Abstract
Major depressive disorder is often comorbid with diabetes and associated with worse glycemic control. Exercise improves glycemic control and depression, and thus could be a parsimonious intervention for patients with comorbid diabetes and major depression. Because patients with diabetes and comorbid depression are often sedentary and lack motivation to exercise, we developed a group exercise intervention that integrates strategies from behavioral activation therapy for depression to increase motivation for and enjoyment of exercise. We conducted a 6-month pilot randomized controlled trial to test the feasibility of the behavioral activation exercise intervention (EX) for women with diabetes and depression. Of the 715 individuals who contacted us about the study, 29 participants were randomized to the EX condition or an enhanced usual care condition (EUC), which represents 4.1% of participants who initially contacted us. Inclusion criteria made recruitment challenging and limits the feasibility of recruiting women with diabetes and depression for a larger trial of the intervention. Retention was 96.5% and 86.2% at 3 and 6 months. Participants reported high treatment acceptability; use of behavioral activation strategies and exercise class attendance was acceptable. No condition differences were observed for glycemic control, depressive symptoms, and physical activity, though depressive symptoms and self-reported physical activity improved over time. Compared to participants in the EUC condition, participants in the EX condition reported greater exercise enjoyment and no increase in avoidance behavior over time. Using behavioral activation strategies to increase exercise is feasible in a group exercise setting. However, whether these strategies can be delivered in a less intensive manner to a broader population of sedentary adults, for greater initiation and maintenance of physical activity, deserves further study.
Keywords: physical activity, behavioral activation, major depressive disorder, Type 2 diabetes mellitus
Type 2 Diabetes Mellitus (T2DM) is a significant public health problem affecting 8.3% of adults in the U.S. (Centers for Disease Control and Prevention., CDC, 2011). Depression is highly comorbid with diabetes; 11% to 27% of adults with T2DM have depression and women with T2DM are 2 to 3 times more likely to have depression than men with T2DM (Ali, Stone, Peters, Davies, & Khunti, 2006; Anderson, Freedland, Clouse, & Lustman, 2001; Roupa et al., 2009). Depression in T2DM is associated with worse glycemic control (Krogh, Nordentoft, Sterne, & Lawlor, 2011; Lustman et al., 2000), poor diabetes self-management (Ciechanowski, Katon, Russo, & Hirsch, 2003; Lin et al., 2004), and elevated risk for all-cause mortality (Pan et al., 2011), making depression a treatment priority.
Approaches to addressing comorbid T2DM and depression include antidepressant medication (Lustman et al., 2006) and psychotherapy like cognitive-behavioral therapy (CBT; e.g., (Lustman & Griffith, 1998). Most studies report improvement in depression, but not glycemic control (Baumeister, Hutter, & Bengel, 2014; Semenkovich, Brown, Svrakic, & Lustman, 2015). Addressing depression with medication and/or psychotherapy may not be sufficient to influence the lifestyle changes (e.g., improvements in dietary quality, exercise) required to facilitate glycemic control. Because exercise is recommended for individuals with T2DM to improve glycemic control (Boulé, Kenny, Haddad, Wells, & Sigal, 2003; Sigal, Kenny, Wasserman, Castaneda-Sceppa, & White, 2006; D. Thomas, Elliott, & Naughton, 2006) and exercise has initial evidence for treating depression (Cooney et al., 2013), integrating structured exercise into depression treatment may be a parsimonious approach for adults with T2DM and depression.
Two studies examined whether combining CBT with structured exercise improved outcomes in adults with T2DM and depression. In a single arm trial, a 12-week, individually based CBT and structured exercise intervention reduced glycosylated hemoglobin (HbA1c), a marker of long-term glycemic control, at 3 months and depressive symptoms at 3 and 6 months in participants with diagnosed depression and diabetes (Groot et al., 2012). The only previously performed randomized controlled trial (RCT) compared CBT plus a walking intervention to usual care in patients with T2DM and elevated depressive symptoms (Piette et al., 2011). Participants in the intervention reported significant improvements in depression and pedometer steps at 12 months compared to usual care, but neither condition improved in glycemic control (Piette et al.). In these studies, CBT strategies were not used specifically to motivate participants to exercise. Tailoring the behavioral strategies of CBT to focus on exercise may bolster the intervention effect on exercise given the sedentary nature of this population, thus enhancing glycemic control and reducing depressive symptoms via a single mechanism: increased exercise.
