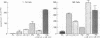Abstract
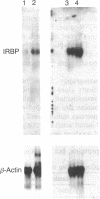
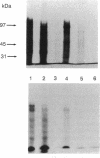
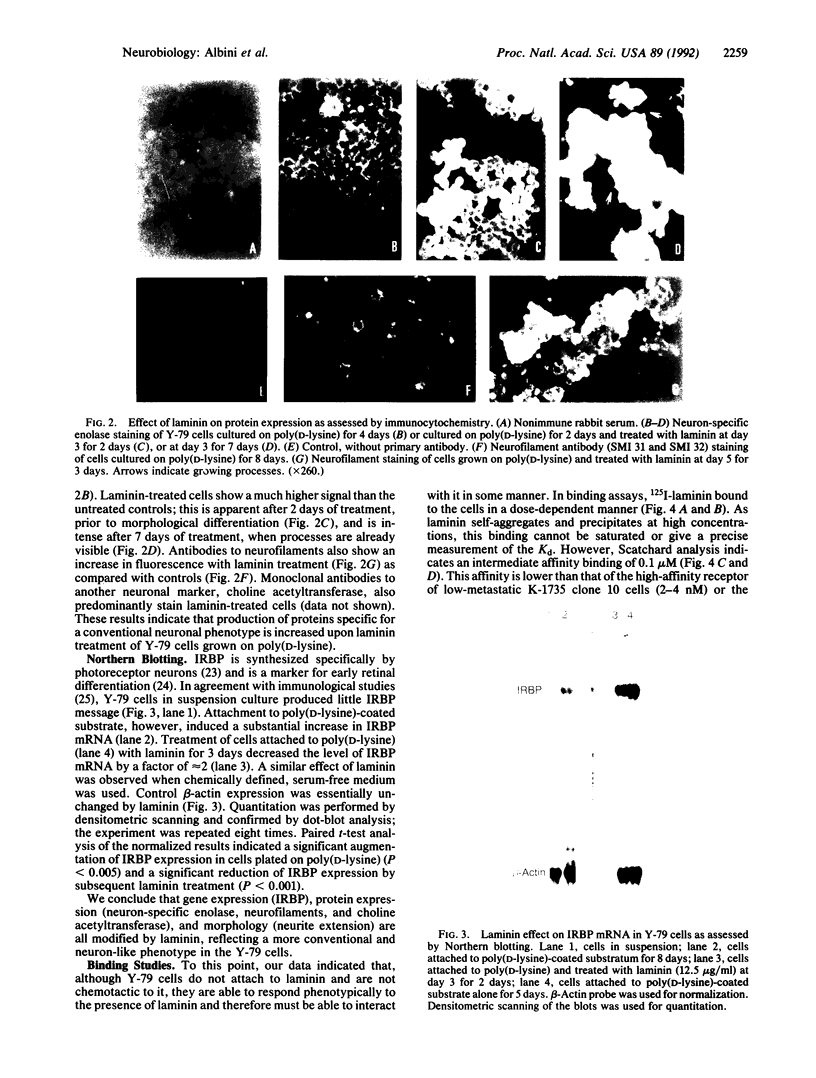
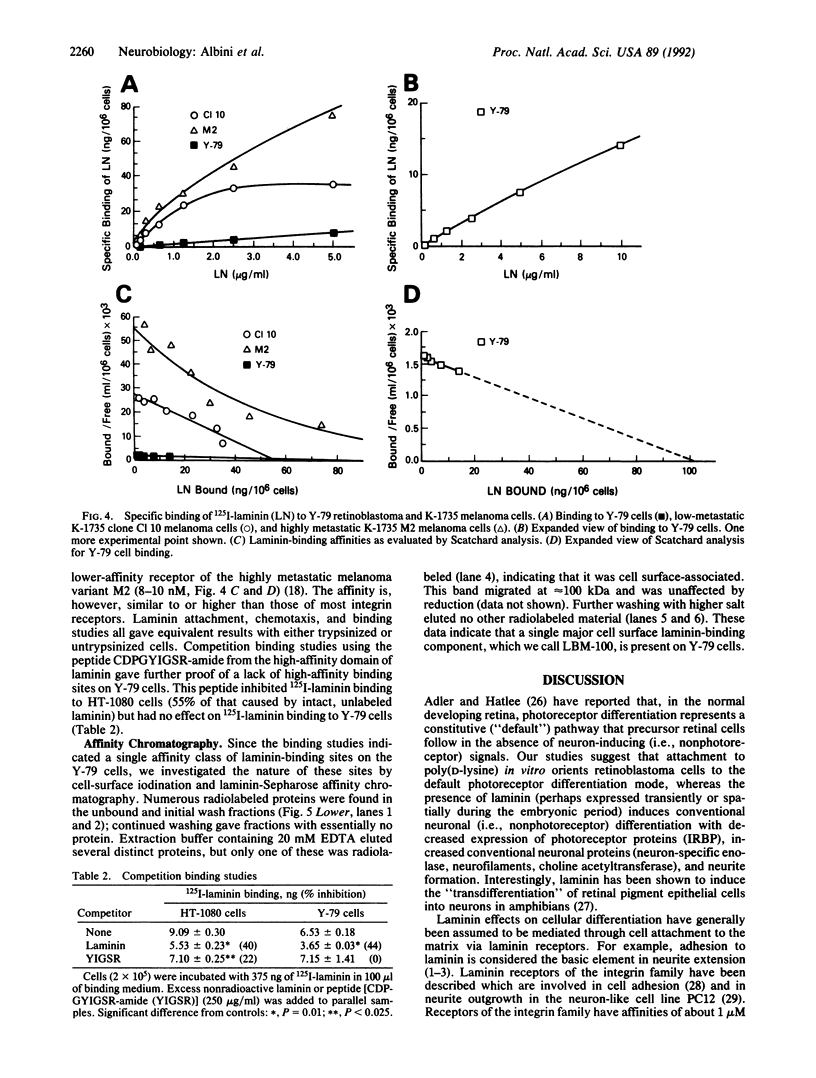
Gene and protein expression of Y-79 retinoblastoma cells growing on poly(D-lysine) is switched from a photoreceptor-like to a conventional neuron-like pathway by the basement membrane glycoprotein laminin. Unlike other cell systems where laminin influences differentiation, Y-79 cells can neither attach to nor chemotactically respond to laminin. However, laminin increases attachment to poly(D-lysine). The laminin effects therefore seem to occur via an adhesion- and chemotaxis-independent mechanism. Moreover, these tumor cells do not exhibit high-affinity laminin binding, having only a single binding site of intermediate affinity. Laminin-Sepharose affinity chromatography of Y-79 cell surface proteins labeled with 125I revealed a single major radiolabeled 100-kDa protein eluted by 20 mM EDTA, with an electrophoretic behavior different from that of integrins. No other proteins were eluted under more stringent conditions. This material, which we call LBM-100 (100-kDa laminin-binding molecule), may be a "differentiative" laminin-binding protein through which laminin influences gene expression and development independently of attachment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989 Jan 20;243(4889):391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- Adler R., Jerdan J., Hewitt A. T. Responses of cultured neural retinal cells to substratum-bound laminin and other extracellular matrix molecules. Dev Biol. 1985 Nov;112(1):100–114. doi: 10.1016/0012-1606(85)90124-1. [DOI] [PubMed] [Google Scholar]
- Albini A., Aukerman S. L., Ogle R. C., Noonan D. M., Fridman R., Martin G. R., Fidler I. J. The in vitro invasiveness and interactions with laminin of K-1735 melanoma cells. Evidence for different laminin-binding affinities in high and low metastatic variants. Clin Exp Metastasis. 1989 Jul-Aug;7(4):437–451. doi: 10.1007/BF01753664. [DOI] [PubMed] [Google Scholar]
- Albini A., Toffenetti J., Zhu Z., Chader G. J., Noonan D. M. Hypomethylation of the interphotoreceptor retinoid-binding protein (IRBP) promotor and first exon is linked to expression of the gene. Nucleic Acids Res. 1990 Sep 11;18(17):5181–5187. doi: 10.1093/nar/18.17.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Chader G. J. Effects of laminin on attachment, growth and differentiation of cultured Y-79 retinoblastoma cells. Invest Ophthalmol Vis Sci. 1988 Oct;29(10):1517–1522. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Codogno P., Doyennette-Moyne M. A., Aubery M. Evidence for a dual mechanism of chick embryo fibroblast adhesion on fibronectin and laminin substrata. Exp Cell Res. 1987 Apr;169(2):478–489. doi: 10.1016/0014-4827(87)90208-4. [DOI] [PubMed] [Google Scholar]
