Abstract
Object
The aim of this study was to examine the brain’s white matter microstructure using magnetic resonance diffusion tensor imaging (DTI) in ice hockey players with a history of clinically symptomatic concussion compared to those players without a history of concussion.
Methods
Sixteen players with a history of concussion (Concussed Group; mean age: 21.7 ± 1.5 years; 6 female) and eighteen players without a history of concussion (Non-Concussed Group; mean age: 21.3 ± 1.8 years, 10 female) underwent 3T DTI at the end of the Canadian Interuniversity Sports ice hockey season 2011–2012. Tract-based spatial statistics (TBSS) was used to test for group differences in fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and trace. Cognitive evaluation was performed using the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT) and the Sport Concussion Assessment Tool-2 (SCAT2).
Results
TBSS revealed a significant increase in FA and AD, and a significant decrease in RD and trace in several brain regions in the Concussed group, compared with the Non-concussed group (p < 0.05). The regions with increased FA and decreased RD and trace included the right posterior limb of the internal capsule, the right corona radiata, and the right temporal lobe. Increased AD was observed in a small area in the left corona radiata. DTI measures neither correlated with the ImPACT nor SCAT2.
Conclusion
The results of the current study indicate that a history of concussion may result in alterations of the brain’s white matter microstructure in ice hockey players. Increased FA based on decreased RD may reflect neuroinflammatory or neuroplastic processes of the brain responding to brain trauma. Future studies are needed that include a longitudinal analysis of the brain’s structure and function following a concussion in order to elucidate further the complex time course of DTI changes and their clinical meaning.
Keywords: concussion, mild traumatic brain injury, diffusion tensor imagin, ice hockey, fractional anisotropy, white matter
INTRODUCTION
Sports-related concussion is an important public health problem given the annual incidence of approximately 300,000 sports-related concussions in the US alone.12,48 Concussion, a subset of mild traumatic brain injury (mTBI),15 is caused by high-speed acceleration-deceleration head motions,44 leading to complex pathophysiological processes affecting the brain’s function and structure.37,38 Common symptoms of concussion include confusion, dizziness, headache, nausea, and balance problems. These symptoms resolve in the majority (80–90%) of individuals within the first 10 days.37 However, in some individuals, a concussion may result in symptoms lasting for more than three months, also known as prolonged postconcussive syndrome.39 Moreover, repeated concussions have been associated with the development of chronic traumatic encephalopathy (CTE).40
To date, diagnosis and management of concussion are largely based on clinically observed or self-reported symptoms. However, this approach is both incomplete and inaccurate because symptoms may either not be reported by the athlete or not be associated with a concussion.9,36 In addition, conventional neuroimaging such as computed tomography (CT) and magnetic resonance imaging (MRI) fail to detect traumatic axonal injury (TAI), the underlying mechanism of mTBI.
Diffusion tensor imaging (DTI) is sensitive for detecting TAI and is therefore expected to improve the diagnosis of mTBI by providing objective parameters to quantify and to localize white matter alterations (see review by Shenton et al.45). DTI measures the movement of water in the brain. In white matter, water molecules move more in directions parallel to the fiber tracts than perpendicular to them. This characteristic, which is referred to as anisotropic diffusion, is most commonly measured by fractional anisotropy (FA), a measure derived from DTI that reflects the coherent microstructural organization of white matter.49 In addition to FA, the measure ‘trace’, or mean diffusivity, denotes the overall average of diffusion. Axial diffusivity (AD) and radial diffusivity (RD) denote the extent of diffusion parallel and perpendicular to the direction of maximal diffusivity, respectively, where AD is purported to be sensitive to axonal damage, while RD is purported to be sensitive to myelin degeneration.47
Alterations of diffusivity following TBI vary in the literature: FA has been shown to either decrease2,19,24,26,27,32,41,42 or increase17,18,25,30,33,49 after head trauma. Moreover, regions with increased and regions with decreased FA have been reported within the same subject following mTBI.3,28 It has been suggested that these diffusivity changes are determined by the severity and/or chronicity of the injury.25,49
Ice hockey is a high-speed collision sport1 for which a high incident rate of concussions is known.8,10,11,34,43 A recent study by Echlin et al. reported the number of concussions per 1000 athlete exposures to be as high as 11.67.10 To date, DTI studies have been performed in players of other contact sports such as American football,16,18,31 boxing,5,6,50,51 and soccer,22,29 with only a small number of studies including ice hockey players.3,7,23,35 However, evaluating the effects of sports-related concussion on the brain’s microstructure among ice hockey players will likely contribute to earlier and more accurate diagnosis, which, in turn, may lead to improved and more specific therapeutic management and decision of when to encourage an athlete to return to play.
