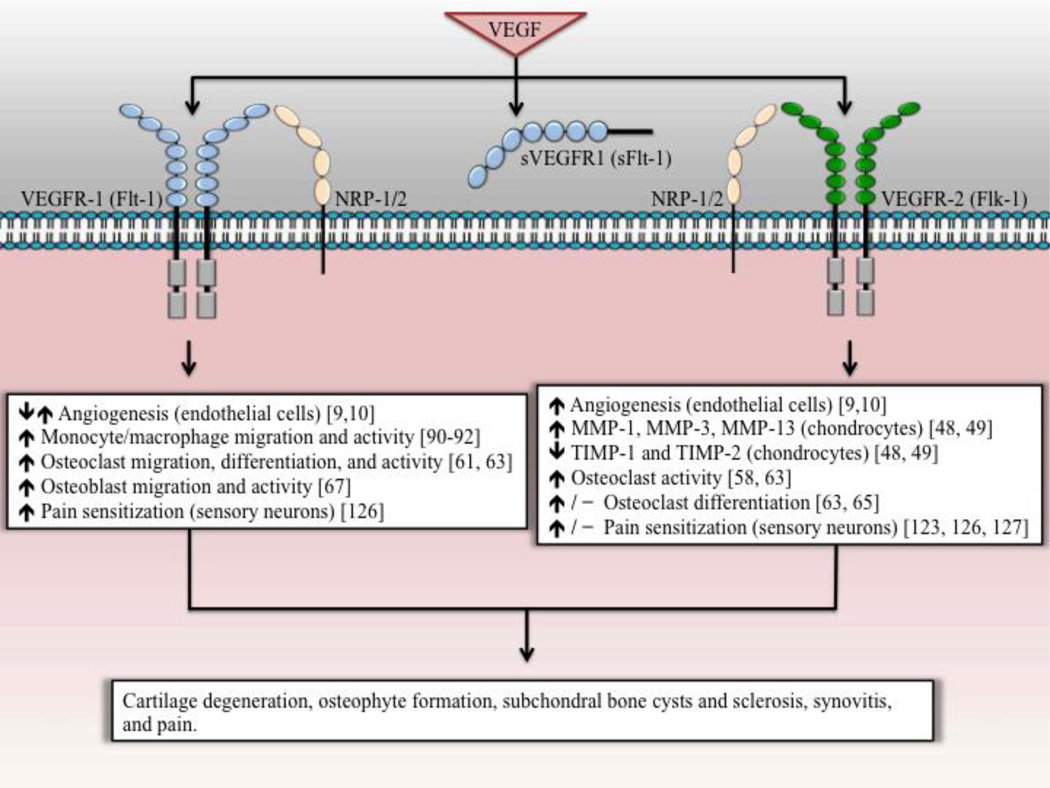
Figure 2.
Specific signaling pathways mediated by VEGFR-1 and VEGFR-2 activation that may lead to OA progression and pain. VEGF binds to both VEGFR-1 and VEGFR-2, inducing RTK signaling. NRP-1 and NRP-2 serve as co-receptors for specific VEGF isoforms to interact with VEGFR-1 and/or VEGFR-2. sVEGFR-1 serves as a soluble decoy receptor for VEGF. VEGFRs are expressed in a wide variety of cell types, mediating different cellular responses. Not shown, VEGFR-1 and VEGFR-2 are suggested to mediate osteoblast differentiation through intracrine signaling.
VEGF Signaling and Pain
A number of studies have evaluated the role of angiogenesis in OA pain. Angiogenesis and inflammation are linked, partly through VEGF, and inflammation is a classical mediator of sensitization of fine unmyelinated sensory nerves, which mediates OA pain.(83) Pathologic angiogenesis promotes sensory nerve ingrowth along the newly formed blood vessels in the osteochondral junction, osteophytes, synovium, and meniscus, facilitating OA pain.(41,43,44,75,83)
Vascular invasion into articular cartilage is associated with increased expression of nerve growth factor (NGF) within the vascular channels.(19) NGF promotes sensory neuron survival, axonal outgrowth, and, importantly, is associated with pain sensitization.(119,120) Therefore, angiogenesis-mediated vascular ingrowth provides a source of NGF-mediated nerve growth and sensitization. NGF induces VEGF expression in sensory neurons in the dorsal root ganglion (DRG),(121) suggesting further cross-talk between sensory neurons and blood vessels. Exogenous, intra-vesicular VEGF administration increased peripheral nerve density and pain.(122) The influence of VEGF on pain may occur indirectly from VEGF-stimulation of angiogenesis and sensory neuron ingrowth or inflammation. Additionally, VEGF may be acting directly on sensory neurons to produce pain sensitization.
VEGF, VEGFR1, and VEGFR2 signaling have appeared to be directly associated with hyperexcitability of sensory neurons, and inhibition of VEGF signaling led to reduction of pain sensitivity.(122–127) Anti-VEGF antibody or inhibition of VEGFR-2 RTK activity by vatalanib attenuates neuropathic pain by decreasing the expression and activity of P2X2/3 and VEGFR-2 receptors in sensory neurons in the DRG.(123,127) More recently, Selvaraj et al. (2015) extensively elaborated the selective role of VEGFR-1 in cancer pain, revealing that: (i) Both VEGFR-1 and VEGFR-2 are broadly expressed in all classes of neurons (i.e., nociceptive and non-nociceptive neurons); (ii) Intraplantar injection of VEGF in mice resulted in both thermal and mechanical hyperalgesia; and (iii) Both thermal and mechanical hyperalgesia were reduced by anti-VEGFR-1 antibody, but not by anti-VEGFR-2 antibody.(126)
Correspondingly, studies using an in vitro preparation of paw skin found that Aδ-nociceptive fibers gave significantly higher discharge rates on mechanical stimulation when exposed to VEGF, which could be reduced with anti-VEGFR-1 antibody.(126) This suggests that VEGF/VEGFR-1 signaling directly sensitizes nociceptors. A significant role for VEGFR-1 in pain was further indicated by studies of transgenic mice. Transgenic mice lacking: an RTK signaling domain of VEGR-1 (Vegfr1-Tk−/−); VEGFR-1 signaling in nociceptive neurons (SNS-Vegfr1−/−); or VEGFR-1 signaling in sensory neurons (Adv-Vegfr1−/−) showed no thermal or mechanical hyperalgesia in response to VEGF stimulation.(126)
VEGF and NGF signaling share common signaling pathways, such as the PI3K–Akt pathway, and VEGF itself may be involved in similar mechanisms of direct nerve sensitization as well as nerve growth that occurs with NGF.(126) Targeting NGF in clinical studies led to a significant reduction in OA pain.(128) In an OA model, anti-VEGF antibody reduced OA pain; (52) however, the effects of anti-VEGF antibody directly targeting nociceptive neuron signaling influenced by VEGF in OA pain remains to be established. Given the role of VEGF in direct pain sensitization, further investigations into the precise roles of VEGFR-1 and VEGFR-2 in pain signaling that are induced by OA are warranted. Figure 1 illustrates a role for VEGF in inflammation and angiogenesis facilitated sensory nerve ingrowth, as well as direct sensitization of sensory nerve fibers by VEGF. Figure 2 summarizes VEGFRs involved in angiogenesis, inflammation, and direct sensitization to pain.