- Gallie B. L., Albert D. M., Wong J. J., Buyukmihci N., Pullafito C. A. Heterotransplantation of retinoblastoma into the athymic "nude" mouse. Invest Ophthalmol Vis Sci. 1977 Mar;16(3):256–259. [PubMed] [Google Scholar]
- Gehlsen K. R., Dillner L., Engvall E., Ruoslahti E. The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science. 1988 Sep 2;241(4870):1228–1229. doi: 10.1126/science.2970671. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fernandez F., Healy J. I. Early expression of the gene for interphotoreceptor retinol-binding protein during photoreceptor differentiation suggests a critical role for the interphotoreceptor matrix in retinal development. J Cell Biol. 1990 Dec;111(6 Pt 1):2775–2784. doi: 10.1083/jcb.111.6.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J., Ogle R. C., Robey F. A., Sasaki M., Martin G. R., Yamada Y., Kleinman H. K. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor. Biochemistry. 1987 Nov 3;26(22):6896–6900. doi: 10.1021/bi00396a004. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Gundersen R. W. Response of sensory neurites and growth cones to patterned substrata of laminin and fibronectin in vitro. Dev Biol. 1987 Jun;121(2):423–431. doi: 10.1016/0012-1606(87)90179-5. [DOI] [PubMed] [Google Scholar]
- Inouye L. N., Albini A., Chader G. J., Redmond T. M., Nickerson J. M. mRNA for interphotoreceptor retinoid-binding protein (IRBP): distribution and size diversity in vertebrate species. Exp Eye Res. 1989 Aug;49(2):171–180. doi: 10.1016/0014-4835(89)90087-0. [DOI] [PubMed] [Google Scholar]
- Kato G., Wakabayashi K. Effect of polylysine-bound laminin on human retinoblastoma cell lines. In Vitro Cell Dev Biol. 1988 Apr;24(4):274–280. doi: 10.1007/BF02628827. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Graf J., Iwamoto Y., Kitten G. T., Ogle R. C., Sasaki M., Yamada Y., Martin G. R., Luckenbill-Edds L. Role of basement membranes in cell differentiation. Ann N Y Acad Sci. 1987;513:134–145. doi: 10.1111/j.1749-6632.1987.tb25004.x. [DOI] [PubMed] [Google Scholar]
- Kyritsis A. P., Fletcher R. T., Chader G. J. Laminin: an initiating role in retinoblastoma cell attachment and differentiation. In Vitro Cell Dev Biol. 1986 Jul;22(7):418–422. doi: 10.1007/BF02623532. [DOI] [PubMed] [Google Scholar]
