Abstract
Aim:
To comparatively evaluate the compressive strength, diametral tensile strength, and shear bond strength of glass ionomer cement type IX, chlorhexidine-incorporated glass ionomer cement, and triclosan-incorporated glass ionomer cement.
Materials and Methods:
In this study, glass ionomer cement type IX was used as a control. Chlorhexidine diacetate, and triclosan were added to glass ionomer cement type IX powder, respectively, in order to obtain 0.5, 1.25, and 2.5% concentrations of the respective experimental groups. Compressive strength, diametral tensile strength, and shear bond strength were evaluated after 24 h using Instron Universal Testing Machine. The results obtained were statistically analyzed using the independent t-test, Dunnett test, and Tukey test.
Results:
There was no statistical difference in the compressive strength, diametral tensile strength, and shear bond strength of glass ionomer cement type IX (control), 0.5% triclosan-glass ionomer cement, and 0.5% chlorhexidine-glass ionomer cement.
Conclusion:
The present study suggests that the compressive strength, diametral tensile strength, and shear bond strength of 0.5% triclosan-glass ionomer cement and 0.5% chlorhexidine-glass ionomer cement were similar to those of the glass ionomer cement type IX, discernibly signifying that these can be considered as viable options for use in pediatric dentistry with the additional value of antimicrobial property along with physical properties within the higher acceptable range.
Keywords: Chlorhexidine, compressive strength, diametral tensile strength, glass ionomer cement, shear bond strength, triclosan
INTRODUCTION
The disease of dental caries dates back to ancient times and is the most common disease besetting human race.[1] In spite of various preventive methods, dental caries still presents a colossal challenge to clinicians.
Once dental caries occurs, restoring the carious lesion becomes mandatory.[1] The most widely used material for restoring the deciduous teeth is glass ionomer cement. It has been shown to be a very useful adjunct to restorative dentistry because of its unique ability to release fluoride, which is mainly responsible for its cariostatic action. Moreover, glass ionomer cement bonds chemically to enamel and dentin, thereby reducing the need for a retentive cavity preparation; thus, also preserving the sound tooth structure following the principle of “Conservation for prevention.”[2]
Because of these properties, glass ionomer cement is the material of choice in atraumatic restorative treatment (ART). Because atraumatic restorative treatment (ART) is practiced using hand instruments only, there is a possibility of insufficient caries removal; therefore, such kind of cavities require a restorative material with good antibacterial efficacy.[3,4]
Thus, therapeutic benefits may be gained by reinforcing glass ionomer cements with additional antibacterial agents.[5,6] Such agents should not affect the physicomechanical properties of the glass ionomer cement.
Among the antiseptics, chlorhexidine diacetate has proven to be safe and effective. A new antibacterial agent of interest is triclosan, a broad spectrum antimicrobial, which has been extensively used in mouthwashes and dentifrices.
Compressive stress results when the body is subjected to two sets of forces in the same straight line but directed toward each other. Shear bond strength is the maximum amount of force required to break the interface between a bonded restoration and the tooth surface with the failure occurring in or near the adhesive interface. Tensile stress results in a body when it is subjected to two sets of forces that are directed away from each other in the same straight line. Considering the importance of compressive strength, diametral tensile strength and shear bond strength of restorative materials, the purpose of the study undertaken was to evaluate and compare the compressive strength, diametral tensile strength, and shear bond strength of GIC Type IX, chlorhexidine-incorporated GIC, and triclosan-incorporated GIC.
MATERIALS AND METHODS
This in vitro study was carried out in the Department of Pedodontics and Preventive Dentistry, D.J. College of Dental Sciences and Research, Modinagar, Ghaziabad, in collaboration with Apex Laboratories, Mohanagar.
Study design
For the evaluation of compressive strength and diametral tensile strength restorative pellets of GIC type IX, 0.5, 1.25, and 2.5% concentrations of chlorhexidine-incorporated GIC and triclosan-incorporated GIC were prepared.
