Summary
Innate immunity is a semi‐specific and widely distributed form of immunity, which represents the first line of defence against pathogens. This type of immunity is critical to maintain homeostasis and prevent microbe invasion, eliminating a great variety of pathogens and contributing with the activation of the adaptive immune response. The components of innate immunity include physical and chemical barriers, humoral and cell‐mediated components, which are present in all jawed vertebrates. The understanding of innate defence mechanisms in non‐mammalian vertebrates is the key to comprehend the general picture of vertebrate innate immunity and its evolutionary history. This is also essential for the identification of new molecules with applications in immunopharmacology and immunotherapy. In this review, we describe and discuss the main elements of vertebrate innate immunity, presenting core findings in this field and identifying areas that need further investigation.
Keywords: defence mechanisms, evolutionary history, infection, innate immunity, vertebrates
Abbreviations
- AMP
antimicrobial peptide
- APP
acute phase protein
- CRP
C‐reactive protein
- FV3
frog virus 3
- IFN
interferon
- IL
interleukin
- ITL
intraepithelial lymphocyte
- NAb
natural antibody
- NK
natural killer
- TGF
transforming growth factor
- TNF
tumour necrosis factor
Introduction
Innate immunity is defined as the first line of defence against pathogens, representing a crucial systemic response to prevent infection and maintain homeostasis.1, 2, 3 This type of immunity also has a critical role in the activation and regulation of adaptive immunity.4 Despite its constitutive elements, innate defence mechanisms have the capacity to develop an induced response during primary infection and create inflammatory conditions.5, 6 This response is specific due to the cell surface expression of pattern recognition receptors, which are capable of recognizing complex polysaccharides, glycolipids, lipoproteins, nucleotides and nucleic acids.4, 7
The elements of innate immunity include external physical barriers, humoral and cellular effector mechanisms, components that are conserved among jawed vertebrates (Table 1) with certain variations and specific characteristics.2, 3, 8 Innate immunity has been extensively studied in mammals in comparison to other vertebrates, because of its significance for human health and veterinary sciences.3, 9, 10, 11 However, a major exploration of these innate mechanisms in non‐mammalian species is essential for comprehension of the complex events involved in human innate immunity and is also crucial for the discovery of new antimicrobial, antitumour and immunomodulatory molecules with therapeutic applications.
Table 1.
General overview of vertebrate innate immunity
| Fish | Amphibians | Reptiles | Birds | Mammals | References | |
|---|---|---|---|---|---|---|
| Physical/chemical barriers | ||||||
| External protective structures | + | − | ++ | ++ | − | 15, 16, 18, 19 |
| External mucous secretions | ++ | ++ | + | + | + | 15, 16, 17, 18 |
| Specialized skin and mucosa | ± | + | + | + | ++ | 2, 16, 20, 24 |
| Humoral innate immunity | ||||||
| Lysozyme | + | + | + | + | + | 20, 21, 33, 34, 35 |
| Antimicrobial peptides | + | ++ | + | + | ++ | 15, 20, 22, 37, 40 |
| Acute phase proteins | ++ | + | + | + | ++ | 9, 10, 47, 49, 51 |
| Complement | ++ | + | + | + | ++ | 49, 52, 53, 56, 60 |
| Cytokines | + | + | + | + | ++ | 65, 66, 68, 70, 71, 73 |
| Natural antibodies | + | + | + | + | + | 60, 93, 96, 97, 100 |
| Cell‐mediated innate immunity | ||||||
| Intraepithelial T lymphocytes | + | + | + | + | + | 11, 25, 26, 27, 28 |
| Myeloid phagocytic cells | + | + | + | + | + | 73, 75, 104, 108, 112 |
| Nuocytes | − | − | − | − | + | 85 |
| Innate lymphoid cells | − | − | − | − | + | 113 |
| Phagocytic B cells | + | + | + | ? | − | 94, 110, 114 |
| Non‐specific cytotoxic cells | + | + | ? | + | + | 116, 118, 121, 123 |
| Mucosa‐associated invariant T cells | − | − | − | − | + | 84 |
Recent studies have evaluated the innate immune mechanisms in pathogen–host interactions and their phylogenetic conservation across animal species, revealing new evidence that is changing the concept of innate immunity. The former view of a non‐inducible and pre‐existing innate immune response is now evolving to the concept of a more specific immunity based on pattern recognition receptor molecular recognition and even memory development, leading to increased responses to secondary infections.6, 7, 12, 13, 14 This innate immunological memory depends mainly on natural killer (NK) cells and macrophages, which provide protection against re‐infection in a T/B‐cell‐independent manner.13 The development of memory responses in innate cells, involves epigenetic changes based on methylation and acetylation, a functional reprogramming that induces cell reactivation upon secondary stimulation.14 The memory in innate immunity has been described as a defence mechanism present not only in vertebrate organisms, but also in invertebrates such as plants and insects.12 In this context, the study of innate immunity in non‐mammalian vertebrates could provide integrative evidence for the understanding of this changing type of immunity and its evolutionary history.
In the current review, we examine the main defence systems of vertebrate innate immunity, describing its components, functions and properties. We also discuss the evolutionary conservation and diversification of these mechanisms, comparing them with their counterpart in mammals and providing a background of what is known and what needs further investigation in vertebrate innate immunity.
Physical and chemical barriers
External structures as a protection strategy against pathogens are the primary mechanism of innate immunity, which prevents microbe spreading and infection.1, 2, 15, 16 Among different species these barriers have morphological and anatomical variations, but they preserve the essential role of isolating the internal environment from external factors, such as harmful substances and pathogenic microorganisms2, 15, 17 (Fig. 1).
Figure 1.
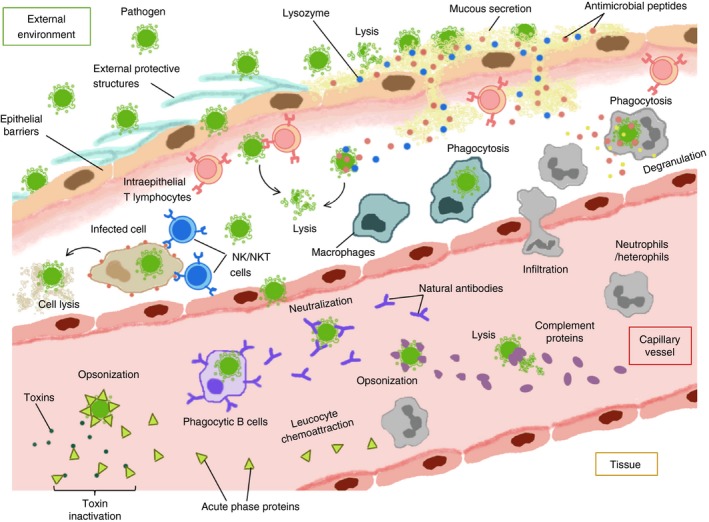
Integrative view of vertebrate innate immunity. Schematic illustration of the main innate defence systems in jawed vertebrates, which represents its physical, humoral and cell‐mediated components and the interactions between them, in a physiological context.
The skin is the main protective barrier in all living vertebrates and consists of stratified epithelial layers with tight junctions and structural proteins that provide mechanical protection, preventing water loss and pathogen infiltration, in addition to other sensory and excretory functions.16, 18 Vertebrate integument contains two multilayered zones or regions: the dermis, which is the most internal and the epidermis, which is the most external and is in direct contact with environmental factors and pathogens.16, 18, 19 This basic morphology is shared by the five classes of vertebrates, but the adaptation to their respective habitats has led to the evolution of certain functional and structural differences. For example, fish epidermis is protected by ossified scales, actin‐rich filaments called microridges and localized keratin filaments, which are adequate mechanical defences for aquatic environments and their associated pathogens.15, 16 The subsequent colonization of terrestrial habitats by tetrapods led to the evolution of a more reinforced and specialized epidermis with an active keratinization process and a resistant outer keratinized layer, the stratum corneum, which is made of dead cells that are continually desquamated.16, 18
In amphibians this corneous envelope contains a few cell layers with α‐keratin and does not form scales, whereas in reptiles it is improved by a hard cornification process that allows the formation of a thicker epidermis with multiple layers of α‐ and β‐keratin and rigid corneal scales that confer additional protection.2, 16, 18, 19 Avian epidermis is similar to its reptilian predecessor and the multiple α‐ and β‐keratin layers, with a predominant β‐keratinized stratum corneum, is conserved.16, 18, 19 Birds do not possess scales but instead they have feathers, which are chemically and structurally resistant to pathogens and are also essential for aerial locomotion.18 Interestingly, mammalian epidermis and its main appendices, the hair, only contain α‐keratin and keratin‐associated proteins, consequently they are mechanically less resistant in comparison with scales or feathers.16, 18, 19 However, this loss of rigidity is compensated with a soft, flexible and moistened stratum corneum, which is necessary for mammalian locomotion and sensory functions. This cornous layer has a specialized desquamation process and a weakly acidic pH condition that prevent infections with high efficiency.16, 19
In addition, the skin of all vertebrates is constantly lubricated with external secretions that maintain the epidermal integrity and moisture and also exhibit important antimicrobial properties.16, 18 These secretions in aquatic and semi‐aquatic anamniotic vertebrates (fish and amphibians) form a mucous cuticle that contains abundant mucopolysaccharides and glycoproteins, whereas in terrestrial amniotes (reptilians, birds and mammals) they form a sebaceous fluid with glycolipids and lipids.15, 17, 18 Despite these differences in chemical composition, the external secretions of all vertebrates are enriched in microbicidal molecules, such as lysozyme and antimicrobial peptides (AMPs), which are an important innate defence mechanism.2, 3, 15, 20, 21, 22 The phylogenetic conservation and functional properties of these molecules are discussed in a separate section.
The vertebrate skin also possesses specific cellular responses to pathogen invasion. For instance, epithelial cells can react to microbe contact by thickening and cellular hyperplasia.8, 18 Likewise, immature dendritic cells, called Langerhans cells, infiltrate into the stratified layers of epidermis where they extend dendritic prolongations, capturing antigens and preventing local infection.2, 16, 20, 23 Langerhans cells have been extensively characterized in mammals but they are present in other vertebrates with similar phenotypic and functional characteristics.16, 23 Other innate cells associated with these epithelial barriers are the specific intraepithelial lymphocytes and macrophages that destroy pathogens as well as transformed or infected cells.1, 2, 23 The role of these innate immune cells is analysed in detail in the Cell‐mediated innate immunity section.
