Summary
Calcineurin inhibitors (CNI) and mammalian target of rapamycin inhibitors (mTORi) are the main immunosuppressants used for long‐term maintenance therapy in transplant recipients to avoid acute rejection episodes. Both groups of immunosuppressants have wide effects and are focused against the T cells, although different impacts on specific T‐cell subsets, such as regulatory T cells, have been demonstrated. A greater knowledge of the impact of immunosuppression on the cellular components involved in allograft rejection could facilitate decisions for individualized immunosuppression when an acute rejection event is suspected. Memory T cells have recently gained focus because they might induce a more potent response compared with naive cells. The impact of immunosuppressants on different memory T‐cell subsets remains unclear. In the present study, we have studied the specific impact of CNI (tacrolimus) and mTORi (rapamycin and everolimus) over memory and naive CD4+ T cells. To do so, we have analysed the proliferation, phenotypic changes and cytokine synthesis in vitro in the presence of these immunosuppressants. The present work shows a more potent effect of CNI on proliferation and cytokine production in naive and memory T cells. However, the mTORi permit the differentiation of naive T cells to the memory phenotype and allow the production of interleukin‐2. Taken together, our data show evidence to support the combined use of CNI and mTORi in transplant immunosuppression.
Keywords: cell differentiation, cell proliferation, cytokines, T cells, transplantation
Abbreviations
- APC
allophycocyanin
- CNI
calcineurin inhibitors
- IFN‐γ
interferon‐γ
- IL
interleukin
- IS
immunosuppression
- mTORi
mammalian target of rapamycin inhibitors
- PE
phycoerythrin
- Tcm
central memory T cells
- Tem
effector memory T cells
- Temra
terminally differentiated memory T cells
- Tn
naive T cells
- Treg
regulatory T cells
Introduction
Helper T cells coordinate the adaptive immune response through cytokine production that modulates not only cellular but also humoral immunity after the challenge against the antigen they are designed for. After the immune response clears the antigen, two different subsets of memory T cells arise; central memory T (Tcm) cells, confined in lymphoid tissues with low proliferative and cytokine production capability, and effector memory T (Tem) cells, which are generally present in non‐lymphoid tissues and are able to be quickly activated with a higher cytokine production than Tcm cells.1 After antigen recall, Tcm cells could differentiate into Tem cells.2 In the transplantation setting, the role of Tcm and Tem subpopulations in acute or chronic rejection of the allograft has not been clearly defined.
A rejection event driven by memory T cells is faster and more potent than that mediated by naive T (Tn) cells. Recently, donor‐specific memory T cells have been associated with early acute cellular rejection in kidney transplantation and increased numbers of circulating CD8 Tem cells before transplantation have been associated with enhanced acute rejection in lung transplant patients.3 On the other hand, there is little evidence about the role of memory T‐cell subsets in human lung transplantation rejection.4 In a cardiac allograft mouse model, neither rapamycin nor tacrolimus alone was able to control accelerated rejection of heart allograft by alloreactive memory CD4+ T cells,5 however, the specific effect of immunosuppression (IS) on memory T cells is not fully described.
With the use of new and potent IS, the acute rejection rate in kidney transplantation has been reduced to < 5% within the first year after kidney transplantation, although the rate of chronic rejection in the long‐term is still far from being reduced.6 The immunosuppression therapy currently used relieves the alloresponse without precisely knowing the targeted cell type.7
The impact of different IS on memory T cells has been previously assessed in an in vitro model of CD8 Tem differentiation,8 whereas studies on CD4 T cells are scarce. The present study addresses the direct impact of the two main IS used for maintenance therapy in transplant patients on different aspects of sorted naive and memory CD4+ T‐cell subsets, such as phenotype, proliferation capability and cytokine production.
Materials and methods
Sample preparation
Peripheral blood mononuclear cells were isolated on a Ficoll gradient from buffy coats gathered at the Regional Blood Donor Bank after given consent. Subsequently, the peripheral blood mononuclear cells were incubated with anti‐CD4 and anti‐CD8 magnetic bead antibodies (Miltenyi Biotech, Bergisch Gladbach, Germany) following the manufacturer's instructions and sorted by magnetic‐automated cell sorting (AutoMACS; Miltenyi Biotech). The cells were split into CD4+ and CD8+ cells and stained with anti‐CCR7‐allophycocyanin (APC; clone G043H7; Biolegend, San Diego, CA), anti‐CD45RO‐phycoerythrin (PE; clone UCHL1), anti‐CD62L‐ FITC (clone Dreg56) and anti‐CD25‐PE (clone 2A3) (both from BD Biosciences, San Jose, CA), anti‐CD27‐APC‐Vio770 (clone M‐T271; Miltenyi Biotech), anti‐CD127‐PE‐cyanin‐7 (PC7; clone R34.34; Beckman Coulter, Marseille, France) during 30 min, washed with PBS and acquired for FACS on a FACS‐Aria‐II (BD Biosciences).
The different T‐cell subpopulations were defined as follows. Tn: CCR7+ CD62L− CD45RO−, Tcm: CCR7− CD62L+ CD45RO+, Tem: CCR7− CD62L− CD45RO+, terminally differentiated memory T (Temra) cells: CCR7− CD62L− CD45RO− and regulatory T (Treg) cells: CD25+ CD27+ CD127−.
The purity of the cell sorting was tested after each experiment, and > 98% efficiency was considered acceptable for the study. All the experimental conditions were replicated at least three times using blood from different donors.
In vitro culture
Upon MACS and FACS sorting, 105 cells/well were stimulated with anti‐CD3 and CD28 beads (Dynabeads; Life Technologies AS, Oslo, Norway) following the manufacturer's instructions for T‐cell activation, and in the presence/absence of different concentrations of tacrolimus, rapamycin and everolimus (Sigma Aldrich, St Louis, MO) in a U‐bottomed 96‐well plate. The final concentrations of the IS used were established at dosages that effectively inhibited the proliferation and cytokine production of Tn cells. The working drug concentration ranges were: tacrolimus (2–0·25 ng/ml), rapamycin (10–0·01 ng/ml) and everolimus (20–2·5 μg/ml).
Phenotypic assay
After 120 hr of culture, cells were collected, washed and stained with CCR7‐APC, CD45RO‐PE, CD62L‐FITC, CCR6‐PE, CD25‐PE and CD103‐PE monoclonal antibodies to assess the phenotypic changes of each subpopulation before acquisition by FACS‐Canto‐II (BD Biosciences) cytometer. The data were analysed on facs‐diva software, 6.3.1 version (BD Biosciences).
