Abstract
The efficacy of the conjunctival application of a crude concentration of stingless bee honey (SBH) for the treatment of bacterial conjunctivitis was investigated in an animal model. Bacterial conjunctivitis caused by Staphylococcus aureus or Pseudomonas aeruginosa was induced in Hartley guinea pigs. The conjunctival application of SBH or gentamicin was used for treatment, and the results of this treatment were compared with control values. Inflammatory signs, duration of infection (ie, positive culture), and time for the complete resolution of infection with S. aureus or P. aeruginosa were shortened by the conjunctival application of 1 drop (70 µL) of crude SBH twice daily. The potency of SBH was comparable with that of gentamicin. SBH may be a rational agent for the treatment of infective conjunctivitis in humans; it is inexpensive and commonly available to the rural population.
Keywords: stingless bee honey, efficacy, bacteria, conjunctivitis, inflammation, infection
Infection of the eye can result in conjunctivitis, keratitis, endophthalmitis, and other infections that are responsible for increased incidences of morbidity and blindness worldwide.1 These infections are common occurrences in the tropics and in resource-poor countries as a result of poor hygiene and environmental contaminants. Furthermore, the problems of resistance, adverse responses, and the high cost of established antibiotic compounds have given rise to the search for new anti-infective agents from natural sources for better therapeutic effects.2,3
Stingless bee honey (SBH) – a natural product from a diverse group of highly eusocial bees (ie, meliponines) that comprise the tribe Meliponini in the family Apidae – has shown therapeutic potential in current research.4,5 Undiluted SBH was found to be bactericidal for many pathogenic organisms, including various Gram-negative and Gram-positive bacteria.4 In another study, SBH was more potent than the standard antibiotics against both Gram-positive and Gram-negative bacteria.6 In a recent study, undiluted SBH and SBH at concentrations of 60% or more were effective against isolated agents of conjunctivitis.7 However, in most of these studies, the inhibition of bacterial growth was demonstrated with the use of in vitro methods. There are very few in vivo studies of the use of SBH for the treatment of eye diseases. In the current study, guinea pig conjunctivae were infected with isolates of Staphylococcus aureus and Pseudomonas aeruginosa from infective human conjunctivitis and treated with SBH or a standard antibiotic, with the aim of investigating the efficacy of SBH for the treatment of infective conjunctivitis.
Materials and methods
Bacterial cultures
Cultures of S. aureus and P. aeruginosa were obtained from the microbiology unit of the Central Regional Hospital, Cape Coast, Ghana. These strains were isolated from patients with infective conjunctivitis who reported to ophthalmologic unit of the hospital. The isolates were identified by standard bacteriological techniques. Suspensions of various isolates were prepared by culturing a specimen (ie, single colony) of each isolate, which was picked up from a pure culture on a plate with a standard loop and placed into 10 mL of nutrient broth. These isolates were then incubated for 24 hours at 37°C.
Collection of honey
A matured honey that had been sealed in pots within stingless bee (Meliponula spp.) hives located at the International Stingless Bee Centre at Abrafo, near Kakum National Park in Ghana, was used for this study. The collection was performed with disposable syringes. During collection, the sealed portion of the pot was carefully broken with a sterile needle. The tip of the sterile disposable syringe was dipped into the opening of the honey pot. The honey was carefully drawn with the syringe, transferred into an empty sterile container, and covered immediately. This container was then kept in a cool, dry place. The pH of the SBH that was collected was determined with a pH meter.
The experimental animals
A total of 30 adult guinea pigs of both sexes between the ages of 4 and 6 months old and weighing 405 g ± 50 g were obtained from the Animal Laboratory of the School of Biological Sciences, University of Cape Coast, Ghana. The animals were housed singly in polyacrylic cages (34 cm × 47 cm × 18 cm) with soft wood shavings as bedding; they were fed with a normal commercial pellet diet (GAFCO, Tema, Ghana), given water ad libitum, and maintained under laboratory conditions (ie, temperature 28°C ± 2°C, relative humidity of 60%–70%, and a normal light–dark cycle). All procedures with animals were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The protocols for the study were approved by the University of Cape Coast’s Departmental Ethics Committee.