Exercise could be used as the sole approach to improve depression and glycemic control. However, adults with T2DM report more exercise barriers, are less likely to engage in exercise, and are more likely to relapse to a sedentary lifestyle after beginning an exercise program, compared to their counterparts without T2DM (Dutton, Johnson, Whitehead, Bodenlos, & Brantley, 2005; Grace, Barry-Bianchi, Stewart, Rukholm, & Nolan, 2007; Morrato, Hill, Wyatt, Ghushchyan, & Sullivan, 2007; Plotnikoff, Brez, & Brunet, 2003). The challenges of exercise are further heightened in adults with T2DM and comorbid depression. Depressive symptoms among individuals with T2DM are associated with less use of relapse prevention behaviors, less ability to restructure plans for exercise, greater perception of barriers relative to benefits of exercise, and lower self-efficacy for exercise (Vickers, Nies, Patten, Dierkhising, & Smith, 2006). Embedding strategies from behavioral activation (BA), an evidence-based treatment for depression (Chartier & Provencher, 2013), into a structured exercise intervention, rather than separating depression treatment from the exercise intervention, could provide a less stigmatizing and more acceptable way of addressing depression and facilitating exercise in a population that struggles with exercise. BA involves structured attempts to increase healthy behaviors that bring the patient into contact with reinforcing environmental contingencies, which produce corresponding improvements in cognitions and mood (Chartier & Provencher; Lejuez, Hopko, Acierno, Daughters, & Pagoto, 2011). BA begins by helping the patient understand the impact of their daily activities on their mood and the alignment between their activities and their core values. BA then focuses on increasing the number of value-driven activities in the patient’s life, with the ultimate goal of creating a more fulfilling life, the key to overcoming depression. These strategies could be applied to increase values-driven physical activities to facilitate improvements in depression and glycemic control, without the need for separate providers to deliver CBT and structured exercise. Additionally, stigma is a barrier to traditional psychological treatment for depression (Livingston & Boyd, 2010), which may limit the acceptability of psychotherapy as a separate component of an exercise program among some individuals.
This pilot RCT aimed to test the feasibility of an intervention that uses BA strategies to increase exercise within a structured exercise intervention (EX), among women with comorbid T2DM and depression. This is the first study to examine whether BA strategies can be delivered by an exercise professional to promote exercise and improve outcomes in women with diagnosed depression and inadequately controlled diabetes. We hypothesized that the EX condition would be feasible as indicated by: (a) a recruitment rate comparable to exercise studies of adults with depression and diabetes; (b) high study retention, intervention fidelity, adherence and intervention acceptability; and (c) greater exercise enjoyment in the EX condition compared to an enhanced usual care condition (EUC). We explored changes in HbA1c, depression, physical activity, weight, exercise self-efficacy, social support, and behavioral activation at 3 and 6 months in the two conditions. We also conducted post-hoc analyses exploring whether EX class adherence was associated with change in outcomes.
Material and Methods
Procedures were approved by the Internal Review Boards of the University of Massachusetts Medical School’s (UMMS) and Rosalind Franklin University of Medicine & Science (Schneider et al., 2011). The trial was registered at clinicaltrials.gov (NCT01024790) before recruitment began and monitored by a Data Safety and Monitoring Board (DSMB) consisting of a biostatistician and an endocrinologist. The DSMB met prior to the onset of study recruitment to review the study aims and protocol, and every 6 months thereafter, to review the aggregated data, recruitment, and adverse events.