Pharmacologic Inhibition of VEGF Signaling Pathways in Cartilage Repair and Arthritis
Pre-clinical studies on anti-VEGF and anti-angiogenic therapy have investigated treatments for OA, chondral defects (CDs, focal defects in the articular cartilage), osteochondral defects (OCDs, defects in both cartilage and bone), and rheumatoid arthritis (RA). Similar to what has been found at OA cartilage, cytokine profiling of symptomatic focal CDs assessing 19 possible soluble mediators demonstrated that only VEGF is significantly elevated compared to healthy cartilage.(23) Clinical treatment of CDs and OCDs with procedures such as Pridie drilling or microfracture (MFX), allowing MSCs to enter the defective sites, or stem cell transplantation for cartilage defects, faces several challenges. For example, endochondral ossification can occur at the site of the defect.(129–131) Pre-clinical studies assessing anti-VEGF and anti-angiogenic therapy in RA will be addressed, since pathways of joint degeneration between RA and OA share similarities through VEGF signaling.(19,83,132) Table 3 summarizes studies that have assessed treatment with VEGF and angiogenesis inhibitors in OCD/CD, OA, and RA animal models.
Table 3.
Anti-VEGF and Anti-Angiogenic Treatments in Chondral Defect, Osteochondral Defect, Osteoarthritis, and Rheumatoid Arthritis Animal Models.
| Treatment | Model | Results | Ref. |
|---|---|---|---|
| VEGF Inhibitor | OCD/CD | ↑ cartilage repair | (133) |
| OA | ↓ OA progression and pain | (52) | |
| RA | ↓ disease severity | (89,137–139,141–143) | |
| VEGFR Inhibitor | RA | ↓ disease severity | (90,138,144,145) |
| PPI-2458 | OA | ↓ OA progression and pain | (149) |
| RA | ↓ disease severity | (150,151) | |
| TNP-470 | RA | ↓ disease severity | (152) |
| TSP-1 | OCD/CD | ↑ cartilage repair | (157) |
| OA | ↓ OA progression | (158) | |
| RA | ↓ disease severity | (159,160) | |
| Chm-1 | OCD/CD | ↑ cartilage repair | (129) |
| Endostatin | RA | ↓ disease severity | (168,170–173,188) |
| Angiostatin | RA | ↓ disease severity | (178) |
| K1–5 | RA | ↓ disease severity | (179) |
| ExTek | RA | ↓ disease severity | (180) |
| Suramin | OCD/CD | ↑ cartilage repair | (186) |
Abbreviations: Chm-1: chondromodulin-1; CD: chondral defect; ExTek: soluble Tie-2 extracellular domain; K1–5: protease-activated kringles 1–5; OA: osteoarthritis; OCD: osteochondral defect; RA: rheumatoid arthritis; VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor.
Bevacizumab is a monocolonal anti-VEGF antibody. Treatment with intravenous (IV) bevacizumab resulted in improved cartilage repair at OCDs in rabbits.(133) In this study, drilling through the articular cartilage and subchondral bone allowed MSCs access to the site of repair. The group receiving IV bevacizumab showed repair sites filled mostly with hyaline cartilage after 3 months, while control groups healed with mostly fibrocartilage and bone. It was suggested that the retention of hyaline cartilage in the OCD in the bevacizumab group was due to inhibition of endochondral ossification at the repair site.
Nagai et al. (2014) demonstrated that early treatment with bevacizumab in an OA rabbit model showed reduction of articular cartilage degeneration, osteophyte formation, synovitis, and pain.(52,134) Results were better overall with lower dose IA injection of bevacizumab in the knee compared to higher dose IV treatment. Compared to control, bevacizumab treatment resulted in: (i) increased expression of collagen type II, aggrecan, and chondromodulin −1 (Chm-1) at the chondrocytes; (ii) decreased Runx2 expression at the subchondral bone; and (iii) suppressed expression of MMP-13 and ADAMTS-5 in the synovium. The authors further speculated that early treatment of IA bevacizumab may have reduced OA progression and associated pain by suppressing angiogenesis and associated nerve ingrowth at the joint.(52)
sFlt-1 is a soluble splice variant of VEGFR-1 and is endogenously produced; it acts as a decoy receptor by binding to and inhibiting VEGF. In an OCD model, it was suggested that sFlt-1 therapy enhanced therapeutic treatment of OCDs with muscle-derived stem cells (MDSCs) by reducing vascularization and bone invasion into the articular cartilage at the site of repair.(47) In experimental OA models, IA administration of sFlt-1-expressing MDSCs worked synergistically with BMP4-expressing MDSCs to reduce OA progression.(135,136)
A number of RA animal studies have assessed specific inhibition of VEGF through various methods including anti-VEGF antibody,(137–140) peptides,(89) sFlt-1,(141,142) as well as vaccination against VEGF.(143) These studies have demonstrated that these treatments resulted in reduction of joint inflammation and joint destruction. Inhibition of VEGFR signaling by anti-VEGFR antibodies or VEGFR RTK inhibitors decreases disease severity in RA models;(90,138,144–146) however, this has not been currently published in OA or CD/OCD models. For RA treatment, inhibiting VEGFR-1 signaling appears to be more effective than targeting VEGFR-2.(90,138,144,145) Given differences between RA and OA, such as a greater component of inflammatory joint destruction seen in RA, determination of the specific pathophysiologic roles of VEGFR-1 and VEGFR-2 in the development of OA and investigation into targeted inhibition of these receptors in the setting of OA is of interest.
PPI-2458 and TNP-470 are fumagillin analogues that inhibit methionine aminopeptidase type 2 (MetAP-2); they inhibit angiogenesis by inhibiting EC proliferation and cell cycle progression.(147,148) In an OA model, oral administration of PPI-2458 decreased synovial angiogenesis, synovitis, vascularization of articular cartilage, cartilage degeneration, osteophyte formation, and pain behavior.(149) This study specifically targeted angiogenesis, rather than VEGF signaling in OA. It showed that angiogenesis alone can reduce OA progression and pain. PPI-2458 also reduces disease severity in RA models.(150,151) TNP-470 reduced disease severity in an RA model.(152)
Thrombospondin-1 (TSP-1) is a glycoprotein found in joints, and can be expressed and secreted by chondrocytes. It is an inhibitor of EC proliferation and migration, and TSP-1 signaling can lead to EC apoptosis.(153) TSP-1 expression level is significantly decreased in cartilage in severe OA.(154) TSP-1 can inhibit VEGF signaling by directly binding to VEGF, competing with VEGF for binding sites on cell-surface heparin sulfate proteoglycans, as well as by inhibiting VEGFR signaling through TSP-1 interaction with CD47.(155,156) In a CD model, TSP-1 inhibited endochondral ossification, leading to significantly better repair when combined with osteogenic protein-1 (OP-1/BMP7).(157) In an OA model, IA injection of an adenoviral vector encoding mouse TSP-1 (AdTSP-1) resulted in decreased cartilage degeneration, osteophyte formation, synovial micro-vessel density and synovitis.(158) IA delivery of TSP-1 has also facilitated reduction of disease severity in RA models.(159,160)
Chondromodulin-1 (Chm-1) is a glycoprotein present in cartilage that inhibits angiogenesis.(161–163) An animal OA study demonstrated that vascular invasion into OA cartilage occurred at sites of decreased ChM-I and increased VEGF expression.(164) In a CD model, it was suggested that both (i) osteochondral progenitor cells expressing Chm-1 implanted into cartilage defects and (ii) direct loading of adeno-associated virus (AAV) vectors expressing Chm-1 into MFX sites, resulted in enhanced cartilage regeneration by inhibiting chondrocyte hypertrophy and endochondral ossification.(129)
Endostatin is a C-terminal fragment of collagen type XVIII.(165) It acts as an inhibitor of angiogenesis and VEGF signaling.(165,166) It has been hypothesized that endostatin at the articular is protective against cartilage destruction in OA by inhibiting VEGF signaling and vascular invasion.(167) No in-vivo studies have been reported using endostatin for OA or cartilage repair. However, endostatin was reported to reduce disease severity in RA animal models.(168–173) MSCs and chondrocytes transfected to express endostatin have been designed for placement into collagen scaffolds and hydrogels for treatment of articular cartilage defects.(174–176)
Angiostatin is an endogenous protein and fragment of plasminogen. Reported effects of angiostatin include reduction of EC proliferation, migration, as well as increased EC apoptosis.(177) Fibroblasts transduced with angiostatin transplanted into the knee cavity reduced disease severity in an RA model.(178) Protease-activated kringles 1–5 (K1–5) is a fragment of plasminogen that has anti-angiogenic properties similar to angiostatin. In an RA model, K1–5 decreased disease severity, reducing inflammation and joint destruction.(179) Use of a soluble Tie-2 receptor (ExTek), which binds to angiopoiten-1 (Ang-1) and inhibits interaction with Tie2 receptor on endothelial cells, led to a reduction of RA progression in an animal model.(180) Endothelial Tie-2 signaling is a critical component of angiogenic remodeling, vascular maturation, and vascular stabilization.(181–183)
Suramin was originally developed as an anti-parasitic drug. It was found to be anti-angiogenic and to inhibit VEGF signaling;(184) however, it has a wide range of biological effects.(185) Suramin has been used as an anti-angiogenic treatment in an OCD model. Local treatment with suramin at the OCD substantially decreased the amount of bone growth into the cartilaginous compartment, and this effect was further enhanced by sustained release of suramin using liposomal delivery.(186) While no studies have directly assessed suramin in OA, it effectively reduced joint destruction in an RA model.(187)
Clinical studies specifically targeting VEGF or angiogenesis in OA, cartilage repair, or RA has not been reported. However, bevacizumab has been used clinically off-label for IA injection at the knee joint for pigmented villonodular synovitis with high efficacy in a case report,(189) and a clinical trial is underway for IA injection of bevacizumab in recurrent hemarthrosis with chronic hemophilic synovitis (clinicaltrials.gov NCT02060305). Hemophilic arthropathy and OA may share similar degenerative pathological pathways of disease progression involving VEGF signaling.(132) These clinical studies assessing IA anti-VEGF treatments may provide some insight into potential efficacy for OA, and will be able to provide an understanding of the potential side-effects with local treatment at the joint.
