Abstract
Classically described as a potent inhibitor of the sodium-potassium adenosine triphosphatase enzyme, ouabain has been further shown to act as an effective immunomodulator in mammals. Recently, our group showed that this hormone downregulates membrane CD14 (mCD14) in human monocytes, though it is not known whether monocyte activation status could modify ouabain influence. Hence, we aimed to investigate ouabain effect during monocyte activation in vitro, analyzing mCD14, CD16 and CD69 expression in total monocytes after two periods of adhesion (2 hours and 24 hours) or in small and large monocyte subpopulations separately. Ouabain (100 nM) inhibited monocyte-size increase, characteristic of activation, only when added to cells immediately after 2 hours’ adhesion. Moreover, downregulation of both mCD14 and CD16 expression by ouabain was more effective in small monocytes and in cells after 2 hours’ adhesion. Since monocytes after 24 hours’ adhesion showed no lack of ouabain binding and no CD69 upregulation, it seems that ouabain is somehow incapable of triggering an appropriate cell-signaling induction once monocytes become activated. Furthermore, though p38 MAPK activation was crucial for the impairment in cell-size progression induced by ouabain, its inhibition did not alter ouabain-induced CD69 upregulation, suggesting that other molecules may participate in the response to this hormone by monocytes. Our data suggest that ouabain inhibits monocyte activation in vitro, preventing both cell-size increase and the appearance of the proinflammatory mCD14+/CD16+ subpopulation. Thus, the findings suggest that individuals suffering from disorders commonly associated with high ouabain plasma levels, like hypertension, may present defective monocyte activation under inflammation or infection.
Keywords: ouabain, human monocytes, p38 MAPK, mCD14, CD16, CD69
Introduction
Inflammation is a protective mechanism that defends the host from distinct sources of harmful stimuli, such as pathogens or damaged cells, in order to perform the clearance of the hazard signal and start the healing process. In general, the first line of defense of the host during inflammation involves the action of pattern-recognition molecules present in immune cells, which are responsible for the detection of antigens commonly present in pathogens.1
In this manner, it is known that both infection and sterile conditions are able to promote strong immune responses. Such ability is possible because the immune system does not simply recognize pathogen-associated molecular patterns but also endogenous molecules induced under stress or injury conditions, named damage-associated molecular patterns (DAMPs).2
In general terms, DAMPs are the endogenous equivalents of pathogen-associated molecular patterns and include intracellular molecules released from necrotic cells, heat-shock proteins, uric acid, defensins, and interleukin (IL)-1α, amongst others. However, in both cases, inflammation can be triggered, for instance, via activation of Toll-like receptors (TLRs), leading to the secretion of proinflammatory cytokines and chemokines, thus demonstrating a fundamental relationship between tissue damage, infection, and inflammation.2,3
Amongst the cells involved in this process, the monocyte/macrophage plays a crucial role, performing a broad spectrum of functions. Monocytes are mononuclear cells with a very short life span in the circulation, from approximately 1 to 3 days.4 Once activated, they migrate into target tissues or organs and undergo activation and differentiation, normally into macrophages. They play a key role in the onset, amplification, and termination of the immune response, participating in several features of immune regulation, such as cytokine production and recognition of pathogen-associated molecular patterns,5 the capture, processing, and antigen presentation to T-lymphocytes,6 the clearance of apoptotic cells,7 and also phagocytosis of either bacteria or fungi.8,9
However, peripheral monocytes are not a homogeneous population. About 20 years ago, Ziegler-Heitbrock and colleagues described the existence of two subsets of monocytes in human blood.10 Before that, monocytes were only classified using the expression of mCD14 on their plasma membrane, but since then monocytes have been able to be further categorized based on the expression of mCD14 and CD16, namely the lipopolysaccharide receptor membrane CD14 and the immunoglobulin Fcγ receptor type III, respectively.
The mCD14high/CD16− monocytes were designated classical monocytes, due to their abundance in peripheral blood, accounting for 90%–95% of the total count of monocytes in healthy individuals. The other subpopulation, mCD14+/CD16+, comprises around 5%–10% of the total monocytes in blood and is commonly referred to as “nonclassical” or “proinflammatory,” as a result of the observation that these monocytes secrete high amounts of tumor necrosis factor-α (TNFα) in response to stimulation with TLR2 or TLR4 agonists.11 The subdivision of these two subpopulations is not merely owing to their phenotypes but also to functional differences, as mCD14+/CD16+ monocytes are increased both in acute and chronic infection12 and in inflammatory diseases or sepsis.13,14 Furthermore, proinflammatory monocytes present higher phagocytic and antigen-presenting activities than the classical subpopulation as well.12
Monocyte differentiation is a complex matter and may vary depending on the composition of the surrounding environment. In this manner, monocytes can give rise to M1 or M2 macrophages. Monocyte differentiation into M1 cells is induced by IFNγ but inhibited by IL-10. On the other hand, differentiation into M2 macrophages is induced by IL-10. These macrophages are distinct both phenotypically and functionally, since M1 macrophages are the typical antigen-presenting cells, expressing both MHC class II and B7 molecules for the stimulation of T cells, and M2 macrophages lack those receptors but express CD16, allowing them to destroy antibody-coated target cells or pathogens.15
Besides the regulation of monocyte and macrophage function by immune mediators, these cells are also strongly regulated by hormones. Amongst them, glucocorticoids are usually employed in clinics to treat inflammatory and autoimmune diseases, based on their ability to shut down the inflammatory response. However, the prolonged use of these hormones is associated with several side effects, including osteoporosis and metabolic disease.16
Nevertheless, another hormone, namely ouabain, was also shown to influence the immune system. Ouabain was first described as a cardiac glycoside extracted from plants with the ability of inhibiting the sodium-potassium adenosine triphosphatase enzyme (Na+/K+-ATPase), thus promoting a positive inotropic effect in the cardiac muscle.17 However, 20 years ago, an endogenous analogue of this compound was firstly described in humans18 and later in other mammals,19–21 whose secretion is performed by adrenal glands and the hypothalamus.19,22 Interestingly, several actions of this hormone are not related to the inhibition of Na+/K+-ATPase activity, since the activation of cell-signaling pathways in different cell types, like kidney cells, cardiomyocytes, and retinal ganglion cells, amongst others, has been described. As expected for a multifaceted hormone, the outcomes induced by ouabain are diverse and may include cell proliferation, survival, or even cell death, depending on the concentration and period of administration.23–26
In the immune system, several actions elicited by ouabain have been described,27 like the inhibition of mitogen-induced lymphocyte proliferation and upregulation of the proinflammatory cytokines IL-1 and TNFα in vivo.28,29 Conversely, it has been reported that repeated in vivo treatment with ouabain reduces the inflammation induced by several inflammatory substances.30 Additionally, our group reported that ouabain also induces mitogen-activated cell death in lymphocytes31,32 and calcium mobilization at nanomolar concentrations in thymocytes, a feature that correlated with increased expression of CD69.33,34 CD69 is a transmembrane glycoprotein expressed in several cell types, classically described as the earliest antigen cell-surface molecule upregulated in lymphocytes following activation. Although its ligands remain unknown in monocytes and macrophages, CD69 acts as a potent trigger of monocyte activation, being rapidly upregulated after stimulation with γ-interferon, TNFα, and lipopolysaccharide.35
Recently, we demonstrated that ouabain induces mCD14 downregulation on human monocytes, a facet that relies on the transactivation of the epidermal growth factor receptor and activation of p38 MAPK.36 However, it is not known whether the degree of cell activation could be important for the onset of its effects. That observation is critical, as monocytes undergo activation in culture over time, becoming morphologically indistinguishable from tissue macrophages.37,38
Hence, the aim of the present work was to study whether ouabain may influence in vitro activation/differentiation of human monocytes and if, in turn, monocyte activation status could modulate ouabain effects. For that, we evaluated activation through phenotypical analysis of cell size and the expression of mCD14 and CD16, receptors that confer critical functional skills to monocytes in the recognition, phagocytosis, and pathogen killing.5,7–9,39 We also studied the cellular mechanisms involved in ouabain actions, particularly the binding of this hormone to the cell surface and the induction of cell-signaling pathways, namely p38 MAPK activation and the induction of CD69.