The animals were divided into two main groups, with animals A through O and 1 through 15 representing the S. aureus and P. aeruginosa groups, respectively. In each group, the animals were subdivided into three groups of five each: the SBH group, the gentamicin group, and the control group. Animals A through E belonged to the gentamicin group, animals F through J were in the SBH group, and animals K through O served as the control group for S. aureus conjunctivitis. Similarly, animals 1 through 15 were equally subdivided into SBH, gentamicin, and control groups to study the treatment of P. aeruginosa conjunctivitis.
Induction of bacterial conjunctivitis
To induce conjunctival infection, the method proposed by Al-Waili8 was adopted. Each animal was anesthetized with a ketamine hydrochloride injection (10 mg/kg intramuscularly), and a drop of 1% ametocaine topical anesthetic agent was instilled into the cornea of the right eye. A small abrasion was then made on the conjunctiva of the right eye of each animal. Specimens from the bacterial suspensions were taken with the standard loop and inoculated onto each animal’s abraded conjunctiva.
Observation
Forty-eight hours after inoculation, swabs of discharge were taken from the eyes of the test animals and cultured to confirm the growth of microorganisms before treatment commenced. The assessment of the conjunctival infection was based on local signs of edema, the size of the conjunctival sac, any redness and discharge that were present, and daily swabbing for culture.
After confirming the presence of infection, the animals in the SBH groups were treated with one drop of SBH twice daily at 12-hour intervals. The animals in the gentamicin groups were treated in the same manner as the SBH group. The animals in the control groups were left untreated.
The presence or absence of conjunctival redness, the narrowing of the palpebral fissure, and swelling or discharge were observed and recorded daily. Swabs were taken every morning to monitor for the number of days of positive cultures for a 14-day period. After the 14th day, animals that still yielded a heavy growth of microorganisms that appeared to threaten their sight were withdrawn from observation, and rescue treatment was administered.
Data analysis
Data was subjected to descriptive analysis with the use of the Statistical Package for Social Sciences software (version 16.0; SPSS Inc, Chicago, IL) to generate frequencies and proportions. Continuous numerical data were captured as means and standard deviations, and categorical data were presented as percentages. The independent t-test was used to compare proportions of variables that were measured between groups. A P value of less than 0.05 was considered to be statistically significant.
Results
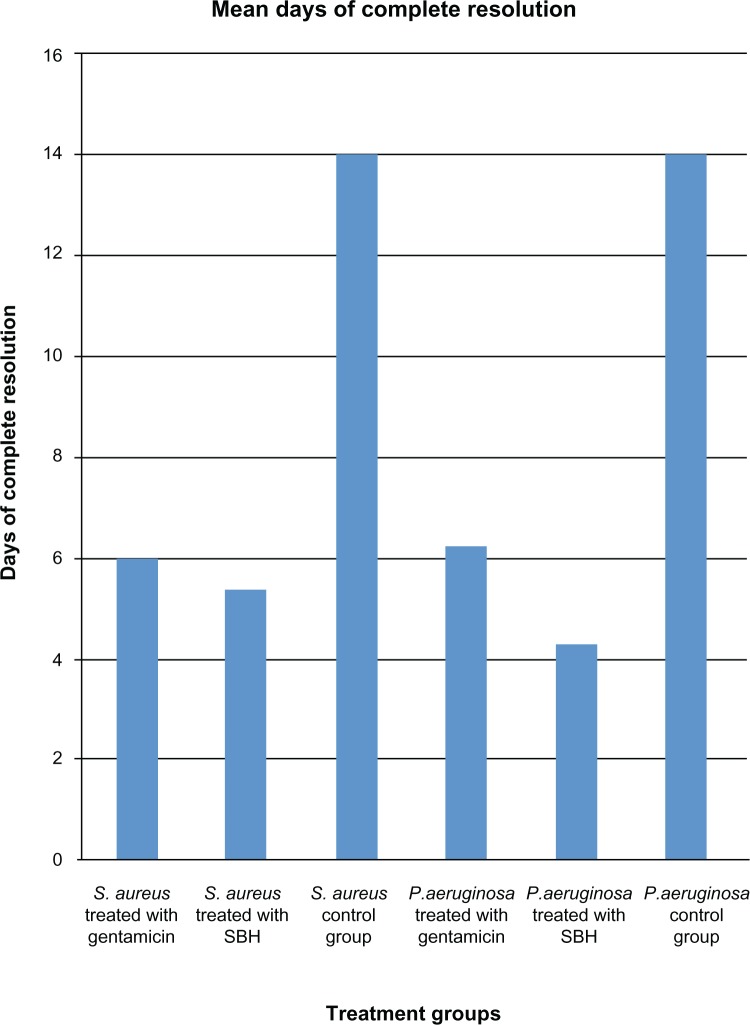
Daily conjunctival swabbing revealed heavy growth of S. aureus and P. aeruginosa 4 days and 2 days after the inoculation of the animals, respectively. The results of the effects of SBH and gentamicin on S. aureus and P. aeruginosa are shown in Tables 1 and 2, respectively. Figure 1 presents the mean days of complete resolution after treatment with gentamicin or SBH, which commenced on the 5th day.
