Summary
Cellular and molecular investigation of parasitic helminth infections has greatly accelerated the understanding of type 2 immune responses. However, there remains considerable debate regarding the specific leucocytes that kill parasites and whether these mechanisms are distinct from those responsible for tissue repair. Herein, we chronicle discoveries over the past decade highlighting current paradigms in type 2 immunity with a particular emphasis upon how CD4+ T helper type 2 cells, type 2 innate lymphoid cells and alternatively activated macrophages coordinately control helminth‐induced parasitism. Primarily, this review will draw from studies of the murine nematode parasite Nippostrongylus brasiliensis, which bears important similarities to the human hookworms Ancylostoma duodenale and Necator americanus. Given that one or more hookworm species currently infect millions of individuals across the globe, we propose that vaccine and/or pharmaceutical‐based cure strategies targeting these affected human populations should incorporate the conceptual advances outlined herein.
Keywords: helminth infection, helminth therapy, innate lymphoid cell, M2 macrophage, T helper type 2
Abbreviations
- CTLA‐4
cytotoxic T‐lymphocyte antigen 4
- E/S
excretory/secretory
- Foxp3
forkhead box P3
- IL‐4
interleukin‐4
- IL‐4Rα
interleukin‐4 receptor α
- ILC
innate lymphoid cell
- LAG‐3
lymphocyte activation gene 3
- M2
alternatively activated macrophage
- PD‐1
programmed cell death protein 1
- PD‐L1
programmed cell death protein 1 ligand
- RELMβ
resistin‐like molecule β
- STAT6
signal transducer and activator of transcription 6
- Th2
T helper type 2
Introduction
Human hookworm infections are primarily caused by two species, Necator americanus and Ancylostoma duodenale, that together cause between 500 and 700 million cases of infection worldwide.1 Mechanistic understanding of hookworm immunobiology has been aided considerably through laboratory‐based studies in rodents using the hookworms Nippostrongylus brasiliensis and Ancylostoma ceylanicum.2 Depending on the species, fecund adult females can produce up to 20 000 eggs per day that, when passed in stool, undergo further development when sanitation conditions and agricultural practices fail to block transmission.3 The circuitous life‐cycle of the hookworm proceeds from free‐living rhabditiform larvae that hatch in warm, damp soil, transiently feed upon bacteria, and after two moults, rapidly develop into the infectious larvae (third stage L3). Third‐stage infective larvae migrate up blades of grass or other foliage to facilitate host entry through skin or oral routes. Migratory larvae travel through the vasculature, enter lung capillaries, breaching these vessels to enter alveoli, and migrate up the trachea and down the esophagus. Immature larvae that are swallowed develop into adult worms ranging from 10 to 20 mm in size that feed upon intestinal villi in the proximal small intestine.4 Blood‐feeding is a distinguishing characteristic of hookworm infections, which, if excessive, causes iron deficiency anaemia in individuals with high worm burdens. Such haematophagous feeding is a major driver of increased disease‐associated life‐year (DALY) scores in countries where hookworms are endemic.5
That hookworms cause persistent injury to host mucosal tissue is most likely the major driving force behind the ensuing type 2 inflammatory response. Notwithstanding the conserved torpedo‐like body, hookworm skin penetration is facilitated by the enzymatic nature of excretory/secretory (E/S) proteins that initiate the type 2 immune response sequelae (Fig. 1a). In fact, enzymatic activity is a common feature of allergens that elicit type 2 inflammation.6 Even though skin pathology induced by migratory larvae and gastrointestinal haemorrhage caused by feeding adults are both well‐documented,7 the pathophysiology of lung pathology caused by hookworms remains less well understood.8, 9 Although some reports have suggested that deficiencies in epithelial repair molecules in the Trefoil factor family can exacerbate damage, whether Trefoils or epidermal growth factor family members (e.g. amphiregulin) drive resolution of damage remains unclear (Fig. 1b). Irrespective of the organ‐system affected, hookworms induce prototypical type 2 inflammation, characterized by a distinct cytokines (e.g. interleukins 4, 5, 13, 25, 33, and thymic stromal lymphopoietin (TSLP), leucocytes (e.g. eosinophils, mast cells, basophils) and soluble factors (e.g. matrix metallo‐proteinases and immunoglobulins G and E).10, 11 Hookworm‐infected individuals can also experience a progressive and generalized immunosuppression, which may explain why adult individuals, instead of children, harbour the highest worm burdens within endemic regions.5, 12 Data showing elevated secretion of interleukin‐10 (IL‐10) and expansion of lymphocytes expressing markers of immune tolerance/suppression [i.e. forkhead box P3 (Foxp3), programmed cell death protein 1 (PD‐1), lymphocyte activation gene 3 (LAG‐3), cytotoxic T‐lymphocyte antigen 4 (CTLA‐4)] may underlie the mechanisms responsible.13, 14 Taken together, given the complex nature of hookworm pathogenesis, a clearer understanding of how certain immune cell types [T helper type 2 (Th2), innate lymphoid cell type 2 (ILC2) and alternatively activated macrophage (M2 macrophage)] are orchestrated in response to this pathogen could promote the eventual eradication of this neglected tropical disease.
Figure 1.
