Summary
Type 1 diabetes (T1D) belongs among polygenic multifactorial autoimmune diseases. The highest risk is associated with human leucocyte antigen (HLA) class II genes, including HLA‐DQA1 gene. Our aim was to investigate DNA methylation of HLA‐DQA1 promoter alleles (QAP) and correlate methylation status with individual HLA‐DQA1 allele expression of patients with T1D and healthy controls. DNA methylation is one of the epigenetic modifications that regulate gene expression and is known to be shaped by the environment.Sixty one patients with T1D and 39 healthy controls were involved in this study. Isolated DNA was treated with sodium bisulphite and HLA‐DQA1 promoter sequence was amplified using nested PCR. After sequencing, DNA methylation of HLA‐DQA1 promoter alleles was analysed. Individual mRNA HLA‐DQA1 relative allele expression was assessed using two different endogenous controls (PPIA, DRA). We have found statistically significant differences in HLA‐DQA1 allele 02:01 expression (PPIA normalization, P corr = 0·041; DRA normalization, P corr = 0·052) between healthy controls and patients with T1D. The complete methylation profile of the HLA‐DQA1 promoter was gained with the most methylated allele DQA1*02:01 and the least methylated DQA1*05:01 in both studied groups. Methylation profile observed in patients with T1D and healthy controls was similar, and no correlation between HLA‐DQA1 allele expression and DNA methylation was found. Although we have not proved significant methylation differences between the two groups, detailed DNA methylation status and its correlation with expression of each HLA‐DQA1 allele in patients with T1D have been described for the first time.
Keywords: DNA methylation, HLA class II genes, HLA‐DQA1 promoter (QAP), mRNA expression, type 1 diabetes mellitus
Abbreviations
- CD14+
cluster of differentiation 14
- CTLA‐4
cytotoxic T‐lymphocyte‐associated protein 4
- IPTG
isopropyl‐β‐d‐thiogalactopyranoside
- HC
healthy controls
- HLA
human leucocyte antigen
- PPIA
peptidylprolyl isomerase A
- QAP
HLA‐DQA1 promoter
- T1D
type 1 diabetes mellitus
- X‐Gal
5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐galacto‐pyranoside
Introduction
Type 1 diabetes (T1D) is an autoimmune disease that leads to the selective destruction of pancreatic β‐cells and to lifelong dependence on exogenous insulin. Its worldwide incidence is increasing at a rate of nearly 3% per year.1 Nearly 40 genes and gene complexes contribute to T1D risk, including protein tyrosine phosphatase, non‐receptor type 22, cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4) and insulin genes.2 The strongest genetic risk (about 50%) is associated with HLA class II complex (particularly with its DQ and DR regions) located on the short arm of chromosome 6. Heterozygotes for DRB1*04‐DQA1*03:01‐HLA‐DQB1*03:02 and DRB1*03‐DQA1*05:01‐DQB1*02:01 haplotypes carry the highest risk for T1D development in the Caucasian population. In contrast, the allele DQB1*06:02 (part of DRB1*15‐DQA1*02:01‐DQB1*06:02 haplotype) is negatively associated with the disease.3 The presence of a single copy of this allele is sufficient to protect from T1D. Although many studies have confirmed the association between T1D and HLA‐DQ and HLA‐DR molecules, their role in T1D aetiopathogenesis is not fully understood. One possibility is that HLA‐DQ risk alleles bind autoantigens with low affinity and that leads to the aberrant selection of T‐cell repertoire in thymus or periphery.4 Another possibility is that autoimmunity could be associated with low expression of HLA molecules. This would result in a general decrease in the number of HLA class II molecules on the cell surface and hence to less efficient presentation of autoantigens to protective regulatory T cells.5
Not only genetics but also an environment can modulate T1D risk in genetically susceptible individuals. Concordance values in monozygotic twins, ranging from 25 to 60%, clearly demonstrate the relation between T1D manifestation and environmental factors.6, 7 Furthermore, in recent years, more people with low risk or even protective HLA genotypes are becoming prone to developing T1D.8, 9 This could be caused by gene–environment interactions leading to aberrant DNA methylation of genes regulating T1D susceptibility, including HLA class II locus, and causing different expression of HLA class II alleles. Recent studies showed a distinct DNA methylation profile in T1D risk genetic regions, like in the insulin promoter or HLA class II region.10, 11, 12 However, it remains to be elucidated how this methylation difference influences T1D susceptibility and whether these changes correlate with changes in the expression of T1D risk genes. So far, this was only observed in the insulin gene, where hypermethylation of one CpG dinucleotide within the promoter region correlated in vitro with low expression of insulin.13
In this study we focused on DNA methylation of HLA‐DQA1 gene promoter (QAP). It has been shown that DQA1 promoter alleles have different strengths and can influence the expression level of the HLA‐DQA1 gene.14, 15, 16 We hypothesized that HLA class II expression could be regulated not only by genetic polymorphisms, but also by epigenetic modifications including DNA methylation within the promoter region. To confirm this hypothesis we analysed the DNA methylation status of individual HLA‐DQA1 promoter alleles. We tried to understand the relationship between allele's methylation and its mRNA expression level. Finally we compared these characteristics between patients with T1D and healthy controls.
Material and methods
Subject
The participants in this study consisted of 61 patients with T1D (45 men and 16 women; median age of 32·5 years; average age 39·8 years) and 39 healthy individuals (17 men and 22 women; median age of 34·5 years; average age of 39·1 years) of Caucasian origin and from the same ethnic background. The study was approved by the Ethical Committee of the Third Faculty of Medicine of Charles University in Prague and the written informed consent was obtained from each subject. All patients with T1D were diagnosed at the University Hospital Kralovske Vinohrady, Prague, Czech Republic. The diagnosis of diabetes was made according to published criteria.17 Autoimmune origin was confirmed by very low levels of C‐peptide and/or positive serum autoantibodies against either insulin, or glutamic acid decarboxylase, or islet antigen islet antigen‐2.