Proliferation assays
Once the different subsets of memory and naive T cells were harvested, the cells (from 5 × 105 to 5 × 107 sorted cells) were suspended in PBS–fetal bovine serum 5% (volume/volume; PAA Laboratories, Pasching, Austria) and stained with 5 nm of carboxyfluorescein diacetate succinimidyl ester, and incubated for 5 min at room temperature. The cells were washed again with PBS–fetal bovine serum 5% and cultured for 120 hr before acquisition on FACS‐Canto‐II and further analysis with flowjo software (Tree Stars, Ashland, OR) was performed. We analysed the total number of divisions, divided by the number of cells that went into division (Proliferation Index), and the average number of cell divisions (% Divided), showing only % Divided data because it facilitates comparisons in mild proliferation samples.
Analysis of cell viability
To assess the apoptosis after 5 days of culture, the cells were stained with 7‐amino‐actinomycin D (Beckman Coulter) before acquisition by FACS‐Canto‐II cytometer. The cell death was estimated from 7‐ amino‐actinomycin D‐positive cells within the lymphocyte gate.
Cytokine production test
After 120 hr of culture at 37° in 5% CO2, for the cytokine production test, the cells were re‐stimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml) in the presence of the stop‐Golgi reagent brefeldin‐A (10 μg/ml; all from Sigma Aldrich) for 4 hr. Subsequent permeabilization (FACS Permeabilizing Solution II; BD Biosciences) and staining with monoclonal antibodies to different cytokines was performed. Interleukin‐2 (IL‐2) ‐APC, interferon‐γ (IFN‐γ) ‐PE and IL‐17‐FITC (all antibodies from BD Biosciences) were incubated for 30 min and cells were washed before acquisition in a FACS‐Canto‐II cytometer.
Statistical analysis
The difference of the means for all parameters was tested by the Student's t‐test and P < 0·05 was considered significant (see Supplementary material, Tables S1–S3). The IS dose–effect was tested using the repeated‐measures two‐way analysis of variance with graphpad software version 5·0 (GraphPad Inc.,San Diego, CA). Bonferroni post hoc test was used when appropriate. The IS dose–effect significance was determined by the interaction factor of each IS treatment compared with stimulated control and expressed in the text as FDOSE (Figs 2, 3, 4).
Figure 2.
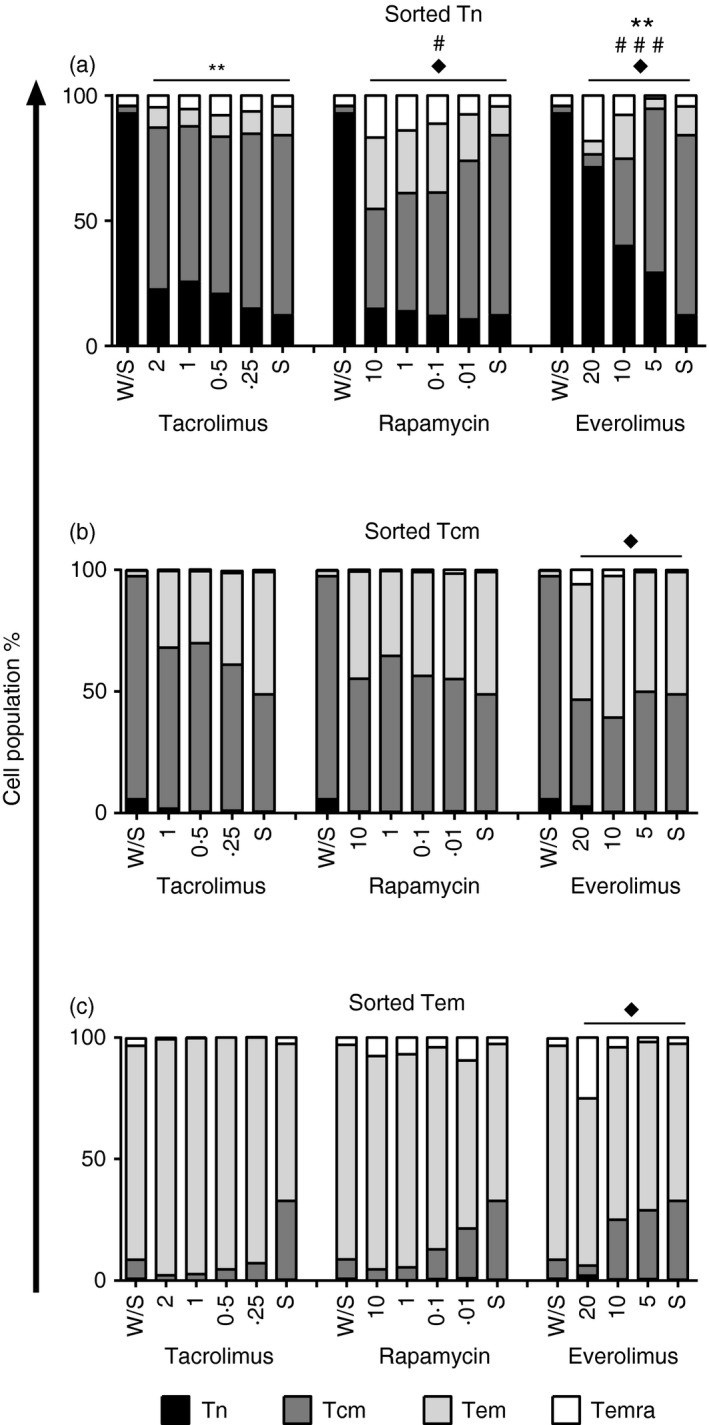
Relative phenotypic changes of naive and memory T‐cell subsets upon immunosuppression treatment. The sorted subpopulations: naive (Tn; n = 4) (a), central memory (Tcm; n = 4) (b) and effector memory (Tem; n = 3) (c) T cells were polyclonally activated with anti‐CD3/CD28 beads (S) or left alone (W/S) for 5 days and stained with CCR7, CD45RO and CD62L as described in the Materials and methods section. Three independent experiments were performed (mean, standard error of mean, and their comparison with stimulated control are shown in the Supplementary material, Table S1). The different subsets of T lymphocytes were co‐cultured with different amounts of immunosuppressants: tacrolimus (Tac, ranged from 2 to 0·25 ng/ml), rapamycin (Rapa, ranged from 10 to 0·01 ng/ml) and everolimus (Eve, ranged from 20 to 5 µg/ml). The interaction between each treatment and stimulated control was assessed by repeated‐measures two way analysis of variance test and expressed in the graphs: sorted Tn–tacrolimus [Tn: FDOSE(3,18) = 9·14, P < 0·01**], sorted Tn‐rapamycin [Tcm: FDOSE(3,15) = 3·93, P < 0·05#; Temra: FDOSE(3,15) = 4·29, P < 0·05♦], sorted Tn‐everolimus [Tn: FDOSE(2,8) = 10·03, P < 0·01**; Tcm: FDOSE(2,8) = 7·02, P < 0·001###; Temra: FDOSE(2,8) = 7·02, P < 0·05♦[, sorted Tcm‐everolimus [Temra: FDOSE(2,8) = 3·08, P < 0·05♦], sorted Tem‐everolimus [Temra: FDOSE(2,15) = 4·2, P < 0·05♦].