PARTICIPANTS
To be eligible, women between the ages of 21–65 years old had to meet the following inclusion criteria: (a) inadequately controlled T2DM (HbA1c level of 7–10%); (b) met criteria for major depressive disorder as defined by the Structured Clinical Interview for DSM-IV disorders (SCID-IV); (c) a body mass index between 18.5–45 kg/m2; and (d) inactive (less than 60 minutes of moderate intensity exercise per week). Exclusion criteria were used to: (a) minimize adverse effects of the intervention (e.g., physical limitations); (b) prevent avoidable dropouts (e.g., plans to move out of the area), and (c) exclude participants who require more intensive psychological intervention (e.g., active suicidal ideation). Participants taking antidepressant medications for more than 3 months with no plans to change the regimen and those currently in psychotherapy were eligible.
PROCEDURES
Participants were recruited in three 4-month waves since the EX intervention involved group exercise (recruitment began in 2/2010 for Wave 1 and ended in 10/2011 for Wave 3). Recruitment targeted women with T2DM who reported depressed mood, wanted to increase their physical activity, and improve diabetes management. Recruitment sources included mailings to diabetes support groups and diabetes educators, postings at pharmacies, community health centers and primary care and diabetes outpatient clinics, newspaper ads and online resources (e.g., craigslist). Interested individuals contacted the study team by phone to receive a description of the study and initial eligibility screening. Those meeting initial criteria were scheduled for a 2-hour screening visit where they provided written informed consent. A clinician then administered the SCID-IV; participants had their blood drawn and height and weight measured by a trained research assistant. Primary care physicians were faxed a form asking for permission for their patient to participate and to confirm the T2DM diagnosis. Participants with physician approval were scheduled for a 1-hour baseline visit where they completed measures and received an accelerometer. Participants who did not have a negative stress test in the past 2 years completed a maximal treadmill exercise stress test in the Cardiology Department at the University of Massachusetts Memorial Medical Center.
RANDOMIZATION
Randomization to conditions (1:1) was stratified based on antidepressant medication use (present, absent) and HbA1c (7–8.5%, 8.6–10%) to ensure that participants who may be less responsive to treatment were evenly distributed between the two conditions. Within each of the four strata, participants were randomized in randomly permuted blocks of size 2 and 4 using the ralloc program in Stata (StataCorp LP, College Station, TX) (Ryan, 2000). The study statistician generated the randomization sequence, which was then integrated into the study database tracking system (IBM Notes: IBM, Armonk, New York). IBM Notes concealed randomization status until the research coordinator clicked a button to randomize a participant.
INTERVENTION
Behavioral Activation and Exercise Condition (EX)
The intervention was pretested in 5 women who met the same eligibility criteria to inform the BA materials, exercise content, and class format (Schneider et al., 2011). The pretesting also provided in vivo training in the BA content to the female group leader. The leader had a Pilates and yoga certification and received weekly supervision from the principal investigator (KLS).
The EX condition involved one orientation session and 38 group exercise classes over 24 weeks at UMMS. At orientation, participants learned how to exercise safely from the study endocrinologist and received an overview of the BA strategies from the group leader. The 90-minute classes occurred 2 times per week for 16 weeks, once a week for 4 weeks, and then once every other week for 4 weeks. Classes contained three parts: (a) warmup; (b) exercise; and (c) cool down and BA. The time devoted to exercise increased (from 20 minutes to 65 minutes) to help participants gradually reach their exercise goal, while the time devoted to BA decreased.
Warm-up
Participants checked their blood glucose to ensure that they were safe for exercise. They then began a 10-minute warm-up exercise.
Exercise
The first 12 weeks featured a different moderate intensity exercise every 2 weeks (walking, Zumba, Pilates, step aerobics, cardio-kickboxing, and power yoga); subsequent activities for the remaining 12 weeks were selected based on participants’ mean exercise enjoyment ratings. Exercise increased in duration to 150 minutes of moderate intensity activity per week, as recommended by the American Diabetes Association (ADA) (Sigal et al., 2006). To achieve the recommended dose, participants were asked to exercise at least one day on their own.