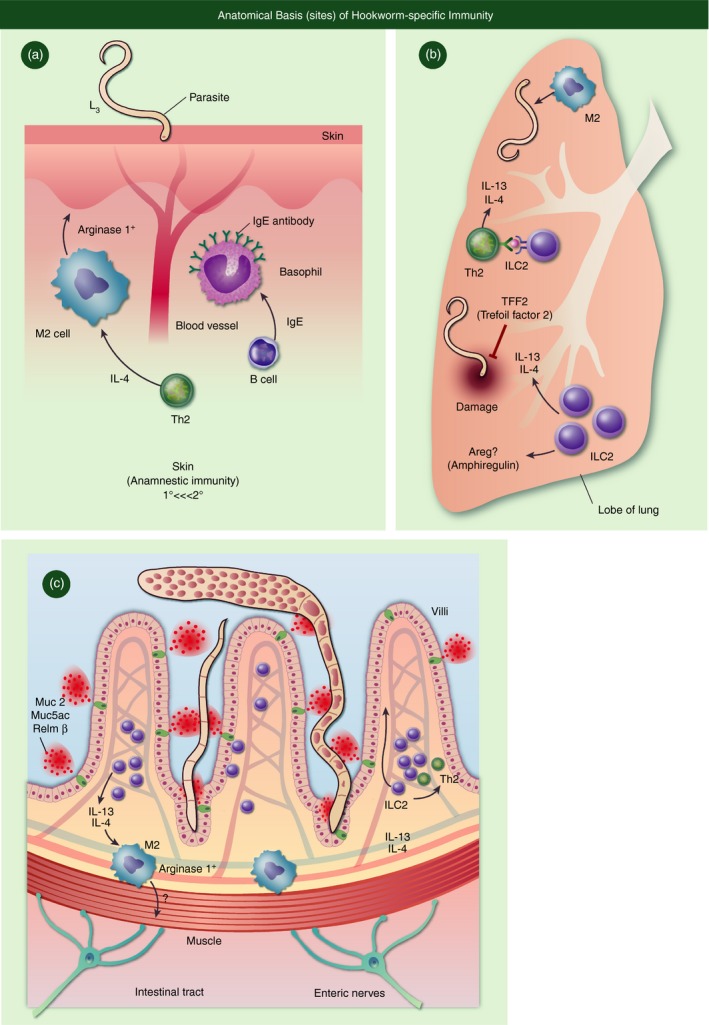
Anatomical basis of Hookworm‐specific Immunity. (a) In the skin, IL‐4 driven M2 cells along with IgE primed basophils drive highly amannestic immunity. (b) In the lung, Trefoil factor 2 and potentially Amphiregulin control lung damage caused by larvae, but coordination between ILC2 and TH2 cells produce high levels of IL‐4/13. (c) In the intestine, clearance of adult worms is mediated by epithelial cell derived factors such as mucins (Muc2 and Muc5ac) and other goblet cell derived proteins such as resistin like molecule beta (RELMb), which function to limit parasite nutrition.
Th2 cytokines
CD4+ Th2 cell differentiation is a highly conserved feature of immune responses against parasitic worms. Th2 cytokine administration accelerates the clearance of N. brasiliensis adult worms and immunity is significantly delayed in IL‐4 receptor α (IL‐4Rα) or signal transducer and activaotr of transcription 6 (STAT6) double‐negative mouse strains or anti‐CD4 monoclonal antibody‐treated wild‐type mice15, 16, 17 Moreover, IL‐5 production in humans positively correlated with acquired resistance to Necator re‐infection.18 Although, infection‐induced Th2 cells were previously considered entirely responsible for IL‐4, IL‐5, IL‐9, IL‐10 and IL‐13 production, current evidence reveals IL‐9‐producing Th9 cells and IL‐10‐producing type 1 regulatory T cells as distinct Th subsets that confer host protective roles during hookworm infection.19, 20, 21 Nonetheless, Th2‐derived cytokines drive host immunity against worms that are migrating through the pulmonary architecture, where larvae may be destroyed more readily by effector mechanisms, particularly during secondary infections22 (Fig. 1b). In secondary infection, IL‐4‐responsive B cells are also critical in mediating immunity through cognate interaction with CD4 Th2 cells, production of IL‐1323 and antibody‐mediated activation of basophils and mast cells.24, 25, 26 .
Within the intestine, expulsion of adult N. brasiliensis worms from the gastrointestinal tract requires IL‐4Rα expression on non‐haematopoietic cells.27 In this context, Th2‐derived IL‐4 and IL‐13 induce intestinal goblet cells to secrete resistin‐like molecule β (RELMβ), which binds to the chemosensory neurons of gastrointestinal nematodes to prevent feeding27, 28 (Fig. 1c). However, this protective mechanism may be unique to hookworm species, because RELMβ did not promote worm expulsion, during infection with Trichinella spiralis or the whipworm Trichuris muris, where instead RELMβ acted on host leucocytes to promote Th1 cytokine production.29 Although IL‐13 is a major driver of RELMβ production, IL‐13 acts to clear T. muris infection through driving excessive epithelial cell proliferation termed an ‘epithelial escalator’ mechanism leading to the sloughing of epithelia and associated worms.30 Interleukin‐13 drives STAT6‐dependent‐changes within intestinal physiology, known as the ‘weep and sweep’ response, which is considered to drive worm clearance through a combination of smooth muscle contraction and excessive mucus production.31, 32, 33 In support of this latter mechanism, hookworm infections in mice with a targeted gene deletion of either Muc2 or Muc5ac result in worms with greater energy content and greater survival ability than parasites within wild‐type mice.28, 34 Regenerative cytokines in the IL‐10 superfamily such as IL‐22 have been shown to promote immunity against hookworms, most likely by enhancing the epithelial effector response (Fig 2a). Excess mucus release is thought to trap worms and prevent access to crypt niches, but additional mechanisms considered responsible for worm clearance include IL‐4/IL‐13‐driven changes in epithelial cell permeability and smooth muscle contractility. Although IL‐4/IL‐13 can act directly on epithelia and smooth muscle to promote immunity,35 these cytokines may also act indirectly through stimulation of enteric nerves (Fig. 1c). Whether the sole lack of IL‐4/IL‐13 responsiveness on enteric nerves in an otherwise immunocompetent host would impair worm expulsion has not been determined. Even so, there is published evidence for STAT6‐responsive enteric nerve receptors such as protease‐activated receptors 1 and 2, muscarinic 3 receptor,36 while immune cells including macrophages and T cells produce and respond to adrenaline and acetylcholine.37 Combined, these data are entirely consistent with the rapidly emerging field of neuroimmunology, which is predicated on data showing that communication between neuronal networks and the immune system has a broad impact upon the outcome of host–pathogen interactions.38 .
Figure 2.