Figure 3.
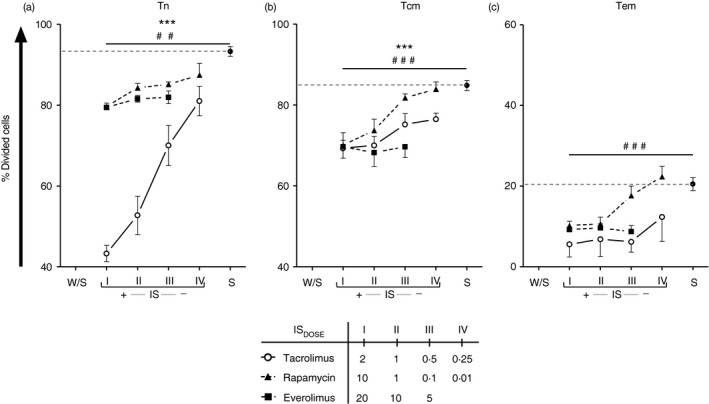
Proliferation of sorted naive and memory T cells with different immunosuppressant. Sorted naive (Tn; n = 4) (a), central memory (Tcm; n = 6) (b) and effector memory (Tem; n = 3) T cells (c) were stained with carboxyfluorescein diacetate succinimidyl ester before polyclonal stimulation with anti‐CD3/CD28 beads, during 5 days. The cells were alone (W/S), stimulated (S) and treated with the indicated dose of tacrolimus (Tac, open circles), rapamycin (Rapa, closed triangle and dotted line) or everolimus (Eve, closed squares and dotted line) (mean, standard error of mean, and their comparison respect stimulated control are shown in the Supplementary material, Table S2). The proliferation was expressed by the percentage of dividing cells (%Divided). The reference of stimulated control is represented with a dotted line in each plot. The interaction between each treatment and stimulated control was assessed by repeated‐measures two‐way analysis of variance test [sorted Tn‐tacrolimus: FDOSE(3,24) = 26·08, P < 0·01**; sorted Tn‐rapamycin: FDOSE(3,24) = 4·78, P < 0·001###; sorted Tcm‐tacrolimus: FDOSE(3,36) = 7·74, P < 0·001***; sorted Tcm‐rapamycin: FDOSE(3,36) = 13·85, P < 0·001###; sorted Tem‐rapamycin: FDOSE(3,6) = 77·86, P < 0·001###].
Figure 4.
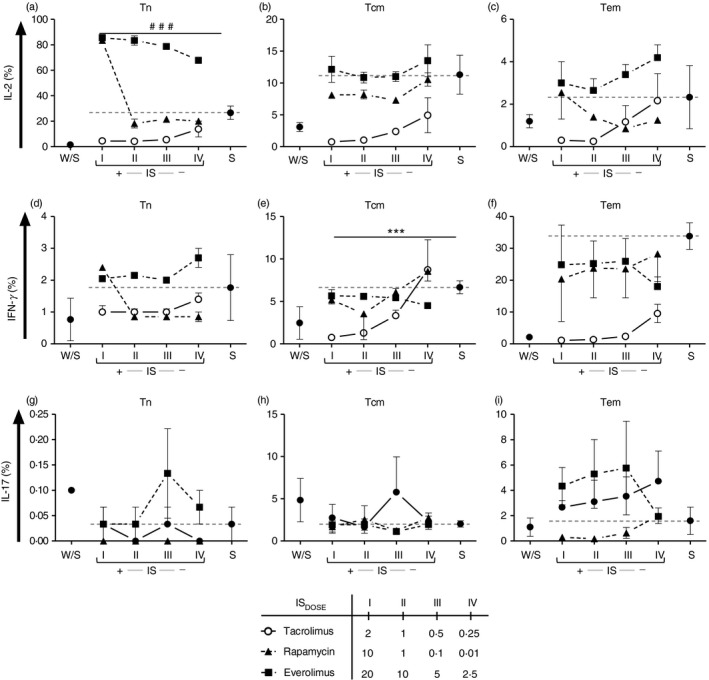
Cytokine production by T‐lymphocyte subpopulations after immunosuppression treatment. The percentage of interleukin‐2 (IL‐2) (a–c), interferon‐γ (IFN‐γ) (d–f) and IL‐17 (g–i) of sorted naive (Tn; n = 3) (a,d), central memory (Tcm; n = 3) (b,e) and effector memory (Tem; n = 3) (c,f) T cells was measured after 5 days of culture with polyclonal stimulation with anti‐CD3/CD28 beads and re‐stimulation with PMA, ionomycin and brefeldin‐A. Each condition came from at least three independent experiments. The cells were alone (W/S), stimulated (S) and treated with the indicated dose of tacrolimus (Tac, open circles), rapamycin (Rapa, closed triangle and dotted lined) or everolimus (Eve, closed squares and dotted line) (mean, standard error of mean, and their comparison respect stimulated control are shown in the Supplementary material, Table S3). The reference of stimulated control is represented with a dotted line in each plot. The interaction between each treatment and stimulated control was assessed by repeated‐measures two‐way analysis of variance test [sorted Tn‐rapamycin, IL‐2: FDOSE: (3,9) = 353·5, P < 0·001###, sorted Tcm‐tacrolimus, IFN‐γ: FDOSE: (3,9) = 23·16, P < 0·001***].