The aim of this study was to examine the brain’s white matter microstructure using DTI in ice hockey players with history of a clinically symptomatic concussion compared to those players without a history of concussion.
METHODS
Participants and clinical information
All participants were part of the Hockey Concussion Education Project (HCEP), a cohort study performed during a CIS (Canadian interuniversity sports) ice hockey season (2011–2012). Thirty-nine players were scanned at the end of the season. None of the included participants had a history of any neurological or psychiatric disorder other than concussion. Players with gross structural MRI abnormalities were excluded. The study protocol was approved by the ethics committee within the universities at which the CIS teams were based. All participants provided written informed consent prior to the beginning of the study.
Five players were excluded due to severe motion artifacts (3 players), a large arachnoidal cyst (1 player), or age greater than 8 standard deviations from the mean (1 player). Therefore, 34 players (18 male and 16 female) were included in the statistical analyses.
Self-report concussion history was obtained from the players prior to the beginning of the season using a questionnaire. At the time the data were collected concussion was defined according to the Zurich consensus statement on concussion from the 3rd International Conference on Concussion in Sport which took place in 2009.37 However, the definition used here also meets the concussion criteria from the 4th International Conference on Concussion in Sport which took place in 2012.38
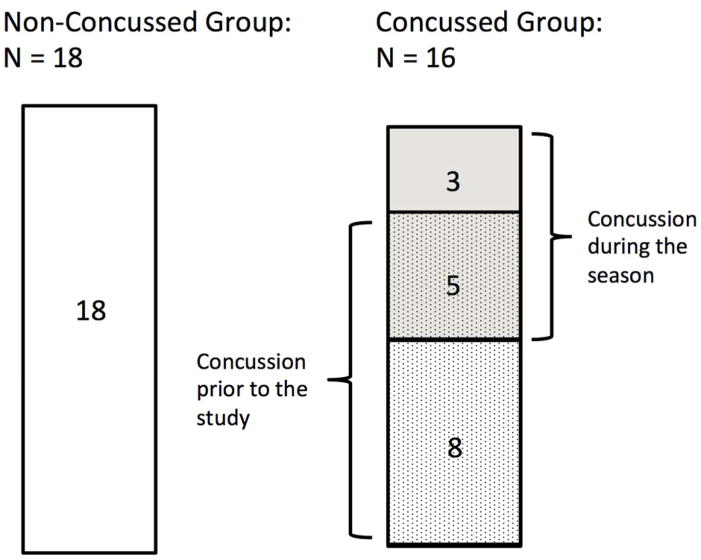
Prior to the current season, team members received physical examinations by the team physician (not study related). Thirteen of the 34 players reported that they had suffered at least one concussion prior to the start of the study (mean number of concussions = 1.46; SD = 0.88, range: 1 – 4). Additionally, 8 of the 34 players experienced at least one clinically symptomatic concussion during the season. Concussions occurring during the season were directly observed and diagnosed by the independent designated specialist physician that attended the game.10 The average concussion-scan interval was 95 ± 45 days (range: 42 – 161 days) for those who suffered from concussion during the study. For those who suffered from a concussion prior to the study, the concussion-scan interval was by definition more than 6 months. In total, 16 players had concussion(s) either prior to the study (n=8), during the study (n=3), or both (n=5) (Concussed group; mean age: 21.7 ± 1.5 years) and 18 players reported no history of concussion (Non-concussed group; mean age: 21.3 ± 1.8 years) (Fig. 1).
Figure 1.
The schematic illustration of grouping: Non Concussed (N = 18) and Concussed (N = 16) group. Within the Concussed group, eight players had a concussion during the season, and thirteen players had a concussion prior to the start of the study. Five players had concussion both during the season and prior to the start of the study.
Cognitive examination
At the end of the CIS season cognitive testing was performed with the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT Applications, Inc., Pittsburgh, PA, USA) and the Sport Concussion Assessment Tool-2 (SCAT2). The ImPACT is a computer-based test battery consisting of a concussion symptom inventory and six modules measuring neurocognitive function. It is the most widely used system for evaluating sports-related concussion, however, to date it has not been independently evaluated. These modules were used to generate four composite scores: verbal memory, visual memory, visual motor speed, and reaction time. SCAT2 is a test battery for the evaluation of concussion which consists of eight component scores. These are designed to assess concussion symptoms, cognition, balance, Glasgow coma scale, and other neurological symptoms.14
Magnetic Resonance Imaging Protocol and Data Acquisition
Data were acquired using a 3T MRI scanner (Achieva, Philips Medical Systems, Best, The Netherlands) with an 8-channel head coil array. A DTI sequence with 2 averages and the following parameters was performed: 60 noncolinear diffusion directions, TR = 7015 msec, TE = 60 msec, b-value = 0 and 700 sec/mm2, and 70 slices. Data were acquired using a 2.2 mm isotropic voxel size and 100 × 100 matrix reconstructed into a 112×112 matrix with a resolution of 2 × 2 × 2.2 mm3.