Age‐matching controls were chosen according to their HLA haplotypes to correspond with HLA haplotypes of patients with T1D. The controls were not tested for the presence of specific autoantibodies, however, only healthy individuals (self‐reported) with neither diabetes and diabetes‐associated symptoms nor any other autoimmune disease were included in the study. Characteristics of the subject population are shown in Table 1. Mann–Whitney non‐parametric test did not prove any statistically significant differences between the ages of both groups (α = 0·05).
Table 1.
Characteristics of study subjects
| Group | Patients with type 1 diabetes mellitusa (n = 61) | Healthy controlsb (n = 39) |
|---|---|---|
| Number of males/females | 45/16 | 17/22 |
|
Age in years range (median and mean) |
21–70 (32·5 and 39·8) | 20–77 (34·5 and 39·1) |
|
Men's age in years range (median and mean; SD) |
21–70 (36·0 and 41·5; 14·8) | 20–77 (40·0 and 40·1; 15·5) |
|
Women's age in years range (median and mean; SD) |
21–68 (31·0 and 33·9; 19·1) | 25–75 (28·0 and 38·3; 17·8) |
|
Duration of T1D in years range (median and mean) |
5–42 (14·0 and 15·2) | – |
Individuals with overt hyperglycaemia and at least one of specific autoantibodies.
Individuals with no overt disease symptoms and with no history of any autoimmune diseases.
DNA isolation and bisulphite sequencing
From each subject, 20 ml of whole peripheral blood was collected into tubes with 3% EDTA. Genomic DNA from whole blood (patients with T1D and healthy controls) and CD14+ monocytes (only patients with T1D) were extracted by the salting out method and treated with sodium bisulphite using an Epitect Bisulfite kit (Qiagen, Hilden, Germany). CD14+ monocytes were isolated using Dynabeads CD14® (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.18 HLA‐DQA1 gene promoter region was amplified by nested PCR in three separate reactions that were mixed together after PCR; target fragments were separated by 1% TAE agarose gel electrophoresis and purified by a MinElute Gel Extraction kit (Qiagen). For amplification, the following sets of primers were used:
F1 5′‐GGT TGT AAG TTA GAA TAT TTT GAA GGA TG‐3′ and R1 5′‐CAA ACC AAA CCC TAC CAA ATC A‐3′ for the first PCR; and F2 5′‐AGG TTG TTT AGA AAT GTT TAT TTT TGG‐3′ and R2 5′‐AAA ATC CCC TAT AAT AAC ATC TCA ATT AC‐3′ for the second reaction. PCR conditions were reported in Zajacova et al.16 The 545‐bp long amplicon was inserted into the pGEM‐T easy vector (Promega, Madison, WI) and cloned into the Escherichia coli DH5α strain. Positive colonies were selected on agar plates containing ampicillin (100 mg/ml), X‐Gal (5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐galacto‐pyranoside, 3%), IPTG (isopropyl‐β‐d‐thiogalactopyranoside, 100 mm) and confirmed by colony PCR using universal SP6 and T7 primers. At least six different positive colonies from each patient were sequenced by Sanger method with fluorescence‐labelled nucleotides in Macrogen (Seoul, Korea). Obtained sequences were aligned according to the reference sequence of HLA‐DQA1 (ENSG00000196735) in bioedit software, version 7.0.9.0 (Carlsbad, CA, USA). Only sequences where bisulphite treatment was at least 95% successful were taken into consideration. Damaged or recombined sequences were removed from analysis.
HLA‐DQA1, DRB1 and DQB1 genotyping
For genotyping, HLA Olerup SSP™ typing kits (Olerup AB, Stockholm, Sweden) were used. First, HLA‐DQ and DR low‐resolution and HLA‐DQA1 genotyping was done. Second, HLA‐DQB1 high‐resolution typing using allele‐specific Olerup SSP™ HLA‐DQB1 typing kits was performed according to the manufacturer's instructions. PCR products were identified by 2% agarose gel electrophoresis and evaluated according to manufacturer's instructions.
RNA isolation and mRNA expression
Total RNA was isolated from whole blood using Gen Elute™ Miniprep Kit (Sigma Aldrich, St Louis, MO). The cDNA was prepared by High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) with 200 ng input RNA. Sequences of primers and probes used in real‐time PCR were reported previously.16 The ‘DQA1 total’ assay was used to quantify total DQA1 mRNA irrespective of alleles present, the ‘DRA’ assay was used as an endogenous control and the ‘DQA1 intron’ assay amplifying intronic segment of DQA1 gene was used to determine genomic DNA contamination of our samples. All measurements were done using 7500 Fast Real time PCR system (Applied Biosystems) with fluorescent Taqman™ probes and primers. All samples were measured in triplicate and contained 200 nM of probe, 300 nM of each primer and 1× Gene Expression Master Mix (Applied Biosystems) using the following cycling conditions: 50° for min and 95° for 10 min, followed by 40 cycles of 95° for 15 seconds and 60° for 1 min. For each assay, an amplification efficiency was determined and ranged between 95% and 102%.