For the evaluation of shear bond strength primary human molar teeth were taken.
Preparation of restorative pellets for compressive strength and diametral tensile strength testing
The desired concentrations of GIC powder, chlorhexidine salt, and triclosan powder were obtained using an analytical digital scale.
Various concentrations of CHX-GIC and T-GIC
Chlorhexidine-GIC (CHX-GIC): Three different proportions of CHX-GIC were prepared based on the concentration of chlorhexidine.
0.5% CHX-GIC: Prepared by adding 0.015 g of chlorhexidine to 2.985 g of glass ionomer powder;
1.25% CHX-GIC: Prepared by adding 0.037 g of chlorhexidine to 2.96 g of glass ionomer powder;
2.5% CHX-GIC: Prepared by adding 0.075 g of chlorhexidine to 2.925 g of glass ionomer powder.
Triclosan-GIC (T-GIC): Similarly, 0.5, 1.25, and 2.5% concentrations of triclosan-incorporated GIC were prepared. All the prepared materials were stored in six amber-colored bottles.
Autoclavable plastic molds of standardized dimensions (4 mm diameter, 6 mm thickness) were used for the preparation of all restorative pellets. Immediately after mixing, the material was placed into plastic molds by a plastic instrument and covered with acetate strips on both sides. This assembly was then placed into an incubator at 37 ± 1°C and 95 ± 5% relative humidity for 1 h to simulate oral conditions. The pellets were then removed from the molds and ground on 500-grit Silicon carbide paper for finishing. The diameter of each pellet was determined using dial calipers. Thereafter, the division of samples was done accordingly [Table 1].
Table 1.
Division of samples

Preparation of extracted human molars for the assessment of shear bond strength
Occlusal dentin samples were obtained from 35 primary human molars (five molars for each group). The buccal enamel was reduced to a flat surface using a diamond disk, and was polished thereafter. The dentin surface was conditioned using polyacrylic acid for 10 s followed by air-water spray for 10 s. Then, the restorative materials from various groups were mixed and placed on the buccal surface of the tooth. A block of glass ionomer cement of dimension 4 × 3 × 2 mm was prepared. After that the specimens were stored in an incubator at 37 ± 1°C and 95 ± 5% relative humidity for 24 h. Thereafter, the division of samples was done accordingly [Table 1].
Evaluation of compressive strength and diametral tensile strength
For the compressive strength testing, restorative pellets were placed with the flat ends up between the plates of the Instron Universal Testing machine (Instron 1500HDX). A compressive load was applied at a crosshead speed of 1 mm/min until the restorative pellet fractured.
For the diametral tensile strength testing, the restorative pellets were placed on the Instron Universal testing machine such that the diameter of the pellet coincided with the direction of the force. A crosshead speed of 1 mm/min was used and the force was applied until the pellets fractured.
Evaluation of shear bond strength
The specimens were placed in the lower assembly of the Instron Universal testing machine one by one. A sharp knife-like mandrel was attached to the upper assembly and was suspended downwards toward the glass ionomer cement block. Crosshead speed of 0.5 mm/min was adjusted. The force with which the restoration block was dislodged was recorded and shear bond strength was calculated.
Statistical analysis
The data was statistically analyzed using independent t-test and intercomparison among various groups was done using Dunnett test and post hoc test.
RESULTS
The mean values of compressive strength, diametral tensile strength, and shear bond strength of various groups are depicted in Table 2. Independent t-test revealed that GIC type IX (Group 1) showed the highest values followed by 0.5% T-GIC (Group 3a) and the least values were found in 0.5% CHX-GIC (Group 2c).
Table 2.