Processing of DTI
Post-processing and statistical analyses were performed by the first author (TS), who was not blinded to the groups. Blind to group membership was not an issue, since the post-processing measures are automated. MRI data sets were examined for image quality. To remove intrascan misalignments due to eddy currents and head motion, an affine registration of the diffusion-weighted images to the baseline image was performed for each participant (FSL 4.1, part of the FMRIB Software Library, The Oxford Centre for Functional MRI of the Brain). Gradient directions were adjusted using the rotational component of the affine transformations. Non-brain tissue and background noise were then removed from the b0 image using 3Dslicer version 3.6.2 (Surgical Planning Laboratory, Brigham and Women’s Hospital). The diffusion tensor for each voxel was estimated using a multivariate linear fitting algorithm, and the 3 pairs of eigenvalues and eigenvectors were obtained. From these tensor volumes, scalar measures including fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and trace for each voxel were calculated as follows:
where λ1, λ2, λ3 are the largest to smallest eigenvalues.
In order to avoid any bias due to head motion in the scanner, we computed a relative motion index. This parameter was then compared between groups using a t test. In addition, we included this index as a covariate in the statistical analysis (see below).
White matter analysis
Whole-brain tract-based spatial statistics version 1.2 (TBSS),46 a voxel-based standard-space group statistical analysis (FMRIB Software Library, FSL 4.1), was used for the investigation of white matter. The TBSS procedure is described in detail by Smith et al.46 In short, FA images from all subjects were coregistered into a template and then linearly aligned into Montreal Neurological Institute (MNI) 152 space. These aligned FA images were then averaged to generate a cross-participant mean FA image. The mean FA image was then thinned to create a mean FA skeleton, which represents the center of all white matter fiber tracts common to the group. The mean FA skeleton was thresholded to contain only voxels with FA > 0.3 to exclude peripheral voxels with significant inter-subject variability and/or partial volume effects with gray matter. Each participant’s aligned FA data were then projected onto the skeleton by searching the local maxima along the perpendicular direction from the skeleton to create a skeletonized FA map. Thus, without prior perfect coregistration, the central course of each subject’s fiber tract is represented on the skeleton. In order to analyze group differences in the other scalar measures (AD, RD, and trace), we applied the nonlinear warps obtained from the FA registration, as well as the skeleton projection of the FA data, to the other diffusion scalar volumes.
Statistical Analyses
Group comparisons for each voxel on the skeleton were performed by a nonparametric permutations-based test (Randomise, FSL). Threshold-free cluster enhancement (TFCE) was used to avoid choosing an arbitrary initial cluster-forming threshold. The data were tested against an empirical null distribution generated by 5000 permutations for each contrast, thus providing statistical maps fully corrected for multiple comparisons across space. A corrected value of p < 0.05 was considered significant. The test was linearly adjusted for age, handedness, sex, and motion, even though there was no significant difference in these variables between the groups (Table 1). Statistical maps plotting the corrected p values were visualized using FSL (TBSS-fill and FSLview). Pearson’s linear analysis was used to assess the correlation between DTI parameters and measures of cognitive function. Spearman’s rank correlation was used when normal distribution was not given. A p < 0.05 was considered statistically significant.
Table 1.
Demographic characteristics and neuropsychological tests
| Non Concussed Group (N = 18) (mean ± SD) |
Concussed Group (N = 16) (mean ± SD) |
Statistics, p value | ||
|---|---|---|---|---|
| Age | 21.25 ± 1.84 | 21.68 ± 1.54 | t32 = 1.22, p = 0.23 | |
| Number of females, n (%) | 10 (56) | 6 (38) | χ2 = 1.11, p = 0.29 | |
| Handedness (right/either/left) | 15/1/2 | 13/0/3 | Fisher’s exact test, p = 0.82 | |
| motion (mm) | 0.78 ± 0.09 | 0.79 ± 0.12 | t32 = −0.368, p = 0.716 | |
| ImPACTa | Verbal memory | 92.3 ± 5.94 | 90.3 ± 9.26 | t31 = 0.78, p = 0.44 |
| Visual memory | 79.7 ± 11.1 | 80.9 ± 13.5 | t31 = 0.28, p = 0.87 | |
| Visual motor speed | 45.7 ± 5.34 | 45.0 ± 6.95 | t31 = 0.33, p = 0.74 | |
| Reaction time | 0.53 ± 0.06 | 0.54 ± 0.07 | t31 = 0.41, p = 0.68 | |
| Sympto scale | 3.4 ± 4.7 | 5.3 ± 13.2 | U = 114.0, p = 0.43 | |
| SCAT2b | Total | 94.4 ± 3.40 | 95.5 ± 1.62 | t28 = −1.05, p = 0.30 |
One subject’s data in Concussed group was not available.