Table 1.
Effect of the conjunctival application of stingless bee honey or gentamicin for the treatment of bacterial conjunctivitis caused by Staphylococcus aureus
| Gentamicin group | Stingless bee honey group | Control group | |
|---|---|---|---|
| Redness (no of guinea pigs) | 5 | 5 | 3 |
| Swelling (no of guinea pigs) | 5 | 5 | 3 |
| Narrowing of palpebral fissure (no of guinea pigs) | 1 | 2 | 1 |
| Pus (no of guinea pigs) | 0 | 0 | 1 |
| Death (no of guinea pigs) | 0 | 0 | 2 |
| Positive culture (mean no of days) | 5 ± 0.45 | 4 ± 0.40 | 14 |
| Complete resolution (mean no of days) | 6 ± 0.707 | 5 ± 0.55 | 0 |
| Rescue method (average no of days) | 0 | 0 | 14 |
Table 2.
Effect of the conjunctival application of stingless bee honey or gentamicin for the treatment of bacterial conjunctivitis caused by Pseudomonas aeruginosa
| Variable | Gentamicin group | Stingless bee honey group | Control group |
|---|---|---|---|
| Redness (no of guinea pigs) | 5 | 5 | 2 |
| Swelling (no of guinea pigs) | 5 | 5 | 2 |
| Narrowing of palpebral fissure (no of guinea pigs) | 1 | 0 | 1 |
| Pus (no of guinea pigs) | 0 | 0 | 0 |
| Death (no of guinea pigs) | 1 | 1 | 3 |
| Positive culture (average no of days) | 5 ± 0.65 | 3 ± 0.45 | 14 |
| Complete resolution (average no of days) | 6.25 ± 0.50 | 4.25 ± 0.50 | 0 |
| Rescue method (average no of days) | 0 | 0 | 14 |
Figure 1.
Mean days of the complete resolution of infection of the various treatment groups.
Abbreviation: SBH, stingless bee honey.
Effect of stingless bee honey or gentamicin on conjunctival infection due to S. aureus
In the S. aureus gentamicin group, all five of the animals developed redness and swelling of the conjunctiva, with one animal having a narrowing of the palpebral fissure. The culturing of the conjunctival swabs revealed positive growth during five days of observation. In the SBH group for this bacteria, all five guinea pigs developed redness and swelling, with two of them having a narrowing of the palpebral fissure. Culture became negative after 4 days of treatment with SBH. The complete resolution of local signs of inflammation was observed on the 6th day after treatment in the gentamicin group and on the 5th day after treatment in the SBH group. However, the difference was not significant (t[0.05, 8] = 1.50) (P = 0.172). In the S. aureus control group, all five guinea pigs developed redness and swelling, with one showing a narrowing of the palpebral fissure and pus discharge. The cultures of the conjunctival swabs were still positive after 14 days. Table 1 shows the effects of SBH or gentamicin eyedrops on S. aureus-induced conjunctival infection.
Effect of stingless bee honey or gentamicin on conjunctival infection due to P. aeruginosa
The results of effects of SBH or gentamicin on conjunctival infection caused by P. aeruginosa are presented in Table 2. Five of the animals in this group died 1 day after the commencement of treatment: one each from the SBH and gentamicin groups and three from the control group. The rest of the animals in each group developed both swelling and redness, with one each from the control and gentamicin groups demonstrating a narrowing of the palpebral fissure. The culturing of the conjunctival swabs yielded no growth on the 3rd day after SBH treatment and on the 5th day after gentamicin treatment. The complete resolution of local signs of inflammation was observed on the 6th day after treatment in the gentamicin group and on the 4th day after treatment in the SBH group; this difference was significant (t[0.05, 6] = 5.657) (P = 0.001). When SBH was administered topically, the mean number of days to the complete resolution of bacterial conjunctivitis caused by S. aureus and P. aeruginosa was 5.40 days ± 0.55 days and 4.25 days ± 0.50 days, respectively; this difference was statistically significant (t[0.05, 7] = 3.248) (P = 0.014).