Statistical analysis
graphpad prism 5·0·4 software was used for statistical assessments (GraphPad Software, La Jolla, CA, USA). Differences in overall DNA methylation of DQA1 promoter alleles were evaluated by a two‐tailed non‐parametric Mann–Whitney test with significance level of α = 0·05. Statistically significant results were corrected using Bonferroni correction (P multiplied by number of promoter alleles tested, P corr = P uncorr *10). Significant differences between allele frequencies of patients with T1D and healthy controls were determined using chi‐square test (α = 0·05) with Bonferroni correction (P corr = P uncorr *10). Differences between individual methylation positions were calculated using Fisher's exact test with α = 0·05 with Bonferroni correction (P corr = P uncorr *10). Methylation differences between whole blood leucocytes and monocytes of patients with T1D were tested using a non‐parametric Kruskal–Wallis test with Dunn's post‐test at the level of significance α = 0·1.
Relative expression of DQA1 alleles was determined with reference to the amount of HLA‐DRA mRNA. Only samples in which the ratio of the sum of both alleles mRNA to the amount of total DQA1 mRNA was in the interval between 0.85 and 1.15 were included in the analyses. Relative expression between all possible pairs of alleles was compared by a two‐tailed Mann–Whitney test with 95% CI. All P‐values were subjected to Bonferroni correction for the number of alleles compared (P corr = P uncorr *10).
Results
Identification of individual DQA1 promoters (QAP alleles)
DQA1 promoter alleles were identified according to the known sequences found in the IMGT/HLA database (http://www.ebi.ac.uk/ipd/imgt/hla/). In total, 10 different promoter alleles in patients with T1D and 11 promoter alleles in healthy controls (HC) were detected. Most of the promoter alleles were in linkage disequilibrium with one respective DQA1 allele, except DQA1*01:02 allele, which was associated with two different QAP alleles 1.2K, and 1.4. While QAP 1.2K was associated with DR*16‐DQA1*01:02‐DQB1*05:02, QAP 1.4 was associated with DR*13‐DQA1*01:02‐DQB1*06:04. The association between DQA1 alleles and promoter alleles (QAP) is depicted in the Supplementary material (Table S1). Two patients were detected with a protective haplotype DRB1*13‐DQA1*01:03‐DQB1*06:03. These patients had manifested disease at 40 and 41 years of age, respectively.
Normalization against two different endogenous controls
As an endogenous control to normalize mRNA expression of HLA‐DQA1 alleles we used another HLA class II gene, the non‐polymorphic HLA‐DRA, which is located in the linkage disequilibrium region together with the DRB1, DQB1 and DQA1 genes. Transcription of all these genes is regulated by the same transcription factors (RFX, X2BP, NF‐Y and the class II transactivator CIITA). Hence, the normalization against the DRA gene allows us to correct for the increase of DQA1 expression caused not by differences in promoter strength, but by inflammation. We hypothesized that expression of HLA class II genes including the DQA1 gene could be changed by the health status of the patient, so we explored this hypothesis by normalizing the expression data against another endogenous control, peptidylprolyl isomerase A (PPIA). This approach allowed us to see the differences in individual DQA1 allele expression between healthy controls and patients with T1D that were caused by altered inflammatory state or by factors other than promoter strength in patients with autoimmune disease.
Relative expression of individual DQA1 alleles HLA‐DRA normalized
In total, mRNA levels of DQA1 alleles in 43 patients with T1D (36 heterozygotes and seven homozygotes) and 39 healthy controls (34 heterozygotes and five homozygotes) were analysed in our study. DQA1*03 allele was significantly more expressed in patients with T1D than all other analysed alleles (DQA1*01:01; 01:02K; 02:01; 03:01 and 05:01). In healthy controls, the DQA1*03 allele was significantly more expressed than 01:01; 01:02L; 01:03; 01:04; 02:01; 03:01; 05:01 and 05:05. Furthermore DQA1*0103 allele was significantly more expressed than 01:01, 02:01, 05:01, 05:05. (Fig. 1).
Figure 1.
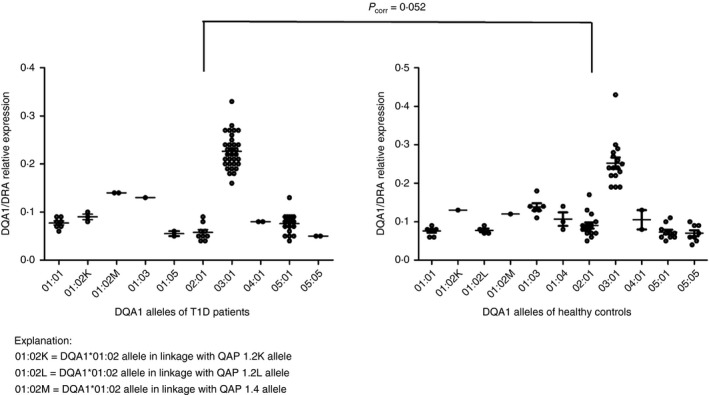
Relative expression of individual DQA1 alleles normalized against HLA‐DRA. Each point in the graph represents normalized value for one individual and one allele. The horizontal line indicates mean relative expression of the allele. Mean values with standard error of the mean are indicated. In total 43 patients with type 1 diabetes mellitus (T1D) and 39 healthy controls were analysed in this study. Difference in expression of DQA1*02:01 in healthy controls and patients with T1D was at the edge of significance (P corr = 0·052). DQA1*03 was significantly more expressed than all other alleles as in patients with T1D so in healthy controls. DQA1*05:05 has the lowest expression in both studied groups. Statistical significance was tested by Mann–Whitney test (α = 0·05) followed by Bonferroni correction (P corr = P uncorr *10).