Cool down and BA content
Participants completed a cool-down activity, followed by a discussion of BA content (Lejuez et al., 2011). During the intervention, participants completed: (a) activity and mood monitoring, which centered on leisure time sedentary activities and exercise; (b) activity experiments, where participants were encouraged to explore ways to make exercise more enjoyable; (c) a behavioral contract in which participants contracted with friends or family to promote exercise; and (d) a values and activities assessment in which participants generated a list of their life values and ways to connect exercise to their values. Participants were encouraged to identify values in all life areas. For example, in the relationships life area, activities that addressed the value of maintaining friendships could include walking during a biweekly telephone call with a friend or meeting weekly with a friend to take an exercise class.
Exercise counseling strategies were also discussed. Strategies included self-monitoring activity using a pedometer, monitoring exercise intensity via perceived exertion and heart rate, identifying facilitators to exercise and engaging in problem solving for exercise barriers.
Enhanced Usual Care Condition (EUC)
EUC condition participants were called to inform them of their condition; they were made aware of their depression diagnosis and encouraged to discuss treatment with their physician. Their physician was contacted and informed of their diagnosis. Depression treatment referrals were also provided. Participants received information available from the ADA on nutrition, exercise, and glucose monitoring. Participants were reminded during this phone call that, upon completion of the final assessment, they would receive a 3-month gym membership and an individual session with the PI to develop a personal exercise plan, which included coaching on the BA strategies.
ASSESSMENTS
Participants attended 90-minute visits at baseline and 3 and 6 months after randomization at UMMS. Three-month visits began in 10/2010; 6-month visits ended in 11/2012. Participants completed measures and received an accelerometer; they received $50 upon returning the accelerometer. Clinicians conducting assessments were blind to condition.
MEASURES
Screening
A master’s- or doctoral-level clinician administered the SCID-IV (Spitzer, Gibbon, Skodol, Williams, & First, 2002) to diagnose depression. Participants completed a medical history questionnaire, the revised physical activity readiness questionnaire (Thomas, Reading, & Shephard, 1992), and the Beck scale for suicidal ideation (BSI; Beck & Steer, 1991). The BSI has high internal reliability (Cronbach α range 0.87 to 0.97) and moderate test-retest reliability (r = 0.54; Beck & Steer, 1991).
Feasibility Measures
Recruitment rate
Recruitment rate was calculated as the number of randomized participants divided by the number of individuals who completed a telephone screen.
Retention rate
Retention at 3 and 6 months was calculated.
Adherence
For EX condition, percent attendance to classes was calculated. After each class the leader completed a checklist in which they recorded participant adherence to the BA content (e.g., daily monitoring form).
Treatment acceptability
A modified version of the valid and reliable diabetes measurement and evaluation tool (Paddock, Veloski, Chatterton, Gevirtz, & Nash, 2000) assessed participants’ satisfaction with the EX intervention.
Adverse events
Events such as increased depressive symptoms and injuries were assessed in EX participants during exercise classes and assessment visits. These issues were identified at assessments for EUC participants. Adverse events were categorized by a study coordinator as unsure if related, unrelated, possibly related, or definitely related.
Exercise enjoyment
Participants completed the reliable and valid exercise enjoyment scale (Kendzierski & DeCarlo, 1991). Coefficient α = 0.93 for the current study.
Concurrent treatment
Participants reported their participation in psychotherapy and use of antidepressant and T2DM medication.
Outcome Measures
Glycosylated Hemoglobin (HbA1c)
Blood samples were taken through venipuncture by a phlebotomist. Serum HbA1c was measured in the University of Massachusetts Memorial Medical Center laboratory. HbA1c values ≥ 7% indicated inadequately controlled diabetes.