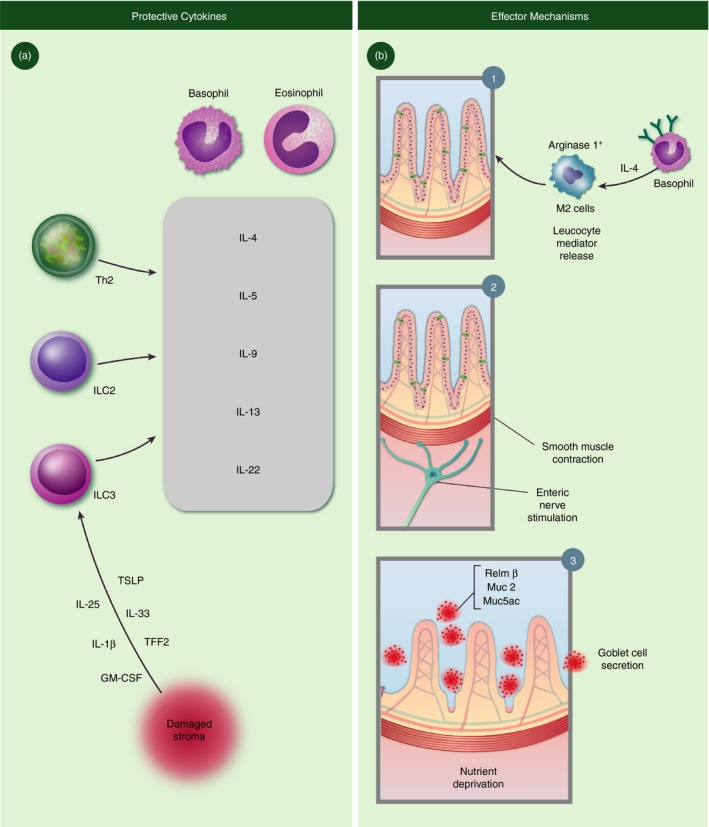
Protective cytokines and effector mechanisms in the context of hookworm infections. (a) The site of tissue injury releases an array of cytokines that, in turn promotes release of host protective cytokines (gray box) from innate and adaptive lymphocytes and myeloid populations. (b) Established mechanisms of worm clearance in murine skin (1) and intestine (2,3).
Although CD4+ T cells are necessary for both primary and secondary immune protection from N. brasiliensis expulsion17 , 22 identifying the nature of the Th2 cells driving immunity, with regards to antigen specificity, and whether CD4+ T‐cell‐intrinsic cytokines are necessary has led to unexpected results. Infection by N. brasiliensis of ovalbumin‐specific T‐cell receptor transgenic mice resulted in higher parasite burdens compared with wild‐type mice with a polyclonal T‐cell receptor repertoire, suggesting that N. brasiliensis antigen‐specific T cells are required.39 Paul and colleagues took an alternative approach and tested if N. brasiliensis‐induced Th2 cells could promote type 2 cytokine responses in an antigen‐independent manner.40 They found that Th2 cells from mice that had been previously infected with N. brasiliensis promoted eosinophil airway inflammation to house dust mite antigen through production of IL‐13 and independently of antigen presentation via MHC class II. This innate function of Th2 cells was dependent on IL‐33 and enhanced by TSLP and IL‐7. Conversely, they tested if Th2 cells generated in response to Ascaris suum, a phylogenetically distant nematode, could confer protection to N. brasiliensis. They found that previous infection with either nematode could protect reciprocally against the other nematode and that this protection was dependent on the CD4+ T cells. Further, they showed that ovalbumin‐specific Th2 cells also conferred some protection against N. brasiliensis infection, suggesting an innate immunological function for Th2 cells that was independent of antigen specificity. Surprisingly, with regards to the importance of responsiveness or production of Th2 cytokines by CD4+ cells, it appears that neither IL‐4Rα expression nor expression of IL‐4 and/or IL‐13 from T cells is necessary for N. brasiliensis expulsion.41, 42 These somewhat conflicting data suggest that CD4+ Th2 cell antigen specificity and cytokine production are important, but not altogether essential for immunity to hookworms. This assertion is entirely consistent with the emergence of ILC2 as a key source of IL‐5, IL‐9 and IL‐13, which even though a relatively small population, on a per cell basis, rivals cytokine release from CD4+ Th2 cells.
Type 2 innate lymphoid cell
Perhaps the most exciting discovery in the immunology community over the past decade has been the formal determination that previously obscure non‐B/non‐T‐cell populations induced by helminth infections43, 44 are a discrete population of lineage‐negative ILC.45 Populations of ILC1, ILC2 and ILC3 are established lineages that elicit distinct cytokine profiles and although ILC3‐derived IL‐22 may serve an important role in driving anti‐helminth immunity, this issue has not been directly addressed (Fig. 2a). On the other hand, the predominance of the data indicates that ILC2 are vital for host immunity against hookworms. Dedicated to type 2 cytokine production, the ILC2 subset secretes high levels of IL‐5 and IL‐13, even before the adaptive immune response has developed.46, 47, 48 Although IL‐5 primarily acts on haematopoietic cells, IL‐13 regulates non‐haematopoietic cells through the type 2 IL‐4R (comprised of IL‐4Rα and IL‐13Rα subunits). Hence, while IL‐5 regulates eosinophil and mast cell activity, IL‐13 specifically drives goblet cell metaplasia, mucus hyper‐secretion, smooth muscle contraction, and presumably enteric nerve stimulation. ILC2 also produce large quantities of IL‐9, a cytokine that contributes to hookworm immunity through promotion of mast cell, eosinophil and goblet cell responses.49 In fact, IL‐9‐producing ILC2 help to curtail lung damage and promote tissue repair following hookworm migration through the pulmonary tract.50 The formal recognition of ILC2 as a biologically important lymphocyte population has led to a re‐evaluation of the notion that Th2 cells are solely responsible for long‐term host protection against hookworms. Recent demonstration by McKenzie and colleagues demonstrates that even in the presence of Th2 cells, the sole absence of ILC2 impairs anamnestic immunity to hookworm infection due to defects in IL‐13‐induced priming of a critical dendritic cell subset necessary for chemokine production.51 Indeed, ILC2 present within the lung parenchyma poised to release canonical cytokines that drive eosinophilopoiesis (IL‐5), mastocytosis and basophil activation. Despite this recent report, due to the historical evidence showing lack of memory in the absence of canonical Th2 cells, the question remains as to whether long‐term host immunity Th2 cells and/or ILC2 work in concert or not.