DQA1 alleles for which fewer than three expression values were obtained (T1D: 01:02M, 01:03, 01:05, 04:01, 05:05; HC: 0102K, 0102M, 04:01) were not included in the statistical analysis. Our data indicate that promoters of DQA1*01:03 and DQA1*01:02M alleles may be stronger than DQA1*01:01, DQA1*01:02L (Fig. 1). Statistical analysis revealed no significant differences between DRA‐normalized DQA1 allele expression in patients with T1D and healthy controls. However, expression of DQA1*02:01 in healthy controls compared with patients with T1D (P corr = 0·052) was at the edge of significance. All results are summarized in Fig. 1.
Relative expression of individual DQA1 alleles normalized against PPIA
Normalization against PPIA revealed significantly higher expression of the DQA1*02:01 allele in healthy controls compared patients with T1D (P corr = 0·041). Higher expression of DQA1*05:01 allele in patients with T1D compared with healthy controls lost significance after correction. All other observations from PPIA normalization were similar to those from HLA‐DRA normalization. In support of our hypothesis for using two endogenous controls, the expression data from normalization against PPIA (Fig. 2) were much more variable than the data from normalization against HLA‐DRA gene, especially for DQA1*03 and DQA1*01:01 alleles.
Figure 2.
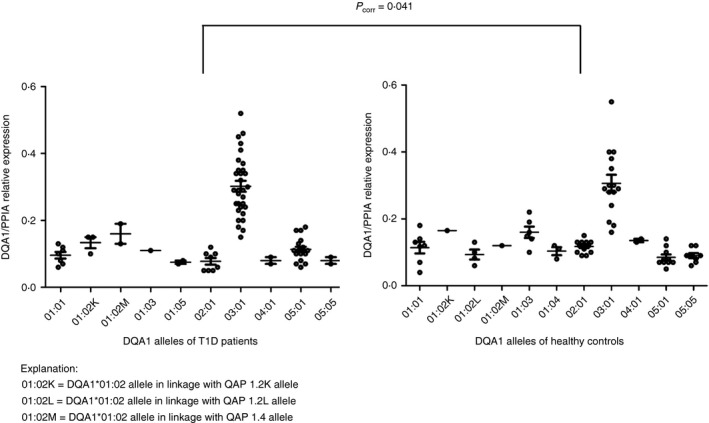
Relative expression of individual DQA1 alleles normalized against peptidylprolyl isomerase A (PPIA). Each point in the graph represents normalized value for one individual and one allele. The horizontal line indicates mean relative expression of the allele. Mean values with standard error of the mean are indicated. DQA1*02:01 was significantly more expressed in healthy controls than in patients with type 1 diabetes mellitus (T1D) (P corr = 0·041). HLA‐DQA1*0501 expression was elevated in patients with T1D compared with healthy controls, but significance was lost after correction (P corr = 0·369). Normalization against PPIA was performed to analyse differences in individual DQA1 allele expression between healthy controls and patients with T1D caused by altered inflammatory state or by other factors different than promoter strength in patients with autoimmune disease.
Unequal expression of DQA1*02:01 allele in DQA1 02:01/03 heterozygotes
Expression of DQA1*02:01 was higher in healthy controls, so we investigated if there was a haplotype combination with the most marked differences in DQA1*02:01 expression. We calculated mean allele expression ratio between different haplotypes of patients with T1D and healthy controls (see Supplementary material, Table S2). We found that the mean allele expression ratio of DQA1 03/02:01 heterozygotes was almost two times higher (not significant) in patients with T1D (4·86) than in healthy controls (2·96). Relative expression of DQA1*02:01 and DQA1*03 alleles of T1D and healthy individuals is illustrated in the Supplementary material (Fig. S1), mean allele expression ratios of all different haplotype combinations are summarized in Table S2 (see Supplementary material).
Methylation variances between whole blood leucocytes and monocytes of patients with T1D
Using the bisulphite sequencing method, we determined methylation status of 9–12 CpGs per sequence depending on QAP allele identity. Methylation profile of CD14+ monocytes and whole blood leucocytes of patients with T1D was obtained. We tested for significant differences between both cell populations including total QAP methylation and specific QAP methylation of individual CpGs. For this purpose, we compared all eight DQA1 alleles between whole blood leucocytes and monocytes using non‐parametric Kruskal–Wallis test with Dunn's post‐test at the level of significance α = 0·1. This test revealed no statistically significant differences between total methylation of individual DQA1 alleles of whole blood leucocytes and monocytes (see Supplementary material, Fig. S2). This approach allowed us to combine the sequences from monocytes and whole blood leucocytes of patients with T1D into one group and compare them with whole blood leucocyte sequences from healthy controls.
When comparing the specific QAP methylation of individual CpGs, we found one statistically significant difference between whole blood leucocytes and monocytes of patients with T1D in DQA1*01:02M promoter at the position −311 (P corr = 0·020). Monocyte DQA1*01:02M sequences were collected from only one patient, who had this position (−311) completely unmethylated. This was not observed in whole blood leucocytes where sequences came from three different people and where the position −311 was more methylated (70%). For this reason we decided to exclude 01:02M monocyte sequences from further analysis and use only sequences from whole blood leucocytes.
HLA‐DQA1 promoter methylation of T1D patients and healthy controls
In both studied groups, the most methylated promoter allele was DQA1*04:01 (part of DRB1*08 haplotype) and DQA1*02:01 (DRB1*07 haplotype). In contrast, the least methylated promoter was DQA1*05:01 (DRB1*03 haplotype) allele. Results are summarized in Fig. 3.
Figure 3.