Results
Phenotypic changes of naive and memory subsets after 5 days of stimulation
The frequency of peripheral blood cell subsets from buffy coat was equivalent to that described in previous studies.1 The naive compartment was the most frequent in both CD4+ and CD8+ T lymphocytes, but a different pattern of memory T‐cell subsets was observed between CD4+ and CD8+ T cells. Hence, the Temra subpopulation was almost absent in CD4+ T cells whereas Tcm cells represented a lower fraction of CD8+ T cells (Fig. 1).1 Due to their low numbers, the Temra CD4+ T cells were discarded in these studies. The next step was to check polyclonal activation of T‐cell subsets with anti‐CD3/CD28 beads at different rates (see Supplementary material, Fig. S1), showing that increasing doses of beads did not improve the proliferation achieved by 1 : 1 rate (recommended by the manufacturer). We then assessed the phenotypic changes of sorted T cells after 5 days of culture (see Supplementary material, Fig. S2). Naive and memory T‐cell subsets conserved their phenotypic profile without stimulation (see Supplementary material, Fig. S2). However, after in vitro activation, the majority of sorted Tn cells changed to a Tcm‐like phenotype whereas < 15% remained with Tn‐like phenotype (see Supplementary material, Fig. S2). Half of the sorted and activated Tcm cells converted to a Tem‐like phenotype and two‐thirds of sorted Tem cells preserved their Tem‐like phenotype after culture (see Supplementary material, Fig. S2). Hence, under the same culture conditions, the phenotypic changes are more evident in Tn than Tcm and Tem.
Figure 1.
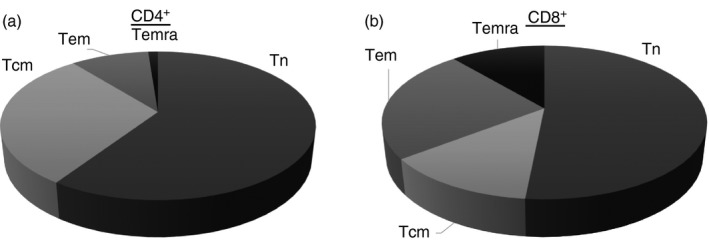
Frequencies of naive and memory T‐cell subsets after CD4+ and CD8+ T‐cell sorting in healthy controls. Differential distribution of T cells subsets based of CCR7, CD45RO and CD62L expression in CD4+ (a) and CD8+ T lymphocytes (b) is shown. The subpopulations were defined naive T cells (Tn): CCR7+ CD62L+ CD45RO−; central memory T cells (Tcm): CCR7+ CD62L+ CD45RO+; effector memory T cells (Tem): CCR7− CD62L− CD45RO+ and terminally differentiated memory T cells (Temra): CCR7− CD62L− CD45RO−.
Impact of calcineurin and mammalian target of rapamycin inhibitors on phenotypic changes of naive and memory CD4+ T‐cell subsets after 5 days of stimulation
As our in vitro activation experiments showed stronger phenotype changes in Tn cells, we assessed other cell surface markers and the impact of different immunosuppressants in activation and cell surface marker expression of the different subsets (see Supplementary material, Fig. S3). No immunosuppressant dose altered the normal cell apoptosis after culture (see Supplementary material, Fig. S4). Within the immunosuppressants, calcineurin inhibitors (CNIs) block the IL‐2 production of Tn cells, whereas mammalian target of rapamycin inhibitors (mTORi) allow IL‐2 production into the media.9 Hence, the interleukin requirement for cell differentiation may differentially affect phenotypic changes on sorted cells. In this regard, the main differences in phenotype changes were observed in sorted Tn cells. The effect of each treatment on phenotypic changes of every sorted subpopulation was studied independently. To assess this effect, the interaction of different dosages with stimuli control was studied. A slightly dose‐dependent effect on sorted Tn cells with tacrolimus and rapamycin was observed for the dosages tested. The higher dose of tacrolimus maintained an increased naive phenotype after stimulation [Fig. 2a, FDOSE(3,18) = 9·14, P < 0·01**], whereas rapamycin induced a lower Tcm [FDOSE(3,15) = 3·93, P < 0·05] higher Temra‐like phenotype differentiation [F3,15 = FDOSE(3,15) = 4·29, P < 0·05]. A significantly stronger dose‐dependent effect was observed with everolimus in naive sorted T cells, at higher doses they retain the naive phenotype [FDOSE(2,8) = 10·03, P < 0·01] with a more Temra‐like phenotype differentiation [FDOSE(2,8) = 7·02, P < 0·05] to the detriment of Tcm‐like phenotype [FDOSE(2,8) = 7·02, P < 0·001].
The dose‐dependent effect in Tcm (Fig. 2b) and Tem (Fig. 2c) sorted cells was only observed after incubation with everolimus. An increased Temra‐like differentiation with higher doses of everolimus was observed in Tcm [FDOSE(2,8) = 3·08, P < 0·05] and Tem [FDOSE(2,15) = 4·2, P < 0·05] sorted cells.
Effect of CNI and mTORi on proliferation of memory T‐cell subsets
Proliferation of T cells after the encounter with their specific antigen is one of the characteristics of the adaptive immune response. The control of T‐cell proliferation could regulate the enhancement of their effector functions and the use of the most effective IS in suppressing T‐cell proliferation might potentially better control the alloresponse. Within the naive and memory T‐cell subsets there are differences in proliferation capability since the Tn and Tcm proliferate better than Tem.1 After 5 days of culture in the absence of IS, the Tn and Tcm subpopulation achieved maximum proliferation, whereas the Tem showed a clearly decreased proliferation index (see Supplementary material, Fig. S5). To perform comparable analysis, the percentage of dividing cells was the parameter of proliferation that was tested. Tacrolimus and rapamycin (Fig. 3) reduced the proliferation capability of sorted Tn [tacrolimus: FDOSE(3,24) = 26·08, P < 0·01; rapamycin: FDOSE(3,24) = 4·78, P < 0·001], Tcm [tacrolimus: FDOSE(3,36) = 7·74, P < 0·001; rapamycin: FDOSE(3,36) = 13·85, P < 0·001] and Tem [rapamycin: FDOSE(3,6) = 77·86, P < 0·001] cells, respectively in a dose‐dependent manner; tacrolimus significantly reduced the sorted Tn cell proliferation to 43·33% versus control 92·7% (P < 0·001; see Supplementary material, Table S2) whereas both mTORi only reduced the cell proliferation to ≈ 79·5% (P < 0·001; see Supplementary material, Table S2). All IS demonstrated the same inhibition of sorted Tcm proliferation at the maximum dose tested (≈ 70% versus control 84·9%; P < 0·001; see Supplementary material, Table S2). However, while tacrolimus and everolimus maintained the percentage of dividing cells <77% at any dose, the use of decreasing doses of rapamycin recovered the frequency of proliferation (Fig. 3b; see Supplementary material, Table S2). Similarly, in sorted Tem, all IS at the highest concentration demonstrated comparable inhibition of the percentage of dividing cells but decreasing doses of rapamycin were unable to control the proliferation of sorted Tem (Fig. 3c; see Supplementary material, Table S2).