Four subject’s data in Concussed group was not available.
ImPACT = Immediate Post-Concussion Assessment and Cognitive Test.
SCAT2 = Sport Concussion Assessment Tool-2.
RESULTS
Demographic characteristics and neuropsychological examination
Concussed and Non-concussed groups did not differ significantly with respect to age, sex, handedness, or motion. There was also no significant difference in ImPACT score between the Concussed and Non-concussed group (Table 1). For one subject in the Concussed group there were no ImPACT test results available. There was no significant difference between the groups for the SCAT2 score. For four subjects in the Concussed group there were no SCAT2 results available.
Analyses within the Concussed group
Subjects who had concussion during the season (N = 8) did not differ from those subjects with a history of concussion only prior to the season regarding number of sustained concussion, ImPACT scores or SCAT2 scores (Table 2). Group comparison using TBSS did not show a significant difference (data not shown).
Table 2.
Analysis within the Concussed group
| Concussed during the season (N = 8) | Concussed pre-season only (N = 8) | Statistics, p value | ||
|---|---|---|---|---|
| age | 21.56 ± 1.5 | 22.16 ± 1.6 | t14 = 0.76, p = 0.46 | |
| Number of females, n (%) | 5 (63) | 1 (13) | Fisher’s exact test, p = 0.12 | |
| Handedness (right/left) | 7/1 | 6/2 | Fisher’s exact test, p = 1.00 | |
| Concussion, n | 2.13 ± 1.4 | 1.25 ± 0.46 | U = 18.0, p = 0.11 | |
| ImPACTa | Verbal memory | 89.9 ± 9.9 | 90.7 ± 9.2 | t13 = 0.17, p = 0.87 |
| Visual memory | 78.5 ± 16.3 | 83.7 ± 9.9 | t13 = 0.73, p = 0.48 | |
| Visual motor | 44.8 ± 7.8 | 45.2 ±6.5 | t13 = 0.1, p = 0.92 | |
| Reaction time | 0.56 ± 0.08 | 0.51 ± 0.04 | t13 = 1.4, p = 0.20 | |
| Total symptom | 8.00 ± 18.0 | 2.39 ± 3.1 | U = 23.0, p = 0.56 | |
| SCAT2b | total | 96.4 ± 1.82 | 94.9 ± 1.22 | t10 = 1.78, p = 0.11 |
One subject’s data was not available.
Four subject’s data was not available.
ImPACT = Immediate Post-Concussion Assessment and Cognitive Test.
SCAT2 = Sport Concussion Assessment Tool-2.
White matter analysis
TBSS revealed a significant increase in FA and AD, and a significant decrease in RD and trace for the Concussed group compared to the Non-concussed group (p < 0.05).
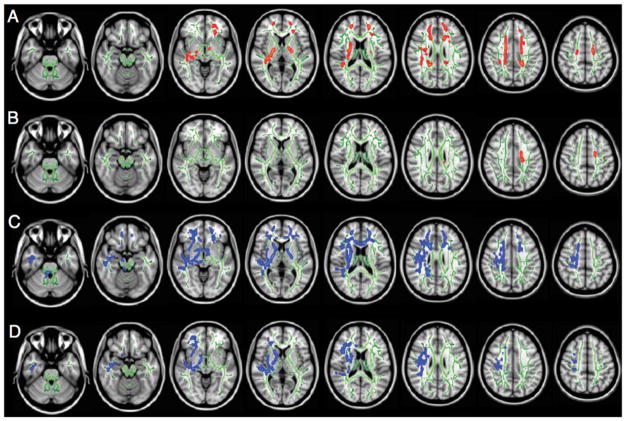
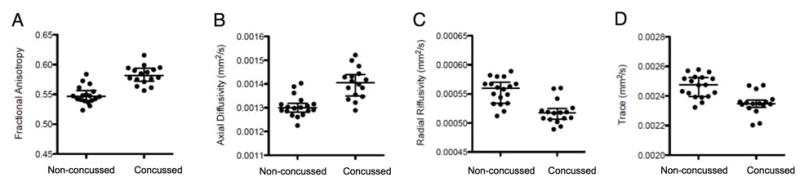
For FA, the Concussed group showed significantly higher values in the bilateral corona radiata, the bilateral posterior limb of the internal capsule, the bilateral superior frontal white matter, and the right superior temporal white matter (Fig. 2A). For AD, the concussed group showed significantly higher values in the left corona radiata (Fig. 2B). For RD, the Concussed group showed significantly lower values in the genu of the corpus callosum, bilateral corona radiata, bilateral posterior limb of the internal capsule, right anterior limb of the internal capsule, right cerebral peduncle, bilateral superior frontal and orbito-frontal white matter, right superior and inferior temporal white matter, and right external capsule (Fig. 2C). For trace, the Concussed group showed significantly lower values in the right corona radiata, the right anterior and posterior limb of the internal capsule, the right superior frontal white matter, and the right inferior temporal white matter (Fig. 2D). Diffusivity measures of the clusters with significant group differences are displayed in the respective scatter plots in Fig. 3A–D. For those clusters, median value and interquartile range (median [interquartile range]) for the Non-concussed and Concussed group were: FA (0.547 [0.017] and 0.582 [0.022], respectively), AD (0.0013 [0.000037] and 0.0014 [0.000091], respectively), RD (0.000560 [0.000037] and 0.000518 [0.000018], respectively), and trace (0.00247 [0.00013] and 0.00235 [0.000049], respectively).