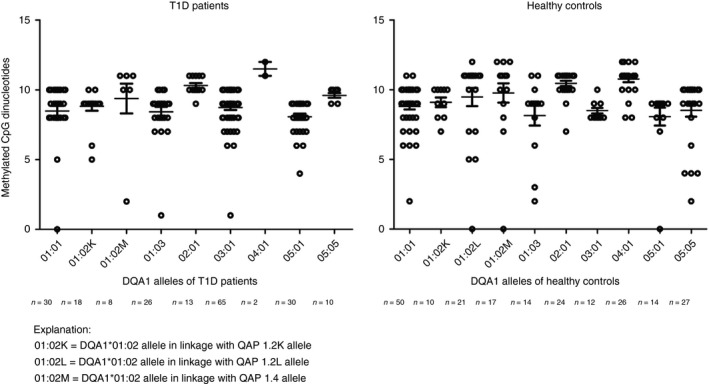
Total methylation profile of DQA1 promoter alleles in patients with type 1 diabetes mellitus (T1D) and healthy controls. Each point in the graph represents number of methylated CpG dinucleotides from single sequence of monocyte or whole blood leucocyte. Mean values with standard error of mean are indicated. Number of methylation positions was dependent on QAP allele identity (9–12 CpGs per sequence). The most methylated allele was DQA1*04:01 allele (12 CpGs per sequence) and DQA1*02:01 allele (11 CpGs per sequence) in both studied groups. The least methylated allele was 05:01 (nine CpGs per sequence). Statistical analysis showed no statistical difference in total methylation of DQA1 promoter alleles between patients with T1D and healthy controls. Statistical significance was tested by Mann–Whitney test (α = 0·05) followed by Bonferroni correction (P corr = P uncorr *10).
When we analysed the specific methylation of individual promoter CpGs in two study groups, we revealed that while CpG dinucleotides from region −641 to −374 are almost completely methylated, as we get closer to the transcription initiation site, DNA methylation level decreases to almost no methylation at position −193 (Fig. 4a,b). Moreover, closer to the transcription initiation site, a more distinct methylation pattern is observed between patients with T1D and healthy controls. The most differences in individual CpG dinucleotide methylation between both groups were found at the position −311. Although the promoter of DQA1*02:01 allele was almost completely methylated (78% HC, 93% T1D) at this position, methylation of other promoter alleles ranged between 33% and 63% (33–63% HC, 37–55% T1D). However, statistical analysis showed no significance regarding the DQA1 promoter methylation status between patients with T1D and healthy controls. We also compared DNA methylation of T1D risk and protective haplotypes of patients with T1D with healthy controls that carry the same T1D risk and protective haplotypes. No methylation differences were found either for T1D protective DR*13‐DQA1*01:03‐DQB1*0603 or for T1D‐risk haplotypes DR*04‐DQA1*03‐DQB1*03:02, DR*03‐DQA1*05:01‐DQB1*02:01.
Figure 4.
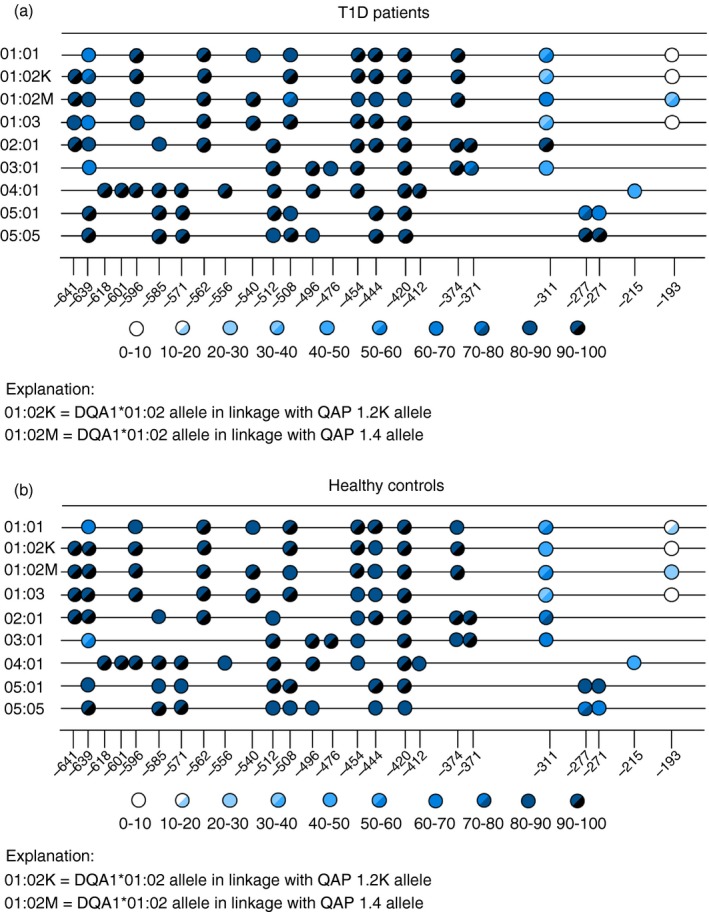
(a) Specific methylation profile of DQA1 promoter CpG sites in patients with type 1 diabetes mellitus (T1D). The matrix represents an amplicon that contains 9–12 CpG sites (number depends on promoter allele identity) obtained for 545 bp region overlapping promoter of the DQA1 gene. Each colour‐coded circle within the matrix corresponds to one CpG site and its colour indicates the percentage of sequences that have the cytosine at the given site methylated. Individual methylation positions under figure marks relative position of CpG dinucleotide before initiation transcription site. CpG dinucleotide at the position −311, −277, −211 was the most variable in methylation between both studied groups. DQA1*02:01 allele at the position −311 was almost completely methylated (93%). Methylation of other alleles ranged between 37 and 55%. (b) Specific methylation profile of DQA1 promoter CpG sites in healthy controls. The matrix represents an amplicon that contains 9–12 CpG sites (number depends on promoter allele identity) obtained for a 545‐bp region overlapping promoter of the DQA1 gene. Each colour‐coded circle within the matrix corresponds to one CpG site and its colour indicates the percentage of sequences that have the cytosine at the given site methylated. Individual methylation positions under figure marks position of CpG dinucleotide before initiation transcription site. CpG dinucleotide at the position −311, −277, −211 was the most variable in methylation between both studied groups. DQA1*02:01 allele at the position −311 was methylated in 78%, whereas methylation of other promoter alleles ranged between 33 and 63%.