Mean values of compressive strength, diametral tensile strength, and shear bond strength (MPa) of various groups
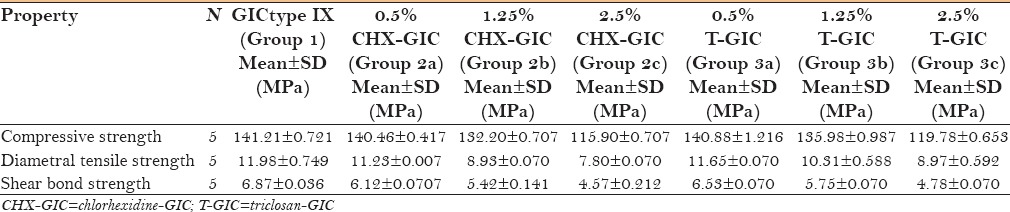
Table 3 depicts the intragroup comparison of compressive strength, diametral tensile strength, and shear bond strength of various concentrations of CHX-GIC. Dunnett test showed that 0.5% CHX-GIC was significantly better (P < 0.05) than 1.25% and 2.5% CHX-GIC.
Table 3.
Intergroup comparison of compressive strength, diametral tensile strength and shear bond strength of various concentrations of CHX-GIC (chlorhexidine-GIC)
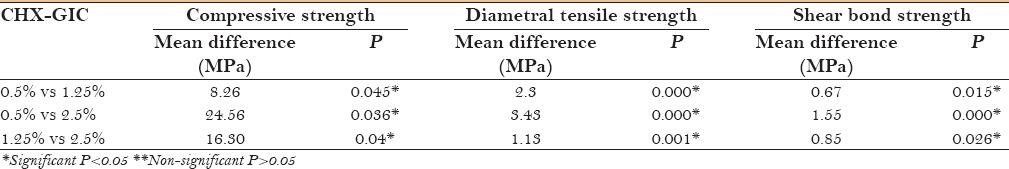
Table 4 depicts the intragroup comparison of compressive strength, diametral tensile strength, and shear bond strength of various concentrations of T-GIC. Dunnett test showed that 0.5% T-GIC was significantly better (P < 0.05) than 1.25% and 2.5% T-GIC.
Table 4.
Intergroup comparison of compressive strength, diametral tensile strength and shear bond strength of various concentrations of T-GIC (triclosan-GIC)
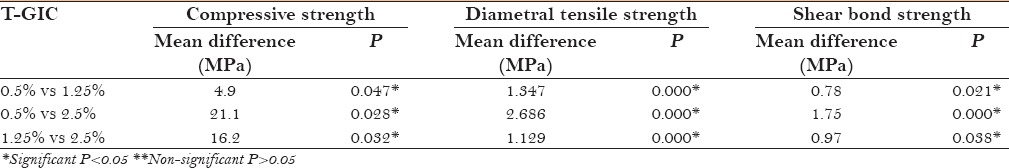
Thus, to evaluate if 0.5% CHX-GIC and 0.5% T-GIC are comparable, in terms of all the abovementioned physical properties, to GIC type IX, an intercomparison of mean values was done using post hoc test [Table 5].
Table 5.
Intergroup comparison of mean difference of compressive strength, diametral tensile strength and shear bond strength of GIC type IX with 0.5% CHX-GIC (chlorhexidine-GIC) and 0.5% T-GIC (triclosan-GIC)
This intercomparison shows that no significant difference (P > 0.05) in compressive strength, diametral tensile strength, and shear bond strength existed between GIC type IX (Group 1), 0.5% CHX-GIC (Group 2a) and 0.5% T-GIC (Group 3a).
DISCUSSION
ART has been practiced using hand instruments only, therefore, there is a possibility of insufficient caries removal. Literature has shown that microorganisms have been found to be viable for at least a period of two years under the glass ionomer cement restoration.[6,7] Thus, therapeutic benefits may be gained by reinforcing glass ionomer cements with additional antibacterial agents.[5] However, such agents should not affect the physical properties of the parent material.[8] Chlorhexidine diacetate is a more stable antibacterial material, not prone to decomposition, and can be easily blended with GIC. Triclosan at low concentrations acts as a bacteriostatic and at high concentrations as bactericidal.