Figure 2.
Figure 2A–D. Results of the TBSS analysis showing the clusters of significantly increased FA (A) and AD (B) (red to yellow), and decreased RD (C) and trace (D) (blue to light blue) for Concussed players compared to Non-concussed players (P < 0.05). Voxels are thickened into local tracts (tbss_fill implemented in FSL) on the FA_skeleton (green) and a T1-weighted template image. Left side on figure corresponds to right hemisphere.
Figure 3.
Figure 3A–D. The scatterplots of average values in the clusters with significant group differences are shown for each DTI parameters: FA (A), AD (B), RD (C), and trace (D). Circles represent individual values, and horizontal bars represent median value and interquartile range.
There were no areas of significant decrease in FA or AD, nor were there any areas of significant increase in RD or trace. DTI measures did not correlate with SCAT2 scores or with any of the composite scores of ImPACT (Table 3).
Table 3.
Correlation analysis between DTI measures and cognitive tests
| ImPACT | SCAT2 | ||||||
|---|---|---|---|---|---|---|---|
| verbal | visual | visualmotor | reaction | symptom | total | ||
| FA | Correlation coefficient | −0.10 | −0.11 | 0.02 | 0.02 | 0.26 | 0.00 |
| p value | 0.58 | 0.53 | 0.93 | 0.91 | 0.89 | 0.98 | |
| AD | Correlation coefficient | 0.16 | −0.03 | 0.08 | 0.11 | −0.52 | 0.19 |
| p value | 0.36 | 0.88 | 0.65 | 0.55 | 0.77 | 0.31 | |
| RA | Correlation coefficient | 0.13 | 0.00 | 0.09 | 0.00 | −0.43 | −0.03 |
| p value | 0.45 | 0.98 | 0.64 | 0.99 | 0.81 | 0.86 | |
| trace | Correlation coefficient | 0.08 | −0.05 | 0.12 | 0.00 | −0.57 | −0.12 |
| p value | 0.66 | 0.77 | 0.50 | 0.99 | 0.75 | 0.53 | |
Correlation coefficients represent Pearson’s r except for symptom subscore of ImPACT where it is Spearman’s rho.
ImPACT = Immediate Post-Concussion Assessment and Cognitive Test.
SCAT2 = Sport Concussion Assessment Tool-2.
DISCUSSION
This study examined varsity level ice hockey players and found a significant difference in DTI measures between the players with and without a history of concussion. We found widespread increase in FA that overlapped with decreased trace and RD in the white matter of the brain of ice hockey players who had a history of concussion compared to those players who did not report a history of concussion. These areas included the right corona radiata, the right posterior limb of the internal capsule, the right superior frontal white matter, and the right superior temporal white matter. Additionally, TBSS revealed a small cluster in the left corona radiata with increased FA and increased AD but no changes in RD or trace. These results suggest possible alterations in white matter microstructure due to concussion. The lack of difference of ImPACT and SCAT2 score between the two groups suggests that DTI is highly sensitive for detecting brain alterations following a concussion even in the absence of clinical symptoms, as evaluated using the ImPACT and SCAT2.