Materials and methods
Obtainment of blood samples and monocyte separation
The ethics committee of the Hospital Universitário Clementino Fraga Filho-UFRJ agreed with the study protocol, which is registered under the approval number 148/09. Blood samples were obtained from buffy coats via a partnership with the Hemotherapy Unit of the Hospital Universitário Clementino Fraga Filho-UFRJ or from peripheral blood samples collected from healthy volunteers using sodium heparin (Roche, Rio de Janeiro, Brazil) as anticoagulant. Peripheral blood mononuclear cells were separated by density gradient, through centrifugation for 30 minutes at 400 g using a Ficoll-Histopaque reagent (GE Healthcare Bio-Sciences, Piscataway, NJ). After separation, mononuclear cells were removed and cells were plated in 24-well plates (Techno Plastic Products, Trasadingen, Switzerland) with culture medium Roswell Park Memorial Institute (RPMI) 1640 (Sigma, St Louis, MO), supplemented with 10% fetal bovine serum (FBS), at a concentration of 2 × 106 cells/mL for 2 hours in a humidified chamber with 5% CO2 atmosphere at 37°C. This procedure was performed in order to allow monocyte adhesion and further separation from lymphocytes, which remained in suspension. To evaluate possible alterations owing to a higher activation state, a prolonged period of 24-hour adhesion was also performed. After those periods, lymphocytes were removed by washing three times with phosphate-buffered saline (PBS) and attached monocytes were incubated again in RPMI medium supplemented with 10% FBS for the analysis in the presence or absence of ouabain.
Incubation with ouabain and p38 MAPK inhibitor
Following adhesion, cells were once more incubated in culture medium with FBS in the presence or absence of 100 nM ouabain, a concentration that was shown to induce a signifcant downregulation in mCD14 expression.36 Moreover, to verify a broader role of p38 MAPK in the modulation induced by ouabain, we made use of the p38 MAPK inhibitor SB202190 (used at 20 μM; Sigma). The experiments were performed with incubation in the presence or absence of ouabain for a period of 24 hours, since short incubations were shown to be ineffective.36
Measurement of mCD14, CD16, and CD69 expression
To assure that only monocytes would be evaluated we employed a gate within the monocyte population, using cell size and complexity or granularity as parameters, as described before.40 For CD16 analysis, only monocytes were evaluated (mCD14-positive cells), as natural killer lymphocytes, a cell type that also expresses CD 16 in the plasma membrane, exhibit cell size comparable with that of monocytes.41
Briefly, after incubation, monocytes were removed using a cell scraper and labeled with human fluorescein isothiocyanate–conjugated anti-CD14 (1:20 dilution factor; eBioscience, San Diego, CA), with human phycoerythrin–conjugated anti-CD 16 (1:15 dilution factor) or human phycoerythrin–conjugated anti-CD69 (1:15 dilution factor), both from BD Pharmingen (San Diego, CA), for 30 minutes at 4°C. Following antibody incubation, cells were washed twice with PBS + FBS 5% solution, resuspended in the same solution and kept in ice until measurement of fluorescence in a flow cytometry apparatus (FACScan, Becton Dickinson, San Diego, CA), equipped with an air-cooled Argon Laser tuned to emit 15 mW at 488 nm.
Determination of large-monocyte subpopulation
Monocytes are known to undergo activation in culture over time, reflecting in an increment in their cell size.37,38 To verify whether in our experimental conditions these cells were indeed undergoing activation induced by adhesion to the substrate, we analyzed the percentage of large monocytes after distinct periods in culture. For that, we made use of the flow cytometry profiles of forward scatter versus side scatter after 2-hour adhesion (always using a gate with mCD14-positive monocytes). Using this moment as a parameter, regions of small and large monocytes were set, and the percentages of large monocytes were evaluated immediately after 2-hour and 24-hour adhesion periods or after 2-hour adhesion followed by maintenance in culture up to 48 hours.
To investigate the effect of ouabain during monocyte activation, the control in the absence of ouabain was used to set the gates of small and large subpopulations. Then, the percentages of large monocytes were determined after the incubation for 24 hours with several ouabain concentrations (1 pM, 1 nM, 10 nM, and 100 nM), comparing the effects in monocytes following 2-hour or 24-hour adhesion.
Measurement of ouabain binding
Flow cytometry was employed to quantify the binding of the fluorescent analogue ouabain-bodipy (Molecular Probes; Life Technologies, Carlsbad, CA). Monocytes were plated on 24-well plates at a concentration of 2 × 106 cells/mL, and the experiments were performed 2 or 24 hours after seeding. Then, the medium containing FBS was removed and a fresh RPMI medium in the absence of FBS was added. Ouabain-bodipy was added in a concentration of 1 µM for 30 minutes, as described elsewhere.42 After that, cells were gently washed with PBS and harvested in PBS solution using cell scraper with the plate on ice. Samples were then kept on ice, and fluorescence was measured by flow cytometry in the FACScan device through a 530-nm-long pass filter. Given that ouabain-bodipy concentration employed was high, we also performed a pretreatment with 100 nM unlabeled ouabain for 30 minutes or 24 hours to verify modulation of the staining induced by Na+/K+-ATPase endocytosis.43
Statistical analysis
Each experiment was repeated at least six times, using different individuals, except for ouabain-bodipy binding, where the experiments were repeated with at least three donors. Flow cytometry analyses were performed using the software Summit 4.3 (Dako, Fort Collins, CO). Data are expressed as means ± standard error of the mean or median and were analyzed using Prism 5.0 software (GraphPad Software, La Jolla, CA) performing Mann–Whitney or paired t-tests for comparison of the differences. Values of P < 0.05 were considered statistically significant.
Results
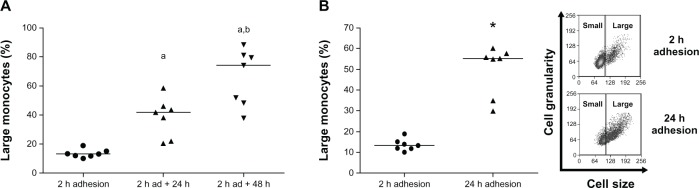
Monocytes are known to undergo activation and differentiation in culture over time in distinct culture conditions.37,38 However, it was important to analyze if the same results would be achieved under our experimental protocol. So we studied monocyte activation by measuring cell size as a function of plating time, using two distinct conditions: after lymphocyte removal following 2-hour adhesion and further maintenance in culture for 24 or 48 hours (Figure 1A); or in the presence of lymphocytes during entire adhesion time, comparing monocytes attached for 2 hours to monocytes after 24-hour adhesion (Figure 1B). Figure 1A shows that after 2-hour adhesion, large monocytes comprised just 15% of the total population. Moreover, after 24 and 48 hours in culture in the absence of lymphocytes, the large-monocyte percentage increased to 40% and 75%, respectively, of the total monocyte population. The increase in monocyte size as a function of time in culture was also observed even in the presence of lymphocytes for 24 hours (Figure 1B), showing that size increase induced by adhesion to the substrate relies on the incubation time, independently of the action of surrounding cells, corroborating the data obtained by other authors.37,38
Figure 1.
(A and B) Percentage of large-monocyte increases in culture. Using 2 hours’ adhesion as a parameter, gates delimiting small- and large-monocyte subpopulations were determined (B, inset), using data obtained from flow cytometric analysis of cell size versus cell granularity, as described in Materials and methods. (A) Comparison of large-cell numbers among monocytes evaluated immediately after 2-hour adhesion and monocytes after 2-hour adhesion, followed by removal of lymphocytes and further maintenance in culture for 24 hours and 48 hours. Values represent the medians of large monocytes in each condition. (B) Comparison of large-cell amount between monocytes after 2-hour and 24-hour adhesion in the presence of lymphocytes. Values represent the medians of large monocytes. Both experiments were performed using seven individuals.
Notes: a,bStatistical difference from 2-hour adhesion or 2-hour adhesion plus 24 hours in culture, respectively (aP < 0.001; bP < 0.05, Mann–Whitney test); *statistical difference from 2-hour adhesion (P < 0.001, Mann–Whitney test).