Perhaps the recent work from McKenzie and colleagues showing how Th2 promote ILC2 function through an MHC II dialogue sheds light on this area.52 In this study, McKenzie and colleagues used two separate transgenic mouse models to delete ILC2 while leaving CD4+ T‐cell function intact, by targeting ILC expression of ICOS for diphtheria toxin‐induced deletion, or by taking advantage of the essential requirement for RORα for ILC2 survival. During N. brasiliensis infection, they showed that deletion of ILC2 led to a significant decrease in CD4+ T‐cell‐derived IL‐5 and IL‐13, culminating in defective parasite expulsion. These data suggested that ILC2 are important in initiating or amplifying the adaptive CD4 Th2 response. Next, they delineated the mechanism by which ILC2 instructed T cells. They showed that ILC2 could take up, process and present antigen via MHC class II to T cells, which in turn provided IL‐2 that promoted ILC2 production of IL‐13 leading to a positive feedback loop for the production of Th2 cytokines. This newly discovered function for CD4+ T cells in providing survival signals to ILC2 may shed light on the unanswered conundrum of why CD4+ T cells are important for protective immunity when other innate cells are the predominant sources of Th2 cytokines. Le Gros and colleagues recently showed cooperative action of ILC2 and T cells in promoting macrophage‐mediated immunity to N. brasiliensis re‐infection in the lung.53 This latter study provides evidence for the likely scenario that ILC2 and M2 macrophages are coordinately regulated to limit hookworm survival and limit tissue damage caused by infection, both during primary and anamnestic immune responses to hookworms.
M2 macrophages
Over two decades ago, Siamon Gordon conceived the idea that IL‐4‐dependent Th2‐driven responses elicit alternatively activated macrophages (i.e. M2 macrophage) as a direct counterpart to Th1‐elicited classically activated macrophages.54, 55 Interleukin‐4 and IL‐13 promote M2 development through IL‐4Rα signalling, presumably to eliminate worm pathogens and/or repair the tissue damage; however, there is emerging evidence that there are also IL‐4Rα‐independent pathways for M2 development, primarily due to helminth‐derived antigens.56 Mice solely lacking IL‐4Rα on tissue macrophages and neutrophils (LysMCre IL4Rαflox) experience lethality during Schistosoma mansoni infection due to microbial flora‐driven inflammatory responses.57 Since then, a considerable amount of published literature has consolidated the M1 versus M2 paradigm as an important governing feature of mechanisms controlling inflammation and tissue repair.58, 59, 60, 61 However, to date the issue of whether M2 cells are needed for promoting repair through regenerative capabilities or whether their role is primarily to thwart the pro‐inflammatory effects of M1 polarization is one that has not been sufficiently addressed. It is likely that there are several, as yet unidentified, effector molecules released from M2 cells that directly promote host protection (Fig. 2b). It is well established that pathogen‐associated molecular patterns (PAMP), outer membrane proteins, lipopolysaccharide (LPS) and interferon‐γ elicit M1 polarization to effect anti‐microbial and tumoricidal activity.62 Even so, M1 cells have immunosuppressive capabilities through interferon‐γ‐induced indoleamine 2,3‐dioxygenase (IDO) production via tryptophan catabolism,63 much like IL‐4/IL‐13‐induced arginase I catabolizes arginine.64 This may be viewed as a nutrient deprivation strategy for inflammation control. Immunosuppression via either M1 or M2 subsets can be afforded by receptor‐mediated pathways such as interferon‐γ‐induced PD‐1 ligand (PD‐L1) versus IL‐4‐induced PD‐L2, both of which can lead to lymphocyte suppression by engaging PD‐1.65, 66 Hence, we are left to speculate whether in the context of hookworm immunopathogenesis, the purported host protective role of M2 cells is due to their ability to promote anti‐inflammatory and/or helminthicidal effector functions. Clearly, M2 cells need to be investigated more thoroughly in the context of helminth infection, chronic protozoan infection and allergic airway inflammation and other biologically relevant contexts.37 .
Delineating the function of macrophages in the immune response to hookworms has been challenging due to difficulties in specifically depleting macrophages at the tissue sites. Two studies using CD11b‐diphtheria‐toxin‐receptor‐specific mice,67, 68 which significantly reduced macrophage frequencies in the lung and peritoneal cavity, showed that CD11b+ macrophages were essential for protecting against N. brasiliensis‐induced acute inflammation in the lung and also promoted effector Th2 cell responses in the intestine for optimal parasite expulsion. Although protective in experimental hookworm infection, CD11b+ macrophages promoted detrimental IL‐13‐driven chronic inflammation and fibrosis in the lung in response two allergic airway inflammation models with house dust mite antigen or Schistosoma mansoni eggs, raising the unanswered question of whether chronic macrophage responses in the lung following hookworm infection reduce or promote secondary allergies.67 Nonetheless, these data suggest that a balanced tissue macrophage response may be necessary for optimal protection against hookworm while alleviating excessive acute inflammation during parasite colonization of the lung. In addition to balancing Th1 and Th2 inflammation, M2 macrophages in the lung also contribute a critical function in protective immunity to secondary N. brasiliensis infection, by binding to and killing the infiltrating larvae.69 In the intestine during primary N. brasiliensis infection M2 macrophages are also effector cells that promote smooth muscle contractility for worm expulsion70 (Fig. 2b). M2 cells require fatty acid oxidation for development71 and recent work demonstrated that CD36‐mediated uptake of triacylglycerol promoted cell survival and M2‐gene expression, all of which required lipolysis via lysosomal acid lipase.72 Aside from classical immune functions in influencing inflammation and immunity to hookworms, a new line of studies implicate hookworm‐induced M2 macrophages in altering host metabolism. In mice fed a high‐fat diet, hookworm infection led to improved glucose homeostasis and reduced obesity through a mechanism that was dependent on M2 macrophages.73 The proposed mechanisms by which M2 macrophages protect from metabolic disorders are potentially numerous and partially involve the production of anti‐inflammatory cytokines.37 In hookworm infection, a more direct mechanism has recently been proposed, in which M2 macrophages directly reduce intestinal epithelial cell glucose uptake leading to a ‘lean’ epithelial cell type.73 .