Anti-VEGF therapy is used clinically as systemic treatment for cancer and has become recently routinely used for local injections into the vitreous cavity of the eye for ocular disease.(190) As compared to cancer therapy, the risk of serious adverse events with local (intravitreal) injection of bevacizumab is thought to be very low, since the intravitreal dose is 300–500 times lower than the IV dose used for cancer and is less frequently administered.(191,192) Clinical studies of intravitreal anti-VEGF treatment correspondingly report a low rate of serious adverse events; however, further assessment of side effects is ongoing.(193–196) Continued clinical use of therapies that target VEGF signaling pathways or angiogenesis in cancer may provide additional insight into potential efficacy of these therapies for OA. For instance, a case report recently suggested inhibition of HGF signaling with crizotinib, which was used as treatment for non-small cell lung cancer, reduced clinical OA symptoms over 16 months by reducing VEGF expression.(197)
Clinical studies have assessed anabolic growth factors, such as BMP-7/OP-1, or platelet-rich plasma (PRP), which contains a combination of growth factors and cytokines for the treatment of OA.(198,199) In animal models, growth factors such as OP-1/BMP7, BMP-4, or a combination of growth factors such as those found in PRP, have been demonstrated to act synergistically with anti-VEGF or anti-angiogenic therapy to facilitate cartilage repair and reduce OA progression.(47,135,136,157,200) Given the presence of VEGF in PRP, it has been suggested that, for the treatment of OA or cartilage defects, a combination of PRP with an anti-VEGF antibody, for instance, could limit a potential negative effect of PRP, while still allowing the potential benefit of other growth factors and cytokines.(200)
Methods of sustained delivery and controlled release of anti-VEGF or anti-angiogenic treatments locally at the joint may prove to enhance efficacy, reduce frequency of injections, and increase safety, which could be of high value in the clinical setting. Methods of extending the release and duration of anti-VEGF and anti-angiogenic therapies have been used for animal models in OA,(136,137,159) RA,(141–143,159,160,168,178) as well as for enhancing cartilage repair.(47,130,158,187) Sustaining the delivery of anti-VEGF and anti-angiogenic treatments in these models for local IA application at the joint have included viral vectors,(129,158,159,168) transduced cells,(47,135,136,178) hydron pellets,(160) and liposome encapsulation.(186) Further considerations of any method of sustained delivery and controlled release in the clinical setting would include safety, costs, feasibility, and efficacy.
Conclusion
Accumulating evidence suggests the pathological involvement of VEGF and its signaling pathways through its cognate receptors, VEGFR-1 and VEGFR-2, in OA progression and associated pain. Inhibition of VEGF signaling pathways and of angiogenesis has emerged as a promising approach in recent pre-clinical studies, demonstrating reduced destruction of joints and of associated pain in OA. Local administration of VEGF or VEGFR inhibitors may significantly reduce adverse effects compared to systemic administration. Widespread clinical use of anti-VEGF treatments administered both systemically and locally and recent clinical use of anti-VEGF therapy targeted to joints, outside of OA, may further facilitate the assessment of risks and benefits of anti-VEGF therapy for OA treatments. Treatments targeting VEGF signaling in OA may be further enhanced by methods of extending the half-life of the drug at the joint as well as combination treatment with anabolic growth factors.
Acknowledgments
This work was supported by NIH grants RO1 AR062136 (to HJI), RO1 AR036819 (to BRO), R21 AR067935 (to HJI), F31 AR070002 (to JLH), a VA BLD&R Merit Award I01BC002647 (to HJI), and Arthritis Foundation (to HJI).
Footnotes
Disclosures
All authors state they have no conflicts of interest.
Authors’ roles
Drafting the manuscript (JLH and HJI); revising the manuscript content and approving the final version of the manuscript (all authors)
Literature Cited
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective: A population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthopedic nursing. 2012;31(2):85–91. doi: 10.1097/NOR.0b013e31824fcd42. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter JA, Stanish WD, Rosier RN, Schenck RC, Jr, Dennis DA, Coutts RD. The increasing need for nonoperative treatment of patients with osteoarthritis. Clinical orthopaedics and related research. 2001;(385):36–45. doi: 10.1097/00003086-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. American journal of public health. 1994;84(3):351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis and rheumatism. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bulletin of the World Health Organization. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 7.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Current opinion in rheumatology. 2010;22(5):533–537. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losina E, Daigle ME, Suter LG, et al. Disease-modifying drugs for knee osteoarthritis: can they be cost-effective? Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(5):655–667. doi: 10.1016/j.joca.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 10.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nature reviews Molecular cell biology. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 11.Bacic M, Edwards NA, Merrill MJ. Differential expression of vascular endothelial growth factor (vascular permeability factor) forms in rat tissues. Growth factors. 1995;12(1):11–15. doi: 10.3109/08977199509003209. [DOI] [PubMed] [Google Scholar]
- 12.Burchardt T, Burchardt M, Chen MW, et al. Expression of VEGF splice variants 144/145 and 205/206 in adult male tissues. IUBMB life. 1999;48(4):405–408. doi: 10.1080/713803545. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine reviews. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 14.Zelzer E, Olsen BR. Multiple roles of vascular endothelial growth factor (VEGF) in skeletal development, growth, and repair. Current topics in developmental biology. 2005;65:169–187. doi: 10.1016/S0070-2153(04)65006-X. [DOI] [PubMed] [Google Scholar]
- 15.Maharaj AS, D'Amore PA. Roles for VEGF in the adult. Microvascular research. 2007;74(2–3):100–113. doi: 10.1016/j.mvr.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JO, Oreffo RO, Clarke NM, Roach HI. Changes in the antiangiogenic properties of articular cartilage in osteoarthritis. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2003;8(6):849–857. doi: 10.1007/s00776-003-0717-8. [DOI] [PubMed] [Google Scholar]
- 17.Pfander D, Kortje D, Zimmermann R, et al. Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Annals of the rheumatic diseases. 2001;60(11):1070–1073. doi: 10.1136/ard.60.11.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pufe T, Petersen W, Tillmann B, Mentlein R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis and rheumatism. 2001;44(5):1082–1088. doi: 10.1002/1529-0131(200105)44:5<1082::AID-ANR188>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Walsh DA, McWilliams DF, Turley MJ, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology. 2010;49(10):1852–1861. doi: 10.1093/rheumatology/keq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honorati MC, Cattini L, Facchini A. IL-17, IL-1beta and TNF-alpha stimulate VEGF production by dedifferentiated chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12(9):683–691. doi: 10.1016/j.joca.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Enomoto H, Inoki I, Komiya K, et al. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. The American journal of pathology. 2003;162(1):171–181. doi: 10.1016/s0002-9440(10)63808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franses RE, McWilliams DF, Mapp PI, Walsh DA. Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18(4):563–571. doi: 10.1016/j.joca.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchida AI, Beekhuizen M, t Hart MC, et al. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis research & therapy. 2014;16(5):441. doi: 10.1186/s13075-014-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray BK, Shakya A, Ray A. Vascular endothelial growth factor expression in arthritic joint is regulated by SAF-1 transcription factor. Journal of immunology. 2007;178(3):1774–1782. doi: 10.4049/jimmunol.178.3.1774. [DOI] [PubMed] [Google Scholar]