Cytokine expression of T‐cell subsets
Together with the assessment of phenotypic markers and proliferation ability, the different T‐cell subpopulations may be further characterized by their different cytokine production profile. Tn cells secrete mainly IL‐2, Tcm have moderate IL‐2 and IFN‐γ secretion, whereas Tem produce low IL‐2 and high amounts of IFN‐γ (see Supplementary material, Fig. S6). The IL‐17, another effector cytokine, was evaluated to better define T‐cell subsets (see Supplementary material, Table S3).
The cytokine expression after activation, together with data shown on proliferation, may help to demonstrate functional impairment of naive/memory T‐cell subsets. To address the effect of IS on cytokine expression in sorted T‐cell subsets, in parallel, after culture and polyclonal re‐stimulation, intracellular cytokine production was assessed (the expressed cytokine profiles of naive and memory T‐cell subsets are depicted in the Supplementary material, Fig. S6). First, activated sorted Tn cells increased the production of IL‐2 but not that of IFN‐γ (P < 0·01; see Supplementary material, Table S3), whereas the Tcm cells did not significantly demonstrate any important changes in the expression of cytokines. Sorted Tem cells produced high amounts of IFN‐γ but not of IL‐2 after in vitro activation (P < 0·05; see Supplementary material, Table S3). Production of IL‐17 was almost null in Tn sorted cells, being slightly higher (≈ 2%) in the case of Tcm and Tem cells. No immunosuppressant tested showed any effect on IL‐17 production (see Supplementary material, Table S3).
When the effect of IS on intracellular cytokine expression was analysed we found a reduction in IL‐2 production in the presence of tacrolimus for both Tn and Tcm cells (see Supplementary material, Table S3) whereas IL‐2 was overproduced at higher doses of mTORi in Tn cells (P < 0·001; see Supplementary material, Table S3). This effect was maintained with everolimus in all doses tested (Fig. 4). Tacrolimus significantly reduced IFN‐γ produced by both sorted Tcm and Tem, whereas this effect was less evident with mTORi (P, NS, Fig. 4).
Discussion
Memory phenotype T cells can arise from canonic antigenic challenge and clearance but functional memory T cells can also result from homeostatic proliferation of Tn cells in lymphopenic states.10 A frequency of 0·1–10% alloreactive T cells within the Tn cell pool has been established.11, 12 This high frequency of alloreactive T cells and their potential transit into the memory pool makes the success of tolerance induction protocols difficult.
The knowledge of the impact of IS used in the clinic on naive and memory T cells could be relevant in the choice of the IS that would better minimize the risk of rejection driven by cellular components. Most importantly, it is difficult to overcome the memory barrier to obtain transplant tolerance, and usually the induction of tolerance protocols based on Treg cells have failed to control alloresponses induced by memory T cells,13 making IS support mandatory to achieve prolonged graft survival.14 Moreover, in cardiac transplant models both CNI and mTORi administered separately failed to prevent accelerated rejection mediated by CD4+ memory T cells.5, 15
In human kidney transplantation without induction therapy, it has been recently demonstrated that the presence of donor‐specific T cells before transplantation is associated with early acute cellular rejection.3 Altogether, these data show the importance of looking for the IS treatment that is able to control memory T‐cell response.
The CNI treatment favours the in vitro generation of memory T cells16 and rapamycin has been demonstrated to have an immunostimulatory effect on the generation of memory CD8+ T cells.17 Here, we confirmed these results with tacrolimus, allowing sorted Tn cells to differentiate to Tcm cells, and rapamycin, which produced not only Tcm but also Tem phenotypes. Strikingly, Tn activated in the presence of high doses of everolimus retained a naive phenotype. There is no clear explanation for such a specific effect of everolimus compared with rapamycin. It is possible that everolimus could induce the apoptosis of activated Tn cells (see Supplementary material, Fig. S7) or that it could facilitate differentiation to a Temra phenotype (Fig. 2). The clinical consequences of such a difference between rapamycin and everolimus remain to be elucidated. On the other hand, the effects of different IS in sorted Tcm and Tem cells were moderate.
The proliferation capability of T‐cell subsets is reduced from Tn to Tcm, Tem and Temra cells.18 Tacrolimus efficiently reduced the proliferation of Tn cells, whereas the control of Tcm and Tem cells was less evident, probably because they have a lower proliferative capacity (Fig. 3). Rapamycin cannot control the proliferation of memory T cells alone, but in combination with bortezomib it synergizes and reduces the proliferation and cytokine production of memory T cells.19 At the doses tested here, rapamycin was the least effective IS in inhibiting the proliferation of Tcm and Tem cells, since a recovery of proliferation was observed with decreasing rapamycin concentration (Fig. 3). We have tested the proliferation of Tcm and Tem cells up to 100 ng/ml of rapamycin with the same results of proliferation control (see Supplementary material, Fig. S8). Hence, our data suggest that the effect of rapamycin on phenotypic changes in Tcm and Tem cells is observed even at low concentrations, but rapamycin can control the proliferation of memory T cells only at a high concentration. It can be hypothesized that rapamycin could be used at low doses in stable clinical transplant patients to avoid differentiation to memory T cells from Tn cells, but in the context of acute rejection, where T‐cell proliferation is crucial and must be blocked, the rapamycin dose should be increased or, instead, IS changes to tacrolimus should be carried out.
The recall response after polyclonal stimulation of memory CD4+ T cells is predominantly based on IL‐2 and IFN‐γ production in mouse models.20 Tacrolimus suppressed not only cytokine production from memory T cells as previously shown,21 but also from Tn cells (Fig. 4). The mTOR pathway is well‐documented to promote the continuous production of IFN‐γ by memory T cells22 and this cytokine regulates differentiation and survival in response to weak T‐cell receptor signals of memory T CD8+ cells.23 Blocking this pathway with mTORi should impair IFN‐γ production, although the intracellular levels of IFN‐γ in Tn, Tcm and Tem cells were not affected after including any mTORi in the culture (Fig. 4). Failure to control IFN‐γ production by memory T cells suggests that mTORi are not suitable to control memory responses driven by IFN‐γ. This type of alloresponse can appear early post‐transplantation, suggesting that the CNI option is preferable in the early stages after transplantation.24 Our in vitro observations on both proliferation and cytokine production support the high acute rejection rate published in clinical trials in patients receiving monotherapy with mTORi compared with CNI.24, 25
Tacrolimus reduced the production of IL‐2 by memory T‐cell subsets, whereas it completely inhibited it in Tn cells. The mTORi induced an over‐production of IL‐2 in Tn cells (Fig. 4a). The differential effect on IL‐2 production that is observed could be explained by different molecular targets of IS. The CNI inhibits activation of the nuclear factor of activated T cells and subsequent IL‐2 production, whereas the mTORi enable IL‐2 production, allowing the differentiation of Tn to Treg cells,26, 27 as confirmed in stable kidney transplant recipients converted to mTORi.28
The CNI has been demonstrated to have a deleterious effect on Treg cells, both in vitro 29 and in vivo,28 whereas mTORi are permissive with Treg cells.28 Potentially, Treg cells are able to control the proliferation of naive and memory T cells in vitro; however, Treg cells were not excluded from our sorted T‐cell subsets because of their low level within CD4+ lymphocytes in healthy subjects (from 2 to 4% in our experience16). Indeed, Treg cells are anergic in vitro,30 which was confirmed in our culture conditions where the percentage of sorted Treg cells was 7–15% (see Supplementary material, Fig. S9) so it is expected that the influence of the Treg cells over the results under our experimental conditions is low.