It is clear that M2 macrophages participate in several important immune and non‐immune functions following hookworm infection; however, the specific contribution of M2‐specific molecules responsible for these functions is less well understood. Epigenetic modification through the histone demethylase Jumonji domain containing 3 (Jmjd3) selectively promotes M2 activation and helminth immunity through interaction with the transcription factor interferon regulatory factor 4 (IRF4).74 Gene expression profiling studies and macrophage‐specific knockout mice have shed light on some of the important M2 ‘signature’ molecules, which include Arginase1, RELMα and Ym1. Anthony et al. demonstrated that Arginase 1 neutralization in Heligmosomoides polygyrus bakeri‐infected mice impaired Th2‐driven worm clearance.75 Arginase1 can play an effector role in N. brasiliensis infection both by affecting larvae trapped in granulomas,75, 76 and by promoting expulsion from the intestine through mediating smooth muscle contraction.70 Arginase also contributes to limiting hookworm‐induced Th2 cytokines, as demonstrated by studies employing macrophage‐specific Arginase1‐deficient mice.64 In contrast to its homologue RELMβ, RELMα does not seem to have any anti‐parasitic effector role, but instead suppresses Th2 cytokine effector responses including parasite expulsion.77 Interestingly, the human RELM protein resistin exhibits similar properties to RELMα, including expression by macrophages following hookworm infection, where it acts to delay parasite expulsion.78 Among the M2‐specific molecules, Ym1, a chitinase‐like protein, is the most highly expressed. Although no Ym1 knockout mice have been generated to‐date, in vivo antibody‐mediated depletion of Ym1 demonstrated that Ym1 contributed to IL‐17‐mediated neutrophil recruitment in the lungs of hookworm‐infected mice. During this early stage of infection, this Ym1‐mediated neutrophil recruitment contributed not only to exacerbating lung inflammation, but also to larval killing.79 Neutrophils themselves were recently shown to express cytokine transcripts for molecules such as IL‐33, which correlated with their ability to promote M2 activation and anamnestic immunity against N. brasiliensis.69 Altogether, these studies highlight the complexity in function of M2‐derived molecules with some regulating infection‐induced inflammation whereas others have anti‐parasitic effector function. It is therefore likely that the functional outcome of M2 macrophages in hookworm infection depends on the expression levels of these proteins, as well as the tissue site and time‐point of infection. Additionally, for the purpose of therapies to improve immunity to hookworms or alleviate immunopathology, it may be important to specifically target individual M2‐derived molecules.
Hookworm therapies
Several epidemiological studies report a correlation between helminth infection and protection against allergy, leading to the attractive concept of helminth therapy to cure inflammatory diseases.80 Although the idea of helminth therapy has existed for several decades, it has only recently been substantiated in controlled studies. Here, we will discuss the epidemiological evidence and recent human experimental hookworm infections that support the therapeutic potential of hookworm infection to treat inflammatory diseases. In contrast to treatment with Trichuris ova, human hookworm therapy stands to provide the additional benefit of establishing a chronic infection and purportedly long‐lasting immunomodulatory effects.
In a meta‐analysis of 33 epidemiological studies with data on intestinal parasite burden and asthma or wheeze, it was found that hookworm infection was the only parasite significantly associated with reduced risk of asthma.81 Anthelminthic drug treatment studies in hookworm‐endemic areas have shown that following hookworm clearance, there is increased skin‐test reactivity to environmental allergens, but not clinical allergy, associated with reduced hookworm‐specific IL‐10.82 It is somewhat counterintuitive that a parasite, which infects and drives inflammation in the skin, lung and intestine, could alleviate allergic symptoms in these organs. Indeed, human hookworm infections are associated with high Th2 responses, characterized by eosinophilia and serum IgE, which are considered to drive pathology in certain allergic diseases. Indeed, in clinical trials conducted in hookworm‐endemic areas aimed at vaccination against re‐infection using a recombinant Necator protein (Ancylostoma‐secreted protein‐2) paradoxically caused significant urticaria (hives) in several volunteers with pre‐existing elevations of Necator‐specific IgE. Hence, on the one hand hookworms suppress prototypical type 2‐driven disease, but on the other they promote those exact types of immune responses. What is the difference between helminth‐driven and allergen‐driven type 2 inflammation? Perhaps the key difference that allows hookworms to ameliorate inflammation is that they drive concomitant regulatory mechanisms such as immune tolerance/suppressive pathways (i.e. Foxp3, PD‐1, LAG‐3 CTLA‐4, IL‐10, PD‐1) that could thwart inflammation against subsequent excessive inflammatory pathologies.
Experimental human hookworm infection studies have been conducted to determine the hookworm doses that can be tolerated without these adverse reactions.83 Although infectious doses of over 50 infective larvae led to skin eruptions, gastrointestinal disturbances and pulmonary symptoms in some volunteers, in contrast, infectious doses of < 50 L3 were well tolerated with minimal discomfort.84, 85 In recent years, controlled trial studies have tested the benefits of experimental hookworm inoculation as therapy against allergic disease. A study of 32 asthmatic volunteers infected with 10 Necator L3 or placebo control, revealed a modest, non‐significant improvement in airway responsiveness.86 In a more promising clinical trial reported this year, Loukas and colleagues showed that infection with 20 Necator larvae could promote tolerance to gluten challenge in patients with coeliac disease.87 This improvement was correlated with reduced Th1 cells and increased regulatory T cells. Along with other experimental hookworm studies, these findings support the therapeutic potential of controlled hookworm doses to alleviate a variety of allergic diseases; however, the continued assessment of the Necator‐induced inflammatory effects will be critical so that the benefits of infection outweigh the potential risk of infection‐induced immunopathology.