- 25.Duan X, Li Q, Lin LJ, et al. [Expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor in the synovium of patients with osteoarthritis] Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2011;31(1):117–120. [PubMed] [Google Scholar]
- 26.Giatromanolaki A, Sivridis E, Maltezos E, et al. Upregulated hypoxia inducible factor-1alpha and −2alpha pathway in rheumatoid arthritis and osteoarthritis. Arthritis research & therapy. 2003;5(4):R193–R201. doi: 10.1186/ar756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haywood L, McWilliams DF, Pearson CI, et al. Inflammation and angiogenesis in osteoarthritis. Arthritis and rheumatism. 2003;48(8):2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- 28.Jackson JR, Minton JA, Ho ML, Wei N, Winkler JD. Expression of vascular endothelial growth factor in synovial fibroblasts is induced by hypoxia and interleukin 1beta. The Journal of Rheumatology. 1997;24(7):1253–1259. [PubMed] [Google Scholar]
- 29.Lambert C, Mathy-Hartert M, Dubuc JE, et al. Characterization of synovial angiogenesis in osteoarthritis patients and its modulation by chondroitin sulfate. Arthritis research & therapy. 2012;14(2):R58. doi: 10.1186/ar3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohn DH, Sokolove J, Sharpe O, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis research & therapy. 2012;14(1):R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saetan N, Honsawek S, Tanavalee A, et al. Relationship of plasma and synovial fluid vascular endothelial growth factor with radiographic severity in primary knee osteoarthritis. International orthopaedics. 2013 doi: 10.1007/s00264-013-2192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fay J, Varoga D, Wruck CJ, Kurz B, Goldring MB, Pufe T. Reactive oxygen species induce expression of vascular endothelial growth factor in chondrocytes and human articular cartilage explants. Arthritis research & therapy. 2006;8(6):R189. doi: 10.1186/ar2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mabey T, Honsawek S, Saetan N, Poovorawan Y, Tanavalee A, Yuktanandana P. Angiogenic cytokine expression profiles in plasma and synovial fluid of primary knee osteoarthritis. International orthopaedics. 2014;38(9):1885–1892. doi: 10.1007/s00264-014-2406-y. [DOI] [PubMed] [Google Scholar]
- 34.Gaballah A, Hussein NA, Risk M, Elsawy N, Elabasiry S. Correlation between synovial vascular endothelial growth factor, clinical, functional and radiological manifestations in knee osteoarthritis. The Egyptian Rheumatologist. (0) [Google Scholar]
- 35.Corrado A, Neve A, Cantatore FP. Expression of vascular endothelial growth factor in normal, osteoarthritic and osteoporotic osteoblasts. Clinical and experimental medicine. 2013;13(1):81–84. doi: 10.1007/s10238-011-0170-5. [DOI] [PubMed] [Google Scholar]
- 36.Neve A, Cantatore FP, Corrado A, Gaudio A, Ruggieri S, Ribatti D. In vitro and in vivo angiogenic activity of osteoarthritic and osteoporotic osteoblasts is modulated by VEGF and vitamin D3 treatment. Regulatory peptides. 2013;184:81–84. doi: 10.1016/j.regpep.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Huh JE, Seo DM, Baek YH, Choi DY, Park DS, Lee JD. Biphasic positive effect of formononetin on metabolic activity of human normal and osteoarthritic subchondral osteoblasts. International immunopharmacology. 2010;10(4):500–507. doi: 10.1016/j.intimp.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Ballara S, Taylor PC, Reusch P, et al. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis and rheumatism. 2001;44(9):2055–2064. doi: 10.1002/1529-0131(200109)44:9<2055::AID-ART355>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Yuan Q, Sun L, Li JJ, An CH. Elevated VEGF levels contribute to the pathogenesis of osteoarthritis. BMC musculoskeletal disorders. 2014;15(1):437. doi: 10.1186/1471-2474-15-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Fontenla C, Calaza M, Evangelou E, et al. Assessment of osteoarthritis candidate genes in a meta-analysis of 9 genome-wide association studies. Arthritis and rheumatism. 2013 doi: 10.1002/art.38300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh DA, Bonnet CS, Turner EL, Wilson D, Situ M, McWilliams DF. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15(7):743–751. doi: 10.1016/j.joca.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Giatromanolaki A, Sivridis E, Athanassou N, et al. The angiogenic pathway "vascular endothelial growth factor/flk-1(KDR)-receptor" in rheumatoid arthritis and osteoarthritis. The Journal of pathology. 2001;194(1):101–108. doi: 10.1002/path.842. [DOI] [PubMed] [Google Scholar]
- 43.Im HJ, Kim JS, Li X, et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis and rheumatism. 2010;62(10):2995–3005. doi: 10.1002/art.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, Walsh DA. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Annals of the rheumatic diseases. 2011;70(3):523–529. doi: 10.1136/ard.2010.137844. [DOI] [PubMed] [Google Scholar]
- 45.Ludin A, Sela JJ, Schroeder A, Samuni Y, Nitzan DW, Amir G. Injection of vascular endothelial growth factor into knee joints induces osteoarthritis in mice. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(3):491–497. doi: 10.1016/j.joca.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Shen P, Jiao Z, Zheng JS, et al. Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Scientific reports. 2015;5:16244. doi: 10.1038/srep16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubo S, Cooper GM, Matsumoto T, et al. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis and rheumatism. 2009;60(1):155–165. doi: 10.1002/art.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pufe T, Harde V, Petersen W, Goldring MB, Tillmann B, Mentlein R. Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. The Journal of pathology. 2004;202(3):367–374. doi: 10.1002/path.1527. [DOI] [PubMed] [Google Scholar]
- 49.Pufe T, Lemke A, Kurz B, et al. Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. The American journal of pathology. 2004;164(1):185–192. doi: 10.1016/S0002-9440(10)63109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. The American journal of pathology. 1995;146(1):75–85. [PMC free article] [PubMed] [Google Scholar]
- 51.Chen XY, Hao YR, Wang Z, Zhou JL, Jia QX, Qiu B. The effect of vascular endothelial growth factor on aggrecan and type II collagen expression in rat articular chondrocytes. Rheumatology international. 2012;32(11):3359–3364. doi: 10.1007/s00296-011-2178-2. [DOI] [PubMed] [Google Scholar]
- 52.Nagai T, Sato M, Kobayashi M, Yokoyama M, Tani Y, Mochida J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis research & therapy. 2014;16(5):427. doi: 10.1186/s13075-014-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Funck-Brentano T, Bouaziz W, Marty C, Geoffroy V, Hay E, Cohen-Solal M. Dkk-1-mediated inhibition of Wnt signaling in bone ameliorates osteoarthritis in mice. Arthritis & Rheumatology. 2014;66(11):3028–3039. doi: 10.1002/art.38799. [DOI] [PubMed] [Google Scholar]