To evaluate which concentration of chlorhexidine and triclosan is best suited for our purpose various concentrations were used, i.e. 0.5%, 1.25%, and 2.5%. All the concentrations have been shown to provide adequate antibacterial property to GIC type IX.[9]
In an in vitro study in 2008, Türkün et al.[5] used 0.5%, 1.25%, and 2.5% concentrations of chlorhexidine-containing GIC to study the long-term antibacterial effects and physical properties. In another study by Deepalakshmi et al.,[6] glass ionomer cements containing chlorhexidine and cetrimide at concentrations of 1% and 2% were used to evaluate their antibacterial and physical properties.
The results of the current study demonstrated that the mean value of compressive strength, diametral tensile strength, and shear bond strength was found to be the highest in GIC type IX. The above result may be attributed to the fact that GIC type IX is characterized by having smaller glass particles and higher powder to liquid ratio. This is said to give the GIC type IX higher strength, greater wear resistance, and increased flexural strength. This result was in accordance to a study done by Ahluwalia et al. in 2012, in which the authors found that the diametral tensile strength of GIC type IX (12.61 MPa) was higher than 1% CHX-GIC (12 MPa).[1]
When the various concentrations of T-GIC and CHX-GIC were compared with each other, it was found that 0.5% T-GIC and 0.5% CHX-GIC have the highest values of compressive strength, diametral tensile strength, and shear bond strength in their respective groups. The common finding in both the intragroup comparisons points out that the physical properties abruptly decrease in a concentration-dependent manner.[10] In addition, the results manifest that 1.5% and 2.5% concentration of antibacterials incorporated into GIC type IX might not be suitable for clinical use because increase in the concentration adversely affects the physical properties of the parent material. Because of the vitrification of GIC with chlorhexidine and triclosan at higher concentrations, many of these carboxylic (COOH) groups are prevented from participating in these coordination complexes.[11,12] A possible reason for the decrease in physical properties at a concentration of more than 2% chlorhexidine can be attributed to the fact that cationic salts hamper the setting reaction of the polyacrylic acid glasses, thereby extending the setting time, because of interfered proton attack and leaching of ions from the glasses.[13,14,15,16]
The intercomparison between GIC type IX (control), 0.5% CHX-GIC (Group 2a), and 0.5% T-GIC (Group 3a) discernibly signified that 0.5% T-GIC and 0.5% CHX-GIC exhibit physical properties comparable to GIC type IX, which indicates that these maybe considered as viable options for use in pediatric dentistry along with physical properties within the higher acceptable range.[14]
CONCLUSIONS
It can be concluded that the compressive strength, diametral tensile strength, and shear bond strength values of 0.5% T-GIC and 0.5% CHX-GIC were comparable to GIC type IX. We recommend that further studies should be conducted to test various antibacterial glass ionomer cements in a randomized clinical trial. Because a smaller sample size was used in this study, further studies with a larger sample size are required to authenticate the results. Clinical impact of various other factors such as occlusal forces needs to be investigated in further studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ahluwalia P, Chopra S, Thomas AM. Strength characteristics and marginal sealing ability of chlorhexidine-modified glass ionomer cement: An in vitro study. J Indian Soc Pedod Prev Dent. 2012;30:41–6. doi: 10.4103/0970-4388.95580. [DOI] [PubMed] [Google Scholar]