Conclusions and future directions
The skin is the primary portal of entry for soil‐transmitted parasitic helminths, but understanding the cutaneous immune response mounted against these organisms remains largely unexplored. Karasuyama and colleagues reveal that anamnestic immunity relies upon IgE‐armed basophils, but whether skin is a critical immune organ for recognition and initiation of prototypical type 2 responses characterized by specific dendritic cell and ILC2 populations remains unclear. Once in the intestine, a culmination of effector mechanisms that include smooth muscle contraction, epithelial‐cell‐derived effector proteins, and M2‐derived arginase have all been described to drive immunity (Fig. 2b). Collectively, the work of many laboratories has revealed an established cadre of leucocytes, cytokines and effector mechanisms that promote clearance of worm infection (Fig. 2). Paradoxically, clinical observations of hookworm‐infected individuals living in endemic regions clearly show that humans rarely, develop secondary immunity. Perhaps further investigation of the molecular and biochemical differences between human and mouse hookworm species will shed light upon this area. Studies aimed to characterize the constellation of neuropeptides released by sensory neurons at the site of hookworm infection may lead to unique insight(s). Could the itch response modulate the development of primary or secondary immunity against migratory larvae? Similar mechanisms of immunoregulation have been described in the context of Staphylococcus aureus infections.88 Upon entry into the pulmonary tract, the coordinated actions of Th2, ILC2 and M2 are thought to limit parasite growth and curtail the injury caused by migratory larvae in this organ system (Fig. 1). Are regulatory macrophage populations responsible for regeneration of damaged lung tissue along with ILC2? Perhaps M2 cells are able to release catecholamines89 to induce smooth muscle contraction in a mechanism that somehow aids repair. Are there any molecules specific for M2 cells that drive regeneration? Finally, once adult hookworms have developed and begun to feed upon the intestinal epithelia, what are the comprehensive mechanisms that function along with goblet cell‐derived mucins and epithelial derived proteins like RELMβ (Fig. 2b)? Are there Th2‐like resident memory T cells that develop in the mouse, but not in the human for some unknown reason? Are B regulatory cells key constituents of generalized immunosuppression, which through aiding the suppression of immunopathology via IL‐10, inadvertently suppress immunity against re‐infection? Taken together, even though we have gained considerable insight through decades of research on murine hookworms, in order for us to eventually eradicate these organisms from humans, there remains much more to be accomplished in the years to come.
Funding
MGN is supported by the National Institutes of Health AI091759. DRH is supported by NIH awards AI095289, GM83204, and Pathogenesis award from the Burroughs Wellcome Fund.
Disclosures
No conflicts of interest have been declared.
References
- 1. Prevention , C. f. D. C. a . 2013. Global health – division of parasitic diseases and malaria.
- 2. Loukas A, Prociv P. Immune responses in hookworm infections. Clin Microbiol Rev 2001; 14:689–703. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matthys B, Tschannen AB, Tian‐Bi NT, Comoe H, Diabate S, Traore M, et al Risk factors for Schistosoma mansoni and hookworm in urban farming communities in western Cote d'Ivoire. Trop Med Int Health 2007; 12:709–23. [DOI] [PubMed] [Google Scholar]
- 4. Hawdon JM, Hotez PJ. Hookworm: developmental biology of the infectious process. Curr Opin Genet Dev 1996; 6:618–23. [DOI] [PubMed] [Google Scholar]
- 5. Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, de Silva N, et al Chapter 24: Helminth Infections: soil‐transmitted Helminth Infections and Schistosomiasis In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, eds. Disease Control Priorities in Developing Countries, 2nd edn. Washington (DC): World Bank, 2006. [Google Scholar]
- 6. Kalsheker NA, Deam S, Chambers L, Sreedharan S, Brocklehurst K, Lomas DA. The house dust mite allergen Der p1 catalytically inactivates alpha 1‐antitrypsin by specific reactive centre loop cleavage: a mechanism that promotes airway inflammation and asthma. Biochem Biophys Res Commun 1996; 221:59–61. [DOI] [PubMed] [Google Scholar]
- 7. Jelinek T, Maiwald H, Nothdurft HD, Loscher T. Cutaneous larva migrans in travelers: synopsis of histories, symptoms, and treatment of 98 patients. Clin Infect Dis 1994; 19:1062–6. [DOI] [PubMed] [Google Scholar]
- 8. Reece JJ, Siracusa MC, Southard TL, Brayton CF, Urban JF Jr, Scott AL. Hookworm‐induced persistent changes to the immunological environment of the lung. Infect Immun 2008; 76:3511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Craig JM, Scott AL. Helminths in the lungs. Parasite Immunol 2014; 36:463–74. [DOI] [PubMed] [Google Scholar]
- 10. Siracusa MC, Kim BS, Spergel JM, Artis D. 2013. Basophils and allergic inflammation. J Allergy Clin Immunol 132: 789–801. quiz 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink‐Kane MM, Wilson MS, et al Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat Immunol 2008; 9:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Behnke JM. Do hookworms elicit protective immunity in man? Parasitology today 1987; 3:200–6. [DOI] [PubMed] [Google Scholar]
- 13. Greenwald RJ, Latchman YE, Sharpe AH. Negative co‐receptors on lymphocytes. Curr Opin Immunol 2002; 14:391–6. [DOI] [PubMed] [Google Scholar]
- 14. Francisco LM, Sage PT, Sharpe AH. The PD‐1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236:219–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urban JF Jr, Maliszewski CR, Madden KB, Katona IM, Finkelman FD. IL‐4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J Immunol 1995; 154:4675–84. [PubMed] [Google Scholar]
- 16. Urban JF Jr, Noben‐Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, et al IL‐13, IL‐4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis . Immunity 1998; 8:255–64. [DOI] [PubMed] [Google Scholar]
- 17. Katona IM, Urban JF Jr, Finkelman FD. The role of L3T4+ and Lyt‐2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis . J Immunol 1988; 140:3206–11. [PubMed] [Google Scholar]
- 18. Quinnell RJ, Pritchard DI, Raiko A, Brown AP, Shaw MA. Immune responses in human necatoriasis: association between interleukin‐5 responses and resistance to reinfection. J Infect Dis 2004; 190:430–8. [DOI] [PubMed] [Google Scholar]
- 19. Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al Transforming growth factor‐β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9‐producing subset. Nat Immunol 2008; 9:1341–6. [DOI] [PubMed] [Google Scholar]