- 54.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 55.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 56.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. Journal of leukocyte biology. 1994;55(3):410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 57.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis and rheumatism. 2010;62(3):647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 58.Yang Q, McHugh KP, Patntirapong S, Gu X, Wunderlich L, Hauschka PV. VEGF enhancement of osteoclast survival and bone resorption involves VEGF receptor-2 signaling and beta3-integrin. Matrix biology : journal of the International Society for Matrix Biology. 2008;27(7):589–599. doi: 10.1016/j.matbio.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y, Berendsen AD, Jia S, et al. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. The Journal of clinical investigation. 2012;122(9):3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakagawa M, Kaneda T, Arakawa T, et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS letters. 2000;473(2):161–164. doi: 10.1016/s0014-5793(00)01520-9. [DOI] [PubMed] [Google Scholar]
- 61.Niida S, Kondo T, Hiratsuka S, et al. VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):14016–14021. doi: 10.1073/pnas.0503544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niida S, Kaku M, Amano H, et al. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. The Journal of experimental medicine. 1999;190(2):293–298. doi: 10.1084/jem.190.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aldridge SE, Lennard TW, Williams JR, Birch MA. Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochemical and biophysical research communications. 2005;335(3):793–798. doi: 10.1016/j.bbrc.2005.07.145. [DOI] [PubMed] [Google Scholar]
- 64.Knowles HJ, Moskovsky L, Thompson MS, et al. Chondroclasts are mature osteoclasts which are capable of cartilage matrix resorption. Virchows Archiv : an international journal of pathology. 2012;461(2):205–210. doi: 10.1007/s00428-012-1274-3. [DOI] [PubMed] [Google Scholar]
- 65.Kim HR, Kim KW, Kim BM, Cho ML, Lee SH. The effect of vascular endothelial growth factor on osteoclastogenesis in rheumatoid arthritis. PloS one. 2015;10(4):e0124909. doi: 10.1371/journal.pone.0124909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan H, Zhou Z, Cao Y, Duan X, Kleinerman ES. VEGF165 promotes the osteolytic bone destruction of ewing's sarcoma tumors by upregulating RANKL. Oncology research. 2009;18(2–3):117–125. doi: 10.3727/096504009789954627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayr-Wohlfart U, Waltenberger J, Hausser H, et al. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30(3):472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 68.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(15):9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Midy V, Plouet J. Vasculotropin/vascular endothelial growth factor induces differentiation in cultured osteoblasts. Biochemical and biophysical research communications. 1994;199(1):380–386. doi: 10.1006/bbrc.1994.1240. [DOI] [PubMed] [Google Scholar]
- 70.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature medicine. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 71.Hashimoto S, Creighton-Achermann L, Takahashi K, Amiel D, Coutts RD, Lotz M. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2002;10(3):180–187. doi: 10.1053/joca.2001.0505. [DOI] [PubMed] [Google Scholar]
- 72.Ziche M, Morbidelli L, Choudhuri R, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. The Journal of clinical investigation. 1997;99(11):2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Zee R, Murohara T, Luo Z, et al. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95(4):1030–1037. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- 74.Fenwick SA, Gregg PJ, Rooney P. Osteoarthritic cartilage loses its ability to remain avascular. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1999;7(5):441–452. doi: 10.1053/joca.1998.0238. [DOI] [PubMed] [Google Scholar]
- 75.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nature reviews Rheumatology. 2012;8(7):390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 76.Fuerst M, Bertrand J, Lammers L, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis and rheumatism. 2009;60(9):2694–2703. doi: 10.1002/art.24774. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka E, Aoyama J, Miyauchi M, et al. Vascular endothelial growth factor plays an important autocrine/paracrine role in the progression of osteoarthritis. Histochemistry and cell biology. 2005;123(3):275–281. doi: 10.1007/s00418-005-0773-6. [DOI] [PubMed] [Google Scholar]
- 78.Lane LB, Villacin A, Bullough PG. The vascularity remodelling of subchondrial bone and calcified cartilage in adult human femoral and humeral heads. An age- and stress-related phenomenon. The Journal of bone and joint surgery British volume. 1977;59(3):272–278. doi: 10.1302/0301-620X.59B3.893504. [DOI] [PubMed] [Google Scholar]
- 79.Lane LB, Bullough PG. Age-related changes in the thickness of the calcified zone and the number of tidemarks in adult human articular cartilage. The Journal of bone and joint surgery British volume. 1980;62(3):372–375. doi: 10.1302/0301-620X.62B3.7410471. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto Y, Tanaka K, Hirata G, et al. Possible involvement of the vascular endothelial growth factor-Flt-1-focal adhesion kinase pathway in chemotaxis and the cell proliferation of osteoclast precursor cells in arthritic joints. Journal of immunology. 2002;168(11):5824–5831. doi: 10.4049/jimmunol.168.11.5824. [DOI] [PubMed] [Google Scholar]
- 81.Sanchez C, Deberg MA, Bellahcene A, et al. Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone. Arthritis and rheumatism. 2008;58(2):442–455. doi: 10.1002/art.23159. [DOI] [PubMed] [Google Scholar]
- 82.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(1):10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology. 2005;44(1):7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 84.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1997;11(6):457–465. [PubMed] [Google Scholar]
- 85.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brkovic A, Sirois MG. Vascular permeability induced by VEGF family members in vivo: role of endogenous PAF and NO synthesis. Journal of cellular biochemistry. 2007;100(3):727–737. doi: 10.1002/jcb.21124. [DOI] [PubMed] [Google Scholar]
- 87.Gille H, Kowalski J, Li B, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1), KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. The Journal of biological chemistry. 2001;276(5):3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 88.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature reviews Rheumatology. 2010;6(11):625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 89.Yoo SA, Bae DG, Ryoo JW, et al. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-alpha and IL-6 by human monocytes. Journal of immunology. 2005;174(9):5846–5855. doi: 10.4049/jimmunol.174.9.5846. [DOI] [PubMed] [Google Scholar]
- 90.Luttun A, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nature medicine. 2002;8(8):831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 91.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87(8):3336–3343. [PubMed] [Google Scholar]
- 92.Clauss M, Weich H, Breier G, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. The Journal of biological chemistry. 1996;271(30):17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 93.Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. The Journal of biological chemistry. 2002;277(12):10445–10451. doi: 10.1074/jbc.M107348200. [DOI] [PubMed] [Google Scholar]
- 94.Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48(5):1131–1137. doi: 10.2337/diabetes.48.5.1131. [DOI] [PubMed] [Google Scholar]
- 95.Taichman NS, Young S, Cruchley AT, Taylor P, Paleolog E. Human neutrophils secrete vascular endothelial growth factor. Journal of leukocyte biology. 1997;62(3):397–400. doi: 10.1002/jlb.62.3.397. [DOI] [PubMed] [Google Scholar]
- 96.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. Journal of immunology. 2004;172(7):4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 97.Sakkas LI, Scanzello C, Johanson N, et al. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clinical and diagnostic laboratory immunology. 1998;5(4):430–437. doi: 10.1128/cdli.5.4.430-437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakkas LI, Platsoucas CD. The role of T cells in the pathogenesis of osteoarthritis. Arthritis and rheumatism. 2007;56(2):409–424. doi: 10.1002/art.22369. [DOI] [PubMed] [Google Scholar]