Other studies investigating the brain’s microstructure using DTI have reported either a decrease2,19,24,26,27,32,41,42 or an increase17,18,25,30,33,49 in FA following TBI. Moreover, and as noted previously, studies have suggested areas with increased and decreased FA within the same individual following mTBI.3,28 Decreases in FA have been associated with demyelination and axonal degeneration disrupting the microstructural coherence.4 As Wilde et al.49 pointed out, the studies reporting decreased FA generally included subjects with more severe cases (e.g., with hemorrhages) 2,19,32,42 and/or cases with a rather long interval between injury and MRI scan.24,26,42 On the other hand, most of the studies reporting an increase in FA have included subjects in the acute and subacute phase following an mTBI.18,33,49 In this context, an increase in FA has been explained by an intracellular edema with consecutive restriction of diffusion in the extracellular space perpendicular to the main axis.33
However, increases in FA have been observed in the chronic phase following mTBI.18,25,28,30,33. This is in line with the results of this study which revealed increased FA in large parts of the brain in subjects who had a history of concussion. Mayer et al. reported that increased FA persisted for 4–6 months in some brain areas including genu of the corpus callosum, the left internal capsule, and the left corona radiata.33 Henry et al. found increased FA in the corticospinal tracts and the corpus callosum of concussed athletes in the acute (1 – 6 days) and chronic (6 months) phase.18 Lo et al. reported increased FA in the posterior limb of the internal capsule more than two years after the head trauma, although, there were also areas with decreased FA.30 Additionally, Lipton et al. reported areas with increased or decreased FA in the brain of individuals following a mTBI. The number of voxels with high FA initially increased from 2 weeks to 3 months, followed by a decrease in the number of voxels with high FA at 6 months.28 While the time course of FA changes following a mTBI is not fully understood, the existing literature and our current results suggest that increased FA may persist for months, or even years following an mTBI. The underlying mechanism of increased FA is, however, not clear. Nonetheless, it is noteworthy that both the increase in FA and the decrease in trace are mathematically linked to the decrease in RD, which is likely due to restriction of diffusion in the extracellular space perpendicular to the main axis. This could either be caused by axonal swelling or due to an increased number of glial cells taking up the extracellular space. Histological studies have reported long-lasting neuroinflammation with persistent microglial activation in the white matter tissue of subjects with a history of traumatic brain injury.13,20 Increased FA has also been interpreted as neuroplastic processes of the brain responding to head trauma.28 However, the evidence for such changes remains tenuous due to the lack of direct, quantitative comparisons between DTI results and histological preparations. Finally, in the current study there was a small area localized in the left corona radiata with increased FA based on an increase in AD, indicating increased diffusivity parallel to the axon. This finding is not easy to explain in the context of a history of concussion. It may reflect axonal swelling due to acute/subacute neuroplastic processes.
Subconcussive head blows (SHBs) may have an additional effect on our results. Koerte et al., for example, found differences in white matter microstructure in soccer players without a history of concussion compared with swimmers.22 Additionally, Lipton et al. reported an association between exposure of heading and both abnormal white matter microstructure and impaired memory.29 Further, comparing pre- and postseason scans of football and ice hockey players, Bazarian et al. reported increased FA and decreased mean diffusivity for the players who had SHBs in the absence of a clinically diagnosed concussion.3 SHBs were positively correlated with the degree of change in diffusivity, suggesting an association between white matter alterations and SHBs.3 All athletes included in this study, both, concussed and non-concussed, likely experienced frequent SHBs during the ice hockey season in the months before the MRI scan. Subjects with a previous concussion may be more vulnerable to the additional effect of SHBs. The increase in FA and AD may, therefore, not only reflect the effects of concussions in the past, but also those of recently sustained SHBs resulting in ongoing repair mechanisms.
The lack of differences in cognition evaluated using ImPACT and SCAT2 measures between the groups indicates that there might be no clinically evident symptoms, despite alterations in white matter microstructure, or that these tests are not sufficiently sensitive to detect subtle differences in cognitive performance. The latter hypothesis is supported by a study by Mayer et al.33 where subjects with a history of mild traumatic brain injury showed differences in DTI parameters but showed no differences in neuropsychological measures, compared with a control group. Further studies are, nonetheless, needed to determine whether or not changes in microarchitecture of white matter in the brain in concussed players might precede symptom and cognitive changes, or if other, more sensitive measures of cognitive and clinical function might be more sensitive and thereby more likely to be correlated to the observed DTI changes.