The main limitation of this work is that the in vitro IS concentrations used are far from those used in the clinics, and justifying the choice of these doses is the saturation of the cell inhibition effect (see Supplementary material, Fig. S8) as well as in vitro cytotoxic effects at higher doses of everolimus (see Supplementary material, Fig. S7).
In summary, at the doses used in the present study, tacrolimus controlled proliferation and IL‐2 and IFN‐γ production of naive and memory T‐cell subsets more efficiently than mTORi. Production of IL‐17 did not change significantly with any immunosuppressants tested. The differences observed between both types of mTORi tested could be relevant, although we do not have a clear explanation for them. Hence, everolimus was more potent than rapamycin in controlling proliferation of the different T‐cell subsets studied. Tacrolimus could be more effective in controlling alloreactive memory T cells responsible for potential rejection episodes and the election therapy in those patients at high risk of rejection. Altogether, our data provide reasoning for the use of CNI and mTORi combination in stable transplants to modulate different aspects of memory/naive T‐cell function.
Disclosure
The authors declare no conflicts of interest.
Supporting information
Figure S1. Proliferation of sorted naive and memory T cells at different rates of CD3/CD28 stimulation. Sorted naive (Tn) (a), central memory (Tcm) (b) and effector memory (Tem) T cells (c) were stained with carboxyfluorescein diacetate succinimidyl ester before polyclonal stimulation with anti‐CD3/CD28 beads, during 5 days. The beads were tested at increasing dose rate cell : bead 1 : 1 (1 ×) 1 : 2 (2 ×) and 1 : 4 (4 ×). The proliferation was expressed by the percentage of dividing cells (%Divided cells).
Figure S2. Phenotypic changes of sorted naive and memory T‐cell subsets. Representative dot plots of sorted naive (Tn), central memory (Tcm) and effector memory (Tem) T cells cultured for 5 days alone (W/S, a–c) or stimulated with anti‐CD3/CD28 beads (S, d–f) are shown. The percentage of each subpopulation is depicted. Each subpopulation was defined based on the membrane CD45RO and CD62L expression as Naive: CD62L+ CD45RO−; Tcm: CD62L+ CD45RO+; Tem: CD62L+ CD45RO+ and terminally differentiated memory T cells (Temra): CD62L− CD45RO−.
Figure S3. Relative expression changes of several phenotypic markers in naive and memory T‐cell subsets. The sorted subpopulations: naive (Tn), central memory (Tcm) and effector memory (Tem) T cells were polyclonally activated with anti‐CD3/CD28 beads (S) or left alone (W/S) for 5 days and stained with CCR7, CD45RO, CD62L, CCR6, CD25 and CD103 as described in the Materials and methods section. The mean fluorescence intensity (MFI) values are shown below.
Figure S4. Cell death of sorted T‐cell subsets with different immunosuppressant. Sorted naive (Tn) (a), central memory (Tcm) (b) and effector memory (Tem) T cells (c) were stained with 7‐amino‐actinomycin D after polyclonal stimulation with anti‐CD3/CD28 beads for 5 days. The cells were alone (W/S), stimulated (S) and treated with the indicated dose of tacrolimus (Tac, open circles), rapamycin (Rapa, closed triangle and dotted line) or everolimus (Eve, closed squares and dotted line). The reference of stimulated control is represented with a dotted line in each plot.
Figure S5. Proliferation of sorted naive and memory T‐cell subsets. Representative histograms of carboxyfluorescein diacetate succinimidyl ester dye to assess the proliferation of sorted naive (Tn) (a), central memory (Tcm) (b) and effector memory (Tem) T cells (c) after 5 days of culture with polyclonal stimulation with anti‐CD3/CD28 (continuous line) and without stimuli (dotted line). The % of divided cells (% Div) and Proliferation Index (PI) are depicted in each histogram.
Figure S6. Cytokine production of sorted naive and memory T‐cell subsets. Representative dot plots of cytokine production by sorted naive (Tn), central memory (Tcm) and effector memory (Tem) T cells, after 5 days of culture alone (W/S, a–c) or with polyclonal stimulation (W, d–f) and intracellular staining for interleukin‐2 (IL‐2) and interferon‐γ (IFN‐γ) after re‐stimulation. The percentage of IL‐2‐ and/or IFN‐γ‐producing cells is depicted in each quadrant.
Figure S7. Scatter plot of sorted of sorted naive T cells treated with everolimus. Forward scatter versus side scatter plot of sorted naive T cells (Tn) alone (W/S), stimulated (S) and treated with the indicated dose of everolimus after 5 days of polyclonal stimulation with anti‐CD3/CD28. The lymph gate is shown with strong dotted circle in W/S and high everolimus dose conditions.
Figure S8. Proliferation of sorted naive T cells after treatment of high dose of immunosupprant. The sorted naive T cells (Tn) were stained with carboxyfluorescein diacetate succinimidyl ester before polyclonal stimulation with anti‐CD3/CD28 beads, for 5 days. The cells were alone (W/S), stimulated (S) and treated with the indicated dose of tacrolimus (open circles), rapamycin (closed triangles and dotted line) or everolimus (closed squares and dotted line). The proliferation was expressed by the percentage of dividing cells (% Divided cells). The reference of stimulated control is represented with a dotted line in each plot. The strong dotted lines show the flat‐fashion curve of proliferation (% Divided cells). The range of dose used is shown in the Materials and methods section.
Figure S9. Proliferation of sorted regulatory T (Treg) cells with different immunosuppressant. Sorted Treg cells were stained with carboxyfluorescein diacetate succinimidyl ester before polyclonal stimulation with anti‐CD3/CD28 beads, for 5 days. The cells were alone (W/S), stimulated (S) and treated with the indicated dose of tacrolimus (Tac, open circles), rapamycin (Rapa, closed triangle and dotted line) or everolimus (Eve, closed squares and dotted line) (mean, standard error of mean, and their comparison respect stimulated control are shown in the Supplementary material, Table S2). The proliferation was expressed as the percentage of dividing cells (% Divided cells). The reference of stimulated control is represented with a dotted line in the plot.