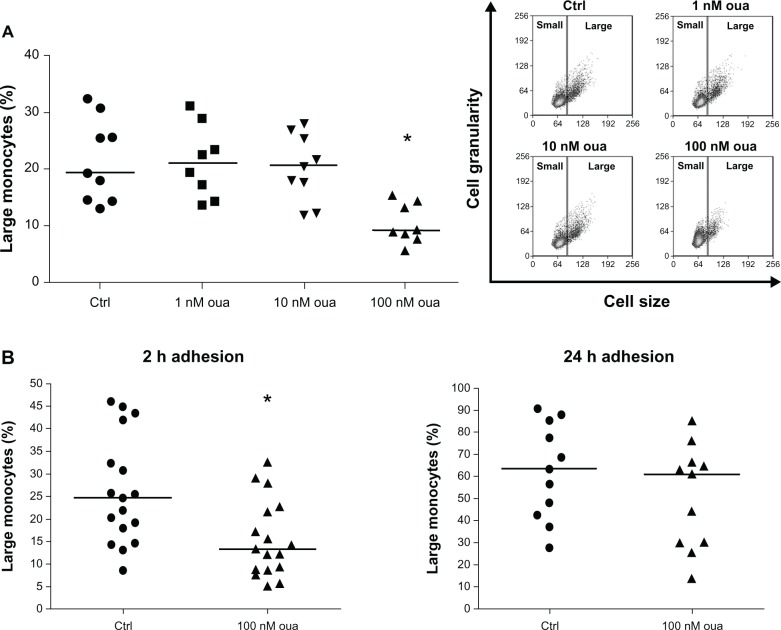
Our next goal was to investigate if ouabain alters monocyte activation. Using the standard protocol of adhesion (2 hours), the influence of physiological (1 nM and 10 nM) and pharmacological (100 nM) concentrations of ouabain on the number of large monocytes after incubation for 24 hours was evaluated. Only 100-nM ouabain promoted a significant difference, almost a 60% decrease in the median of large-monocyte percentage, when compared to the control (Figure 2A). Moreover, in some experiments we also employed a concentration of 1 pM ouabain, but no alteration was detected (data not shown).
Figure 2.
(A and B) Ouabain (Oua) inhibits the appearance of large monocytes in culture. (A) After 2-hour adhesion, cultures containing peripheral blood mononuclear cells were washed for lymphocyte removal, and monocytes were incubated in the presence or absence of several Oua concentrations for a further 24 hours. Next, the number of large monocytes was determined in each condition, using the control (Ctrl) as a parameter to create gates delimiting small- and large-monocyte subpopulations. inset portrays a representative experiment showing the dot plot of cell size versus cell granularity obtained by flow cytometry. (B) Monocytes were allowed to attach to the substrate for either 2 hours or 24 hours. Then, cultures were washed for lymphocyte removal, and monocytes were incubated for a further 24 hours in the presence or absence of 100 nM Oua. Analysis of large-monocyte percentage was similar to that of Figure 2A.
Notes: Values in A and B refer to the medians of large monocytes; *statistical difference from the respective Ctrl (P < 0.01, Mann–Whitney test).
Considering that monocytes after 2-hour and 24-hour adhesion present a remarkable difference in the amount of large monocytes, thus denoting alterations in the cellular activation status, the question of whether ouabain could produce the same effects on cell-size increment in both conditions was addressed. For that, experiments were performed to compare the percentage of large monocytes in cultures after two periods of adhesion: 2 and 24 hours. Then, following adhesion, lymphocytes were removed, and monocytes were incubated for further 24 hours in the presence or absence of 100 nM ouabain. As shown in Figure 2B (left panel), ouabain promoted a significant decrease in the number of large monocytes only in cultures following 2-hour adhesion, as the median of large-monocyte percentage was nearly 45% lower than that seen in control. On the other hand, no significant changes in the median of large monocytes were observed when ouabain was given to monocytes after 24-hour adhesion (Figure 2B, right panel). This finding suggests that monocytes with a higher degree of activation are less susceptible to ouabain influence. Moreover, as cultures immediately evaluated after 2-hour adhesion presented nearly 15% of large monocytes (Figure 1), the decreased amount of large cells seen after incubation with ouabain may be interpreted as an impairment of the normal monocyte-activation process.
Besides phenotypical peculiarities, monocyte subpopulations can also be distinguished by means of expression of functional surface receptors, especially mCD14 and CD16.11 These receptors are important for proper monocyte response and activation, since they are involved in the recognition and response against distinct pathogens, including bacteria, fungi, and antibody-coated particles, culminating in pathogen phagocytosis and killing.8,9,39
To test whether these molecules could be differentially modulated by ouabain, depending on the degree of monocyte activation, we also performed a comparison between monocytes attached to the substrate for 2 and 24 hours, followed by incubation with 100 nM ouabain for a further 24 hours, given that only this concentration induced a significant inhibition in the appearance of large monocytes in vitro.
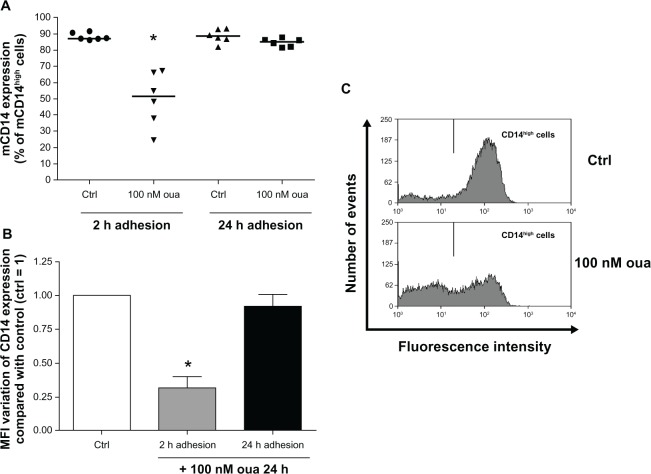
The evaluation of mCD14 expression was performed via measurement of mCD14high cells, denoting cells with high expression of this protein, separated by gates delimiting the peak of flow cytometry histograms obtained in control monocytes (Figure 3C, upper panel). Figure 3A shows that after 2-hour or 24-hour adhesion, the vast majority of monocytes (nearly 90%) were mCD14high. However, it can be seen that treatment with 100 nM ouabain induced a remarkable decline in mCD14 expression in monocytes attached for only 2 hours prior to ouabain treatment. This effect was verified both in the percentage of mCD14high cells (Figure 3A and C), where a 40% decrease was detected, and also in the mean of fluorescence intensity (MFI) of mCD14 expression, whose decline in the presence of ouabain was around 70% (Figure 3B). Although mCD14 downregulation was also observed in a previous work from our group using the same experimental protocol,36 the observation that cells allowed to attach to the substrate for 24 hours do not present any alteration in mCD14 levels induced by ouabain was surprising. Noteworthy was the fact that both protocol conditions differed only in the period of attachment to the substrate (2 or 24 hours) prior to the incubation with ouabain. So such disparity might be explained by the fact that monocytes after 24-hour adhesion display morphological changes indicating a higher degree of activation, when compared to cells attached for only 2 hours.
Figure 3.
(A–C) Ouabain (Oua) modulates mCD14 expression only in monocytes after 2-hour adhesion. Monocytes were allowed to attach to the substrate for either 2 hours or 24 hours. After that, cultures were washed for lymphocyte removal, and monocytes were then incubated with 100 nM Oua for a further 24 hours or left untreated. Next, monocytes were incubated with antihuman CD14, as described in Materials and methods. (A) Medians of mCD14high cell percentages, denoting cells with a high expression of mCD14 in their surface; *difference from the respective control (Ctrl) (P < 0.01, Mann–Whitney test). (B) Variation of the means of fluorescence intensities (MFI) of mCD14 expression from monocytes incubated with Oua after 2-hour or 24-hour adhesion, compared with the Ctrl (set as 1); *statistically different from Ctrl (P < 0.0005, paired t-test). (C) Representative experiment showing the decrease in mCD14high cells (gate located in the right) after treatment with Oua. These experiments were performed using six individuals.