- 2.Palma-Dibb RG, de Castro CG, Ramos RP, Chimello DT, Chinelatti MA. Bond strength of glass-ionomer cements to caries-affected dentin. J Adhes Dent. 2003;5:57–62. [PubMed] [Google Scholar]
- 3.Sainulabdeen S, Neelakantan P, Ramesh S, Subbarao CV. Antibacterial activity of triclosan incorporated glass ionomer cements - An in vitro pilot study. J Clin Pediatr Dent. 2010;35:157–62. doi: 10.17796/jcpd.35.2.96747l52725n608x. [DOI] [PubMed] [Google Scholar]
- 4.Botelho MG. Inhibitory effects on selected oral bacteria of antibacterial agents incorporated in a glass ionomer cement. Caries Res. 2003;37:108–14. doi: 10.1159/000069019. [DOI] [PubMed] [Google Scholar]
- 5.Türkün IS, Türkün M, Ertuðul F, Ateþ M, Brugger S. Long-term antibacterial effects and physical properties of a chlorhexidine-containing glass ionomer cement. J Esthet Restor Dent. 2008;20:29–44. doi: 10.1111/j.1708-8240.2008.00146.x. [DOI] [PubMed] [Google Scholar]
- 6.Deepalakshmi M, Poorni S, Miglani R, Rajamani I, Ramachandran S. Evaluation of the antibacterial and physical properties of glass ionomer cements containing chlorhexidine and cetrimide: An in vitro study. Indian J Dent Res. 2010;21:552–6. doi: 10.4103/0970-9290.74217. [DOI] [PubMed] [Google Scholar]
- 7.Weerheijm KL, Kreulen CM, de Soet JJ, Groen HJ, van Amerongen WE. Bacterial counts in carious dentine under restorations: 2-year in vivo effects. Caries Res. 1999;33:130–4. doi: 10.1159/000016506. [DOI] [PubMed] [Google Scholar]
- 8.Hook ER, Owen OJ, Bellis CA, Holder JA, O’Sullivan DJ, Barbour ME. Development of a novel antimicrobial-releasing glass ionomer cement functionalized with chlorhexidine hexametaphosphate nanoparticles. J Nanobiotechnol. 2014;12:3. doi: 10.1186/1477-3155-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sainulabdeen S, Neelakantan P, Ramesh S, Subbarao CV. Antibacterial activity of triclosan incorporated glass ionomer cements - An in vitro pilot study. J Clin Pediatr Dent. 2010;35:157–61. doi: 10.17796/jcpd.35.2.96747l52725n608x. [DOI] [PubMed] [Google Scholar]
- 10.Becci ACO, Marti LM, Zuanon ACC, Brighenti FL, Spolidório DMP, Giro EMA. Influence of the addition of chlorhexidine diacetate on bond strength of a high-viscosity glass ionomer cement to sound and artificial caries-affected dentin. Rev Odontol. 2014;43:109–16. [Google Scholar]
- 11.Crisp S, Wilson AD. Reactions in glass ionomer cements: I. Decomposition of the Powder. J Dent Res. 1974;53:1408–13. doi: 10.1177/00220345740530061901. [DOI] [PubMed] [Google Scholar]
- 12.Mittal S, Soni H, Sharma DK, Mittal K, Pathania V, Sharma S. Comparative evaluation of the antibacterial and physical of conventional glass ionomer cement containing chlorhexidine and antibiotics. J Int Soc Prev Community Dent. 2015;5:268–75. doi: 10.4103/2231-0762.161754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prabhakar AR, Agarwal S, Basappa N. Comparative evaluation of antibacterial effect and physical properties of conventional glass-ionomer cement containing 1% chlorhexidine and 1% xylitol. Int J Oral Health Sci. 2014;4:63–9. [Google Scholar]
- 14.Marti LM, Mata MD, Ferraz-Santos B, Azevedo ER, Giro EM, Zuanon AC. Addition of chlorhexidine gluconate to a glass ionomer cement: A study on mechanical, physical and antibacterial properties. Braz Dent J. 2014;25:33–7. doi: 10.1590/0103-6440201302328. [DOI] [PubMed] [Google Scholar]
- 15.Ewoldsen N, Covey D, Lavin M. The physical and adhesive properties of dental cements used for atraumatic restorative treatment. Spec Care Dentist. 1997;17:19–24. doi: 10.1111/j.1754-4505.1997.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 16.Kerby RE, Knobloch L. Strength characteristics of glass-ionomer cements. Oper Dent. 1992;17:170–4. [PubMed] [Google Scholar]