- 20. Roncarolo MG, Gregori S, Bacchetta R, Battaglia M. Tr1 cells and the counter‐regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol 2014; 380:39–68. [DOI] [PubMed] [Google Scholar]
- 21. Anthony RM, Rutitzky LI, Urban JF Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol 2007; 7:975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harvie M, Camberis M, Tang SC, Delahunt B, Paul W, Le Gros G. The lung is an important site for priming CD4 T‐cell‐mediated protective immunity against gastrointestinal helminth parasites. Infect Immun 2010; 78:3753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Logan E, Chetty A, Horsnell WG. The role of antibody in parasitic helminth infections. Adv Exp Med Biol 2014; 828:1–26. [DOI] [PubMed] [Google Scholar]
- 24. Herbst T, Esser J, Prati M, Kulagin M, Stettler R, Zaiss MM, et al Antibodies and IL‐3 support helminth‐induced basophil expansion. Proc Natl Acad Sci USA 2012; 109:14954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horsnell WG, Darby MG, Hoving JC, Nieuwenhuizen N, McSorley HJ, Ndlovu H, et al IL‐4Rα‐associated antigen processing by B cells promotes immunity in Nippostrongylus brasiliensis infection. PLoS Pathog 2013; 9:e1003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 2010; 33:364–74. [DOI] [PubMed] [Google Scholar]
- 27. Urban JF Jr, Noben‐Trauth N, Schopf L, Madden KB, Finkelman FD. Cutting edge: IL‐4 receptor expression by non‐bone marrow‐derived cells is required to expel gastrointestinal nematode parasites. J Immunol 2001; 167:6078–81. [DOI] [PubMed] [Google Scholar]
- 28. Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, et al Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med 2011; 208:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nair MG, Guild KJ, Du Y, Zaph C, Yancopoulos GD, Valenzuela DM, et al Goblet cell‐derived resistin‐like molecule β augments CD4+ T cell production of IFN‐γ and infection‐induced intestinal inflammation. J Immunol 2008; 181:4709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 2005; 308:1463–5. [DOI] [PubMed] [Google Scholar]
- 31. Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, et al Enteric nematodes induce stereotypic STAT6‐dependent alterations in intestinal epithelial cell function. J Immunol 2004; 172:5616–21. [DOI] [PubMed] [Google Scholar]
- 32. Finkelman FD, Shea‐Donohue T, Morris SC, Gildea L, Strait R, Madden KB, et al Interleukin‐4‐ and interleukin‐13‐mediated host protection against intestinal nematode parasites. Immunol Rev 2004; 201:139–55. [DOI] [PubMed] [Google Scholar]
- 33. Zhao A, McDermott J, Urban JF Jr, Gause W, Madden KB, Yeung KA, et al Dependence of IL‐4, IL‐13, and nematode‐induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol 2003; 171:948–54. [DOI] [PubMed] [Google Scholar]
- 34. Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, et al Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 2010; 138:1763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horsnell WG, Vira A, Kirstein F, Mearns H, Hoving JC, Cutler AJ, et al IL‐4Rα‐responsive smooth muscle cells contribute to initiation of TH2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections. Mucosal Immunol 2011; 4:83–92. [DOI] [PubMed] [Google Scholar]
- 36. Shea‐Donohue T, Sun R, Bohl JA, McLean LP, Zhao A. Enteric nematodes and the path to up‐regulation of type 2 cytokines IL‐4 and IL‐13. Cytokine 2015; 75:62–7. [DOI] [PubMed] [Google Scholar]
- 37. Barnes MA, Carson MJ, Nair MG. Non‐traditional cytokines: how catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine 2015; 72:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin‐23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 2015; 43:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seidl A, Panzer M, Voehringer D. Protective immunity against the gastrointestinal nematode Nippostrongylus brasiliensis requires a broad T‐cell receptor repertoire. Immunology 2011; 134:214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo L, Huang Y, Chen X, Hu‐Li J, Urban JF Jr, Paul WE. Innate immunological function of TH2 cells in vivo . Nat Immunol 2015; 16:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oeser K, Schwartz C, Voehringer D. Conditional IL‐4/IL‐13‐deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol 2015; 8:672–82. [DOI] [PubMed] [Google Scholar]
- 42. Schmidt S, Hoving JC, Horsnell WG, Mearns H, Cutler AJ, Brombacher TM, et al Nippostrongylus‐induced intestinal hypercontractility requires IL‐4 receptor α‐responsiveness by T cells in mice. PLoS ONE 2012; 7:e52211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheever AW, Williams ME, Wynn TA, Finkelman FD, Seder RA, Cox TM, et al Anti‐IL‐4 treatment of Schistosoma mansoni‐infected mice inhibits development of T cells and non‐B, non‐T cells expressing Th2 cytokines while decreasing egg‐induced hepatic fibrosis. J Immunol 1994; 153:753–9. [PubMed] [Google Scholar]
- 44. Kullberg MC, Berzofsky JA, Jankovic DL, Barbieri S, Williams ME, Perlmann P, et al T cell‐derived IL‐3 induces the production of IL‐4 by non‐B, non‐T cells to amplify the Th2‐cytokine response to a non‐parasite antigen in Schistosoma mansoni‐infected mice. J Immunol 1996; 156:1482–9. [PubMed] [Google Scholar]
- 45. Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells–how did we miss them? Nature reviews . Immunology 2013; 13:75–87. [DOI] [PubMed] [Google Scholar]
- 46. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al Innate production of TH2 cytokines by adipose tissue‐associated c‐Kit+ Sca‐1+ lymphoid cells. Nature 2010; 463:540–4. [DOI] [PubMed] [Google Scholar]
- 47. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature 2010; 464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF Jr, Tocker JE, et al IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature 2010; 464:1362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fallon PG, Smith P, Richardson EJ, Jones FJ, Faulkner HC, Van Snick J, et al Expression of interleukin‐9 leads to Th2 cytokine‐dominated responses and fatal enteropathy in mice with chronic Schistosoma mansoni infections. Infect Immun 2000; 68:6005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, et al IL‐9‐mediated survival of type 2 innate lymphoid cells promotes damage control in helminth‐induced lung inflammation. J Exp Med 2013; 210:2951–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, et al Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol 2016; 17:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al MHCII‐mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014; 41:283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bouchery T, Kyle R, Camberis M, Shepherd A, Filbey K, Smith A, et al ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat Commun 2015; 6:6970. [DOI] [PubMed] [Google Scholar]