Table S1. Comparison of the mean percentage of each sorted T‐cell subpopulation after culture with different immunosuppressants. The means were compared using Student's t‐test. Different levels of significance were observed: *P < 0·05, **P < 0·01 and ***P < 0·001.
Table S2. Comparison of the mean percentage of dividing cells of each sorted T‐cell subpopulation after culture with different immunosuppressants. The means were compared using Student's t‐test. Different levels of significance were observed: *P < 0·05, **P < 0·01 and ***P < 0·001.
Table S3. Comparison of the mean percentage of interleukin‐2‐ (IL‐2), interferon‐γ‐ (IFN‐γ) and IL‐17‐producing cells of each sorted T‐cell subpopulation after culture with different immunosuppressants. The means were compared using Student's t‐test. Different level of significance was observed: *P < 0·05, **P < 0·01 and ***P < 0·001.
Acknowledgements
We are grateful to Carolina Santa Cruz (IDIVAL, Santander, Spain) for her technical support at the Flow cytometry and Cell Sorting Unit. This work was supported by grants from the FIS‐ISCIII (PI11/00990; RD12/0021/0007).
References
- 1. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745–63. [DOI] [PubMed] [Google Scholar]
- 2. Geginat J, Sallusto F, Lanzavecchia A. Cytokine‐driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med 2001; 194:1711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crespo E, Lucia M, Cruzado JM, Luque S, Melilli E, Manonelles A et al Pre‐transplant donor‐specific T‐cell alloreactivity is strongly associated with early acute cellular rejection in kidney transplant recipients not receiving T‐cell depleting induction therapy. PLoS One 2015; 10:e0117618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. San Segundo D, Ballesteros MA, Naranjo S, Zurbano F, Miñambres E, López‐Hoyos M. Increased numbers of circulating CD8 effector memory T cells before transplantation enhance the risk of acute rejection in lung transplant recipients. PLoS One 2013; 8:e80601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang H, Liao C, Qi Z, Sha C, Xie B, Chen J et al Rapamycin or tacrolimus alone fails to resist cardiac allograft accelerated rejection mediated by alloreactive CD4+ memory T cells in mice. Transpl Immunol 2010; 22:128–36. [DOI] [PubMed] [Google Scholar]
- 6. Ojo AO, Morales JM, González‐Molina M, Steffick DE, Luan FL, Merion RM et al Comparison of the long‐term outcomes of kidney transplantation: USA versus Spain. Nephrol Dial Transplant 2013; 28:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Descotes J. Methods of evaluating immunotoxicity. Expert Opin Drug Metab Toxicol 2006; 2:249–59. [DOI] [PubMed] [Google Scholar]
- 8. Jones DL, Sacks SH, Wong W. Controlling the generation and function of human CD8+ memory T cells in vitro with immunosuppressants. Transplantation 2006; 82:1352–61. [DOI] [PubMed] [Google Scholar]
- 9. Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004; 351:2715–29. [DOI] [PubMed] [Google Scholar]
- 10. Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X et al Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med 2004; 10:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Tuka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol 2001; 166:973–81. [DOI] [PubMed] [Google Scholar]
- 12. Heeger PS. T‐cell allorecognition and transplant rejection: a summary and update. Am J Transplant 2003; 3:525–33. [DOI] [PubMed] [Google Scholar]
- 13. Yang J, Brook MO, Carvahlo‐Gaspar M, Zhang J, Ramon HE, Sayegh MH et al Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci USA 2007; 104:19954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant 2002; 2:501–9. [DOI] [PubMed] [Google Scholar]
- 15. Shan Z, Li Q, Zhou Y, Zeng X, Fan Q, Liao C et al Effect of CD4+ memory T cells on rejection response of ectopic heart transplantation in mice. Transplant Proc 2011; 43:1989–93. [DOI] [PubMed] [Google Scholar]
- 16. Motta I, Galelli A, Colle JH, Truffa‐Bachi P. FK 506 favors the generation of memory T cells in vitro . Transplant Proc 1991; 23:2953–4. [PubMed] [Google Scholar]
- 17. Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF et al mTOR regulates memory CD8 T‐cell differentiation. Nature 2009; 460:108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T‐cell subsets in response to antigen or homeostatic cytokines. Blood 2003; 101:4260–6. [DOI] [PubMed] [Google Scholar]
- 19. Kim JS, Lee JI, Shin JY, Kim SY, Shin JS, Lim JH et al Bortezomib can suppress activation of rapamycin‐resistant memory T cells without affecting regulatory T‐cell viability in non‐human primates. Transplantation 2009; 88:1349–59. [DOI] [PubMed] [Google Scholar]
- 20. Tang AL, Bingaman AW, Kadavil EA, Leeser DB, Farber DL. Generation and functional capacity of polyclonal alloantigen‐specific memory CD4 T cells. Am J Transplant 2006; 6:1275–84. [DOI] [PubMed] [Google Scholar]
- 21. Tsuda K, Yamanaka K, Kitagawa H, Akeda T, Naka M, Niwa K et al Calcineurin inhibitors suppress cytokine production from memory T cells and differentiation of naive T cells into cytokine‐producing mature T cells. PLoS One 2012; 7:e31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Setoguchi R, Matsui Y, Mouri K. mTOR signaling promotes a robust and continuous production of IFN‐γ by human memory CD8 T cells and their proliferation. Eur J Immunol 2015; 45:893–902. [DOI] [PubMed] [Google Scholar]
- 23. Stoycheva D, Deiser K, Starck L, Nishanth G, Schluter D, Uckert W et al IFN‐γ regulates CD8+ memory T cell differentiation and survival in response to weak, but not strong, TCR signals. J Immunol 2015; 194:553–9. [DOI] [PubMed] [Google Scholar]
- 24. Ekberg H, Bernasconi C, Tedesco‐Silva H, Vítko S, Hugo C, Demirbas A et al Calcineurin inhibitor minimization in the Symphony study. Am J Transplant 2009; 9:1876–85. [DOI] [PubMed] [Google Scholar]
- 25. Vicenti F, Ramos E, Brattstrom C, Cho S, Ekberg H, Grinyo J et al Multicenter trial exploring CNI avoidance in renal transplantation. Transplantation 2001; 71:1282–7. [DOI] [PubMed] [Google Scholar]
- 26. Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol 2007; 7:1819–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+ CD25+ Foxp3+ regulatory T cells. Blood 2005; 105:4743–8. [DOI] [PubMed] [Google Scholar]
- 28. Segundo DS, Ruiz JC, Izquierdo M, Fernández‐Fresnedo G, Gómez‐Alamillo C, Merino R et al Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+ CD25+ FOXP3+ regulatory T cells in renal transplant recipients. Transplantation 2006; 82:550–7. [DOI] [PubMed] [Google Scholar]
- 29. Gallon L, Traitanon O, Yu Y, Shi B, Leventhal JR et al Differential effects of calcineurin and mammalian target of rapamycin inhibitors on alloreactive Th1, Th17, and regulatory T cells. Transplantation 2015; 99:1774–84. [DOI] [PubMed] [Google Scholar]
- 30. Thornton AM, Shevach EM. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 1998; 188:287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Proliferation of sorted naive and memory T cells at different rates of CD3/CD28 stimulation. Sorted naive (Tn) (a), central memory (Tcm) (b) and effector memory (Tem) T cells (c) were stained with carboxyfluorescein diacetate succinimidyl ester before polyclonal stimulation with anti‐CD3/CD28 beads, during 5 days. The beads were tested at increasing dose rate cell : bead 1 : 1 (1 ×) 1 : 2 (2 ×) and 1 : 4 (4 ×). The proliferation was expressed by the percentage of dividing cells (%Divided cells).