- 99.Lajeunesse D, Reboul P. Subchondral bone in osteoarthritis: a biologic link with articular cartilage leading to abnormal remodeling. Current opinion in Rheumatology. 2003;15(5):628–633. doi: 10.1097/00002281-200309000-00018. [DOI] [PubMed] [Google Scholar]
- 100.Imhof H, Breitenseher M, Kainberger F, Rand T, Trattnig S. Importance of subchondral bone to articular cartilage in health and disease. Topics in magnetic resonance imaging : TMRI. 1999;10(3):180–192. doi: 10.1097/00002142-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 101.Sokoloff L. Microcracks in the calcified layer of articular cartilage. Archives of pathology & laboratory medicine. 1993;117(2):191–195. [PubMed] [Google Scholar]
- 102.Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2010;18(4):419–433. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- 103.Murata M, Yudoh K, Nakamura H, et al. Distinct signaling pathways are involved in hypoxia- and IL-1-induced VEGF expression in human articular chondrocytes. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2006;24(7):1544–1554. doi: 10.1002/jor.20168. [DOI] [PubMed] [Google Scholar]
- 104.Li J, Huang J, Dai L, et al. miR-146a, an IL-1beta responsive miRNA, induces vascular endothelial growth factor and chondrocyte apoptosis by targeting Smad4. Arthritis research & therapy. 2012;14(2):R75. doi: 10.1186/ar3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turpaev K, Litvinov D, Dubovaya V, Panasyuk A, Ivanov D, Prassolov V. Induction of vascular endothelial growth factor by nitric oxide in cultured human articular chondrocytes. Biochimie. 2001;83(6):515–522. doi: 10.1016/s0300-9084(01)01280-9. [DOI] [PubMed] [Google Scholar]
- 106.Beckmann R, Houben A, Tohidnezhad M, et al. Mechanical forces induce changes in VEGF and VEGFR-1/sFlt-1 expression in human chondrocytes. International journal of molecular sciences. 2014;15(9):15456–15474. doi: 10.3390/ijms150915456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zelzer E, Glotzer DJ, Hartmann C, et al. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mechanisms of development. 2001;106(1–2):97–106. doi: 10.1016/s0925-4773(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 108.Murata M, Yudoh K, Masuko K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: how the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(3):279–286. doi: 10.1016/j.joca.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 109.Saito T, Fukai A, Mabuchi A, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nature medicine. 2010;16(6):678–686. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 110.Inoue H, Arai Y, Kishida T, et al. Hydrostatic pressure influences HIF-2 alpha expression in chondrocytes. International journal of molecular sciences. 2015;16(1):1043–1050. doi: 10.3390/ijms16011043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and cellular biology. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis and rheumatism. 2007;56(10):3297–3306. doi: 10.1002/art.22878. [DOI] [PubMed] [Google Scholar]
- 113.Juffer P, Jaspers RT, Lips P, Bakker AD, Klein-Nulend J. Expression of muscle anabolic and metabolic factors in mechanically loaded MLO-Y4 osteocytes. American journal of physiology Endocrinology and metabolism. 2012;302(4):E389–E395. doi: 10.1152/ajpendo.00320.2011. [DOI] [PubMed] [Google Scholar]
- 114.Nakahara H, Song J, Sugimoto M, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis and rheumatism. 2003;48(6):1521–1529. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 115.Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS letters. 1995;372(1):83–87. doi: 10.1016/0014-5793(95)00956-a. [DOI] [PubMed] [Google Scholar]
- 116.Koch AE. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis and rheumatism. 1998;41(6):951–962. doi: 10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 117.Ryu S, Lee JH, Kim SI. IL-17 increased the production of vascular endothelial growth factor in rheumatoid arthritis synoviocytes. Clinical Rheumatology. 2006;25(1):16–20. doi: 10.1007/s10067-005-1081-1. [DOI] [PubMed] [Google Scholar]
- 118.Cho CS, Cho ML, Min SY, et al. CD40 engagement on synovial fibroblast up-regulates production of vascular endothelial growth factor. Journal of immunology. 2000;164(10):5055–5061. doi: 10.4049/jimmunol.164.10.5055. [DOI] [PubMed] [Google Scholar]
- 119.McKelvey L, Shorten GD, O'Keeffe GW. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. Journal of neurochemistry. 2013;124(3):276–289. doi: 10.1111/jnc.12093. [DOI] [PubMed] [Google Scholar]
- 120.Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. 2011;115(1):189–204. doi: 10.1097/ALN.0b013e31821b1ac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakamura K, Tan F, Li Z, Thiele CJ. NGF activation of TrkA induces vascular endothelial growth factor expression via induction of hypoxia-inducible factor-1alpha. Molecular and cellular neurosciences. 2011;46(2):498–506. doi: 10.1016/j.mcn.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Malykhina AP, Lei Q, Erickson CS, et al. VEGF induces sensory and motor peripheral plasticity, alters bladder function, and promotes visceral sensitivity. BMC physiology. 2012;12:15. doi: 10.1186/1472-6793-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin J, Li G, Den X, et al. VEGF and its receptor-2 involved in neuropathic pain transmission mediated by P2X(2)(/)(3) receptor of primary sensory neurons. Brain research bulletin. 2010;83(5):284–291. doi: 10.1016/j.brainresbull.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 124.Kiguchi N, Kobayashi Y, Kadowaki Y, Fukazawa Y, Saika F, Kishioka S. Vascular endothelial growth factor signaling in injured nerves underlies peripheral sensitization in neuropathic pain. Journal of neurochemistry. 2014;129(1):169–178. doi: 10.1111/jnc.12614. [DOI] [PubMed] [Google Scholar]
- 125.Nesic O, Sundberg LM, Herrera JJ, Mokkapati VU, Lee J, Narayana PA. Vascular endothelial growth factor and spinal cord injury pain. Journal of neurotrauma. 2010;27(10):1793–1803. doi: 10.1089/neu.2010.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Selvaraj D, Gangadharan V, Michalski CW, et al. A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain. Cancer cell. 2015;27(6):780–796. doi: 10.1016/j.ccell.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu S, Xu C, Li G, et al. Vatalanib decrease the positive interaction of VEGF receptor-2 and P2X2/3 receptor in chronic constriction injury rats. Neurochemistry international. 2012;60(6):565–572. doi: 10.1016/j.neuint.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 128.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. The New England journal of medicine. 2010;363(16):1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Klinger P, Surmann-Schmitt C, Brem M, et al. Chondromodulin 1 stabilizes the chondrocyte phenotype and inhibits endochondral ossification of porcine cartilage repair tissue. Arthritis and rheumatism. 2011;63(9):2721–2731. doi: 10.1002/art.30335. [DOI] [PubMed] [Google Scholar]
- 130.Steinert AF, Proffen B, Kunz M, et al. Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthritis research & therapy. 2009;11(5):R148. doi: 10.1186/ar2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zimmermann P, Boeuf S, Dickhut A, Boehmer S, Olek S, Richter W. Correlation of COL10A1 induction during chondrogenesis of mesenchymal stem cells with demethylation of two CpG sites in the COL10A1 promoter. Arthritis and rheumatism. 2008;58(9):2743–2753. doi: 10.1002/art.23736. [DOI] [PubMed] [Google Scholar]
- 132.Valentino LA. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. Journal of thrombosis and haemostasis : JTH. 2010;8(9):1895–1902. doi: 10.1111/j.1538-7836.2010.03962.x. [DOI] [PubMed] [Google Scholar]