Depressive symptoms
A trained clinician administered the HRSD (Beck, Steer, & Brown, 1996; Hamilton, 1960) and participants completed the Beck Depression Inventory-II (Beck et al., 1996). HRSD scores ≥ 8 and BDI-II ≥ 14 indicate mild or greater symptoms of depression. The HRSD has high internal consistency (coefficient α = 0.92) (Reynolds & Kobak, 1995), strong test-retest reliability (r = 0.65 to 0.98) and good concurrent validity with the depression in diabetes self-rating scale (Kokoszka, 2008; Trajković, Starčević, Latas, Leštarević, Ille, Bukumirić, Marinković, 2011). Internal consistency for the BDI-II is high in adults (Smarr & Keefer, 2011) and adults with diabetes (Lee et al., 2009) and the BDI-II demonstrates adequate test-retest reliability (r = 0.73 to 0.96) (Wang & Gorenstein, 2013). BDI-II coefficient α = 0.80 for the current study.
Physical activity
Participants wore an accelerometer (Actigraph, LLC, Fort Walton Beach, FL; model 7164 WAM) for 7 days to measure physical activity. Valid days were defined using wear counts indicating that the participant wore the accelerometer for at least 10 hours/day; the cutpoint ≥ 1,952 counts × min (−1), was used to define moderate intensity activity or greater (Freedson, Melanson, & Sirard, 1998). Participants received instructions on the placement (i.e., hip) and wear time of the accelerometer (i.e., put it on immediately upon waking). Participants were required to have at least 3 valid days of data to calculate moderate to vigorous intensity physical activity minutes.
Body Mass Index (BMI)
Body weight and height were measured by a trained research assistant with the participant wearing only light clothing and no shoes. BMI was calculated as weight (kg)/height(m)2. A BMI of 25.0 – 29.9 kg/m2 is categorized as overweight and a BMI of 30 kg/m2 is classified as obese.
Self-Efficacy for Exercise Scale
Participants rated their confidence that they will exercise in response to 9 exercise barriers. Internal consistency is high (coefficient α = 0.91) in adults (Resnick & Jenkins, 2000) and adults with diabetes (Gleeson-Kreig, 2006). This measure has adequate test-retest reliability (r = 0.89) (Steinhardt & Dishman, 1989) and concurrent validity with exercise behavior (r = 0.56) (Resnick & Jenkins, 2000). Coefficient α = 0.87 for the current study.
Social support
General social support from family, friends, and a significant other was measured using the reliable and valid multidimensional scale of perceived social support (Zimet, Powell, Farley, Werkman, & Berkoff, 1990). Participants rated their perception that friends and family support their engagement in exercise using the social support and exercise survey (Sallis, Grossman, Pinski, Patterson, & Nader, 1987). It consists of three valid and reliable subscales: family participation, friend participation, and family rewards and punishment (Sallis et al., 1987). Internal consistency is high in adults (coefficient α = 0.87–0.94) (Stanley, Beck, & Zebb, 1998) and adults with diabetes (Parada & Rivera, 2011). This measure has adequate test-retest reliability (r =0.73 to 0.88) and convergent validity with the self-efficacy for diabetes scale (r = 0.26) (Duru, 2007; Park, Nguyen, & Park, 2012; Stanley et al., 1998; Zimet, Dahlem, Zimet, & Farley, 1988). In the current study, coefficient α ranged from 0.91 to 0.97 for the subscales.
Behavioral Activation for Depression Scale (BADS)
The BADS contains factor-analytically derived subscales for activation, avoidance/rumination, work/school impairment and social impairment subscale (Kanter, Mulick, Busch, Berlin, & Martell, 2007). The BADS has adequate test-retest reliability (r = 0.74) (Kanter et al., 2007) and internal consistency in a clinical sample was high (coefficient α = 0.75 to 0.92; (Kanter, Rusch, Busch, & Sedivy, 2009). In the current sample, coefficient α ranged from 0.82 to 0.89 for activation, avoidance/rumination and social impairment. Coefficient α = 0.53 for the work/school impairment subscale; thus this subscale was not analyzed.