Mucosal membranes form another important epithelial barrier conserved in vertebrate evolution that protects the body cavities from invading pathogens.2, 20, 24 In fish, the mucosal epithelium of the gills and the alimentary tract protects the areas that are most vulnerable to microbe penetration in aquatic environments.8 On the other hand, more specialized mucosa of respiratory and gastrointestinal tracts in terrestrial amniotes, prevent the infiltration of foreign particles and air‐associated or ingested pathogens.16, 20 In general, the mucosa of vertebrates exhibit basic biochemical defences against microbes and parasites such as the low pH, hydrolytic enzymes, the internal mucus layer and the secretion of bioactive molecules that include lysozyme, AMPs and pro‐inflammatory cytokines.1, 2, 20, 22, 25 Other immune‐related tissues, like the gut‐associated lymphoid tissue, are also present in all jawed vertebrates and contain high levels of infiltrating intraepithelial lymphocytes that destroy pathogens and preserve epithelial integrity.3, 11, 26, 27, 28, 29 In addition, the presence of symbiont microbes, organized in communities called microbiomes, have been reported in the majority of vertebrates; where they are associated with the mucosal surfaces and the skin.17, 20 They inhibit the colonization by pathogenic microorganisms, competing with them for attachment sites and producing antimicrobial metabolites.17, 20, 24
Humoral innate immunity
Lysozyme
The mucous secretions and the plasma of all vertebrates are enriched in proteins with antimicrobial activity (Table 2). One of the most active is the lysozyme (muramidase or N‐acetyl muramide glycanohydrolase), an enzyme that lyses bacteria.1, 3, 21, 30 This protein has been found in fish,30 generally associated with the mucous cuticle,8, 15, 31 whereas in amphibians like Rana pipiens eight lysozyme isoforms were identified by chromatographic and electrophoretic methods in the skin, serum, liver, kidney, ovary and spleen, demonstrating lytic capacity on a Micrococcus lysodeikticus‐agar suspension.32 This enzyme has also been identified in anurans like Xenopus laevis 33 and Xenopus tropicalis.31 In addition, different lysozyme homologues have been isolated from reptiles such as the Chinese soft‐shelled turtle (Pelodiscus sinensis), Asian soft‐shelled turtle (Amyda cartilagenea) and green sea turtle (Cheloni amydas), demonstrating lytic ability against several strains of Gram‐positive and Gram‐negative bacteria.34 In the same way, using an improved lytic assay on Micrococcus lysodeikticus‐agar plates, lysozyme has been detected and quantified in the plasma of captive birds, such as chickens (Gallus gallus), American kestrels (Falco sparverius), cockatiels (Nymphicus hollandicus) and zebra finches (Taeniopygia guttata) and free‐living birds, such as Clay‐coloured thrushes (Turdus grayi), blue‐grey tanagers (Thraupis episcopus), crimson‐backed tanagers (Ramphocelus dimidiatus), variable seedeaters (Sporophila americana) and white‐tipped doves (Leptotila verreauxi).35 The bactericidal activity of these avian plasma samples against Staphylococcus aureus and Escherichia coli was evaluated, demonstrating lytic capacity against both bacterial strains with higher levels for Escherichia coli in all avian species.35 This enzyme is also expressed in different mouse, rat and human tissues and is secreted by glandular serous cells, surface epithelial cells, and macrophages into the airway lumen.20, 21 Mammalian lysozyme has been characterized at gene and protein levels in rodents, where different isoforms have been identified.21, 36 The bacteriolytic capacity of rat lysozyme has been evaluated in vitro and in vivo, in transgenic mice, demonstrating a major activity against Gram‐positive bacteria like Pseudomonas aeruginosa and Staphylococcus aureus.21 In humans, high concentrations of lysozyme can be found in the airway surface liquid, where it contributes to the microbicidal properties of nasal secretions.20 This protein is also produced by neutrophils, macrophages and specialized intestinal secretory cells (Paneth cells), but can be secreted by gastrointestinal epithelial cells during inflammatory and infectious conditions, such as ulcerative colitis and Crohn's disease.20
Table 2.
Detailed humoral immunity in jawed vertebrates
| Molecule | Function | Organisma | References |
|---|---|---|---|
| α‐Defensins | Antimicrobial peptides | B,M | 20, 22, 37 |
| β‐Defensins | Antimicrobial peptides | R,B,M | 20, 22, 37, 38 |
| θ‐Defensins | Antimicrobial peptides | M | 22, 37 |
| Cathelicidins | Antimicrobial peptides | F, R, M | 15, 20, 37, 39 |
| Hepcidin | Antimicrobial peptide | F, A, R, M | 40 |
| Cationic peptides | Antimicrobial peptides | F, A, R, M | 15, 22, 37, 43, 44 |
| C‐reactive protein | Acute phase protein | F, A, M | 9, 10, 48, 49 |
| Serum amyloid P | Acute phase protein | F, M | 10, 48, 49 |
| Serum amyloid A | Acute phase protein | F, R, B, M | 9, 10, 49, 51 |
| Haptoglobin | Acute phase protein | F, B, M | 10, 46, 52 |
| α‐1 acid glycoprotein | Acute phase protein | B, M | 9, 10 |
| α‐2 macroglobulin | Acute phase protein | F, M | 9, 10, 52 |
| Fibrinogen | Acute phase protein | F, R, B, M | 9, 10, 49, 51, 52 |
| C3 | Complement protein | F, A, R, B, M | 23, 49, 51, 52, 53, 55 |
| B factor | C3 convertase | F, A, B, M | 49, 51, 52, 61 |
| C4 | Complement protein | F, B, M | 49, 51, 52, 53 |
| C5 | Complement protein | F, A, B, M | 23, 49, 51, 52 |
| C6 | Complement proteins | M | 52, 53, 55 |
| C1, C2, C7, C8, C9 | Complement proteins | F, M | 52, 53, 55 |
| Type I interferons | Antiviral cytokines | F, A, B, M | 65, 66, 67, 70 |
| Type II interferons | Phagocytosis stimulators | F, A, R, B, M | 65, 70, 73, 74 |
| Type III interferons | Antiviral cytokines | A, M | 71 |
| Interleukins | Immunomodulatory cytokines | F, A, R, B, M | 64, 70, 75, 77, 80 |
| Tumour necrosis factor‐α | Pro‐inflammatory cytokine | F, A, B, M | 49, 64, 70, 75 |
| Transforming growth factor‐β | Immunomodulatory cytokine | F, A, R, B, M | 25, 80, 89, 90 |
| IgM | Natural antibody | F, A, R, B, M | 93, 96, 97, 98, 100 |
F, fish; A, amphibians; R, reptiles; B, birds; M, mammals.
Recent phylogenetic analyses demonstrate the wide distribution of this enzyme across the whole animal kingdom.33 The amino acid sequences of different lysozyme types have been identified in vertebrates such as humans, mice (Mus musculus), chickens (Gallus gallus), turtles (Pelodiscus sinensis), frogs (Xenopus laevis), fish (Epinephelus coioides and Paralichthys olivaceus), in cephalochordates like amphioxus (Branchiostoma japonicum) and in several invertebrates and plants.33 The high conservation of this protein in multicellular organisms is strong evidence of its crucial role in innate immunity.
Antimicrobial peptides
The AMPs are another innate humoral defence that is highly conserved in vertebrates. These molecules exhibit antibacterial, antifungal and antiparasitic activity through a wide variety of mechanisms including destabilization and disruption of microbial membrane, pore formation, protein aggregation, inhibition of intracellular targets, interference in DNA transcription and blockade of protein synthesis and folding.22, 37 The most conserved peptides include defensins, cathelicidins and hepcidin. Defensins and cathelicidins are present in the mucous secretions of fish15 and reptiles like the European pond turtle (Emys orbicularis)38 and the banded krait (Bungarus fasciatus).39 The β‐defensin and cathelicidin‐BF, isolated from reptiles exhibit bactericidal capacity against Salmonella typhimurium, Listeria monocytogenes, Escherichia coli and methicillin‐resistant Staphylococcus aureus and antifungal activity against Candida albicans.38, 39 Likewise, the defensin‐like peptides and cathelicidins isolated from fish have potent antimicrobial activity against different pathogens, such as bacteria, fungi, viruses or parasites.15 The α‐ and β‐defensins are also found in birds and different mammals, such as cows, goats, pigs, rats and mice.22, 37 In humans, specific β‐defensins such as the human β‐defensins 1, 2 and 3, produced by epithelial cells and neutrophils, have been identified.20, 37 Other neutrophil‐derived antimicrobial peptides include azurocidins and α‐defensins like the human neutrophil peptides 1, 2 and 3.20 On the other hand, in some mammals specific cathelicidins have been found, such as PR‐39 in pigs and LL‐37 in humans20, 37 and a unique type of defensin, the θ‐defensins, has been isolated from the rhesus macaque (Macaca mulatta).22, 37 The mammalian defensins and cathelicidins have been shown to be an effective mechanism against bacterial infections with Samonella typhimurium, group A streptococcus, some Staphylococcus species and Pseudomonas aeruginosa.20 Besides, they display multiple immunomodulatory functions that include the stimulation of chemokine production, inhibition of pro‐inflammatory cytokines, direct leucocyte chemotaxis and modulation of host cell gene expression.20
Hepcidin, is possibly the most conserved AMP across vertebrates. This small peptide enriched in cysteine residues is present in > 51 vertebrate species, where it acts as an acute phase reactant synthesized by the liver (reviewed in ref. 40). The presence of hepcidin in birds is debatable, but experiments based on mRNA expression and searches in the EST and HTGS databases, did not reveal any evidence that confirms the presence of this gene in birds.40
The EST database (http://www.ncbi.nlm.nih.-gov/nucest/) is a collection of short single‐read transcript sequences from GenBank. whereas the HTGS database (http://www.ncbi.nlm.nih.gov/genbank/htgs) contains unfinished DNA sequences generated by the high‐throughput sequencing centers using traditional clone‐based sequencing.
Other cationic peptides, with linear or helical structure, have been found in different vertebrates. For example, piscidin and pleurocidin have been isolated from fish,15, 37 while pelovaterins are present in the eggs of the Chinese soft‐shelled turtle (Pelodiscus sinensis).41 Several AMPs have been identified in amphibians, such as ascaphine‐1 and ascaphine‐8, isolated from Ascaphus truei and Ascaphus montanus, 42, 43 bombinin H6, isolated from Bombina bombina, Bombina orientalis and Bombina variegata 44 and alyteserin‐1 and alyteserin‐2, obtained from Alytes obstetricans.45 Other examples are magainin 2, dermaseptin I, brevinin IT, esculentin I, ranalexin and ranateurin I, which were isolated from different amphibians.22, 37 In mammals, cationic peptides have also been found, such as profenins in pigs and histatins in the saliva of humans and some primates.37.