- 133.Nagai T, Sato M, Kutsuna T, et al. Intravenous administration of anti-vascular endothelial growth factor humanized monoclonal antibody bevacizumab improves articular cartilage repair. Arthritis research & therapy. 2010;12(5):R178. doi: 10.1186/ar3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barranco C. Osteoarthritis: Animal data show VEGF blocker inhibits post-traumatic OA. Nature reviews Rheumatology. 2014;10(11):638. doi: 10.1038/nrrheum.2014.173. [DOI] [PubMed] [Google Scholar]
- 135.Matsumoto T, Cooper GM, Gharaibeh B, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis and rheumatism. 2009;60(5):1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mifune Y, Matsumoto T, Takayama K, et al. The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(1):175–185. doi: 10.1016/j.joca.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 137.Sone H, Kawakami Y, Sakauchi M, et al. Neutralization of vascular endothelial growth factor prevents collagen-induced arthritis and ameliorates established disease in mice. Biochemical and biophysical research communications. 2001;281(2):562–568. doi: 10.1006/bbrc.2001.4395. [DOI] [PubMed] [Google Scholar]
- 138.De Bandt M, Ben Mahdi MH, Ollivier V, et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. Journal of immunology. 2003;171(9):4853–4859. doi: 10.4049/jimmunol.171.9.4853. [DOI] [PubMed] [Google Scholar]
- 139.Lu J, Kasama T, Kobayashi K, et al. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. Journal of immunology. 2000;164(11):5922–5927. doi: 10.4049/jimmunol.164.11.5922. [DOI] [PubMed] [Google Scholar]
- 140.Wang Y, Da G, Li H, Zheng Y. Avastin exhibits therapeutic effects on collagen-induced arthritis in rat model. Inflammation. 2013;36(6):1460–1467. doi: 10.1007/s10753-013-9687-y. [DOI] [PubMed] [Google Scholar]
- 141.Miotla J, Maciewicz R, Kendrew J, Feldmann M, Paleolog E. Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Laboratory investigation; a journal of technical methods and pathology. 2000;80(8):1195–1205. doi: 10.1038/labinvest.3780127. [DOI] [PubMed] [Google Scholar]
- 142.Afuwape AO, Feldmann M, Paleolog EM. Adenoviral delivery of soluble VEGF receptor 1 (sFlt-1) abrogates disease activity in murine collagen-induced arthritis. Gene therapy. 2003;10(23):1950–1960. doi: 10.1038/sj.gt.3302104. [DOI] [PubMed] [Google Scholar]
- 143.Semerano L, Duvallet E, Belmellat N, et al. Targeting VEGF-A with a vaccine decreases inflammation and joint destruction in experimental arthritis. Angiogenesis. 2015 doi: 10.1007/s10456-015-9487-0. [DOI] [PubMed] [Google Scholar]
- 144.Choi ST, Kim JH, Seok JY, Park YB, Lee SK. Therapeutic effect of anti-vascular endothelial growth factor receptor I antibody in the established collagen-induced arthritis mouse model. Clinical Rheumatology. 2009;28(3):333–337. doi: 10.1007/s10067-008-1075-x. [DOI] [PubMed] [Google Scholar]
- 145.Grosios K, Wood J, Esser R, Raychaudhuri A, Dawson J. Angiogenesis inhibition by the novel VEGF receptor tyrosine kinase inhibitor, PTK787/ZK222584, causes significant anti-arthritic effects in models of rheumatoid arthritis. Inflammation research : official journal of the European Histamine Research Society [et al] 2004;53(4):133–142. doi: 10.1007/s00011-003-1230-4. [DOI] [PubMed] [Google Scholar]
- 146.Furuya K, Kaku Y, Yoshida K, Joh K, Kurosaka D. Therapeutic effects of sunitinib, one of the anti-angiogenetic drugs, in a murine arthritis. Modern rheumatology / the Japan Rheumatism Association. 2014;24(3):487–491. doi: 10.3109/14397595.2013.844295. [DOI] [PubMed] [Google Scholar]
- 147.Griffith EC, Su Z, Turk BE, et al. Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chemistry & biology. 1997;4(6):461–471. doi: 10.1016/s1074-5521(97)90198-8. [DOI] [PubMed] [Google Scholar]
- 148.Kruger EA, Figg WD. TNP-470: an angiogenesis inhibitor in clinical development for cancer. Expert opinion on investigational drugs. 2000;9(6):1383–1396. doi: 10.1517/13543784.9.6.1383. [DOI] [PubMed] [Google Scholar]
- 149.Ashraf S, Mapp PI, Walsh DA. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis and rheumatism. 2011;63(9):2700–2710. doi: 10.1002/art.30422. [DOI] [PubMed] [Google Scholar]
- 150.Bainbridge J, Madden L, Essex D, Binks M, Malhotra R, Paleolog EM. Methionine aminopeptidase-2 blockade reduces chronic collagen-induced arthritis: potential role for angiogenesis inhibition. Arthritis research & therapy. 2007;9(6):R127. doi: 10.1186/ar2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lazarus DD, Doyle EG, Bernier SG, et al. An inhibitor of methionine aminopeptidase type-2, PPI-2458, ameliorates the pathophysiological disease processes of rheumatoid arthritis. Inflammation research : official journal of the European Histamine Research Society [et al] 2008;57(1):18–27. doi: 10.1007/s00011-007-7075-5. [DOI] [PubMed] [Google Scholar]
- 152.de Bandt M, Grossin M, Weber AJ, et al. Suppression of arthritis and protection from bone destruction by treatment with TNP-470/AGM-1470 in a transgenic mouse model of rheumatoid arthritis. Arthritis and rheumatism. 2000;43(9):2056–2063. doi: 10.1002/1529-0131(200009)43:9<2056::AID-ANR17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 153.Bornstein P. Thrombospondins function as regulators of angiogenesis. Journal of cell communication and signaling. 2009;3(3–4):189–200. doi: 10.1007/s12079-009-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pfander D, Cramer T, Deuerling D, Weseloh G, Swoboda B. Expression of thrombospondin-1 and its receptor CD36 in human osteoarthritic cartilage. Annals of the rheumatic diseases. 2000;59(6):448–454. doi: 10.1136/ard.59.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3(2):147–158. doi: 10.1023/a:1009018702832. [DOI] [PubMed] [Google Scholar]
- 156.Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. The Journal of biological chemistry. 2010;285(50):38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gelse K, Klinger P, Koch M, et al. Thrombospondin-1 prevents excessive ossification in cartilage repair tissue induced by osteogenic protein-1. Tissue engineering Part A. 2011;17(15–16):2101–2112. doi: 10.1089/ten.TEA.2010.0691. [DOI] [PubMed] [Google Scholar]
- 158.Hsieh JL, Shen PC, Shiau AL, et al. Intraarticular gene transfer of thrombospondin-1 suppresses the disease progression of experimental osteoarthritis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28(10):1300–1306. doi: 10.1002/jor.21134. [DOI] [PubMed] [Google Scholar]
- 159.Jou IM, Shiau AL, Chen SY, et al. Thrombospondin 1 as an effective gene therapeutic strategy in collagen-induced arthritis. Arthritis and rheumatism. 2005;52(1):339–344. doi: 10.1002/art.20746. [DOI] [PubMed] [Google Scholar]
- 160.Koch AE, Szekanecz Z, Friedman J, Haines GK, Langman CB, Bouck NP. Effects of thrombospondin-1 on disease course and angiogenesis in rat adjuvant-induced arthritis. Clinical immunology and immunopathology. 1998;86(2):199–208. doi: 10.1006/clin.1997.4480. [DOI] [PubMed] [Google Scholar]
- 161.Hiraki Y, Tanaka H, Inoue H, Kondo J, Kamizono A, Suzuki F. Molecular cloning of a new class of cartilage-specific matrix, chondromodulin-I, which stimulates growth of cultured chondrocytes. Biochemical and biophysical research communications. 1991;175(3):971–977. doi: 10.1016/0006-291x(91)91660-5. [DOI] [PubMed] [Google Scholar]
- 162.Hiraki Y, Kono T, Sato M, Shukunami C, Kondo J. Inhibition of DNA synthesis and tube morphogenesis of cultured vascular endothelial cells by chondromodulin-I. FEBS letters. 1997;415(3):321–324. doi: 10.1016/s0014-5793(97)01151-4. [DOI] [PubMed] [Google Scholar]
- 163.Shukunami C, Iyama K, Inoue H, Hiraki Y. Spatiotemporal pattern of the mouse chondromodulin-I gene expression and its regulatory role in vascular invasion into cartilage during endochondral bone formation. The International journal of developmental biology. 1999;43(1):39–49. [PubMed] [Google Scholar]
- 164.Hayami T, Funaki H, Yaoeda K, et al. Expression of the cartilage derived anti-angiogenic factor chondromodulin-I decreases in the early stage of experimental osteoarthritis. The Journal of Rheumatology. 2003;30(10):2207–2217. [PubMed] [Google Scholar]