Limitations of this study include the small sample size and the lack of a control group consisting of athletes taking part in non-contact sports. A further limitation of the study is that information regarding the history of concussion before the season is based on the athlete’s self-report. Self-report of sports-related concussion may not be reliable.21 Future studies should also include information on the frequency of SHBs. Furthermore, group-wise analysis by TBSS may not be sensitive to variable spatial location of abnormal DTI measures in heterogeneous conditions such as mTBI. Thus future studies should include analysis of subject-specific changes such as tractography. Additionally, future studies should also include free-water corrected DTI measures. The free-water method as used by Pasternak et al. (in this special issue) estimates the extracellular portion of diffusion. Accordingly, free-water corrected DTI parameters provide more specific information about the brain’s tissue. Finally, subjects included in this study were also scanned before the start of the season. However, a gradient coil change occurred during the season and a possible bias could not be entirely ruled out and so we refrained from comparing pre- and postseason scans in this sample.
Conclusions
The results of the current study indicate that a history of concussion may result in alterations of the brain’s white matter microstructure in ice hockey players. The increase in FA due to a decrease in RD may reflect neuroinflammatory or neuroplastic processes of the brain responding to brain trauma. Future studies are needed that include longitudinal analyses of the brain’s structure and function following a concussion, in order to elucidate the complex time course of DTI changes and their clinical meaning.
Acknowledgments
The authors acknowledge the players and staffs of two CIS varsity hockey teams for their participation in the HCEP, and also the participating researchers, physicians, observers, and volunteers for their contributions to the HCEP. We thank Toru Nishikawa, M.D., PhD, Department of Psychiatry and Behavioral Sciences, Tokyo Medical and Dental University, for his helpful advice.
Footnotes
Disclosure
This work was funded by the Ontario Trillium Foundation, the Dave Irwin Foundation for Brain Injury, the Ontario Neurotrauma Foundation, and Air Canada, all provided to Paul Echlin. Takeshi Sasaki is supported by Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation from Japan Society for the Promotion of Science (S2301). Inga Koerte is supported by the Else Kröner-Fresenius Stiftung, Germany. Michael Mayinger is supported by the Petraeic Legate foundation. Ofer Pasternak was partially supported by a NARSAD young investigator grant from the Brain & Behavior Research Foundation. This work was part of a Michael Mayinger’s doctoral thesis.
References
- 1.Agel J, Harvey EJ. A 7-year review of men’s and women’s ice hockey injuries in the NCAA. Can J Surg. 2010;53:319–323. [PMC free article] [PubMed] [Google Scholar]
- 2.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 3.Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging. 2012;30:171–180. doi: 10.1016/j.mri.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappell MH, Brown JA, Dalrymple-Alford JC, Ulug AM, Watts R. Multivariate analysis of diffusion tensor imaging data improves the detection of microstructural damage in young professional boxers. Magn Reson Imaging. 2008;26:1398–1405. doi: 10.1016/j.mri.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Chappell MH, Ulug AM, Zhang L, Heitger MH, Jordan BD, Zimmerman RD, et al. Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study. J Magn Reson Imaging. 2006;24:537–542. doi: 10.1002/jmri.20656. [DOI] [PubMed] [Google Scholar]
- 7.Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28:189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaldson L, Asbridge M, Cusimano MD. Bodychecking rules and concussion in elite hockey. PLoS One. 2013;8:e69122. doi: 10.1371/journal.pone.0069122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echlin PS. A prospective study of physician-observed concussion during a varsity university ice hockey season. Part 1 of 4. Neurosurg Focus. 2012;33(E1):1–7. doi: 10.3171/2012.9.FOCUS12287. [DOI] [PubMed] [Google Scholar]
- 10.Echlin PS, Skopelja EN, Worsley R, Dadachanji SB, Lloyd-Smith DR, Taunton JA, et al. A prospective study of physician-observed concussion during a varsity university ice hockey season: incidence and neuropsychological changes. Part 2 of 4. Neurosurg Focus. 2012;33(E2):1–11. doi: 10.3171/2012.10.FOCUS12286. [DOI] [PubMed] [Google Scholar]
- 11.Echlin PS, Tator CH, Cusimano MD, Cantu RC, Taunton JE, Upshur RE, et al. A prospective study of physician-observed concussions during junior ice hockey: implications for incidence rates. Neurosurg Focus. 2010;29:E4. doi: 10.3171/2010.9.FOCUS10186. [DOI] [PubMed] [Google Scholar]
- 12.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2000. [Google Scholar]
- 13.Gentleman SM, Leclercq PD, Moyes L, Graham DI, Smith C, Griffin WS, et al. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146:97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Guskiewicz KM, Register-Mihalik J, McCrory P, McCrea M, Johnston K, Makdissi M, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47:289–293. doi: 10.1136/bjsports-2013-092225. [DOI] [PubMed] [Google Scholar]