In the same way, AMPs derived from domains of other proteins have also been described in vertebrates. Some examples include hipposin, a histone‐derived peptide from Atlantic halibut (Hippoglossus hippoglossus L),30 lactoferricin from mammalian lactoferrin I, casodicin I from human casein, buforin II, derived from the histone 2A, which is highly conserved among vertebrates, and soluble antimicrobial domains of bovine lactoalbumin, human hemoglobin, lysozyme and ovalbumin.22, 37
Acute phase proteins
The vertebrate plasma and epithelial secretions contain several proteins that are synthesized during infection and inflammation, playing a crucial role in innate immunity and host defence9, 10, 46 (Fig. 1). The main acute phase proteins (APPs) described in mammals are C‐reactive protein (CRP), serum amyloid A and P, haptoglobin, α1‐acid glycoprotein, α2‐macroglobulin, ceruloplasmin, fibrinogen and transferrin.10 Serum amyloid P and CRP are pentraxins implicated in pathogen recognition, agglutination, opsonization and complement activation.10, 47 CRP is a major and moderate APP in many mammals, such as sheep, cows, rabbits, dogs, mice, rats, humans and other primates9, 10 and different CRP homologues have been identified in teleost fish,47, 48 but not in reptiles or birds. On the other hand, serum amyloid P seems to be a CRP counterpart in some species.10, 49 This lectin is a major APP in mice, but has not been further identified in other mammals.10 More recently, both CRP and serum amyloid P were purified from the serum of the iridescent shark (Pangasianodon hypophthalmus), demonstrating the capacity to agglutinate rabbit erythrocytes and pathogenic bacteria like Edwardsiella ictaluri and Aeromonas hydrophila.48 On the other hand, different pentraxins with CRP domains have been described in amphibians of Xenopus genus.47 Serum amyloid A is a more conserved APP that has been identified in fish,49, 50 reptiles,51 birds and mammals,9, 10 but not in amphibians. The expression of the gene encoding the serum amyloid A protein is increased in zebrafish (Danio rerio), rainbow trout (Onchorhyncus mykiss) and Chinese soft‐shelled turtle (Pelodiscus sinensis) during bacterial infections.49, 50, 51 Serum amyloid A is a major APP in mammals such as sheep, goats, cows, horses, cats, dogs, pigs, mice and humans, but is a moderate APP in chickens and non‐human primates.10 This protein is implicated in leucocyte chemoattraction and regulation of the inflammatory response10 and is classified as the major APP due to its high plasma levels during inflammation.9, 10 Interestingly, a trypsin inhibitor only present in pigs is known as major acute phase protein and is increased > 10‐fold during infections.10
Haptoglobin and α1‐acid glycoprotein are other important acute phase reactants that are not present in all vertebrates. Haptoglobin binds to free haemoglobin to prevent oxidative damage and also displays antimicrobial and immunomodulatory effects.10 This protein has only been detected in fish and mammals,9, 46, 50, 52 but recently an avian counterpart of mammalian haptoglobin, called PIT 54, was identified, which is induced in Escherichia coli‐infected chickens.46 Likewise, the α1‐acid glycoprotein has only been described in birds and mammals.9, 10 Its main function is the inactivation of harmful molecules like lipopolysaccharide or different types of drugs.10
Other plasma proteins such as α2‐macroglobulin, ceruloplasmin, fibrinogen and transferrin, in addition to their normal activity, can act as APPs and increase their plasma levels during inflammation and infection.9, 10 The protease inhibitor α2‐macroglobulin inactivates toxins and proteolytic enzymes10 and has been identified in teleost fish like Ictalurus punctatus 52 and mammals such as rats and primates,9, 10 whereas ceruloplasmin is a copper‐binding protein that scavenges free radicals,10 and has been identified as a positive APP in fish,52 birds46 and mammals.10 Fibrinogen is involved in tissue repair and is up‐regulated during acute infections in fish,52 reptiles,51 birds46 and mammals,9, 10 while transferrin acts as a negative APP in mammals,10 but is a positive acute phase reactant in other vertebrates like fish52 and birds.10 In contrast, albumin decreases its plasma levels during acute infections or inflammatory conditions, representing a negative APP among domestic animals like chickens and mammals.10 The loss of albumin is caused by renal and gastrointestinal changes during infection and its hepatic synthesis decreases, representing a strategy to preserve the unused amino acids for the production of positive APPs.10
Complement system
The complement system is composed of serum proteins that react against pathogens through a molecular cascade, resulting in opsonization, enhanced inflammatory response and formation of a membrane‐penetrating macromolecular pore, known as the membrane attack complex, which produces microbial lysis1, 3, 53 (Fig. 1). This system is better characterized in mammals, where three activation pathways have been identified: the classical, the alternative and the lectin pathways.1, 53, 54, 55 In the classical pathway the proteolytic cascade is activated by antigen–antibody immune complexes (generally with IgG or IgM), whereas in the lectin pathway the recognition of microbial surface carbohydrates by mannose‐binding lectin triggers the activation of mannose‐binding‐lectin‐associated serine proteases that initiate the complement proteolytic cascade.53 The alternative pathway, on the other hand, is self‐activated by spontaneous hydrolysis of the complement protein C3 at the microbial membrane.1, 53 Different studies in vitro on immobilized proteins and in vivo in mouse models have revealed a novel role of properdin in the activation of the alternative pathway.53 Other evidence demonstrates the involvement of coagulation‐associated proteases like kallikrein and thrombin in the complement proteolytic cascade, indicating a functional connection between these molecular pathways.53 In immunodeficient patients and specific knockout mice, the relevance of the complement system for mammalian immunity has been evaluated, demonstrating that several complement proteins or their proteolytic fragments are capable of activating B‐ or T‐cell function and are also crucial for the innate immune response against bacterial and viral pathogens, such as Streptococcus pneumonia, Pseudomonas aeruginosa, Neisseria meningitidis, Haemophilus influenza, herpes simplex virus and West Nile virus (reviewed in ref. 53).The reactivity and antimicrobial properties of the serum complement has also been evaluated in different vertebrates. For instance, complement‐mediated opsonization and antibody‐mediated complement activation have been described in teleost fish, such as common carp (Cyprinus carpio), catfish (Ictalurus punctatus) and salmonids.8 Likewise, the serum haemolytic capacity has been studied in different amphibians like Rana pipiens and Lithobates berlandieri.56 This study demonstrates that lytic activity against heterologous cells is stimulated by antibodies, reaches the levels reported for mammals and is influenced by heat, EDTA, hydrazine, carrageenan and antigen–antibody precipitate to a similar extent as the complement of some mammals, like pigs.56
The serum of reptiles also exhibits antimicrobial properties. Some comparative studies between the human serum complement and the complement of the American alligator (Alligator mississippiensis) demonstrate a major range of action against Gram‐negative bacteria for the alligator serum.57 The complement of Alligator mississippiensis displays antiviral activity against human immunodeficiency virus type 1, West Nile virus and herpes simplex virus,58 and additionally, amoebicidal properties against various species of Naegleria and Acanthamoeba that have been reported as resistant to human complement lysis.59 In birds, evidence shows that the complement system is also responsible for the lysis of heterologous cells. Matson et al. in 200560 evaluated the haemolytic capacity on rabbit erythrocyte suspension of the sera from several bird species, including American kestrel (Falco sparverius), Japanese quail (Coturnix coturnix japonica), zebra finch (Taeniopygia guttata), mourning dove (Zenaida macroura), waved albatross (Phoebastria irrorata), grey catbird (Dumetella carolinensis) and common grackle (Quiscalus quiscula). This work demonstrates that haemolysis decreases with heat, which indicates that it is mediated by the complement system. A comparison between subjects of different age and species also indicates that complement‐mediated lysis is affected by age and varies among species.60
The mammalian complement system has been extensively studied from a molecular perspective and nine complement proteins have been identified: C1, C2, C3, C4, C5, C6, C7, C8 and C9.1, 53, 55 Several complement inhibitors detected in mammals impair the complement cascade activation on host cell membranes and ensure the specificity of this innate mechanism.53 The identified mammalian inhibitors include Factor I, involved in C3b and C4b proteolysis, the decay‐accelerating factor, complement receptor 1 (CD35), Factor H and C4‐binding protein, which are enzymatic inhibitors of C3 convertase activity.53 Other inhibitors that impair the assembly of the membrane attack complex, preventing C9 binding and polymerization are CD59, vitronectin and S protein.53 In other vertebrates the presence of complement proteins has been described, confirming that this mechanism is preserved among vertebrates. Key components of the complement system, such as C3 and C5, have been isolated from some anurans of the genus Xenopus 23 and have been detected as down‐regulated genes in teleosts like Onchorhyncus mykiss during infection with Yersinia ruckeri O1.49 In other osteichthyes like catfish (Ictalurus punctatus), several complement proteins such as C1, C3, C4, C5, C7, C8 and C9 have been identified.52 In addition, the B factor that is a crucial element of the C3 convertase cascade implicated in alternative C3 activation has been identified in Onchorhyncus mykiss,49 Ictalurus punctatus,52 Silurana (Xenopus) tropicalis and Xenopus laevis.61
Similarly, different proteins of this enzymatic cascade like C3, factor B, C4 and C5, have been identified in domestic birds like chickens.62 In contrast, the molecular composition of the complement cascade of reptiles remains elusive and has only been identified the complement protein C3.51
Cytokines
Cytokines are proteins that mediate the communication between cells and are essential for the immune response.63 These soluble factors are one of the most conserved elements of vertebrate innate immunity. Cytokines are subdivided into different classes according to their genomic encoding regions, target receptors, associated signalling pathways and biological functions; these classes include interferons (IFNs), interleukins (ILs), tumour necrosis factors (TNFs) and transforming growth factors (TGFs).1, 63, 64 Although the cytokines characterized to date have been cloned and extensively studied in mice, many of them are present in other vertebrates with similar functions.