- Kyritsis A. P., Tsokos M., Triche T. J., Chader G. J. Retinoblastoma--origin from a primitive neuroectodermal cell? Nature. 1984 Feb 2;307(5950):471–473. doi: 10.1038/307471a0. [DOI] [PubMed] [Google Scholar]
- Kyritsis A. P., Wiggert B., Lee L., Chader G. J. Butyrate enhances the synthesis of interphotoreceptor retinoid-binding protein (IRBP) by Y-79 human retinoblastoma cells. J Cell Physiol. 1985 Aug;124(2):233–239. doi: 10.1002/jcp.1041240210. [DOI] [PubMed] [Google Scholar]
- Kyritsis A., Tsokos M., Chader G. Attachment culture of human retinoblastoma cells: long-term culture conditions and effects of dibutyryl cyclic AMP. Exp Eye Res. 1984 Apr;38(4):411–421. doi: 10.1016/0014-4835(84)90196-9. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Reh T. A., Nagy T., Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987 Nov 5;330(6143):68–71. doi: 10.1038/330068a0. [DOI] [PubMed] [Google Scholar]
- San Antonio J. D., Tuan R. S. Chondrogenesis of limb bud mesenchyme in vitro: stimulation by cations. Dev Biol. 1986 Jun;115(2):313–324. doi: 10.1016/0012-1606(86)90252-6. [DOI] [PubMed] [Google Scholar]
- Stamatoglou S. C., Ge R. C., Mills G., Butters T. D., Zaidi F., Hughes R. C. Identification of a novel glycoprotein (AGp110) involved in interactions of rat liver parenchymal cells with fibronectin. J Cell Biol. 1990 Nov;111(5 Pt 1):2117–2127. doi: 10.1083/jcb.111.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Timpl R. Antibodies to collagens and procollagens. Methods Enzymol. 1982;82(Pt A):472–498. doi: 10.1016/0076-6879(82)82079-x. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J., Damsky C. H., Reichardt L. F. Interactions of a neuronal cell line (PC12) with laminin, collagen IV, and fibronectin: identification of integrin-related glycoproteins involved in attachment and process outgrowth. J Cell Biol. 1987 Nov;105(5):2347–2358. doi: 10.1083/jcb.105.5.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen T., Katial A., Shinohara T., Barrett D. J., Wiggert B., Chader G. J., Nickerson J. M. Retinal photoreceptor neurons and pinealocytes accumulate mRNA for interphotoreceptor retinoid-binding protein (IRBP). FEBS Lett. 1986 Nov 10;208(1):133–137. doi: 10.1016/0014-5793(86)81547-2. [DOI] [PubMed] [Google Scholar]
- von der Mark K., Risse G. Isolation and characterization of laminin receptors. Methods Enzymol. 1987;144:490–507. doi: 10.1016/0076-6879(87)44197-9. [DOI] [PubMed] [Google Scholar]