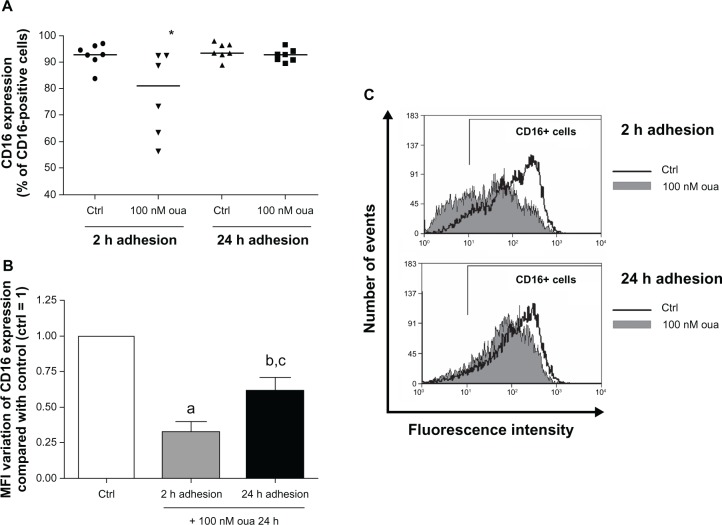
Next, the same comparison was performed to analyze the modulation of CD16 expression by ouabain, particularly in the population mCD14+, in order to ensure that only monocytes would be evaluated. Similarly to that seen for mCD14 analysis, CD16 expression was more efficiently modulated by ouabain in monocytes attached for only 2 hours to the substrate (Figure 4A). In this case, the median of CD16+ monocytes was about 13% lower in cells treated with ouabain when compared to the control. However, no significant difference was detected in monocytes after 24-hour adhesion (Figure 4A and C). Conversely, it was observed that ouabain induced a decrease in the MFI of CD16 expression even in monocytes treated with ouabain after the prolonged adhesion, approximately 40% lower than the control. Nevertheless, cells attached for only 2 hours exhibited a much higher variation, with a 67% decrease in the MFI values (Figure 4B).
Figure 4.
(A–C) Ouabain (Oua) modulates CD16+ cell percentage only in monocytes after 2-hour adhesion. Monocytes were allowed to attach to the substrate either for 2 hours or 24 hours. After that, cultures were washed for lymphocyte removal, and monocytes were then incubated with 100 nM Oua for a further 24 hours or left untreated. After that, monocytes were incubated with antihuman CD16, as described in Materials and methods. (A) Medians of CD16+ cell percentages; *statistical difference from the control (Ctrl) (P < 0.05, Mann–Whitney test). (B) Variation in the means of fluorescence intensities (MFI) of CD16 expression from monocytes incubated with Oua after 2-hour or 24-hour adhesion, compared with the control (set as 1); a,bdifferences from the Ctrl (P < 0.001 and P < 0.01, respectively, paired t-test); cstatistical difference from 2-hour adhesion (P < 0.05, paired t-test). (C) Representative experiment showing CD16 fluorescence histograms obtained by flow cytometry. Black curves indicate control staining, and gray-filled histograms designate monocytes treated with 100 nM Oua for 24 hours after 2-hour adhesion (upper panel) or 24-hour adhesion (lower panel). These experiments were performed using six individuals.
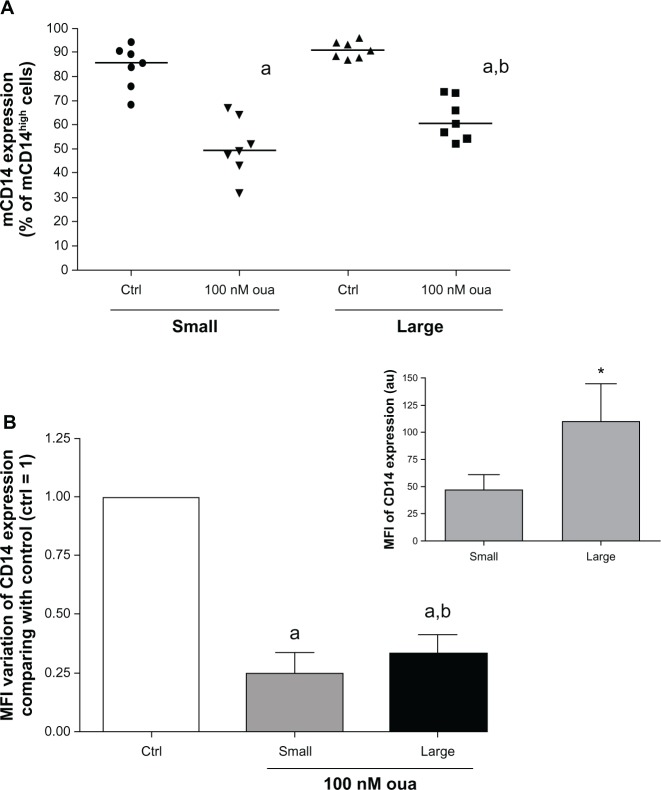
As mentioned before, the results shown in Figure 1 demonstrate that in our experimental protocol, human monocytes become larger after 24-hour adhesion, indicating that after this period monocytes are indeed more activated. Therefore, it was important to assess whether small- and large-monocyte subpopulations display different degrees of susceptibility to this glycoside. For that, the expression of both mCD14 and CD16 was analyzed in monocytes after 2-hour adhesion, evaluating these two monocyte subpopulations separately.
Examining firstly the mCD14high cell percentage, it can be seen that small and large monocytes both showed a decrease in the number of mCD14high cells when exposed to ouabain (Figure 5A). However, the mCD14high cell median decrease was slightly but significantly minor in large monocytes (approximately 10%, when compared to monocytes after 2-hour adhesion). This feature might possibly occur owing to the elevated mCD14 expression levels observed in these cells under control conditions, roughly twofold higher than seen in small monocytes (Figure 5B, inset). In addition to mCD14high cell percentage, small and large subpopulations also presented a similar pattern of modulation in mCD14 MFI induced by ouabain treatment (Figure 5B).
Figure 5.
(A and B) Ouabain (Oua) modulation of mCD14 expression in small and large monocytes. Monocytes were maintained for adhesion in culture for 2 hours, and mCD14 expression was evaluated as described in Figure 3, analyzing small and large subpopulations separately. (A) Medians of mCD14high cell percentages in small and large monocytes; astatistical difference from control (Ctrl) (P < 0.001, paired t-test); bstatistical difference from small monocytes treated with Oua (P < 0.01, paired t-test). (B) Variation in the means of fluorescence intensities (MFI) of mCD14 expression from small and large monocytes incubated with Oua, compared with the Ctrl (set as 1); astatistical difference from Ctrl (P < 0.001, paired t-test); bstatistical difference from small monocytes treated with Oua (P < 0.05, paired t-test). inset: MFI values of mCD14 expression in small and large monocytes; *statistically different (P < 0.05, paired t-test). These experiments were performed using seven individuals.
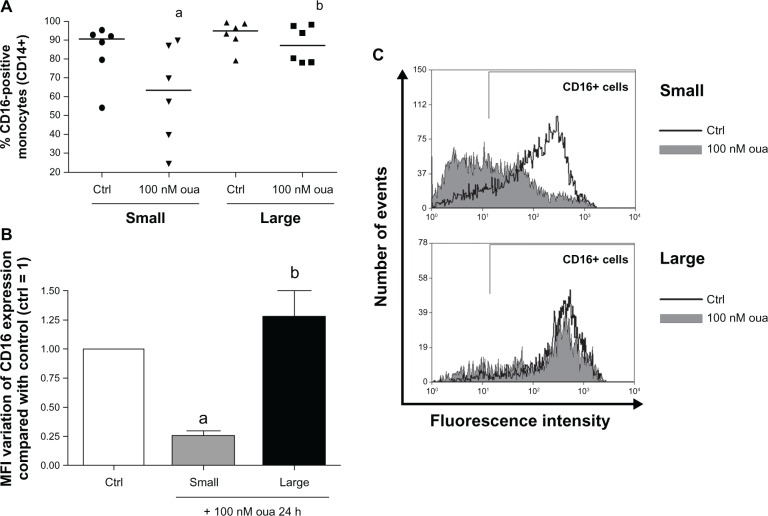
Evaluating CD16 expression after ouabain treatment in these two groups, it is apparent that small monocytes are highly more susceptible than the large subpopulation. The median of CD16+ monocyte percentage in small cells is about 30% lower in the presence of ouabain, but no significant alteration was observed in the large-size subpopulation (Figure 6A and C). This scenario was similarly seen when evaluating the MFI of CD16 expression in monocytes treated with this hormone (Figure 6B), where small monocytes showed a loss in CD16 expression of approximately 75%, but the expression of this receptor in large cells was not statistically different from the control.
Figure 6.
(A–C) Ouabain (Oua) differentially modulates CD16 expression in small and large monocytes. Monocytes were maintained for adhesion in culture for 2 hours, and CD16 expression was evaluated as described in Figure 4, analyzing small and large subpopulations separately. (A) Medians of CD16-positive cell percentages in small and large monocytes; astatistical difference from control (Ctrl) (P < 0.05, paired t-test); bstatistical difference from small monocytes treated with Oua (P < 0.05, paired t-test). (B) Variation in the means of fluorescence intensities (MFI) of CD16 expression from small and large monocytes incubated with Oua, compared with the Ctrl (set as 1); astatistical difference from Ctrl (P < 0.05, paired t-test); bstatistical difference from small monocytes treated with Oua (P < 0.01, paired t-test). (C) Representative experiment showing CD16 fluorescence histograms obtained by flow cytometry. Black curves indicate control staining, and gray-filled histograms designate treatment with 100 nM Oua for 24 hours in small monocytes (upper panel) and large monocytes (lower panel). These experiments were performed using six individuals.