Interferons are mainly involved in antiviral defence, although they display additional immunomodulatory function.63, 64, 65 In mammals, three types of IFNs have been identified so far: type I IFNs, which include IFN‐α, IFN‐β, IFN‐ω, IFN‐κ and IFN‐ε (reported in humans), type II IFNs (IFN‐γ) and type III IFNs (IFN‐λ).65 Type I IFNs represent a conserved antiviral system in vertebrates but they have a different genomic organization in lower and higher vertebrates.66 Fish and amphibian type I IFNs are encoded by characteristic five‐exon/four‐intron transcripts, in contrast to reptilian, avian and mammalian type I IFNs, which are encoded by intronless transcripts.66, 67 The type I IFNs in lower vertebrates could not be phylogenetically classified as α or β, but a detailed structural analysis in teleost fish has subdivided it into groups I and II, based on the amount and position of cysteine residues.67 The role of type I IFNs in antiviral immunity has been corroborated in different vertebrates. In teleost fish like the crucian carp (Carassius auratus L.) these cytokines provide strong antiviral protection against grass carp haemorrhagic virus infection and mediate the Poly I:C‐induced antiviral response, acting through the signal transducer and activator of transcription 1 pathway, as occurs in mammals.68 Likewise, type I IFNs have been identified as repressors of viral protein synthesis and stimulators of NK cells in amphibians69 and have been recently evaluated in frog virus 3 (FV3)‐infected frogs (Xenopus laevis), demonstrating its capacity to inhibit the replication of FV3 and expand the mean survival time of infected tadpoles.66 In reptilians, IFN‐like activity have been reported in virus‐infected animals and cell lines,3 but a detailed evaluation of type I IFNs in these vertebrates is still missing. On the other hand, this type of IFN has been characterized in birds, at genetic and molecular levels (reviewed in ref 70). The presence of an IFN‐α homologue and other closely related genes was reported in chickens, where they are encoded by a single intronless transcript, like in mammals.70 These IFN‐like molecules showed significant antiviral properties in virus‐infected chicken embryos and cell lines and were also identified in ducks and turkeys.25, 70 A second type I IFN, homologue to mammalian IFN‐β and encoded by an independent intronless transcript, was further identified in chickens demonstrating antiviral activity in cell cultures.71, 72
Type II IFNs are also conserved among vertebrates and their main functions include antiviral defence, regulation of MHC expression, phagocytosis stimulation, inhibition of cell growth and apoptosis.63, 64, 65, 70 Interferon‐γ is the only type II IFN identified in mammals, whereas in lower vertebrates like teleost fish multiple type II IFNs have been found.71 In Takifugu rubripes and other teleosts, IFN‐γ homologues have been identified and they share the antiviral properties and genomic organization of mammalian IFN‐γ.72, 73 Likewise, in the amphibian Xenopus tropicalis the expression of the IFN‐γ gene has been corroborated.74 This result was confirmed by Guanchun and Jacques in Xenopus laevis, demonstrating that the IFN‐γ gene is up‐regulated by FV3 infection in tadpoles and adult frogs.75 New evidence demonstrates the presence of an IFN‐γ gene in the North American green anole lizard (Anolis carolinensis) and its expression in different tissues.65 On the other hand, in different birds such as chickens, ducks, turkeys, pheasants, quails and Guinea fowls the presence of IFN‐γ genes with high sequence homology to mammalian IFN‐γ and strong antiviral activity as well as macrophage‐stimulating capacity has been reported.70, 76 An increased expression of IFN‐γ gene has also been detected in chickens during coccidial infections.25 In contrast, type III IFNs (IFN‐λ) have only been identified in mammals and recently in the amphibian Xenopus laevis.71 In both species IFN‐λ seems to be involved in antiviral defence and displays common functions with type I IFNs.65, 71 Although type III IFNs are not present in teleost fish,71 the existence of IFN‐λ genes in reptilians or birds has not been properly evaluated. Hence, a genomic screening for IFN‐λ in amniotes is crucial to determine the evolution of type III IFNs in jawed vertebrates.
Interleukins are another class of cytokines conserved in vertebrate immunity, but they are not equally represented in all classes of vertebrates. Interleukin‐1, IL‐2 and IL‐8 are among the most preserved ILs in amphibians, reptiles, birds and mammals.3, 61, 63, 64, 70, 77 Additionally, IL‐1 has been identified in teleost fish with the same functions that it exhibits in other vertebrate species.8, 49 The mammalian IL‐1 cytokine family has three characterized members: IL‐1α and IL‐1β, which mediate inflammatory responses and leucocyte stimulation and a third member, the IL‐1 receptor antagonist (IL‐1Ra) that inhibits the biological activity of IL‐1α and IL‐1β.1, 64 Of these three genes, only the IL‐1β has been identified in other vertebrates. This gene has been detected in 13 teleost species,8, 50 in amphibians75 and in chickens,25, 70 were it preserves the pro‐inflammatory and cell‐stimulating capacities that have been observed in mammals. In reptiles an IL‐1‐like molecule was detected but it could not be classified as α or β.78 This cytokine is produced by splenic macrophages of the wall lizard (Hemidactylus flaviviridis) in response to lipopolysaccharide stimulation.78 On the other hand, IL‐2 that is involved in lymphoid and myeloid cell differentiation in mammals63 has been identified in reptilians like diadem snake (Spalerosophis diadema) as a thymocyte proliferating factor3 and in chickens associated to the nuclear factor‐κB to regulate cell differentiation.70 Furthermore, the IL‐2 gene is over‐expressed in amphibians like Xenopus tropicalis, during Batrachochytrium dendrobatidis infection.61
Interleukin‐8 is another cytokine conserved in vertebrate immunity. This molecule acts as a chemokine that participates in macrophage and leucocyte chemoattraction.1, 70, 75, 79 It has been identified in elasmobranchs like dogfish (Triakis scyllia)80 and teleosts like Onchorhyncus mykiss, 81 reptiles like Pelodiscus sinensis, 77 domestic birds71 and mammals.1, 79, 80 However, no IL‐8 homologue gene has been reported in amphibians. Interleukin‐6 is another important cytokine in the immune response that stimulates the proliferation of B lymphocytes and the production of fibronectin and APPs in hepatocytes.64, 82 It has been found in fish,8, 50 amphibians like Rana esculenta, 80 birds and mammals,70, 79, 80, 82 but not in reptiles. Other ILs such as IL‐15, IL‐16, IL‐17 and IL‐18 seem to be restricted to birds and mammals and their functions include cell differentiation, chemoattraction of T lymphocytes, induction of cytokine expression and stimulation of IFN‐γ secretion.25, 70, 79, 80, 83, 84 Additional ILs that display a variety of functions in innate and adaptive immunity have been identified in mammals. For example, IL‐4, IL‐5, IL‐10 and IL‐13, which are secreted during T helper type 2 response and regulate lymphoid cell activation and cytokine secretion.64, 79, 85 Likewise, IL‐12, IL‐20,64 IL‐21,86 IL‐22, IL‐26,73, 87 IL‐25 and IL‐3385 have been characterized in mice or human tissues, where they exhibit specific biological properties related with inflammation, leucocyte proliferation and lymphocyte differentiation. Interestingly, IL‐12, IL‐22 and IL‐26 were also discovered in teleost fish,73, 80 suggesting that some of these cytokines could be present in other vertebrates but remain unidentified.
Among pro‐inflammatory cytokines, TNF‐α is the second most conserved across vertebrate species after IL‐1β and is implicated in leucocyte chemoattraction and macrophage stimulation.8, 25, 63, 64, 79 This cytokine has been detected in teleosts such as rainbow trout (Onchorhynchus mykiss), sea bream (Sparus aurata), goldfish (Carassius auratus) and catfish (Ictalurus punctatus),8, 49 in amphibians like Xenopus laevis 75 and mammals.63, 64, 79 A recent work identified the TNFSF13 gene (a member of the TNF superfamily) in Xenopus laevis and demonstrated that its encoding protein promotes the proliferation of Xenopus B lymphocytes and mouse splenic B cells.88 The similar structure, tissue expression and bioactivity of TNFSF13 between mammals and Xenopus laevis, suggest that this anuran could be an interesting model to evaluate different immunological disorders caused by this cytokine.88 On the other hand, although TNF‐α‐like activity was reported in chickens, the sequence of a chicken TNF‐α homologue gene has not been identified or described in any database.25, 70 Similarly, the presence of this molecule or its encoding gene has not been reported in reptiles.
Other immunoregulatory cytokines like TGF‐β 80 are also well‐preserved across vertebrate species and are present in early metazoans and protozoans.89 The mammalian TGF‐β family have been extensively characterized and include three related isoforms encoded by independent genes, that regulate cell growth and differentiation and are involved in oocyte maturation, wound repair, modulation of inflammatory response, immunosuppression and tolerance.90 In the same way, the presence of TGF‐β in other vertebrates has been reported and the ubiquitous expression of this pleiotropic cytokine among animals could be more related to its function in reproduction and embryonic development than to its role in immunity.80 In 2009, a genomic screening of 33 animal species of different taxa identified the TGF‐β gene in humans, primates such as Pan troglodytes and Macaca mulatta, rodents like Mus musculus and Rattus norvegicus, in dogs (Canis domesticus), birds like roosters (Gallus gallus), osteichthyes (bony fish) such as Oryzias latipes, Danio rerio, Tetraodon nigroviridis and Takifugu rubripes.89 The TGF‐β is also secreted by the placenta of viviparous squamate reptiles like Chalcides chalcides.80 Additionally, the three mammalian isoforms of TGF‐β were identified in chickens as regulators of embryonic development and haematopoiesis.70 Interestingly, the mRNA expression of a fourth isoform, TGF‐β 4, is induced by coccidial infection in the intestinal epithelia of chickens.25, 70
Natural antibodies
In the plasma of vertebrates there are circling antibodies known as natural antibodies (NAbs) (Fig. 1), which are mainly of IgM isotype, possess a limited genetic repertoire and are synthesized by B cells in absence of pathogens.91 These pre‐existent immunoglobulins are essential components of humoral innate immunity; they react against foreign antigens and microbe‐derived substances and can activate the complement cascade by the classical pathway.60, 91
The NAbs have been described as part of humoral immunity in fish, reacting with proteins exposed on the microbial surface and providing protection against pathogens like Aeromonas salmonicida.92 This natural IgM is found in chondrichthyes (sturgeons and sharks) and some osteichthyes such as Onchorhynchus mykiss and Pagrus auratus.93 Such IgM isotype NAbs have also been identified in amphibians,94 where they are implicated in the inactivation of modified bacteriophages and other viral particles,95 as well as haemoagglutination of foreign red blood cells from chicken, sheep, rat and human.96 High levels of NAbs have been detected in anurans like Bufo regularis, Bufo arenarum and Xenopus laevis.96
The production of NAbs is also present in reptiles, as an essential element of the humoral immune response that has been found in some species, e.g. water python (Liasis fuscus).97 This study demonstrates that NAbs increase with age for tropical pythons, possibly to compensate for the immunosenescence and loss of effectiveness in the adaptive humoral immune response.97 Natural immunoglobulins like IgM and IgG, are also produced by the gut‐associated lymphoid tissue of birds, due to stimulation by the intestinal microbiota.98 These NAbs eliminate bacteria and spirochaetes in vivo and promote the clearance of bacterial substances like lipopolysaccharides.60 They represent an important humoral defence mechanism that correlates with resistance to pathogens, like Amblyceran lice in some bird species such as Galápagos hawks (Buteo galapagoensis).99 In addition, NAbs against parasites like Plasmodium lophurae have been identified in White Leghorn chickens (Gallus domesticus).60
The NAbs are present in mammals as well, representing a conserved element from lower vertebrates involved in humoral innate immunity; they neutralize pathogens and helminth parasites2 and confer resistance against influenza virus.91 These antibodies also display new modulatory functions in complex vertebrates like mammals. For example, the binding to oxidized proteins like oxidized low‐density lipoprotein, in a mechanism that reduces its uptake by macrophages and attenuates the atherogenic process.100 Additionally, some reports demonstrate that these natural IgMs participate in immune surveillance, recognizing tumour‐associated or tumour‐specific antigens such as CFR‐1, expressed in malignant cells of different epithelial cancers, and even inducing apoptosis.101
Cell‐mediated innate immunity
Intraepithelial T lymphocytes
The mucosa and epithelial barriers of vertebrates are infiltrated by a specific type of lymphocyte – intraepithelial T lymphocytes (ITLs) (Fig. 1), which recognize and destroy pathogens.102 These cells have been better characterized in mammals and are phenotypically classified as γδ T lymphocytes.11, 102 However, they have more characteristics in common with the effector cells of innate immunity. For example, its limited antigenic repertoire and the low variability of its T‐cell receptors make them different from the typical lymphocytes present in the adaptive immune response.11, 102
Intraepithelial lymphocytes have been isolated from the intestinal epithelial tissue of fish, like Onchorhynchus mykiss, and its cytotoxic capacity against EL4 mouse thymoma cells has been demonstrated.27 Likewise, in some specialized epithelia of amphibians, such as the gut‐associated lymphoid tissue, the presence of intraepithelial lymphocytes has been reported; these are similar to those found in fish and share many phenotypic characteristics with the ITLs of mammals.26