Discussion
HLA‐DQA1 expression is not well mapped and only a few studies have characterized its expression in patients with T1D and healthy controls. A study by Maffei et al., confirmed by Donner et al., revealed higher expression of DQA1*03 in DQA1*03/DQA1*05:01 healthy heterozygotes.19, 20 These studies were followed by study from Fernandez et al., where in contrast to other works the highest DQA1 expression was found for DQA1*04 allele and the lowest for DQA1*02, 03 and 05 alleles.21 So far the most extensive work was published by Britten et al., where not only individual allele expression was performed but also promoter activity assays were carried out.15 In general, we found the highest expression for DQA1*03 allele and the lowest for DQA1*05:05 allele in both groups, which is in accordance with the work of Britten and Donner. DQA1*03 was significantly more expressed than the other DQA1 alleles in both studied groups. Alleles where expression values for only two samples were obtained could not be statistically analysed. The most variable expression was found for DQA1*01 allele, where DQA1 expression was dependent on the specific DQA1 promoter (QAP). We did not observe HLA‐DQA1 expression differences in T1D risk or protective haplotypes between patients with T1D and healthy controls. However, we found a statistically significant increase in expression of HLA‐DQA1*02:01 allele in healthy controls. To our knowledge, we are the first to report this observation. This allele is in linkage disequilibrium (part of haplotype) with HLA‐DRB1*07 and HLA‐DQB1*02:02 and is neither a risk nor protective for T1D.
HLA class II expression is mainly regulated at the transcription level by the SXY module, which is localized −150 to −300 bp before the transcription initiation site. All MHC class II genes include this motif and this is where the enhanceosome forms. Moreover, additional SXY modules are scattered across the MHC class II locus at distal positions and can function as enhancers.22, 23 Therefore it is possible that increased DQA1 expression could be related to cis and trans acting elements upstream of the promoter. Epigenetic mechanisms can also be involved in this type of gene expression regulation, for example, Mio et al. demonstrated that the upstream region of HLA‐DRB1 and HLA‐DQB1 genes has an increased response to interferon‐γ and tumour necrosis factor‐α that is accompanied by histone H3 lysine 9 acetylation.24
The link between decreased expression of DQA1*02:01 in patients with T1D and T1D pathogenesis is not clear. Possible explanation is that decreased DQA1 expression can influence the spectrum of T‐cell‐produced cytokines and possibly the shape of immune response. It was shown that the amount of the particular HLA–peptide complex on a cell's surface influences the amount of the cytokines interleukin‐4 and interferon‐γ produced by the triggered T cells and consequently the T helper type 1/type 2 response balance.25 It is interesting that by far the most expressed allele DQA1*03 is part of the DRB1*04‐DQA1*03‐DQB1*03 haplotype, which is highly predisposing to many autoimmune diseases (like T1D, dominated by a T helper type 1 response). To explore this hypothesis, mRNA expression analysis of the DQA1 partner molecule DQB1 should be performed, and also the total amount of DQαβ dimer on the cells’ surfaces should be assessed, because the total amount of the DQαβ–peptide trimers on the cell surface does not depend only on the availability of subunits α, β and peptide, but also on the stability of the resulting trimer. As we have performed whole blood leucocyte expression analysis, expression in more immunologically T1D‐relevant cell populations (monocytes and B lymphocytes) should be assessed. Another possibility is that autoimmunity could be related to low expression of HLA class II alleles, which results in less efficient presentation of autoantigen to protective regulatory T cells, as published by Swanberg et al.5 Swanberg et al. also described a polymorphism (168A→G) in the MHC2TA gene that is associated with reduced MHC class II expression.5 Interestingly, in our study, higher DQA1*02:01 expression was mainly noticeable in 02:01/03 healthy heterozygotes where the ratios between these allele combinations was almost two times lower in healthy controls in comparison with patients with T1D. We are not the first to report different 02:01/03 ratios between healthy controls and patients with T1D. A similar trend was already described in Donner's work, but likewise in our work, allele ratios were not significant due to the small amount of data.20 DQA1*04:01 allele tends to be more expressed in patients with T1D, but only when normalizing against PPIA. As explained earlier, this difference can be caused by inflammatory processes present in patients with T1D but not in healthy controls, and therefore not visible in DRA normalization. This tendency to higher allele expression in patients with T1D was visible also for other DQA1 alleles, but none of the differences was significant.
We also analysed two heterozygous diabetic patients with DRB1*13‐DQA1*01:03‐DQB1*06:03 and DRB1*04‐DQA1*03:01‐DQB1*03:02 haplotypes. It is known that the DRB1*13‐DQA1*01:03‐DQB1*06:03 haplotype is protective for T1D development, even though some studies state that the protection is lost when in combination with the DRB1*04‐DQA1*03:01‐DQB1*03:02 haplotype.26 Despite this fact, both patients have developed autoimmunity relatively late in life, at around 40 years of age. Expression analysis of DQA1*01:03 and DQA1*03:01 did not show aberrant expression compared with the same haplotypes of the controls.