The data obtained so far suggest that activated monocytes present some degree of resistance to ouabain effects, at least in terms of mCD14 and CD16 modulation. Supporting this idea, it is known that increased CD16 expression is associated with a higher degree of monocyte response, either in phagocytosis or antigen presentation.12 Interestingly, in our experimental protocol, monocytes evaluated immediately after 2-hour adhesion presented CD16 levels 47 times lower than monocytes after 24-hour adhesion. Moreover, only 45% of monocytes were CD16+ promptly after 2-hour adhesion, contrasting with a vast proportion seen after 24-hour adhesion (90% of monocytes; data not shown).
Thus, two major hypotheses could explain this decreased response in either large monocytes and/or monocytes attached to the substrate for 24 hours: (1) activated monocytes might exhibit a decrease in ouabain-binding sites on their surface or (2) ouabain is unable to trigger cell-signaling events once monocytes become activated.
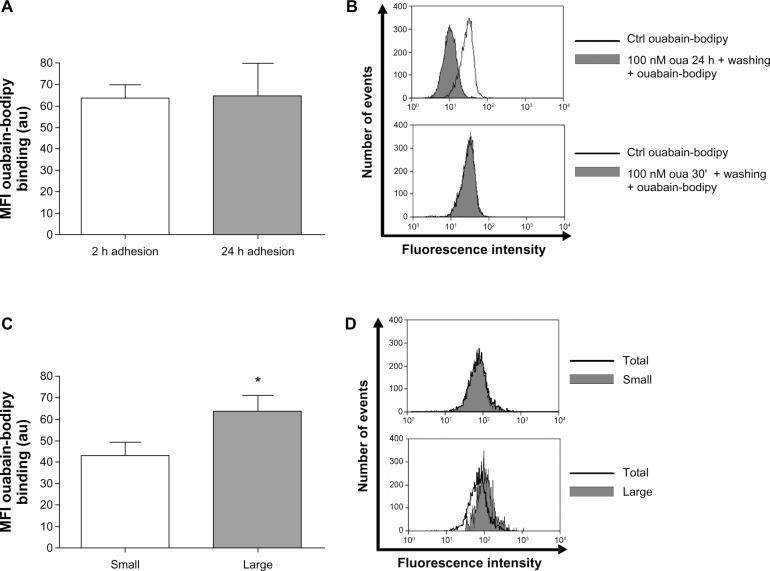
To test the first conjecture, the fluorescent analogue ouabain-bodipy was used. This probe is useful to measure ouabain binding to its receptor, which is the Na+/K+-ATPase alpha subunit.42 If the first assumption were correct, activated monocytes would display minor ouabain-bodipy fluorescence in their plasma membrane, compared to nonactivated cells. However, monocytes after 2-hour or 24-hour adhesion presented the same fluorescence levels for this compound (Figure 7A). Furthermore, evaluating small and large monocytes after 2-hour adhesion, it is apparent that large monocytes present even higher levels of ouabain-bodipy binding (Figure 7C and D), refuting the idea that these cells do not respond properly to ouabain owing to a decrease in their Na+/K+-ATPase content.
Figure 7.
(A–D) Large monocytes do not lack ouabain (Oua)-binding sites. Monocytes were incubated with 1 µM Oua-bodipy for 30 minutes in Roswell Park Memorial institute medium, as described in Materials and methods. Values refer to the means of fluorescence intensities of Oua-bodipy labeling in (A) monocytes after 2-hour and 24-hour adhesion, and (C) small and large monocytes after 2-hour adhesion; *statistical difference (P < 0.05, paired t-test). (B and D) Representative experiments showing Oua-bodipy fluorescence histograms obtained by flow cytometry. (B) Black curves indicate control (Ctrl) Oua-bodipy staining in total monocyte population, and gray-filled histograms indicate monocytes pretreated with 100 nM unlabeled Oua for 24 hours or 30 minutes, followed by washing and further staining with Oua-bodipy. (D) Black curves indicate total population binding levels, and gray-filled histograms designate fluorescence levels in small monocytes (upper panel) and large monocytes (lower panel). These experiments were performed at least three times.
Though the concentration of ouabain-bodipy used in the assay was high (1 µM) and could possibly provoke unspecific binding, lower concentrations did not allow fluorescence measurement. Nevertheless, in order to verify the specificity of the method, we performed experiments pretreating monocytes with 100 nM unlabeled ouabain for 24 hours or 30 minutes prior to ouabain-bodipy staining. Only pretreatment with ouabain for 24 hours induced a significant decline in ouabain-bodipy fluorescence in human monocytes, when compared to control cells (Figure 7B). Such a result is not surprising, as prolonged incubation with ouabain is known to induce Na+/K+-ATPase endocytosis,43 but that is an important control to assure that the staining is not related to nonspecific ouabain-bodipy binding to the cell surface.
Our group has previously shown that ouabain increases CD69 expression both in thymocytes and in peripheral blood lymphocytes.33,44 This protein seems to be the earliest inducible cell-surface glycoprotein acquired during lymphoid activation and functions as a signal-transmitting receptor in lymphocytes.45 Although CD69 expression is induced in vitro in cells of most hematopoietic lineages, its constitutive expression has been described in monocytes.46
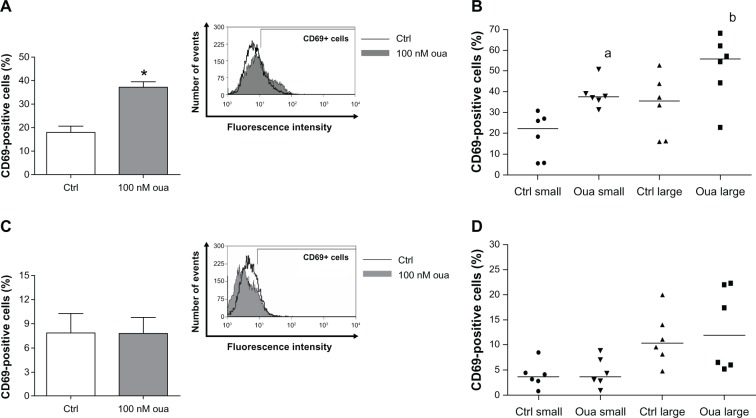
To check the second hypothesis, ie, whether ouabain is unable to trigger cell-signaling responses in large monocytes, the expression of CD69 was assessed in human monocytes, comparing cells after 2-hour and 24-hour adhesion as well as small and large monocytes after 2-hour adhesion. As depicted in Figure 8A, incubation with ouabain induced a twofold increase in the percentage of CD69+ monocytes after 2-hour adhesion. Interestingly, this effect was observed even when analyzing small- and large-monocyte subpopulations separately. The medians of CD69+ monocytes incubated with ouabain were approximately 70% and 60% higher, for small and large monocytes, respectively (Figure 8B). However, ouabain did not exert any effect on CD69 expression in monocytes after 24-hour adhesion (Figure 8C and D).
Figure 8.
(A–D) Ouabain fails to induce CD69 expression in monocytes after 24-hour adhesion. Monocytes were maintained for adhesion in culture for 2 hours (A and B) or 24 hours (C and D), and then incubated with 100 nM Oua for 24 hours or left untreated. After that, monocytes were incubated with antihuman CD69, as described in Materials and methods.
Notes: (A and C) Means of CD69-positive cells in total population; *statistical difference from control (Ctrl) (P < 0.001, Mann–Whitney test). (B and D) Medians of CD69-positive cells in small and large monocytes; a,bstatistical difference from small and large monocyte Ctrl, respectively (aP < 0.01 and bP < 0.05, Mann–Whitney test). These experiments were performed using six individuals.
Despite the fact that CD69 is well known as a receptor engaged in lymphocyte activation,45 the data obtained in Figure 8 indicate that the number of monocytes expressing this receptor declines about 50% in monocytes after 24-hour adhesion + 24 hours in culture when compared to monocytes after 2-hour adhesion + 24 hours in culture. Thus, this result suggests that CD69 might be transiently expressed in human monocytes in vitro.