These lymphocytes have also been identified in reptiles, as an element of protective immunity, in intestinal epithelium and gut‐associated lymphoid tissue,28 a characteristic that is preserved from fish and amphibians. They are present in some species, such as platypus (Ornithorhynchus anatinus), in the lamina propria of intestinal mucosa, and are phenotypically similar to mammalian ITLs.28 The gut‐associated lymphoid tissue of birds is also infiltrated by intraepithelial lymphocytes, which diversify into various subsets of T lymphocytes that include CD8+ T cells (CD8αα), and γδ T CD8αβ cells.29 These cells represent a primary active barrier against pathogen dissemination, and constitute the main component of protective immunity to avian coccidiosis caused by the intestinal parasitic pathogen Eimeria.25 Some investigations indicate that this cellular component of epithelium increases in number after infection with intestinal parasites such as Eimeria acervulina and Eimeria tenella.103
In humans, two subsets of γδ T lymphocytes have been identified: Vδ1 and Vδ2 γδ T cells. Vδ1 T cells reside mainly in the epithelial mucosa, whereas Vδ2 T cells are abundant in the lymphatic system and peripheral blood.11 These cells can recognize non‐peptidic antigens, soluble proteins and MHC molecules, displaying multiple functions, such as cytokine secretion, activation of dendritic cell maturation, macrophage recruitment, cytotoxic activity against transformed cells, maintenance of epidermal integrity and B‐ or T‐cell cooperation, among others.11
Myeloid phagocytic cells
The main cellular components of vertebrate innate immunity are myeloid phagocytic cells, also called ‘professional’ phagocytes3, 94 (Fig. 1). This type of leucocyte includes monocyte–macrophages, neutrophils/heterophils, basophils and eosinophils.1, 2, 3, 94 These cells phagocytose and destroy pathogens, secrete cytokines and release soluble mediators like histamine, reactive oxygen species, reactive nitrogen species, lysozyme and AMPs.2, 3, 79, 104 They recognize senescent cells and microbes by pattern recognizing receptors and present phagocytosed antigens to B or T cells, acting as the first line of defence against infection and contributing with the activation of adaptive immune response.1, 79, 104 The main pattern recognizing receptors expressed in vertebrate phagocytes are the Toll‐like receptors, C‐type lectin receptors, NOD‐like receptors and Rig I‐helicases, which recognize chemical structures that are present in all microbes, such as lipopolysaccharides, lipoproteins, complex glycans, formylmethionine and even nucleotides and nucleic acids.5, 6, 7, 12 These receptors are able to distinguish between chemical isomers and can also recognize a variety of polysaccharides, lipids or nucleotides, cooperating with each other to selectively activate different signalling pathways that induce specific pro‐inflammatory and antimicrobial innate responses.5, 6
Myeloid phagocytes represent a potent defence mechanism in mammals, essential against infections with Gram‐negative (Escherichia coli, Salmonella typhimurium, Yersinia enterocolitica) and Gram‐positive (Listeria monocytogenes, Staphylococcus aureus) bacteria.104, 105 The immune functions of mammalian innate myeloid cells have been evaluated in mouse models and they include phagocytosis, antigen presentation, releasing of histamine, pro‐inflammatory cytokines, AMPs and granule‐derived proteins that destroy microorganisms.1, 104, 105 These cells are also involved in wound healing and normal tissue functions.106 Among myeloid phagocytic cells, neutrophils represent the earliest innate response in humans, phagocytosing microbes, cooperating with other immune cells and releasing AMPs, proteases, hydrolytic enzymes and phospholipases.105, 107
In other vertebrates, leucocytes such as macrophages, heterophils (homologues of mammalian neutrophils), basophils and eosinophils are crucial for the effectiveness of innate immunity.3, 94, 108 These cells have been described in fish,8, 108, 109 amphibians23, 75 and reptiles,3, 78, 110 preserving their functions in phagocytosis, inflammation and the release of soluble mediators. In these ectothermic vertebrates, the phagocytosis plays an important role in immune response, because it is less influenced by temperature than adaptive immune mechanisms.111 Recent imaging techniques in fish model organisms like Danio rerio, reveal new insights into the phagocytotic process and immune cell recruitment during inflammation, from a unique in vivo perspective.108 These approaches combined with genetic manipulation have allowed the discovery of novel inflammatory pathways, danger signals and myeloid development‐related genes, which are integrative findings for the comprehension of the mammalian immune system.108
Innate myeloid cells have also been characterized in birds. They include macrophages, heterophils, eosinophils and basophils, which are preserved from the early vertebrates and whose main function is the recognition and phagocytosis of pathogens.112 Its pattern recognizing receptor‐mediated recognition stimulates phagocytosis and activates destruction of pathogens, antigen presentation, stimulation of the adaptive immune response and production of pro‐inflammatory cytokines.112
On the other hand, the lymphoid lineage of vertebrate leucocytes has recently been a focus of research in innate immunity to comprehend the developmental relationships between the various lineages that have been identified to date as well as their functionality. A new type of leucocyte, known as a nuocyte, has been identified in mammals, implicated in type 2 immunity against helminth parasites.85 These cells were characterized in the IL13‐eGFP reporter mice, demonstrating an IL‐25/IL‐33‐dependent proliferation and a capacity to secrete IL‐13 during infection with the helminth Nippostrongylus brasiliensis.85 In addition, recent reports describe the existence of lineage‐marker‐negative innate lymphoid cell subsets that do not express cell surface markers associated with other immune cell lineages. These newly identified members of the lymphoid lineage, which include nuocytes, have emerging roles in mediating immune responses and in regulating tissue homeostasis and inflammation.113
Phagocytic B cells
The B lymphocytes are considered another type of cell involved in vertebrate innate immunity (Fig. 1). In lower vertebrates these cells display phagocytic capacity and activation of degradative pathways that result in phagolysosome fusion and killing of internalized bacteria.94, 114 B cells seem to act like leucocytes; however, they are able to produce antibodies of IgG and IgA isotype, involved in classical adaptive immune response, and also NAbs of IgM isotype.92, 110
Phagocytic B cells have been identified in teleost fish like Onchorhynchus mykiss and Ictalurus punctatus, in amphibians like Xenopus laevis and in reptiles like the red‐eared slider (Trachemys scripta).110, 114 In mammals, the B cells do not exhibit phagocytic capacity and their immune functions seem to be limited to adaptive immunity.94, 110, 114 However, a mammalian subpopulation of B lymphocytes known as B‐1 lymphocytes, retains the phagocytic capacity and antimicrobial properties of the B cells from lower vertebrates.114 These cells reside mainly in the peritoneal and pleural cavities91 where they produce NAbs and present phagocytosed antigens to CD4+ T cells, playing an important role in innate and adaptive immune responses.114 The phagocytic ability of non‐mammalian B cells which is preserved in B‐1 cells, suggests an evolutionary connection between both cell types. In fact, the discovery of a bipotent B macrophage precursor in fetal liver and adult bone marrow of mammals, supports the idea of a common origin for B cells and macrophages, from a phagocytic ancestor.94, 114 In contrast, the phagocytic capacity of B cells in birds has not been evaluated, making it hard to determine the evolution of this function into the higher amniotes.110 However, subsets of B cells, similar to B‐1 lymphocytes of mammals, are found in birds, in the gut‐associated lymphoid tissue where they produce NAbs.98
Non‐specific cytotoxic cells
Non‐specific cytotoxicity is one of the most important cellular mechanisms in vertebrate innate immunity (Fig. 1), playing an essential role in antiviral defence and immune surveillance.115, 116 This function is carried out in mammals mainly by NK lymphocytes, which are involved in the cytotoxic response against cells that are transformed or infected by virus or intracellular microbes.115 In addition, NK cells secrete cytokines with antiviral activity117 and are also involved in hepatic defence and tissue regeneration and remodelling.87
The presence of non‐specific cytotoxic cells similar to NK cells has been described in lower vertebrates. These cells have been identified in fish like Ictalurus punctatus where they are distinguishable by their small, non‐granular morphology and their abundance in lymphoid tissues, such as kidney and spleen.8, 118 In addition, the identification of a new gene family known as novel immune‐type receptors in zebrafish (Danio rerio) is strong evidence for the presence of NK‐like cells in teleosts.108 These proteins are structurally similar to mammalian NK receptors, are differentially expressed in fish lymphoid cells but not in myeloid cells, and confer lytic capacity on the cells in which they are expressed.108
Natural killer‐like lymphocytes have also been reported in amphibians, with an essential role in cytotoxic response against virus‐infected cells.69, 75 Many NK candidates have been identified in the intraepithelial layer and the spleen tissue of Xenopus laevis, using a staining method with specific anti‐NK monoclonal antibodies 1F8, 1G5 and 4D4.119 This study demonstrates the existence of at least three subsets of NK lymphocytes in Xenopus laevis: 1F8+ CD5– splenocytes, 1F8+ CD5lo gut cells and 1F8+ F17– lymphocytes, which exhibit strong in vitro cytotoxic activity, against the Xenopus thymus lymphoid tumour cell line B3B7.119 These results have been confirmed by Goyos and Robert in 2009,116 through the identification of 1F8+ NK cells and NKT‐like cells (with NK‐ and T‐cell‐associated markers), which are similar to those found in mammals and are involved in in vivo anti‐tumour immune responses in Xenopus laevis. Different studies in this anuran species have revealed the connection between tumorigenesis and embryonic development and are also providing evidence for the innate cellular responses to oncogenic processes.116 In comparison to murine models, anurans like Xenopus laevis and Xenopus tropicalis provide advantages in the genetic manipulation and constitute a powerful alternative for the identification of new genes involved in tumorigenesis and anti‐tumour immune responses.116
In birds, non‐specific cytotoxic activity against infected or transformed cells has also been reported, carried out by NK‐like lymphocytes called TRC0 cells.120 These cells are homologues to mammalian NK lymphocytes and express their typical surface antigens, such as CD3.120 This kind of lymphocyte has been identified in the intestinal mucosa of chickens, where they are subdivided into two distinct populations: 28‐4+ CD3– NK cells (detected with 28‐4 monoclonal antibody) and 28‐4+ CD3+ NK cells, which are homologues to the NKT cells of mammals.121 They represent a conserved cellular element from the lower vertebrates that plays an important role in anti‐parasitic immune response and mucosal immunity.25
In contrast with other vertebrates, non‐specific cytotoxic cells or NK cells are not found in reptiles, or at least have not been identified and studied thoroughly. They are present in the other classes of vertebrates, suggesting that they should exist in reptiles as well. This theory can be supported by the finding of NK‐mediated cytotoxicity against tumour cells in the turtle Mauremys caspica.122 This activity was identified in thymic cells, but was not defined in a specific subset.122
In addition, a particular type of NK cell, with many phenotypic characteristics of a T lymphocyte, known as NKT lymphocyte, has been described in mammals.117, 123 These cells recognize glycolipid antigens in the context of the CD1 molecule and release cytokines implicated in the innate immune response.123 The presence of NKT functional analogues has been reported in amphibians116 and birds,25, 121 suggesting that this subset of NK lymphocytes is a conserved component among vertebrates, which represents an evolutionary link between innate and adaptive immunity. The main functions of these cells include cytotoxic response, antitumour activity123 and the secretion of antiviral cytokines like type I IFNs117 and IL‐21.86 Other types of invariant T cell, similar to NKT lymphocytes, are known as mucosal‐associated invariant T cells and are also implicated in the mammalian innate immune response by the release of IL‐17, mainly in the gastrointestinal tract84 and the liver.124
Recent comparative studies across animal species have revealed new functional properties for NK lymphocytes and other innate cells, like monocyte–macrophages.13, 14 These novel findings indicate that innate immune cells display enhanced responses to secondary infections in a T/B‐cell‐independent manner, through epigenetic mechanisms and a biochemical reprogramming that predisposes cells to a rapid activation by secondary stimulation.13, 14 This innate immunological memory termed as ‘trained immunity’ is an interesting new feature of innate cells that is changing the concept of innate immunity.13, 14
Conclusions
Across jawed vertebrates, innate immunity displays a diversity of mechanisms that adapt to the anatomy and behaviour of different species, while preserving its core elements and main functions. Physical barriers are conserved across all vertebrates, nevertheless they have anatomical and chemical variations that exhibit a tendency to specialization of the skin and mucosa. Besides, among humoral components lysozyme, AMPs, complement system and NAbs are the most conserved, showing the same basic functions and sharing their main properties. On the other hand, the acute phase response and cytokine release are conserved mechanisms, triggered in the presence of pathogens, even though the involved molecules differ among vertebrate species. In general, humoral components are poorly characterized in amphibians and reptiles, making it difficult to understand the evolution of humoral innate immunity in vertebrates.