- 15.Harmon KG, Drezner JA, Gammons M, Guskiewicz KM, Halstead M, Herring SA, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- 16.Hart J, Kraut MA, Womack KB, Strain J, Didehbani N, Bartz E, et al. Neuroimaging of Cognitive Dysfunction and Depression in Aging Retired National Football League Players: A Cross-sectional Study. JAMA Neurol. 2013:1–10. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartikainen KM, Waljas M, Isoviita T, Dastidar P, Liimatainen S, Solbakk AK, et al. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. J Clin Exp Neuropsychol. 2010;32:767–774. doi: 10.1080/13803390903521000. [DOI] [PubMed] [Google Scholar]
- 18.Henry LC, Tremblay J, Tremblay S, Lee A, Brun C, Lepore N, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28:2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- 19.Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 20.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr ZY, Marshall SW, Guskiewicz KM. Reliability of concussion history in former professional football players. Med Sci Sports Exerc. 2012;44:377–382. doi: 10.1249/MSS.0b013e31823240f2. [DOI] [PubMed] [Google Scholar]
- 22.Koerte IK, Ertl-Wagner B, Reiser M, Zafonte R, Shenton ME. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA. 2012;308:1859–1861. doi: 10.1001/jama.2012.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koerte IK, Kaufmann D, Hartl E, Bouix S, Pasternak O, Kubicki M, et al. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg Focus. 2012;33(E3):1–7. doi: 10.3171/2012.10.FOCUS12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 25.Ling JM, Pena A, Yeo RA, Merideth FL, Klimaj S, Gasparovic C, et al. Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain. 2012;135:1281–1292. doi: 10.1093/brain/aws073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma. 2008;25:1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- 27.Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252:816–824. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- 28.Lipton ML, Kim N, Park YK, Hulkower MB, Gardin TM, Shifteh K, et al. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging Behav. 2012;6:329–342. doi: 10.1007/s11682-012-9175-2. [DOI] [PubMed] [Google Scholar]
- 29.Lipton ML, Kim N, Zimmerman ME, Kim M, Stewart WF, Branch CA, et al. Soccer Heading Is Associated with White Matter Microstructural and Cognitive Abnormalities. Radiology. 2013 doi: 10.1148/radiol.13130545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo C, Shifteh K, Gold T, Bello JA, Lipton ML. Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. J Comput Assist Tomogr. 2009;33:293–297. doi: 10.1097/RCT.0b013e31817579d1. [DOI] [PubMed] [Google Scholar]
- 31.Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J, et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS One. 2013;8:e56805. doi: 10.1371/journal.pone.0056805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsushita M, Hosoda K, Naitoh Y, Yamashita H, Kohmura E. Utility of diffusion tensor imaging in the acute stage of mild to moderate traumatic brain injury for detecting white matter lesions and predicting long-term cognitive function in adults. J Neurosurg. 2011;115:130–139. doi: 10.3171/2011.2.JNS101547. [DOI] [PubMed] [Google Scholar]
- 33.Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAllister TW, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, Tosteson TD, et al. Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology. 2012;78:1777–1784. doi: 10.1212/WNL.0b013e3182582fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAllister TW, Ford JC, Ji S, Beckwith JG, Flashman LA, Paulsen K, et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann Biomed Eng. 2012;40:127–140. doi: 10.1007/s10439-011-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCrea M, Hammeke T, Olsen G, Leo P, Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14:13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 37.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med. 2009;43(Suppl 1):i76–90. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- 38.McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 39.McCrory P, Meeuwisse WH, Kutcher JS, Jordan BD, Gardner A. What is the evidence for chronic concussion-related changes in retired athletes: behavioural, pathological and clinical outcomes? Br J Sports Med. 2013;47:327–330. doi: 10.1136/bjsports-2013-092248. [DOI] [PubMed] [Google Scholar]
- 40.McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2012 doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22:115–122. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- 42.Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- 43.Rishiraj N, Lloyd-Smith R, Lorenz T, Niven B, Michel M. University men’s ice hockey: rates and risk of injuries over 6-years. J Sports Med Phys Fitness. 2009;49:159–166. [PubMed] [Google Scholar]
- 44.Seifert TD. Sports concussion and associated post-traumatic headache. Headache. 2013;53:726–736. doi: 10.1111/head.12087. [DOI] [PubMed] [Google Scholar]
- 45.Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012 doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Thurman DJ, Branche CM, Sniezek JE. The epidemiology of sports-related traumatic brain injuries in the United States: recent developments. J Head Trauma Rehabil. 1998;13:1–8. doi: 10.1097/00001199-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM. Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol. 2006;27:2000–2004. [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Ravdin LD, Relkin N, Zimmerman RD, Jordan B, Lathan WE, et al. Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? AJNR Am J Neuroradiol. 2003;24:52–57. [PMC free article] [PubMed] [Google Scholar]