In a previous report, our group revealed that p38 MAPK had a central role in the modulation of mCD14 expression induced by ouabain.36 After the observation in the present work that ouabain induces a broader effect in monocytes, preventing the activation induced by adhesion, it was important to verify whether this kinase could play a role in this ouabain facet.
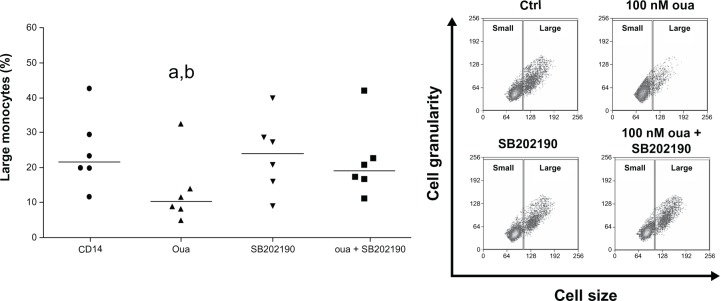
As seen in Figure 9, though 100 nM ouabain significantly reduced the median of large-monocyte percentage in culture (from 21.71% in the control to 10.78% in the presence of ouabain), incubation with the p38 MAPK inhibitor SB202190 significantly reverted this effect (medians: SB202190 = 24.03 and SB202190 + ouabain = 19.15).
Figure 9.
Role of p38MAPK inhibition in the modulation of monocyte cell-size progression induced by ouabain (Oua).
Notes: Monocytes were maintained for adhesion in culture for 2 hours and then incubated with 100 nM Oua for 24 hours, in the presence or absence of the p38MAPK inhibitor SB202190 (used at 20 µM). Values refer to the medians of large monocytes in each condition (evaluating only CD14+ cells); astatistical difference from control (P < 0.01, paired t-test); bstatistical difference from Oua + SB202190 (P < 0.05, paired t-test). Inset: Representative experiment showing monocyte cell-size and cell-granularity profiles obtained by flow cytometry, where gates delimiting small and large subpopulations are depicted, as well as both Oua and SB202190 effects on large-monocyte subpopulation number. These experiments were performed using six individuals.
Thus, our findings suggest that p38 MAPK might have a central role in the effects triggered by ouabain in monocytes, also controlling the effects of this hormone in cell-size increase. In order to confirm this hypothesis, we tested whether p38 MAPK function could also be important for the ouabain-induced CD69 upregulation in human monocytes, given that the pattern of modulation observed for this receptor is the opposite of that observed for both mCD14 and CD16. Therefore, monocytes after 2-hour adhesion were incubated with 100 nM ouabain in the presence or absence of the p38 MAPK inhibitor, and the expression of CD69 was subsequently assessed. As described in Table 1, p38 MAPK inhibition did not alter CD69 induction triggered by ouabain in monocytes, refuting the theory that p38 MAPK could act in regulating all effects triggered by ouabain in human monocytes.
Table 1.
Effect of the p38 MAPK inhibitor SB202190 on ouabain-induced CD69 upregulation in human monocytes
| Treatment | CD69+ monocytes (means ± SEM) |
|---|---|
| Control | 17.51 ± 3.04 |
| Ouabain 100 nM | 41.19 ± 7.58* |
| SB202190 | 23.19 ± 7.40 |
| SB202190 + ouabain 100 nM | 38.80 ± 7.79* |
Notes: Monocytes were maintained for adhesion in culture for 2 hours and then incubated with 100 nM ouabain for 24 hours, in the presence or absence of the p38 MAPK inhibitor SB202190 (used at 20 µM). Values refer to the percentage of large monocytes in each condition ± standard error of mean (SEM);
statistical difference from the respective controls (P < 0.05, paired t-test). These experiments were performed using six individuals.
Discussion
The modulation of human monocytes by ouabain is a complex matter and involves the activation of several signaling pathways, varying with each cell type. In our experimental protocol, the concentration of 100 nM ouabain was relatively higher than the physiological range for this hormone in human plasma, from approximately 100 pM to nanomolar concentrations, depending on the experimental conditions and possibly on the individuals tested.18,47 However, contrasting with several observations from other authors, which made use of excessively high quantities of this hormone when studying the regulation of cardiac hypertrophy and renal cell toxicity induced by ouabain,23–25 the concentration used in the present study was observed in the plasma of individuals suffering from cardiac dysfunction treated with ouabain or even under acute stress conditions.47,48 Moreover, taking into account that adrenocorticotropic hormone stimulation induces the secretion of both corticosterone/cortisol and ouabain by adrenal glands,49,50 it has been suggested that ouabain also acts as a stress hormone,51 whose secretion is likely to be above its physiological range in any type of situation where plasma cortisol levels are elevated.
Though monocytes seemed to be robustly modulated by ouabain in terms of mCD14 expression,36 the present data support the idea that ouabain influence may fluctuate according to the extent of cellular activation, as cells after 2-hour and 24-hour adhesion are distinctly regulated. Interestingly, both mCD14 and CD16 downregulation induced by ouabain were significantly more pronounced in cells after short periods of adhesion (2 hours). At this time, monocytes were clearly less activated, reflected in the low number of large monocytes, when compared to cells after the prolonged adhesion of 24 hours.
Furthermore, our data showed that CD16 modulation induced by ouabain was considerably higher in the small-monocyte subpopulation. Taking into account that the amount of CD16+ monocytes increases in culture, our results suggest that ouabain prevents the appearance of the mCD14+/CD16+ subpopulation in vitro. However, it is not known so far whether in vivo administration of ouabain could induce a similar effect, preventing the increase of mCD14+/CD16+ monocytes in either infection or inflammatory diseases. That is an important issue, as the rise of the mCD14+/CD16+ subpopulation is related to the aggravation of several immune disorders, such as inflammatory arthritis or sepsis, by means of the secretion of proinflammatory cytokines.12–14 Nevertheless, there is growing evidence in the literature suggesting that ouabain is able to inhibit the secretion of the proinflammatory cytokines TNFα, IL-1β, and IL-6 induced by lipopolysaccharide in vitro and even to protect mice against lipopolysaccharide-induced lethal endotoxemia and inflammation induced by zymosan,29,30 thus supporting the hypothesis that the mCD14+/CD16+ subpopulation may be modulated by ouabain even in vivo. Additionally, it is not known whether ouabain treatment in vivo could evoke preferential differentiation towards M1-type macrophages, which lack CD16 in their surface and play an important role in antigen presentation and facilitating the onset of Th1 cell response.
Additionally, we found that ouabain action was not merely restricted to the modulation of cell-surface molecules. Analyzing the percentage of large monocytes after 24-hour incubation with 100 nM ouabain (Figure 2A and B), it was clear that this subpopulation is almost 50% lower in the presence of ouabain than that observed in the control. This finding raised the possibility that this hormone could modulate monocyte size due to impairment of Na+/K+-ATPase ion transport, which could result in cell shrinking, as seen in other experimental models.52,53
Nevertheless, some important observations reject this proposition. First, the studies observing cell shrinkage induced by Na+/K+-ATPase inhibition were performed with ouabain concentrations far higher than that employed in the present work, ranging from 50 to 100 micromolars.52,53 Moreover, prolonged stimulation with high ouabain concentrations was shown to be associated with cell death, but viability assay using annexin V and propidium iodide, which stain cells undergoing apoptosis or necrosis, respectively, indicated the opposite in our experimental protocol. In this manner, incubation with 100 nM ouabain led to only a slight increase in the amount of cells stained with annexin V (nearly 7%) in monocytes after 2-hour and 24-hour adhesion (data not shown), corroborating previous results.36
Second, the number of large monocytes after incubation with ouabain for 24 hours (Figure 2) is almost the same as that seen in monocytes immediately observed after 2-hour adhesion (Figure 1), indicating that this hormone is indeed preventing cell-size progression stimulated by adhesion to the substrate, instead of inducing cell shrinkage. Furthermore, flow cytometry analyses of size versus granularity patterns did not reveal any significant alteration in the size of small monocytes treated with ouabain (Figure 2A, inset). Finally, the observation in Figure 9 that the p38 MAPK inhibitor practically reverted the ouabain effect on cell size reinforces the idea that this facet has no association with ion-transport inhibition.