T1D monozygotic concordance values (25–65%) point in favour of an environmental contribution to T1D, which can be expressed through distinct epigenetic profile. Since we have found higher DQA1*02:01 expression in healthy controls, we explored a hypothesis that lower 02:01 expression in patients with T1D could be related to altered DNA methylation within HLA‐DQA1 promoter and might increase predisposition to disease, particularly in DQA1*02:01/03 heterozygotes. We have not found simple correlation for HLA‐DQA1 expression and DNA methylation. The low expressed alleles 05:05 and 02:01 tend to be highly methylated, but the ‘expression level inversely correlated with methylation density’ principle could not be applied to the most expressed DQA1*03 allele, which has average methylation level. Comparison of total DNA methylation status of HLA‐DQA1 promoter alleles between healthy controls and patients with T1D did not reveal any significant differences. Since we used a very fine technique for analysing DNA methylation status, we were able to analyse individual methylation positions in detail. The more we approached the transcription initiation site, the lower the methylation level was. Similar methylation pattern (at individual CpG methylation positions) was detected in patients with T1D and healthy controls, with the most variable methylation site being located at positions −311 (in DQA1*01:01, 01:02K, 01:02M, 01:03, 02:01, 03:01 alleles) and −277, −271 (in DQA1*05:01, 05:05) before transcription initiation site. Kuroda et al. showed that insulin expression can be influenced by methylation of one particular CpG site.13 If this is the case, then DNA methylation mark could regulate binding of transcription factors to their target sequences. CpG methylation at the position −311, −277, −271 seems to be the best candidate for this type of regulation.
In recent years, increasing attention is focused on epigenetic modifications in T1D. Stefan et al. described differences in DNA methylation profiles between T1D concordant and discordant monozygotic twins in HLA and insulin genes.12 Moreover, a distinct DNA methylation profile was detected in study by Fradin et al., where patients with T1D showed significantly decreased methylation at three CpG positions in the promoter region of the insulin gene.11 Another study by Rakyan et al. analysed DNA methylation of CD14+ monocytes and found 132 T1D methylation variable positions associated with various genes, including HLA‐DQB1 and RFXAB, an HLA class II regulating element.10 Regarding this information, Majumder et al. showed that two important transcription factors that help to form enhanceosome, RFX and CIITA, did not bind to the hypermethylated promoter proximal regions of HLA‐DQA1 and DQB1 genes. Inhibition of methyltransferases restored binding of both factors and led to high HLA‐DQA1 and DQB1 expression.27, 28 These results suggest an importance of the promoter proximal regions of these genes and their methylation status. However, this observation was made in the cell lines derived from acute lymphocytic leukaemia, where different types of regulation can be involved.
In conclusion, this study maps DNA methylation status of the HLA‐DQA1 promoter region and evaluates HLA‐DQA1 expression differences between patients with T1D and healthy controls. The study contributes to the epigenetic research, analyses in detail DQA1 promoter methylation, and extends the knowledge about epigenetic modification in T1D susceptible gene. Although we have not proved significant methylation differences between the two groups, detailed DNA methylation status and its correlation with expression of each HLA‐DQA1 allele in patients with T1D have been described for the first time.
Disclosure
The authors declare that they have no financial or commercial conflict of interests.
Author contributions
Marie Cerna is author of the research idea, designed the experiments and performed the publication revision. Elena Silhova participated in organization of the collection of the clinical database. Anna Kotrbova‐Kozak designed experiments and participated in publication revision and data analysis. Marta Zajacova participated in laboratory work, data analysis and publication revision. Pavel Cepek participated in laboratory work, data analysis and prepared the publication.
Supporting information
Figure S1. Relative expression of HLA DQA1*02:01/03 alleles in heterozygotes.
Figure S2. Methylation profile of whole blood leucocytes and monocytes of patients with type 1 diabetes mellitus.
Table S1. DRB1–QAP–DQA1–DQB1 haplotypes in patients with type 1 diabetes mellitus and healthy controls.
Table S2. Expression ratios of two alleles in DQA1 heterozygotes and their comparison between patients with type 1 diabetes mellitus and healthy controls.
Acknowledgements
This survey was funded by the Research programme of Charles University in Prague: PRVOUK P31: Initial stadia of diabetes, obesity and other metabolic diseases. SVV/2016: Molecular, endocrine and genetic aspects of diabetes mellitus development. A.K.K. gratefully acknowledges the support of the Charles University through University Research Centre programme UNCE 204015.