SAMPLE SIZE
The primary goal of this feasibility pilot RCT was to examine the feasibility and acceptability of the intervention. This study was not powered to estimate effect sizes for a larger trial since effect sizes from pilot studies result in imprecise estimates of the treatment effect (Leon, 2008). We planned to enroll 30 patients per condition. A sample size of 60 ensured that the 95% confidence interval (CI) for the estimated retention rate (80%) in the EX condition will be within +/− 18%. A priori, we decided that a retention rate in the EX condition lower than 55% would indicate that intervention was not feasible and acceptable. Thus, the lower limit of the 95% CI for the observed retention rate should not be lower than 55%.
ANALYTIC PLAN
Data were plotted using histograms and skewness and kurtosis was formally assessed to ensure that data met assumptions required for analysis. Descriptive statistics were used to describe the sample overall and by condition. Chi-square tests were used to examine differences in adverse events by condition. Logistic regressions were conducted to examine whether retention rate differed by condition at 3 and 6 months, adjusted for recruitment wave. Recruitment wave was included as a covariate to control for the use of a group to deliver the EX intervention. Linear mixed models (Littell, Stroup, Milliken, Wolfinger, & Schabenberger, 2006) were conducted to estimate the effect of the EX condition on the outcomes over time (baseline, 3- and 6-months) using maximum likelihood and an identity covariance structure. Models included recruitment wave, time, condition and the time-by-condition interaction. Models included a random intercept and were adjusted to account for repeated observations of participants over time. Intent-to-treat analyses were conducted; all participants were included in the analysis in their assigned condition, even if they were missing data at the 3- or 6-month assessments, since mixed models allow for the inclusion of participants with missing data as long as participants have data at one or more time points. A main advantage of mixed models is that missing data can remain missing. Last observation carried forward is an imputation strategy that assumes no change from the previous assessment, which eliminates the possibility that participants with missing data improved or worsened after the previous data point. Elobeid et al. (2009) examined different strategies for dealing with missing data, including mixed models and last observation carried forward in weight loss trials. They recommended mixed models or multiple imputation for handling missing data instead of last observation carried forward as the former strategies are less likely to produce biased estimates than the strategy of using the last observation carried forward. Effect sizes were calculated to indicate change from baseline to the 6-month assessment by condition.
Post-hoc Pearson correlations between percent exercise group attendance and change in the outcome measures from baseline to 6 months were computed with participants in the EX condition to explore whether compliance with the exercise group was associated with improvement in outcomes. Only participants in the EX condition with complete data at 6 months were included in this analysis so that change scores (6-month – baseline) could be calculated. A guideline of interpreting correlations (r = > 0.20) was used instead of the significance level since the sample size was modest and these analyses were exploratory (Hemphill, 2003).
With the exception of the post-hoc analyses examining group attendance and change in outcomes, an alpha level of 0.05 was used to determine statistical significance. SPSS (Version 20.0; IBM Corporation) was used for analysis.
Results
PARTICIPANTS
Table 1 contains demographics. Participants were mostly non-Hispanic White, with an average age of 53.4 years (SD = 7.1) and an average BMI of 34.6 kg/m2 (SD = 5.6). Participants had an average HbA1c of 7.9% (SD = 0.7); HRSD depression scores were in the moderate range.
Table 1.