The association between monocyte size and activation/differentiation process in vitro has long been known. Even under distinct experimental conditions, monocytes may undergo activation by adhesion to the substrate and differentiate into macrophages.37,38 In this fashion, the more monocytes are able to attach to a certain surface, the faster the activation/differentiation process is. To exemplify this relationship, Hsu et al54 investigated the ability of human monocytes to attach to distinct polyester-type surfaces covered with different amounts of gold nanoparticles or left untreated. They observed that the presence of activated monocytes displaying large size and spread morphology was significantly higher in the untreated polyester-type surface after 96 hours in culture. Moreover, the subcutaneous implantation of this material in rats also induced a higher formation of a fibrous capsule after 19 days, when compared to the gold-covered surfaces. Thus, the authors confirmed that the size of the fibrous capsule in vivo was consistent with the degree of monocyte activation/differentiation in vitro, demonstrating unequivocally the close relationship between monocyte size and activation induced by adhesion.54
The observation that p38 MAPK inhibition influences both ouabain-induced downregulation of mCD1436 and ouabain-induced cell size–progression restraint in culture, seen in the present work, raised the idea that p38 MAPK might play a central role in the cell-signaling events triggered by this hormone in monocytes. However, as described in Table 1, p38 MAPK inhibition showed no effect on the regulation of CD69 expression by ouabain, refuting the notion that all ouabain effects in monocytes may be a result of p38 MAPK function.
These data reinforce the idea that the response of human monocytes to ouabain relies on the activation of several signaling pathways, which may act together to evoke a broad modification in the phenotype, morphology, and possibly function of monocytes. Corroborating this assumption, several authors have postulated that other important signaling events are also induced by ouabain, like ERK1/2 activation and reactive oxygen species generation,24 tyrosine-kinase receptors and PKC activation,26 cytosolic calcium mobilization,33,34 production and secretion of the proinflammatory cytokines TNFα and IL-1β,29 amongst others. Hence, it is likely that one or more of those molecules might be involved in CD69 upregulation induced by ouabain.
Aiming to understand why activated cells failed to respond to ouabain, we firstly analyzed whether those cells presented fewer binding sites for this hormone, namely the Na+/K+-ATPase enzyme. However, the binding of the fluorescent analogue ouabain-bodipy was similar in monocytes after either 2-hour or 24-hour adhesion. Additionally, large cells after 2-hour adhesion exhibited a slight increase in ouabain binding compared to small monocytes, demonstrating that activated monocytes are not less responsive due to a decline in ouabain-binding sites. The reason for the rise in ouabain-binding sites observed in large monocytes is not known at the moment. However, it is possible that large cells may require more copies of Na+/K+-ATPase in order to maintain a proper ion homeostasis or that, in turn, activated cells might express another Na+/K+-ATPase alpha-subunit presenting higher affinity for ouabain.
The observation that altered Na+/K+-ATPase activity and/or content in the plasma membrane is/are not the cause for the lack of response to ouabain seen in activated cells are not surprising, because it is well known that several actions of this hormone are intrinsically related to the activation of cell-signaling molecules.23–26 Corroborating this assumption, CD69 expression was only induced by ouabain in monocytes after 2-hour adhesion, although this result was obtained both in small and large monocytes. So these data suggest that monocytes after 24-hour adhesion fail to respond to ouabain owing to an impediment on the activation of one or more cell-signaling pathways triggered by this hormone.
Our data show, for the first time, that similarly to that observed in lymphocytes, ouabain also induces CD69 upregulation in human monocytes. Though CD69 function is still unknown in monocytes, this molecule acts as a signal-transmitting receptor in lymphocytes, being the earliest cellular glycoprotein acquired under activation.45 However, given that control monocytes after 24-hour adhesion plus 24 hours in culture displayed half the percentage of CD69+ cells of that seen in control monocytes after 2-hour adhesion plus 24 hours in culture, it seems that CD69 expression in monocytes is transitory in vitro. Therefore, CD69 expression levels could not be effectively related to monocyte-activation status.
The results obtained in the present work show that cell size is not the only attribute for the evaluation of whether monocytes are ouabain-sensitive or not. It is clear that the period of adhesion to the substrate is crucial, probably requiring alterations in gene-expression patterns. Though alterations in total protein expression by ouabain were not assessed in the present study, the differential expression induced by ouabain of CD16 and CD69 in monocytes after 2-hour and 24-hour adhesion confirms this assumption. Moreover, CD69 upregulation induced by ouabain clearly indicates that monocytes chronically treated with this hormone do not become unresponsive, but probably perform different physiological roles.
In conclusion, adhesion of monocytes to endothelial cells and their subsequent migration into tissues is one of the earliest changes detectable during immune responses.55 So as ouabain preferentially modulates monocytes displaying a phenotype comparable to that found in circulation, our results suggest that this hormone could act in preventing the exacerbation of inflammatory responses either in inflammatory diseases or infection. Alternatively, it is likely that individuals presenting one of the pathophysiological conditions where ouabain plasma levels are constantly elevated, like chronic stress or essential hypertension, may possibly exhibit deficient monocyte activation.
Acknowledgments
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Conselho Nacional de Desenvolvimento Científco e Tecnológico (CNPq). The authors are indebted to the Hemotherapy Unit from Hospital Universitário Clementino Fraga Filho-UFRJ for the kind donation of buffy coats used in the present study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147(2):227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich S. Monocyte CD14: a multifunctional receptor engaged in apoptosis from both sides. J Leukoc Biol. 1999;65(6):737–743. doi: 10.1002/jlb.65.6.737. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85(2):85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 6.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203(3):583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory CD. CD14-dependent clearance of apoptotic cells: relevance to the immune system. Curr Opin Immunol. 2000;12(1):27–34. doi: 10.1016/s0952-7915(99)00047-3. [DOI] [PubMed] [Google Scholar]
- 8.Schiff DE, Kline L, Soldau K, et al. Phagocytosis of gram-negative bacteria by a unique CD14-dependent mechanism. J Leukoc Biol. 1997;62(6):786–794. doi: 10.1002/jlb.62.6.786. [DOI] [PubMed] [Google Scholar]
- 9.Netea MG, Gijzen K, Coolen N, et al. Human dendritic cells are less potent at killing Candida albicans than both monocytes and macrophages. Microbes Infect. 2004;6(11):985–989. doi: 10.1016/j.micinf.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler-Heitbrock HW, Passlick B, Flieger D. The monoclonal antimonocyte antibody My4 stains B lymphocytes and two distinct monocyte subsets in human peripheral blood. Hybridoma. 1988;7(6):521–527. doi: 10.1089/hyb.1988.7.521. [DOI] [PubMed] [Google Scholar]
- 11.Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82(2):244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- 12.Nockher WA, Scherberich JE. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun. 1998;66(6):2782–2790. doi: 10.1128/iai.66.6.2782-2790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu YG, Shao T, Feng C, et al. CD16 (FcRgammaIII) as a potential marker of osteoclast precursors in psoriatic arthritis. Arthritis Res Ther. 2010;12(1):R14. doi: 10.1186/ar2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82(10):3170–3176. [PubMed] [Google Scholar]
- 15.Kummerle-Deschner JB, Hoffmann MK, Niethammer D, Dannecker GE. Pediatric rheumatology: autoimmune mechanisms and therapeutic strategies. Immunol Today. 1998;19(6):250–253. doi: 10.1016/s0167-5699(98)01263-8. [DOI] [PubMed] [Google Scholar]