The current evidence reveals non‐mammalian vertebrates as a source of potent antimicrobial and antiviral proteins and peptides, which could also have antitumour or immunomodulatory activity. There are several examples of antimicrobial molecules isolated from invertebrates and plants with potential applications in biomedicine; however, vertebrates are an interesting alternative for the development of new therapeutic agents to deal with antibiotic resistance and chemotherapy toxicity.
Cell‐mediated components of innate immunity are preserved across vertebrates and they include ITLs, myeloid phagocytes and non‐specific cytotoxic cells. Nevertheless, the existence of reptilian non‐specific cytotoxicity remains unclear. Further investigation and characterization of cell‐mediated immunity in reptiles is required to elucidate the transition of non‐specific cytotoxic cells into the amniotes. On the other hand, the B lymphocytes are also described as innate effector cells, a recent concept in vertebrate immunity. B cells produce NAbs and display phagocytic capacity and antimicrobial properties in lower vertebrates, a characteristic that remains in mammalian B‐1 lymphocytes. These findings suggest an evolutionary connection between macrophages and B cells, which could have evolved from a common phagocytic ancestor. However, the phagocytic capacity of B cells from birds has not been examined. A detailed evaluation of phagocytic and antimicrobial characteristics of avian B cells is the key to trace the evolution of B‐cell‐mediated phagocytosis into the higher amniotes.
In spite of the evident physiological and morphological differences among the five classes of jawed vertebrates, that determine the kinetics and overall effectiveness of immune responses, the molecules and cells involved in the innate response are conserved and maintain their functionality throughout vertebrates. Hence, even if the immunity in higher vertebrates is more complex than in lower vertebrates, they share some of the basic mechanisms involved in cutaneous protection, serum antimicrobial activity, acute phase response, opsonization, pathogen recognition, phagocytosis and cytotoxicity, among others. From this point of view, non‐mammalian vertebrates can be considered as models for the study of the immune system. In this sense, different zebrafish mutants have been used to examine key aspects of immune system ontogeny, phagocytosis, cell recruitment and inflammatory pathways, while the frog Xenopus laevis is a potent model of tumorigenesis, tumour immunity and other immune‐related pathophysiological conditions. Likewise, chickens and other domestic birds constitute models for the study of the gut‐associated lymphoid tissue and the pathogen–host interactions at the intestinal epithelium.
Further investigation is required to achieve the complete characterization of innate immune mechanisms and their evolution across vertebrates. This could lead to a better understanding of the cellular and molecular processes that determine the dynamic functionality of innate immunity.
Disclosures
The authors declare that there are no financial or commercial conflicts of interest associated with this work.
References
- 1. Abbas AK, Litchman AH, Pillai S. Cellular and molecular immunology. Philadelphia: Elsevier/Saunders, 2012. [Google Scholar]
- 2. de Veer MJ, Kemp JM, Meeusen EN. The innate host defence against nematode parasites. Parasite Immunol 2007; 29:1–9. [DOI] [PubMed] [Google Scholar]
- 3. Zimmerman LM, Vogel LA, Bowden RM. Understanding the vertebrate immune system: insights from the reptilian perspective. J Exp Biol 2010; 213:661–71. [DOI] [PubMed] [Google Scholar]
- 4. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 2015; 16:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 2012; 4: pii:a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose‐binding lectin and innate immunity. Immunol Rev 2009; 230:9–21. [DOI] [PubMed] [Google Scholar]
- 7. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140:805–20. [DOI] [PubMed] [Google Scholar]
- 8. Uribe C, Folch H, Enriquez R, Moran G. Innate and adaptive immunity in teleost fish: a review. Vet Med 2011; 56:486–503. [Google Scholar]
- 9. Petersen HH, Nielsen JP, Heegaard PM. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res 2004; 35:163–87. [DOI] [PubMed] [Google Scholar]
- 10. Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comp Med 2009; 59:517–26. [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Y, Ding Y, Tanaka Y, Shen L, Wei C, Minato N et al γδ T cells and their potential for immunotherapy. Int J Biol Sci 2014; 10:119–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brehélin M, Roch P. Specificity, learning and memory in the innate immune response. ISJ Vision Perspect 2008; 5:103–9. [Google Scholar]
- 13. Netea MG. Training innate immunity: the changing concept of immunological memory in innate host defence. Eur J Clin Invest 2013; 43:881–4. [DOI] [PubMed] [Google Scholar]
- 14. Netea MG, Latz E, Mills KH, O'Neill LA. Innate immune memory: a paradigm shift in understanding host defense. Nat Immunol 2015; 16:675–9. [DOI] [PubMed] [Google Scholar]
- 15. Rakers S, Niklasson L, Steinhagen D, Kruse C, Schauber J, Sundell K et al Antimicrobial peptides (AMPs) from fish epidermis: perspectives for investigative dermatology. J Invest Dermatol 2013; 133:1140–9. [DOI] [PubMed] [Google Scholar]
- 16. Matsui T, Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol 2015; 27:269–80. [DOI] [PubMed] [Google Scholar]
- 17. Kueneman JG, Parfrey LW, Woodhams DC, Archer HM, Knight R, McKenzie VJ. The amphibian skin‐associated microbiome across species, space and life history stages. Mol Ecol 2014; 23:1238–50. [DOI] [PubMed] [Google Scholar]
- 18. Lillywhite HB. Water relations of tetrapod integument. J Exp Biol 2005; 209:202–26. [DOI] [PubMed] [Google Scholar]
- 19. Alibardi L, Toni M, Valle LD. Hard cornification in reptilian epidermis in comparison to cornification in mammalian epidermis. Exp Dermatol 2007; 16:961–76. [DOI] [PubMed] [Google Scholar]
- 20. Bowdish DM, Davidson DJ, Hancock RE. A re‐evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci 2005; 6:35–51. [DOI] [PubMed] [Google Scholar]
- 21. Akinbi HT, Epaud R, Bhatt H, Weaver TE. Bacterial killing is enhanced by expression of lysozyme in the lungs of transgenic mice. J Immunol 2000; 165:5760–6. [DOI] [PubMed] [Google Scholar]
- 22. Zasloff M. Antimicrobial peptides of multicellular organism. Nature 2002; 415:389–95. [DOI] [PubMed] [Google Scholar]
- 23. Mescher A, Neff A. Limb regeneration in amphibians: immunological considerations. Sci World J 2006; 6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Immerseel F, Methner U, Rychlik I, Nagy B, Velge P, Martin G et al Vaccination and early protection against non‐host‐specific Salmonella serotypes in poultry: exploitation of innate immunity and microbial activity. Epidemiol Infect 2005; 133:959–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lillehoj HS, Min W, Dalloul RA. Recent progress on the cytokine regulation of intestinal immune responses to Eimeria . Poult Sci 2004; 83:611–23. [DOI] [PubMed] [Google Scholar]
- 26. Chin KN, Wong WC Some ultrastructural observations on the intestinal mucosa of the toad (Bufo melanostictus). J Anat 1977; 123:331–9. [PMC free article] [PubMed] [Google Scholar]
- 27. Mcmillan DN, Secobes CJ. Isolation of rainbow trout (Oncorhynchus mykiss) intestinal intraepithelial lymphocytes (IEL) and measurement of their cytotoxic activity. Fish Shellfish Immunol 1997; 7:527–41. [Google Scholar]
- 28. Osogoe B, Tsujii T, Liszczynsky HR, Naora H, Seno S. Intestinal mucosa and intra‐abdominal lymphoid tissues of the platypus, Ornithorhynchus anatinus . Okajimas Folia Anat Jpn 1991; 67:457–66. [DOI] [PubMed] [Google Scholar]
- 29. Tregaskes CA, Kong FK, Paramithiotis E, Chen CL, Ratcliffe MJ, Davison TF et al Identification and analysis of the expression of CD8 αβ and CD8 αα isoforms in chickens reveals a major TCR‐γδ CD8 αβ subset of intestinal intraepithelial lymphocytes. J Immunol 1995; 154:4485–94. [PubMed] [Google Scholar]
- 30. Birkemo GA, Luders T, Andersen O, Nes IF, Nissen‐Meyer J. Hipposin, a histone‐derived antimicrobial peptide in Atlantic halibut (Hippoglossus hippoglossus L.). Biochim Biophys Acta 2003; 1646:207–15. [DOI] [PubMed] [Google Scholar]
- 31. Irwin DM, Biegel JM, Stewart CB. Evolution of the mammalian lysozyme gene family. BMC Evol Biol 2011; 11:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ostrovsky D, Snyder JA, Iwata T, Izaka K, Maglott DS, Nace GW. Frog lysozyme. I. Its identification, occurrence as isozymes, and quantitative distribution in tissues of the leopard frog, Rana pipiens . J Exp Zool 1976; 195:279–90. [DOI] [PubMed] [Google Scholar]
- 33. Xu N, Pan J, Liu S, Xue Q, Zhang S. Three in one: identification, expression and enzymatic activity of lysozymes in amphioxus. Dev Comp Immunol 2014; 46:508–17. [DOI] [PubMed] [Google Scholar]
- 34. Thammasiriak S, Ponkham P, Preecharram S, Khanchuan R, Phonyothee P, Daduang S et al Purification, characterization and comparison of reptile lysozymes. Comp Biochem Physiol 2006; 143:209–17. [DOI] [PubMed] [Google Scholar]
- 35. Mille S, Bennetta J, Leeb K, Haub M, Klasing K. Quantifying and comparing constitutive immunity across avian species. Dev Comp Immunol 2007; 31:188–201. [DOI] [PubMed] [Google Scholar]
- 36. White TJ, Mross GA, Osserman EF, Wilson AC. Primary structure of rat lysozyme. Biochemistry 1977; 16:1430–6. [DOI] [PubMed] [Google Scholar]