- 165.O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 166.Kim YM, Hwang S, Kim YM, et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. The Journal of biological chemistry. 2002;277(31):27872–27879. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- 167.Pufe T, Kurz B, Petersen W, et al. The influence of biomechanical parameters on the expression of VEGF and endostatin in the bone and joint system. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2005;187(5–6):461–472. doi: 10.1016/j.aanat.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 168.Yin G, Liu W, An P, et al. Endostatin gene transfer inhibits joint angiogenesis and pannus formation in inflammatory arthritis. Molecular therapy : the journal of the American Society of Gene therapy. 2002;5(5 Pt 1):547–554. doi: 10.1006/mthe.2002.0590. [DOI] [PubMed] [Google Scholar]
- 169.Hu W, Xia LJ, Chen FH, et al. Recombinant human endostatin inhibits adjuvant arthritis by down-regulating VEGF expression and suppression of TNF-alpha, IL-1beta production. Inflammation research : official journal of the European Histamine Research Society [et al] 2012;61(8):827–835. doi: 10.1007/s00011-012-0477-z. [DOI] [PubMed] [Google Scholar]
- 170.Kurosaka D, Yoshida K, Yasuda J, et al. The effect of endostatin evaluated in an experimental animal model of collagen-induced arthritis. Scandinavian journal of Rheumatology. 2007;36(6):434–441. doi: 10.1080/03009740701605913. [DOI] [PubMed] [Google Scholar]
- 171.Yue L, Shen YX, Feng LJ, et al. Blockage of the formation of new blood vessels by recombinant human endostatin contributes to the regression of rat adjuvant arthritis. European journal of pharmacology. 2007;567(1–2):166–170. doi: 10.1016/j.ejphar.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 172.Yue L, Wang H, Liu LH, Shen YX, Wei W. Anti-adjuvant arthritis of recombinant human endostatin in rats via inhibition of angiogenesis and proinflammatory factors. Acta pharmacologica Sinica. 2004;25(9):1182–1185. [PubMed] [Google Scholar]
- 173.Kurosaka D, Yoshida K, Yasuda J, et al. Inhibition of arthritis by systemic administration of endostatin in passive murine collagen induced arthritis. Annals of the rheumatic diseases. 2003;62(7):677–679. doi: 10.1136/ard.62.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun XD, Jeng L, Bolliet C, Olsen BR, Spector M. Non-viral endostatin plasmid transfection of mesenchymal stem cells via collagen scaffolds. Biomaterials. 2009;30(6):1222–1231. doi: 10.1016/j.biomaterials.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 175.Jeng L, Olsen BR, Spector M. Engineering endostatin-producing cartilaginous constructs for cartilage repair using nonviral transfection of chondrocyte-seeded and mesenchymal-stem-cell-seeded collagen scaffolds. Tissue engineering Part A. 2010;16(10):3011–3021. doi: 10.1089/ten.tea.2009.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jeng L, Olsen BR, Spector M. Engineering endostatin-expressing cartilaginous constructs using injectable biopolymer hydrogels. Acta biomaterialia. 2012;8(6):2203–2212. doi: 10.1016/j.actbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 177.Claesson-Welsh L, Welsh M, Ito N, et al. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(10):5579–5583. doi: 10.1073/pnas.95.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim JM, Ho SH, Park EJ, et al. Angiostatin gene transfer as an effective treatment strategy in murine collagen-induced arthritis. Arthritis and rheumatism. 2002;46(3):793–801. doi: 10.1002/art.10113. [DOI] [PubMed] [Google Scholar]
- 179.Sumariwalla PF, Cao Y, Wu HL, Feldmann M, Paleolog EM. The angiogenesis inhibitor protease-activated kringles 1–5 reduces the severity of murine collagen-induced arthritis. Arthritis research & therapy. 2003;5(1):R32–R39. doi: 10.1186/ar608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen Y, Donnelly E, Kobayashi H, Debusk LM, Lin PC. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis and rheumatism. 2005;52(5):1585–1594. doi: 10.1002/art.21016. [DOI] [PubMed] [Google Scholar]
- 181.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 182.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovascular research. 2001;49(3):507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 183.Hansen TM, Singh H, Tahir TA, Brindle NP. Effects of angiopoietins-1 and −2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cellular signalling. 2010;22(3):527–532. doi: 10.1016/j.cellsig.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Waltenberger J, Mayr U, Frank H, Hombach V. Suramin is a potent inhibitor of vascular endothelial growth factor. A contribution to the molecular basis of its antiangiogenic action. Journal of molecular and cellular cardiology. 1996;28(7):1523–1529. doi: 10.1006/jmcc.1996.0142. [DOI] [PubMed] [Google Scholar]
- 185.Larsen AK. Suramin: an anticancer drug with unique biological effects. Cancer chemotherapy and pharmacology. 1993;32(2):96–98. doi: 10.1007/BF00685609. [DOI] [PubMed] [Google Scholar]
- 186.Hunziker EB, Driesang IM. Functional barrier principle for growth-factor-based articular cartilage repair. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2003;11(5):320–327. doi: 10.1016/s1063-4584(03)00031-1. [DOI] [PubMed] [Google Scholar]
- 187.Sahu D, Saroha A, Roy S, Das S, Srivastava PS, Das HR. Suramin ameliorates collagen induced arthritis. International immunopharmacology. 2012;12(1):288–293. doi: 10.1016/j.intimp.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 188.Matsuno H, Yudoh K, Uzuki M, et al. Treatment with the angiogenesis inhibitor endostatin: a novel therapy in rheumatoid arthritis. The Journal of Rheumatology. 2002;29(5):890–895. [PubMed] [Google Scholar]
- 189.Nissen MJ, Boucher A, Brulhart L, Menetrey J, Gabay C. Efficacy of intra-articular bevacizumab for relapsing diffuse-type giant cell tumour. Annals of the rheumatic diseases. 2014;73(5):947–948. doi: 10.1136/annrheumdis-2013-204589. [DOI] [PubMed] [Google Scholar]
- 190.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nature reviews Drug discovery. 2016 doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 191.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature reviews Drug discovery. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 192.Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. The Annals of pharmacotherapy. 2007;41(4):614–625. doi: 10.1345/aph.1H316. [DOI] [PubMed] [Google Scholar]
- 193.van der Reis MI, La Heij EC, De Jong-Hesse Y, Ringens PJ, Hendrikse F, Schouten JS. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31(8):1449–1469. doi: 10.1097/IAE.0b013e3182278ab4. [DOI] [PubMed] [Google Scholar]
- 194.Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye. 2013;27(7):787–794. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Diabetic Retinopathy Clinical Research N, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. The New England journal of medicine. 2015;372(13):1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Survey of ophthalmology. 2011;56(2):95–113. doi: 10.1016/j.survophthal.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 197.Remon J, Gazzah A, Besse B, Soria JC. Crizotinib Improves Osteoarthritis Symptoms in a ROS1-Fusion Advanced Non-Small Cell Lung Cancer Patient. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10(8):e72–e73. doi: 10.1097/JTO.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 198.Hunter DJ, Pike MC, Jonas BL, Kissin E, Krop J, McAlindon T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC musculoskeletal disorders. 2010;11:232. doi: 10.1186/1471-2474-11-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. The American journal of sports medicine. 2015 doi: 10.1177/0363546515580787. [DOI] [PubMed] [Google Scholar]
- 200.Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(11):1627–1637. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]