- 16.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haustein KO. Digitalis. Pharmacol Ther. 1982;18(1):1–89. doi: 10.1016/0163-7258(82)90026-2. [DOI] [PubMed] [Google Scholar]
- 18.Hamlyn JM, Blaustein MP, Bova S, et al. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991;88(14):6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludens JH, Clark MA, Robinson FG, DuCharme DW. Rat adrenal cortex is a source of a circulating ouabainlike compound. Hypertension. 1992;19(6 Pt 2):721–724. doi: 10.1161/01.hyp.19.6.721. [DOI] [PubMed] [Google Scholar]
- 20.Tymiak AA, Norman JA, Bolgar M, et al. Physicochemical characterization of a ouabain isomer isolated from bovine hypothalamus. Proc Natl Acad Sci U S A. 1993;90(17):8189–8193. doi: 10.1073/pnas.90.17.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrandi M, Manunta P, Balzan S, Hamlyn JM, Bianchi G, Ferrari P. Ouabain-like factor quantification in mammalian tissues and plasma: comparison of two independent assays. Hypertension. 1997;30(4):886–896. doi: 10.1161/01.hyp.30.4.886. [DOI] [PubMed] [Google Scholar]
- 22.Yamada K, Goto A, Omata M. Modulation of the levels of ouabain-like compound by central catecholamine neurons in rats. FEBS Lett. 1995;360(1):67–69. doi: 10.1016/0014-5793(95)00078-n. [DOI] [PubMed] [Google Scholar]
- 23.Contreras RG, Shoshani L, Flores-Maldonado C, Lazaro A, Cereijido M. Relationship between Na(+),K(+)-ATPase and cell attachment. J Cell Sci. 1999;112(Pt 23):4223–4232. doi: 10.1242/jcs.112.23.4223. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275(36):27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 25.Valente RC, Capella LS, Monteiro RQ, Rumjanek VM, Lopes AG, Capella MA. Mechanisms of ouabain toxicity. FASEB J. 2003;17(12):1700–1702. doi: 10.1096/fj.02-0937fje. [DOI] [PubMed] [Google Scholar]
- 26.de Rezende Correa G, Araujo dos Santos A, Frederico Leite Fontes C, Giestal de Araujo E. Ouabain induces an increase of retinal ganglion cell survival in vitro: the involvement of protein kinase C. Brain Res. 2005;1049(1):89–94. doi: 10.1016/j.brainres.2005.04.082. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues-Mascarenhas S, Da Silva de Oliveira A, Amoedo ND, Affonso-Mitidieri OR, Rumjanek FD, Rumjanek VM. Modulation of the immune system by ouabain. Ann NY Acad Sci. 2009;1153:153–163. doi: 10.1111/j.1749-6632.2008.03969.x. [DOI] [PubMed] [Google Scholar]
- 28.Quastel MR, Kaplan JG. Inhibition by ouabain of human lymphocyte transformation induced by phytohaemagglutinin in vitro. Nature. 1968;219(5150):198–200. doi: 10.1038/219198a0. [DOI] [PubMed] [Google Scholar]
- 29.Matsumori A, Ono K, Nishio R, et al. Modulation of cytokine production and protection against lethal endotoxemia by the cardiac glycoside ouabain. Circulation. 1997;96(5):1501–1506. doi: 10.1161/01.cir.96.5.1501. [DOI] [PubMed] [Google Scholar]
- 30.de Vasconcelos DI, Leite JA, Carneiro LT, et al. Anti-inflammatory and antinociceptive activity of ouabain in mice. Mediators Inflamm. 2011;2011:912925. doi: 10.1155/2011/912925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olej B, dos Santos NF, Leal L, Rumjanek VM. Ouabain induces apoptosis on PHA-activated lymphocytes. Biosci Rep. 1998;18(1):1–7. doi: 10.1023/a:1022259832207. [DOI] [PubMed] [Google Scholar]
- 32.Esteves MB, Marques-Santos LF, Affonso-Mitidieri OR, Rumjanek VM. Ouabain exacerbates activation-induced cell death in human peripheral blood lymphocytes. An Acad Bras Cienc. 2005;77(2):281–292. doi: 10.1590/s0001-37652005000200008. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues Mascarenhas S, Echevarria-Lima J, Fernandes dos Santos N, Rumjanek VM. CD69 expression induced by thapsigargin, phorbol ester and ouabain on thymocytes is dependent on external Ca2+ entry. Life Sci. 2003;73(8):1037–1051. doi: 10.1016/s0024-3205(03)00377-1. [DOI] [PubMed] [Google Scholar]
- 34.Echevarria-Lima J, de Araujo EG, de Meis L, Rumjanek VM. Ca2+ mobilization induced by ouabain in thymocytes involves intracellular and extracellular Ca2+ pools. Hypertension. 2003;41(6):1386–1392. doi: 10.1161/01.HYP.0000072801.90600.C2. [DOI] [PubMed] [Google Scholar]
- 35.Marzio R, Jirillo E, Ransijn A, Mauel J, Corradin SB. Expression and function of the early activation antigen CD69 in murine macrophages. J Leukoc Biol. 1997;62(3):349–355. doi: 10.1002/jlb.62.3.349. [DOI] [PubMed] [Google Scholar]
- 36.Valente RC, Nascimento CR, Araujo EG, Rumjanek VM. mCD14 expression in human monocytes is downregulated by ouabain via transactivation of epithelial growth factor receptor and activation of p38 mitogen-activated protein kinase. Neuroimmunomodulation. 2009;16(4):228–236. doi: 10.1159/000212383. [DOI] [PubMed] [Google Scholar]
- 37.Carrel A, Ebeling AH. The fundamental properties of the fibroblast and the macrophage: II. The macrophage. J Exp Med. 1926;44(3):285–305. doi: 10.1084/jem.44.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCullough KC, Basta S, Knotig S, et al. Intermediate stages in monocyte-macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98(2):203–212. doi: 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagarajan S, Chesla S, Cobern L, Anderson P, Zhu C, Selvaraj P. Ligand binding and phagocytosis by CD16 (Fc gamma receptor III) isoforms. Phagocytic signaling by associated zeta and gamma subunits in Chinese hamster ovary cells. J Biol Chem. 1995;270(43):25762–25770. doi: 10.1074/jbc.270.43.25762. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman RA, Kung PC, Hansen WP, Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci USA. 1980;77(8):4914–4917. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trinchieri G, Valiante N. Receptors for the Fc fragment of IgG on natural killer cells. Nat Immun. 1993;12(4–5):218–234. [PubMed] [Google Scholar]
- 42.Valente RC, Capella LS, Oliveira MM, et al. Diverse actions of ouabain and its aglycone ouabagenin in renal cells. Cell Biol Toxicol. 2010;26(3):201–213. doi: 10.1007/s10565-009-9136-8. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, Shapiro JI. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66(1):227–241. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 44.Pires V, Harab RC, Olej B, Rumjanek VM. Ouabain effects on activated lymphocytes: augmentation of CD25 expression on TPA-stimulated cells and of CD69 on PHA-and TPA-stimulated cells. Int J Immunopharmacol. 1997;19(3):143–148. doi: 10.1016/s0192-0561(96)00070-7. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Cabrera M, Santis AG, Fernandez-Ruiz E, et al. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J Exp Med. 1993;178(2):537–547. doi: 10.1084/jem.178.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzio R, Mauel J, Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol. 1999;21(3):565–582. doi: 10.3109/08923979909007126. [DOI] [PubMed] [Google Scholar]
- 47.Schoner W, Bauer N, Muller-Ehmsen J, et al. Ouabain as a mammalian hormone. Ann NY Acad Sci. 2003;986:678–684. doi: 10.1111/j.1749-6632.2003.tb07282.x. [DOI] [PubMed] [Google Scholar]
- 48.Brennan FJ, McCans JL, Chiong MA, Parker JO. Effects of ouabain on myocardial potassium and sodium balance in man. Circulation. 1972;45(1):107–113. doi: 10.1161/01.cir.45.1.107. [DOI] [PubMed] [Google Scholar]
- 49.Sophocleous A, Elmatzoglou I, Souvatzoglou A. Circulating endogenous digitalis-like factor(s) (EDLF) in man is derived from the adrenals and its secretion is ACTH-dependent. J Endocrinol Invest. 2003;26(7):668–674. doi: 10.1007/BF03347027. [DOI] [PubMed] [Google Scholar]
- 50.Hinson JP, Harwood S, Dawnay AB. Release of ouabain-like compound (OLC) from the intact perfused rat adrenal gland. Endocr Res. 1998;24(3–4):721–724. doi: 10.3109/07435809809032675. [DOI] [PubMed] [Google Scholar]
- 51.Goto A, Yamada K, Nagoshi H, Terano Y, Omata M. Stress-induced elevation of ouabainlike compound in rat plasma and adrenal. Hypertension. 1995;26(6 Pt 2):1173–1176. doi: 10.1161/01.hyp.26.6.1173. [DOI] [PubMed] [Google Scholar]
- 52.Smith TW, Rasmusson RL, Lobaugh LA, Lieberman M. Na+/K+ pump inhibition induces cell shrinkage in cultured chick cardiac myocytes. Basic Res Cardiol. 1993;88(5):411–420. doi: 10.1007/BF00795408. [DOI] [PubMed] [Google Scholar]
- 53.Xiao AY, Wei L, Xia S, Rothman S, Yu SP. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J Neurosci. 2002;22(4):1350–1362. doi: 10.1523/JNEUROSCI.22-04-01350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu SH, Tang CM, Tseng HJ. Biostability and biocompatibility of poly(ester urethane)-gold nanocomposites. Acta Biomater. 2008;4(6):1797–1808. doi: 10.1016/j.actbio.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 55.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4(6):432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]