- 54. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 1992; 176:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doyle AG, Herbein G, Montaner LJ, Minty AJ, Caput D, Ferrara P, et al Interleukin‐13 alters the activation state of murine macrophages in vitro: comparison with interleukin‐4 and interferon‐γ . Eur J Immunol 1994; 24:1441–5. [DOI] [PubMed] [Google Scholar]
- 56. Du L, Wei H, Li L, Shan H, Yu Y, Wang Y, et al Regulation of recombinant Trichinella spiralis 53‐kDa protein (rTsP53) on alternatively activated macrophages via STAT6 but not IL‐4Rα in vitro . Cell Immunol 2014; 288:1–7. [DOI] [PubMed] [Google Scholar]
- 57. Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, et al Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 2004; 20:623–35. [DOI] [PubMed] [Google Scholar]
- 58. Van Dyken SJ, Locksley RM. Interleukin‐4‐ and interleukin‐13‐mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol 2013; 31:317–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity‐induced insulin resistance. Nat Clin Pract Endocrinol Metab 2008; 4:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Odegaard JI, Ricardo‐Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, et al Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity‐induced insulin resistance. Cell Metab 2008; 7:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Odegaard JI, Ricardo‐Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al Macrophage‐specific PPARγ controls alternative activation and improves insulin resistance. Nature 2007; 447:1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 1999; 189:1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pesce JT, Ramalingam TR, Mentink‐Kane MM, Wilson MS, El Kasmi KC, Smith AM, et al Arginase‐1‐expressing macrophages suppress Th2 cytokine‐driven inflammation and fibrosis. PLoS Pathog 2009; 5:e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, et al Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 2014; 123:e110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Loke P, Allison JP. PD‐L1 and PD‐L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA 2003; 100:5336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Borthwick LA, Barron L, Hart KM, Vannella KM, Thompson RW, Oland S, et al Macrophages are critical to the maintenance of IL‐13‐dependent lung inflammation and fibrosis. Mucosal Immunol 2016; 9:38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, et al An essential role for TH2‐type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 2012; 18:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, et al Neutrophils prime a long‐lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol 2014; 15:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhao A, Urban JF Jr, Anthony RM, Sun R, Stiltz J, van Rooijen N, et al Th2 cytokine‐induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 2008; 135(217–225):e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. O'Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med 2016; 213:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, et al Cell‐intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 2014; 15:846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wu D, Molofsky AB, Liang HE, Ricardo‐Gonzalez RR, Jouihan HA, Bando JK, et al Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011; 332:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al The Jmjd3‐Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol 2010; 11:936–44. [DOI] [PubMed] [Google Scholar]
- 75. Anthony RM, Urban JF Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, et al Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med 2006; 12:955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Obata‐Ninomiya K, Ishiwata K, Tsutsui H, Nei Y, Yoshikawa S, Kawano Y, et al The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med 2013; 210:2583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pesce JT, Ramalingam TR, Wilson MS, Mentink‐Kane MM, Thompson RW, Cheever AW, et al Retnla (relmα/fizz1) suppresses helminth‐induced Th2‐type immunity. PLoS Pathog 2009; 5:e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jang JC, Chen G, Wang SH, Barnes MA, Chung JI, Camberis M, et al Macrophage‐derived human resistin is induced in multiple helminth infections and promotes inflammatory monocytes and increased parasite burden. PLoS Pathog 2015; 11:e1004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sutherland TE, Logan N, Ruckerl D, Humbles AA, Allan SM, Papayannopoulos V, et al Chitinase‐like proteins promote IL‐17‐mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol 2014; 15:1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science 2002; 296:490–4. [DOI] [PubMed] [Google Scholar]
- 81. Leonardi‐Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta‐analysis. Am J Respir Crit Care Med 2006; 174:514–23. [DOI] [PubMed] [Google Scholar]
- 82. Flohr C, Tuyen LN, Quinnell RJ, Lewis S, Minh TT, Campbell J, et al Reduced helminth burden increases allergen skin sensitization but not clinical allergy: a randomized, double‐blind, placebo‐controlled trial in Vietnam. Clin Exp Allergy 2010; 40:131–42. [DOI] [PubMed] [Google Scholar]
- 83. Gaze S, Bethony JM, Periago MV. Immunology of experimental and natural human hookworm infection. Parasite Immunol 2014; 36:358–66. [DOI] [PubMed] [Google Scholar]
- 84. Maxwell C, Hussain R, Nutman TB, Poindexter RW, Little MD, Schad GA, et al The clinical and immunologic responses of normal human volunteers to low dose hookworm (Necator americanus) infection. Am J Trop Med Hyg 1987; 37:126–34. [DOI] [PubMed] [Google Scholar]
- 85. Mortimer K, Brown A, Feary J, Jagger C, Lewis S, Antoniak M, et al Dose‐ranging study for trials of therapeutic infection with Necator americanus in humans. Am J Trop Med Hyg 2006; 75:914–20. [PubMed] [Google Scholar]
- 86. Feary JR, Venn AJ, Mortimer K, Brown AP, Hooi D, Falcone FH, et al Experimental hookworm infection: a randomized placebo‐controlled trial in asthma. Clin Exp Allergy 2010; 40:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Croese J, Giacomin P, Navarro S, Clouston A, McCann L, Dougall A, et al Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol 2015; 135:508–16. [DOI] [PubMed] [Google Scholar]
- 88. Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, et al Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013; 501:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, et al Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011; 480:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