- 37. Tellez GA, Castaño JC. Péptidos antimicrobianos. Infectio 2010; 14:55–67. [Google Scholar]
- 38. Stegemann C, Kolobov A Jr, Leonova YF, Knappe D, Shamova O, Ovchinnikova TV et al Isolation, purification and de novo sequencing of TBD‐1, the first β‐defensin from leukocytes of reptiles. Proteomics 2009; 9:1364–73. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Hong J, Liu X, Yang H, Liu R, Wu J et al Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotic. PLoS ONE 2008; 3:e3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hilton KB, Lambert LA. Molecular evolution and characterization of hepcidin gene products in vertebrates. Gene 2008; 415:40–8. [DOI] [PubMed] [Google Scholar]
- 41. Lakshminarayanan R, Vivekanandan S, Samy RP, Banerjee Y, Chi‐Jin EO, Teo KW et al Structure, self‐assembly, and dual role of a β‐defensin‐like peptide from the Chinese soft‐shelled turtle eggshell matrix. J Am Chem Soc 2008; 130:4660–8. [DOI] [PubMed] [Google Scholar]
- 42. Colon JM, Sonnevend A, Davidson C, Smith DD, Nielsen PF. The ascaphins: a family of antimicrobial peptides from the skin secretions of the most primitive extant frog, Ascaphus truei . Biochem Biophys Res Commun 2004; 320:170–5. [DOI] [PubMed] [Google Scholar]
- 43. Colon JM, Bevier CR, Coquet L, Leprince J, Jouenne T, Vandry H. Peptidomic analysis of skin secretions supports separate species status for the tailed frogs, Ascaphus truei and Ascaphus montanus . Comp Biochem Physiol 2007; 2:121–5. [DOI] [PubMed] [Google Scholar]
- 44. Mangoni ML, Marcellini HG, Simmaco M. Biological characterization and modes of action of temporins and bombinins H, multiple forms of short and mildly cationic anti‐microbial peptides from amphibian skin. J Pept Sci 2007; 13:603–13. [DOI] [PubMed] [Google Scholar]
- 45. Colon JM, Demandt A, Nielsen F, Leprince J, Vaundry H, Woodhams D. The alyteserins: two families of antimicrobial peptides from the skin secretions of the midwife toad Alytes obstetricans (Alytidae). Peptides 2009; 30:1069–73. [DOI] [PubMed] [Google Scholar]
- 46. Georgieva TM, Koinarski VN, Urumova VS, Marutsov PD, Christov TT, Nikolov J et al Effects of Escherichia coli infection and Eimeria tenella invasion on blood concentrations of some positive acute phase proteins (haptoglobin (PIT 54), fibrinogen and ceruloplasmin) in chickens. Revue Méd Vét 2010; 161:84–9. [Google Scholar]
- 47. Vasta GR, Ahmed H, Odom EW. Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates. Curr Opin Struct Biol 2004; 14:617–30. [DOI] [PubMed] [Google Scholar]
- 48. Huong Giang DT, Van Driessche E, Vandenberghe I, Devreese B, Beeckmans S. Isolation and characterization of SAP and CRP, two pentraxins from Pangasianodon (Pangasius) hypophthalmus . Fish Shellfish Immunol 2010; 28:743–53. [DOI] [PubMed] [Google Scholar]
- 49. Raida MK, Buchmann K. Innate immune response in rainbow trout (Oncorhynchus mykiss) against primary and secondary infections with Yersinia ruckeri O1. Dev Comp Immunol 2009; 33:35–45. [DOI] [PubMed] [Google Scholar]
- 50. Lin B, Chen S, Cao Z, Lin Y, Mo D, Zhang H et al Acute phase response in zebrafish upon Aeromonas salmonicida and Staphylococcus aureus infection: striking similarities and obvious differences with mammals. Mol Immunol 2007; 44:295–301. [DOI] [PubMed] [Google Scholar]
- 51. Zhou X, Wang L, Feng H, Guo Q, Dai H. Acute phase response in Chinese soft‐shelled turtle (Trionyx sinensis) with Aeromonas hydrophila infection. Dev Comp Immunol 2011; 35:441–51. [DOI] [PubMed] [Google Scholar]
- 52. Peatman E, Baoprasertkul P, Terhune J, Xu P, Nandi S, Kucuktas H et al Expression analysis of the acute phase response in channel catfish (Ictalurus punctatus) after infection with a Gram‐negative bacterium. Dev Comp Immunol 2007; 31:1183–96. [DOI] [PubMed] [Google Scholar]
- 53. Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res 2010; 20:34–50. [DOI] [PubMed] [Google Scholar]
- 54. Zhang S, Wang C, Wang Y, Wei R, Jiang G, Ju H. Presence and characterization of complement‐like activity in the amphioxus Branchiostoma belcheri tsingtauense . Zoolog Sci 2003; 20:1207–14. [DOI] [PubMed] [Google Scholar]
- 55. Kang YH, Tan LA, Carroll MV, Gentle ME, Sim RB. Target pattern recognition by complement proteins of the classical and alternative pathways. Adv Exp Med Biol 2009; 653:117–28. [DOI] [PubMed] [Google Scholar]
- 56. Legler D, Evans E. Comparative Immunology. Haemolytic Complement in Amphibia. Proc Soc Exp Biol Med 1966; 121:1158–62. [DOI] [PubMed] [Google Scholar]
- 57. Merchant ME, Roche C, Elsey RM, Prudhomme J. Antibacterial properties of serum from the American alligator (Alligator mississippiensis). Comp Biochem Physiol B Biochem Mol Biol 2003; 136:505–13. [DOI] [PubMed] [Google Scholar]
- 58. Merchant ME, Pallansch M, Paulman RL, Wells JB, Nalca A, Ptak R. Antiviral activity of serum from the American alligator (Alligator mississippiensis). Antiviral Res 2005; 66:35–8. [DOI] [PubMed] [Google Scholar]
- 59. Merchant M, Thibodeaux D, Loubser K, Elsey RM. Amoebicidal effects of serum from the American alligator (Alligator mississippiensis). J Parasitol 2004; 90:1480–3. [DOI] [PubMed] [Google Scholar]
- 60. Matson KD, Ricklefsa R, Klasing KC. A hemolysis–hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev Comp Immunol 2005; 29:275–86. [DOI] [PubMed] [Google Scholar]
- 61. Ribas L, Li MS, Doddington BJ, Robert J, Seidel JA, Kroll JS et al Expression profiling the temperature‐dependent amphibian response to infection by Batrachochytrium dendrobatidis . PLoS ONE 2009; 4:e8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parmentier HK, Baelmans R, Nieuwland MG, Dorny P, Demey F. Haemolytic complement activity, C3 and Factor B consumption in serum from chickens divergently selected for antibody responses to sheep red blood cells. Vet Immunol Immunopathol 2002; 90:91–100. [DOI] [PubMed] [Google Scholar]
- 63. O'Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol 2002; 2:37–45. [DOI] [PubMed] [Google Scholar]
- 64. Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res 2008; 79:360–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen SN, Huang B, Zhang XW, Li Y, Zhao LJ, Li N et al IFN‐γ and its receptors in a reptile reveal the evolutionary conservation of type II IFNs in vertebrates. Dev Comp Immunol 2013; 41:587–96. [DOI] [PubMed] [Google Scholar]
- 66. Grayfer L, De Jesus Andino F, Robert J. The amphibian (Xenopus laevis) type I interferon response to frog virus 3: new insight into ranavirus pathogenicity. J Virol 2014; 88:5766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zou J, Tafalla C, Truckle J, Secombes CJ. Identification of a second group of type I IFNs in fish sheds light on IFN evolution in vertebrates. J Immunol 2007; 179:3859–71. [DOI] [PubMed] [Google Scholar]
- 68. Yu FF, Zhang YB, Liu TK, Liu Y, Sun F, Jiang J et al Fish virus‐induced interferon exerts antiviral function through Stat1 pathway. Mol Immunol 2010; 47:2330–41. [DOI] [PubMed] [Google Scholar]
- 69. Goodbourn S, Didcock L, Randall E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol 2000; 81:2341–64. [DOI] [PubMed] [Google Scholar]
- 70. Staeheli P, Puehler F, Schneider K, Gobel TW, Kaspers B. Cytokines of birds: conserved functions – a largely different look. J Interferon Cytokine Res 2001; 21:993–1010. [DOI] [PubMed] [Google Scholar]
- 71. Grayfer L, De Jesus Andino F, Robert J. Prominent amphibian (Xenopus laevis) tadpole type III interferon response to the frog virus 3 ranavirus. J Virol 2015; 89:5072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zou J, Yoshiura Y, Dijkstra JM, Sakai M, Ototake M, Secombes C. Identification of an interferon γ homologue in Fugu, Takifugu rubripes . Fish Shellfish Immunol 2004; 17:403–9. [DOI] [PubMed] [Google Scholar]
- 73. Igawa D, Sakai M, Savan R. An unexpected discovery of two interferon γ‐like genes along with interleukin (IL)‐22 and 26 from teleost: IL‐22 and 26 genes have been described for the first time outside mammals. Mol Immunol 2006; 43:999–1009. [DOI] [PubMed] [Google Scholar]
- 74. Qi ZT, Nie P. Comparative study and expression analysis of the interferon γ gene locus cytokines in Xenopus tropicalis . Immunogenetics 2008; 60:699–710. [DOI] [PubMed] [Google Scholar]
- 75. Guanchun C, Jacques R. Antiviral Immunity in Amphibians. Viruses 2011; 3:2065–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lowenthal JW, Staeheli P, Schultz U, Sekellick MJ, Marcus PI. Nomenclature of avian interferon proteins. J Interferon Cytokine Res 2001; 21:547–9. [DOI] [PubMed] [Google Scholar]
- 77. Zhou X, Guo Q, Dai H. Molecular characterization and expression profiles in response to bacterial infection of Chinese soft‐shelled turtle interleukin‐8 (IL‐8), the first reptilian chemokine gene. Dev Comp Immunol 2009; 33:838–47. [DOI] [PubMed] [Google Scholar]
- 78. Mondal S, Rai U. In vitro effect of temperature on phagocytic and cytotoxic activities of splenic phagocytes of the wall lizard, Hemidactylus flaviviridis . Comp Biochem Physiol A Mol Integr Physiol 2001; 129:391–8. [DOI] [PubMed] [Google Scholar]
- 79. Laskin L, Weinberger B, Laskin J. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol 2001; 70:163–70. [PubMed] [Google Scholar]
- 80. Bubanovic I, Najman S. Diversification of Cytokines Across Vertebrate Immune System Evolution, Reproductive Efficacy and Tumor Escape. 12th International Congress of Immunology and 4th annual Conference of FOCIS. Montreal, Canada, 2004:369‐74.