In the control group, the culture was still positive after 14 days. All of the animals in the control group were eventually treated with gentamicin for 4 days as rescue measure.
Discussion
Few studies have reported about the use of honey for the treatment of eye diseases. Bacterial corneal ulcers, blepharitis, and catarrhal conjunctivitis have been treated with natural honey.9,10 There is evidence from in vitro studies that SBH could be as effective as first-line antibiotic for the treatment of common ocular diseases.4,5,8 In this study, we investigated the antimicrobial activity of SBH in experimental animals. The main objective of our study was to compare the efficacy of SBH to that of a first-line antibiotic agent for the treatment of infective conjunctivitis.
The results demonstrated that SBH has potent antimicrobial activity against common ocular pathogens and that this activity is comparable to that of gentamicin. The topical application of SBH to infected conjunctiva eradicated bacterial infections and reduced both sign of inflammation and the duration of infection. The SBH was more effective than gentamicin against P. aeruginosa: the time to the complete resolution of inflammation was significantly shortened when SBH was used for the treatment of bacterial conjunctivitis caused by this agent. These results were observed with the application of one drop of SBH on the conjunctiva twice daily at 12-hour intervals. Our findings corroborate the previous report by Al-Waili,8 in which bacterial conjunctivitis caused by a variety of human pathogens – including Proteus spp, S. aureus, Escherichia coli, P. aeruginosa, and Klebsiella spp – was treated successfully with the topical application of honey.
The microorganisms used in this study are common ocular pathogens. S. aureus, is the most common and most frequently isolated pathogen found in patients with infective conjunctivitis.8,11 P. aeruginosa is notoriously resistant to antibiotics,12 and it is commonly found in patients with corneal ulcers13 and contact lens wearers.13–15 The results of these studies represent an encouraging trend in the ongoing search for easily accessible, nonresistant, anti-infective agents.
The antimicrobial activity of SBH has been attributed to several properties of honey, including its osmotic effect and acidity as well as its inclusion of hydrogen peroxide, phytochemical factors, and several tetracycline derivatives.16 Gentamicin is an aminoglycoside that has been shown to be an effective first-line, broad-spectrum antibiotic.17 It is rapidly bactericidal, and it inhibits protein synthesis by combining with mRNA. It is promising to find that gentamicin’s potency is comparable to that of SBH and to know that SBH may serve as an effective substitute in the event of the therapeutic failure of the drug.
The pH of the SBH collected from the International Stingless Bee Center was 3.8. Our results are in the range that has been reported by other studies;18,19 these studies also mentioned that honey is characteristically quite acidic, with its pH being between 3.2 and 4.5. The acidity was found to be caused by gluconolactone or gluconic acid. Acidification has been shown to promote healing by causing oxygen to be released from the body’s hemoglobin.19 The pH of the ocular tear film is 7.4; however, the eye can tolerate eye medications in lower ranges, with slight discomfort.20 This may explain why the animals in the SBH group showed signs of discomfort; they attempted to use their forelegs to wipe the substance off of their eyes. However, with the appropriate standardization of the minimum inhibition concentration, the therapeutic application of honey for eye infections could effectively complement standard antibiotics with minimal irritation or stinging of the eyes.
The cause of death of some of the animals could not be readily ascertained. However, it cannot be attributed to the effect of the SBH, because there were more deaths in the control and gentamicin groups than the SBH group. However, a major limitation of this study was that the lengths of the abrasions made on the conjunctivae of the animals were not equal, and this may have influenced the extent of infection in each animal. Nevertheless, these findings expand the potential therapeutic uses of SBH for eye diseases and provide a basis for further study.