Figure S2. Phenotypic changes of sorted naive and memory T‐cell subsets. Representative dot plots of sorted naive (Tn), central memory (Tcm) and effector memory (Tem) T cells cultured for 5 days alone (W/S, a–c) or stimulated with anti‐CD3/CD28 beads (S, d–f) are shown. The percentage of each subpopulation is depicted. Each subpopulation was defined based on the membrane CD45RO and CD62L expression as Naive: CD62L+ CD45RO−; Tcm: CD62L+ CD45RO+; Tem: CD62L+ CD45RO+ and terminally differentiated memory T cells (Temra): CD62L− CD45RO−.
Figure S3. Relative expression changes of several phenotypic markers in naive and memory T‐cell subsets. The sorted subpopulations: naive (Tn), central memory (Tcm) and effector memory (Tem) T cells were polyclonally activated with anti‐CD3/CD28 beads (S) or left alone (W/S) for 5 days and stained with CCR7, CD45RO, CD62L, CCR6, CD25 and CD103 as described in the Materials and methods section. The mean fluorescence intensity (MFI) values are shown below.
Figure S4. Cell death of sorted T‐cell subsets with different immunosuppressant. Sorted naive (Tn) (a), central memory (Tcm) (b) and effector memory (Tem) T cells (c) were stained with 7‐amino‐actinomycin D after polyclonal stimulation with anti‐CD3/CD28 beads for 5 days. The cells were alone (W/S), stimulated (S) and treated with the indicated dose of tacrolimus (Tac, open circles), rapamycin (Rapa, closed triangle and dotted line) or everolimus (Eve, closed squares and dotted line). The reference of stimulated control is represented with a dotted line in each plot.
Figure S5. Proliferation of sorted naive and memory T‐cell subsets. Representative histograms of carboxyfluorescein diacetate succinimidyl ester dye to assess the proliferation of sorted naive (Tn) (a), central memory (Tcm) (b) and effector memory (Tem) T cells (c) after 5 days of culture with polyclonal stimulation with anti‐CD3/CD28 (continuous line) and without stimuli (dotted line). The % of divided cells (% Div) and Proliferation Index (PI) are depicted in each histogram.
Figure S6. Cytokine production of sorted naive and memory T‐cell subsets. Representative dot plots of cytokine production by sorted naive (Tn), central memory (Tcm) and effector memory (Tem) T cells, after 5 days of culture alone (W/S, a–c) or with polyclonal stimulation (W, d–f) and intracellular staining for interleukin‐2 (IL‐2) and interferon‐γ (IFN‐γ) after re‐stimulation. The percentage of IL‐2‐ and/or IFN‐γ‐producing cells is depicted in each quadrant.
Figure S7. Scatter plot of sorted of sorted naive T cells treated with everolimus. Forward scatter versus side scatter plot of sorted naive T cells (Tn) alone (W/S), stimulated (S) and treated with the indicated dose of everolimus after 5 days of polyclonal stimulation with anti‐CD3/CD28. The lymph gate is shown with strong dotted circle in W/S and high everolimus dose conditions.
Figure S8. Proliferation of sorted naive T cells after treatment of high dose of immunosupprant. The sorted naive T cells (Tn) were stained with carboxyfluorescein diacetate succinimidyl ester before polyclonal stimulation with anti‐CD3/CD28 beads, for 5 days. The cells were alone (W/S), stimulated (S) and treated with the indicated dose of tacrolimus (open circles), rapamycin (closed triangles and dotted line) or everolimus (closed squares and dotted line). The proliferation was expressed by the percentage of dividing cells (% Divided cells). The reference of stimulated control is represented with a dotted line in each plot. The strong dotted lines show the flat‐fashion curve of proliferation (% Divided cells). The range of dose used is shown in the Materials and methods section.
Figure S9. Proliferation of sorted regulatory T (Treg) cells with different immunosuppressant. Sorted Treg cells were stained with carboxyfluorescein diacetate succinimidyl ester before polyclonal stimulation with anti‐CD3/CD28 beads, for 5 days. The cells were alone (W/S), stimulated (S) and treated with the indicated dose of tacrolimus (Tac, open circles), rapamycin (Rapa, closed triangle and dotted line) or everolimus (Eve, closed squares and dotted line) (mean, standard error of mean, and their comparison respect stimulated control are shown in the Supplementary material, Table S2). The proliferation was expressed as the percentage of dividing cells (% Divided cells). The reference of stimulated control is represented with a dotted line in the plot.
Table S1. Comparison of the mean percentage of each sorted T‐cell subpopulation after culture with different immunosuppressants. The means were compared using Student's t‐test. Different levels of significance were observed: *P < 0·05, **P < 0·01 and ***P < 0·001.
Table S2. Comparison of the mean percentage of dividing cells of each sorted T‐cell subpopulation after culture with different immunosuppressants. The means were compared using Student's t‐test. Different levels of significance were observed: *P < 0·05, **P < 0·01 and ***P < 0·001.
Table S3. Comparison of the mean percentage of interleukin‐2‐ (IL‐2), interferon‐γ‐ (IFN‐γ) and IL‐17‐producing cells of each sorted T‐cell subpopulation after culture with different immunosuppressants. The means were compared using Student's t‐test. Different level of significance was observed: *P < 0·05, **P < 0·01 and ***P < 0·001.