- 81. Laing KJ, Zou JJ, Wang T, Bols N, Hirono I, Aoki T et al Identification and analysis of an interleukin 8‐like molecule in rainbow trout Oncorhynchus mykiss . Dev Comp Immunol 2002; 26:433–44. [DOI] [PubMed] [Google Scholar]
- 82. Lynagh GR, Bailey M, Kaiser P. Interleukin 6 is produced during both murine and avian Eimeria infections. Vet Immunol Immunopathol 2000; 76:89–102. [DOI] [PubMed] [Google Scholar]
- 83. Schneider K, Puehler F, Baeuerle D, Elvers S, Staeheli P, Kaspers B et al cDNA cloning of biologically active chicken interleukin‐18. J Interferon Cytokine Res 2000; 20:879–83. [DOI] [PubMed] [Google Scholar]
- 84. Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D et al Human MAIT cells are xenobiotic‐resistant, tissue‐targeted, CD161hi IL‐17‐secreting T cells. Blood 2011; 117:1250–9. [DOI] [PubMed] [Google Scholar]
- 85. Neil DR, Heng S, Bellosi A, Flynn RJ, Daly M, Langford TK et al Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature 2010; 464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Snyth MJ et al IL‐21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 2007; 178:2827–34. [DOI] [PubMed] [Google Scholar]
- 87. Vivier E, Spits H, Cupedo T. Interleukin‐22 producing innate immune cells: new players in mucosal immunity and tissue repair. Nat Rev Immunol 2009; 9:229–34. [DOI] [PubMed] [Google Scholar]
- 88. Liu X, Ji XM, Du XN, Zong XC, Liang DF, Ma L et al Molecular cloning, expression, bioinformatics analysis, and bioactivity of TNFSF13 (APRIL) in the South African clawed frog (Xenopus laevi): a new model to study immunological diseases. OMICS 2013; 17:384–92. [DOI] [PubMed] [Google Scholar]
- 89. Huminiecki L, Goldovsky L, Freilich S, Moustakas A, Ouzounis C, Heldin CH. Emergence, development and diversification of the TGF‐β signalling pathway within the animal kingdom. BMC Evol Biol 2009; 9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Taylor AW. Review of the activation of TGF‐β in immunity. J Leukoc Biol 2009; 85:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol 2005; 26:347–62. [DOI] [PubMed] [Google Scholar]
- 92. Sinyakov MS, Dror M, Zhevelev HM, Margel S, Avatalion R. Natural antibodies and their significance in active immunization and protection against a defined pathogen in fish. Vaccine 2002; 20:3668–74. [DOI] [PubMed] [Google Scholar]
- 93. Morrison RN, Bruce Lyons A, Nowak BF, Hayball JD. Assessment of snapper (Pagrus auratus) natural IgM binding to bromelain treated sheep erythrocytes. Fish Shellfish Immunol 2005; 18:91–9. [DOI] [PubMed] [Google Scholar]
- 94. Li J, Barreda DR, Zhang Y, Boshira H, Gelman AE, LaPatra S et al B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol 2006; 7:1116–24. [DOI] [PubMed] [Google Scholar]
- 95. Haimovich J, Du Pasquier L. Specificity of antibodies in amphibian larvae possessing a small number of lymphocytes. Proc Natl Acad Sci U S A 1973; 70:1898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rosenberg CE, Salibian A, Fink NE. An enzyme‐linked immunosorbent assay for measuring anti‐sheep red blood cells antibodies in lead‐exposed toads. J Pharmacol Toxicol Methods 2002; 47:121–8. [DOI] [PubMed] [Google Scholar]
- 97. Ujvari B, Madsen T. Do natural antibodies compensate for humoral immunosenescence in tropical pythons? Funct Ecol 2011; 25:813–7. [Google Scholar]
- 98. Haghighi HR, Gong J, Gyles CL, Hayes MA, Zhou H, Sanei B et al Probiotics stimulate production of natural antibodies in chickens. Clin Vaccine Immunol 2006; 13:975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Whiteman NK, Matson KD, Bollmer JL, Parker PG. Disease ecology in the Galapagos Hawk (Buteo galapagoensis): host genetic diversity, parasite load and natural antibodies. Proc Biol Sci 2006; 273:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shaw PX, Horkko S, Chang M, Curtiss L, Palinski W, Silverman GJ et al Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest 2000; 105:1731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vollmers HP, Brandlein S. The, “early birds”: natural IgM antibodies and immune surveillance. Histol Histopathol 2005; 20:927–37. [DOI] [PubMed] [Google Scholar]
- 102. Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol 2001; 2:997–1003. [DOI] [PubMed] [Google Scholar]
- 103. Klasing KC, Adler KL, Remus JC, Calvert CC. Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidia‐infected chicks and increases functional properties of phagocytes. J Nutr 2002; 132:2274–82. [DOI] [PubMed] [Google Scholar]
- 104. Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect 2007; 9:1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Levy O. Antimicrobial proteins and peptides of blood: templates for novel antimicrobial agents. Blood 2000; 96:2664–72. [PubMed] [Google Scholar]
- 106. Bielecki T, Dohan Ehrenfest DM, Everts PA, Wiczkowski A. The role of leukocytes from L‐PRP/L‐PRF in wound healing and immune defense: new perspectives. Curr Pharm Biotechnol 2012; 13:1153–62. [DOI] [PubMed] [Google Scholar]
- 107. Nausee WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 2007; 219:88–102. [DOI] [PubMed] [Google Scholar]
- 108. Renshaw SA, Trede NS. A model 450 million years in the making: zebrafish and vertebrate immunity. Dis Model Mech 2012; 5:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Neumann NF, Stafford JL, Barreda D, Ainsworth AJ, Belosevic M. Antimicrobial mechanisms of fish phagocytes and their role in host defense. Dev Comp Immunol 2001; 25:807–25. [DOI] [PubMed] [Google Scholar]
- 110. Zimmerman LM, Vogel LA, Edwards KA, Bowden RM. Phagocytic B cells in a reptile. Biol Lett 2010; 6:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ellis AE. Innate host defense mechanism of fish against viruses and bacteria. Dev Comp Immunol 2001; 25:827–39. [DOI] [PubMed] [Google Scholar]
- 112. Buehler DM, Encinas‐Viso F, Petit M, Vezina F, Tieleman BI, Piersma T. Limited access to food and physiological trade‐offs in a long‐distance migrant shorebird. II. Constitutive immune function and the acute‐phase response. Physiol Biochem Zool 2009; 82:561–71. [DOI] [PubMed] [Google Scholar]
- 113. Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells – how did we miss them? Nat Rev Immunol 2013; 13:75–87. [DOI] [PubMed] [Google Scholar]
- 114. Parra D, Rieger A, Li J, Zhang Y, Randall L, Hunter C et al Pivotal Advance: peritoneal cavity B‐1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol 2012; 91:525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Caligiuri MA. Human natural killer cells. Blood 2008; 112:461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Goyos A, Robert J. Tumorigenesis and anti‐tumor immune responses in Xenopus . Front Biosci 2009; 14:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kakimia K, Guidottia L, Koezukab Y, Chisaria F. Natural killer T cell activation inhibits hepatitis B virus replication in vivo . J Exp Med 2000; 192:921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Utke K, Bergmann S, Lorenzen N, Kollner B, Ototake M, Fischer U. Cell‐mediated cytotoxicity in rainbow trout, Oncorhynchus mykiss, infected with viral haemorrhagic septicaemia virus. Fish Shellfish Immunol 2007; 22:182–96. [DOI] [PubMed] [Google Scholar]
- 119. Horton TL, Minter R, Stewart R, Ritchie P, Watson MD, Horton JD. Xenopus NK cells identified by novel monoclonal antibodies. Eur J Immunol 2000; 30:604–13. [DOI] [PubMed] [Google Scholar]
- 120. Gobel T, Chen C, Shrimpflo J, Grossin C, Bernoto A, Bucy P et al Characterization of avian natural killer cells and their intracellular CD3 protein complex. J Immunol 1994; 24:1685–01. [DOI] [PubMed] [Google Scholar]
- 121. Gobel TW, Kaspers B, Stangassinger M. NK and T cells constitute two major, functionally distinct intestinal epithelial lymphocyte subsets in the chicken. Int Immunol 2001; 24:757–62. [DOI] [PubMed] [Google Scholar]
- 122. Muñoz F, De La Fuente M. The immune response of thymic cells from the turtle Mauremys caspica . J Comp Physiol 2001; 171:195–200. [DOI] [PubMed] [Google Scholar]
- 123. Bendelac A, Savage P, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007; 25:297–336. [DOI] [PubMed] [Google Scholar]
- 124. Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V et al Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue‐homing properties. Proc Natl Acad Sci U S A 2010; 107:3006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