Acknowledgments
The support of Mr David Larbi Simpong of the Department of Medical Laboratory, University of Cape Coast, Ghana, and the staff of the microbiology unit of the Central Regional Hospital, Cape Coast, Ghana, are gratefully acknowledged.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chirambo MC, Tielsch JM, West KP, Katz J. Blindness and visual impairment in South Malawi. Bull World Health Organ. 1986;64:567–572. [PMC free article] [PubMed] [Google Scholar]
- 2.Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century – a clinical super-challenge. N Engl J Med. 2009;360(5):439–443. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Use of antimicrobials outside human medicine and resultant antimicrobial resistance in humans. 2002. [Accessed on March 27, 2012]. Available from: http://www.who.int/mediacentre/factsheets/fs268/en/index.html.
- 4.Temaru E, Shimura S, Amano K, Karasawa T. Antibacterial activity of honey from stingless honeybees (Hymenoptera; Apidae; Meliponinae) Pol J Microbiol. 2007;56(4):281–285. [PubMed] [Google Scholar]
- 5.Garedew A, Schmolz E, Lamprecht I. Microcalorimetric investigation on the antimicrobial activity of honey of the stingless bee Trigona spp. and comparison of some parameters with those obtained with standard methods. Thermochimica Acta. 2004;415(2):99–106. [Google Scholar]
- 6.Muli E, Maingi J, Macharia J. Antimicrobial properties of propolis and honey from the Kenyan stingless bee, Dactylurina Schimidti. Apiacta. 2005;43(4):49–61. [Google Scholar]
- 7.Ilechie A, Kwapong P, Kusi R. Comparative antimicrobial activity of stingless bee honey and standard antibiotics against common eye pathogens. The Optometric Educator, Journal of the Association of Nigeria Optometric Educators. 2010;3(3):1–8. [Google Scholar]
- 8.Al-Waili NS. Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. J Med Food. 2004;7(2):210–222. doi: 10.1089/1096620041224139. [DOI] [PubMed] [Google Scholar]
- 9.Molan P. The antibacterial activity of honey. The nature of the antibacterial activity. Bee World. 1992;73:59–75. [Google Scholar]
- 10.Emarah H. A clinical study of the topical use of bee honey in the treatment of ocular diseases. Bull Islamic Med. 1982;2:422–425. [Google Scholar]
- 11.Leith EC, Harmis NY, Corrigan KM, Wilcox MD. Identification and enumeration of staphylococci from the eye during soft contact lens wear. Optom Vis Sci. 1998;75:258–265. doi: 10.1097/00006324-199804000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Gelender H, Rettich C. Gentamicin-resistant Pseudomonas aeruginosa corneal ulcers. Cornea. 1984;3(1):21–26. [PubMed] [Google Scholar]
- 13.Mayo WLC, Schlitzer RL, Ward MA, Wilson LA, Ahearn DG. Antibiograms, serotypes and plasmid profiles of Pseudomonas aeruginosa associated with corneal ulcers and contact lens wear. J Clin Microbiol. 1986;24(3):372–376. doi: 10.1128/jcm.24.3.372-376.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donzis PB, Mundino BJ, Weissman BA, Bruckner DA. Microbial contamination of contact lens care systems. Am J Ophthalmol. 1987;104:325–333. doi: 10.1016/0002-9394(87)90219-4. [DOI] [PubMed] [Google Scholar]
- 15.Fleiszig SMJ, Efron N. Microbial flora in the eyes of current and former contact lens wearers. J Clin Microbiol. 1992;30:1156–1161. doi: 10.1128/jcm.30.5.1156-1161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molan PC. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol. 2001;2:13–19. doi: 10.2165/00128071-200102010-00003. [DOI] [PubMed] [Google Scholar]
- 17.White JW. Physical characteristics of honey. In: Crane E, editor. Honey, A Comprehensive Survey. London, UK: Heinemann; 1975. pp. 207–239. [Google Scholar]
- 18.Leveen HH, Falk KB, Borek B, et al. Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Ann Surg. 1973;187:745–753. doi: 10.1097/00000658-197312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duvall B, Kershner R. Ophthalmic Medications and Pharmacology. 2nd ed. Thorofare, NJ: Slack Incorporated; 2002. pp. 1–6. [Google Scholar]
- 20.Stein HA, Slatt BJ, Stein RM. The Ophthalmic Assistant: A Guide for Ophthalmic Medical Personnel. 7th ed. New York: Mosby; 2000. p. 59. [Google Scholar]