References
- 1. Gan MJ, Albanese‐O'Neill A, Haller MJ. Type 1 diabetes: current concepts in epidemiology, pathophysiology, clinical care, and research. Curr Probl Pediatr Adolesc Health Care 2012; 42:269–91. [DOI] [PubMed] [Google Scholar]
- 2. Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al Genome‐wide association study and meta‐analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009; 41:703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moustakas AK, Papadopoulos GK. Molecular properties of HLA‐DQ alleles conferring susceptibility to or protection from insulin‐dependent diabetes mellitus: keys to the fate of islet beta‐cells. Am J Med Genet 2002; 115:37–47. [DOI] [PubMed] [Google Scholar]
- 4. Cerna M. Genetics of autoimmune diabetes mellitus. Wien Med Wochenschr (1946) 2008; 158:2–12. [DOI] [PubMed] [Google Scholar]
- 5. Swanberg M, Lidman O, Padyukov L, Eriksson P, Akesson E, Jagodic M, et al MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat Genet 2005; 37:486–94. [DOI] [PubMed] [Google Scholar]
- 6. Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 2008; 359:2849–50. [DOI] [PubMed] [Google Scholar]
- 7. Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow‐up study. Diabetes 2003; 52:1052–5. [DOI] [PubMed] [Google Scholar]
- 8. Fourlanos S, Varney MD, Tait BD, Morahan G, Honeyman MC, Colman PG, et al The rising incidence of type 1 diabetes is accounted for by cases with lower‐risk human leukocyte antigen genotypes. Diabetes Care 2008; 31:1546–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, et al The rising incidence of childhood type 1 diabetes and reduced contribution of high‐risk HLA haplotypes. Lancet 2004; 364:1699–700. [DOI] [PubMed] [Google Scholar]
- 10. Rakyan VK, Beyan H, Down TA, Hawa MI, Maslau S, Aden D, et al Identification of type 1 diabetes‐associated DNA methylation variable positions that precede disease diagnosis. PLoS Genet 2011; 7:e1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fradin D, Le Fur S, Mille C, Naoui N, Groves C, Zelenika D, et al Association of the CpG methylation pattern of the proximal insulin gene promoter with type 1 diabetes. PLoS One 2012; 7:e36278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stefan M, Zhang W, Concepcion E, Yi Z, Tomer Y. DNA methylation profiles in type 1 diabetes twins point to strong epigenetic effects on etiology. J Autoimmun 2014; 50:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuroda A, Rauch TA, Todorov I, Ku HT, Al‐Abdullah IH, Kandeel F, et al Insulin gene expression is regulated by DNA methylation. PLoS One 2009; 4:e6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morzycka‐Wroblewska E, Harwood JI, Smith JR, Kagnoff MF. Structure and evolution of the promoter regions of the DQA genes. Immunogenetics 1993; 37:364–72. [DOI] [PubMed] [Google Scholar]
- 15. Britten AC, Mijovic CH, Barnett AH, Kelly MA. Differential expression of HLA‐DQ alleles in peripheral blood mononuclear cells: alleles associated with susceptibility to and protection from autoimmune type 1 diabetes. Int J Immunogenet 2009; 36:47–57. [DOI] [PubMed] [Google Scholar]
- 16. Zajacova M, Kotrbova‐Kozak A, Cepek P, Cerna M. Differences in promoter DNA methylation and mRNA expression of individual alleles of the HLA class II DQA1 gene. Immunol Lett 2015; 167:147–54. [DOI] [PubMed] [Google Scholar]
- 17. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15:539–53. [DOI] [PubMed] [Google Scholar]
- 18. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maffei A, Harris PE, Reed EF, Del Pozzo G, Ciullo M, Suciu‐Foca N, et al Differential expression of insulin‐dependent diabetes mellitus‐associated HLA‐DQA1 alleles in vivo . Eur J Immunol 1997; 27:1549–56. [DOI] [PubMed] [Google Scholar]
- 20. Donner H, Seidl C, Rau H, Herwig J, Seifried E, Usadel KH, et al Unbalanced amounts of HLA‐DQA1 allele mRNA: DQA1*03 shows high and DQA1*0501 low amounts of mRNA in heterozygous individuals. Eur J Immunogenet 2002; 29:321–30. [DOI] [PubMed] [Google Scholar]
- 21. Fernandez S, Wassmuth R, Knerr I, Frank C, Haas JP. Relative quantification of HLA‐DRA1 and ‐DQA1 expression by real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR). Eur J Immunogenet 2003; 30:141–8. [DOI] [PubMed] [Google Scholar]
- 22. Reith W, LeibundGut‐Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol 2005; 5:793–806. [DOI] [PubMed] [Google Scholar]
- 23. Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell 2002; 109(Suppl):S21–33. [DOI] [PubMed] [Google Scholar]
- 24. Miao F, Chen Z, Zhang L, Liu Z, Wu X, Yuan YC, et al Profiles of epigenetic histone post‐translational modifications at type 1 diabetes susceptible genes. J Biol Chem 2012; 287:16335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DiMolfetto L, Neal HA, Wu A, Reilly C, Lo D. The density of the class II MHC T cell receptor ligand influences IFN‐γ/IL‐4 ratios in immune responses in vivo . Cell Immunol 1998; 183:70–9. [DOI] [PubMed] [Google Scholar]
- 26. Hermann R, Turpeinen H, Laine AP, Veijola R, Knip M, Simell O, et al HLA DR‐DQ‐encoded genetic determinants of childhood‐onset type 1 diabetes in Finland: an analysis of 622 nuclear families. Tissue Antigens 2003; 62:162–9. [DOI] [PubMed] [Google Scholar]
- 27. Majumder P, Boss JM. DNA methylation dysregulates and silences the HLA‐DQ locus by altering chromatin architecture. Genes Immun 2011; 12:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scharer CD, Choi NM, Barwick BG, Majumder P, Lohsen S, Boss JM. Genome‐wide CIITA‐binding profile identifies sequence preferences that dictate function versus recruitment. Nucleic Acids Res 2015; 43:3128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Relative expression of HLA DQA1*02:01/03 alleles in heterozygotes.
Figure S2. Methylation profile of whole blood leucocytes and monocytes of patients with type 1 diabetes mellitus.
Table S1. DRB1–QAP–DQA1–DQB1 haplotypes in patients with type 1 diabetes mellitus and healthy controls.
Table S2. Expression ratios of two alleles in DQA1 heterozygotes and their comparison between patients with type 1 diabetes mellitus and healthy controls.