Participant demographics for the full sample and split by condition
| Split By Condition |
||||
|---|---|---|---|---|
| All Participants (N = 29) |
EX (N = 15) |
EUC (N = 14) |
Condition comparisons |
|
| M (SD) | M (SD) | M (SD) | p | |
| Age (years) | 53.4 (7.1) | 53.3 (6.0) | 53.6 (8.4) | 0.91 |
| Hamilton Rating Scale for Depression | 16.5 (4.5) | 15.7 (4.6) | 17.4 (4.3) | 0.34 |
| Beck Depression Inventory-II | 20.0 (6.9) | 18.5 (8.2) | 21.6 (4.9) | 0.23 |
| Glycosylated hemoglobin (%) | 7.9 (0.7) | 7.9 (0.8) | 7.9 (0.6) | 0.88 |
| Body mass index (kg/m2) | 34.6 (5.6) | 34.5 (4.6) | 34.7 (6.0) | 0.92 |
| Accelerometer moderate/vigorous minutes | 12.9 (12.2) | 12.6 (12.7) | 13.2 (12.1) | 0.90 |
| Race | N (%) | N (%) | N (%) | 0.27 |
| White | 25 (86.2) | 13 (86.7) | 12 (85.7) | |
| Black or African American | 1 (3.4) | 1 (6.7) | 0 (0) | |
| American Indian/Alaskan Native | 2 (6.9) | 0 (0) | 2 (14.3) | |
| Other race | 1 (3.4) | 1 (6.7) | 0 (0) | |
| Ethnicity | 0.16 | |||
| Hispanic | 3 (10.3) | 0 (0) | 3 (21.4) | |
| Non-Hispanic | 25 (86.2) | 14 (93.3) | 11 (78.6) | |
| I don’t know | 1 (3.4) | 1 (6.7) | 0 (0) | |
| Education | 0.96 | |||
| Less than college degree | 14 (48.2) | 8 (53.3) | 6 (42.8) | |
| College degree | 8 (27.5) | 3 (20.0) | 5 (35.7) | |
| More than college degree | 7 (24.1) | 4 (26.7) | 3 (21.4) | |
| Marital status | 0.79 | |||
| Married | 18 (62.1) | 10 (66.7) | 8 (57.1) | |
| Living with a partner | 2 (6.9) | 1 (6.7) | 1 (7.1) | |
| Single | 3 (10.3) | 2 (13.3) | 1 (7.1) | |
| Separated | 1 (3.4) | 0 (0) | 1 (7.1) | |
| Divorced | 5 (17.2) | 2 (13.3) | 3 (21.4) | 0.94 |
| Psychotherapy | ||||
| Receiving treatment at baseline | 4 (13.8) | 2 (13.3) | 2 (14.3) | |
| Current smoker | 0.96 | |||
| Yes | 2 (6.9) | 1 (6.7) | 1 (7.1) | |
EX = Behavioral activation and exercise condition; EUC = Enhanced usual care condition.
FEASIBILITY
Recruitment
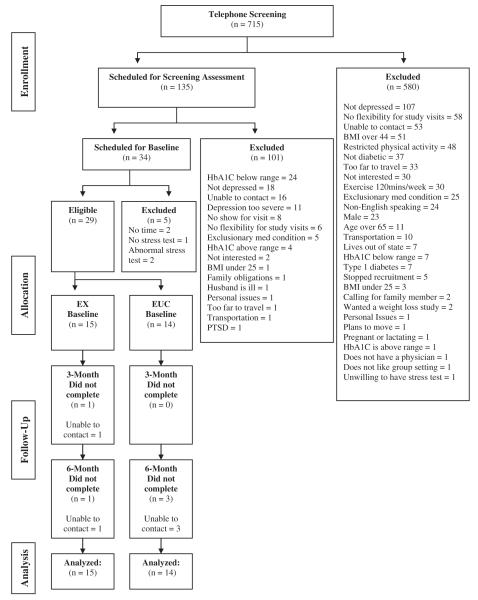
The recruitment target of N = 60 was not met; 29 participants were randomized. Of the 715 individuals who contacted us, 19% (n = 135) met telephone eligibility criteria; of those 135, 25% (n = 34) were eligible after the in-person screening and 85% (n = 29) were then randomized (Figure 1). The 29 participants represent 4.1% of participants who initially contacted us.
FIGURE 1.
CONSORT diagram of participant flow through the study.
Retention
Retention rates were 96.5% and 86.2% at the 3- and 6-month assessments. Logistic regression analyses demonstrated that retention did not differ by condition at the 3- (p = 0.99) or 6-month assessments (p = 0.27; see Figure 1 for retention rate by condition).
Adherence
Average percent attendance to the 38 classes was 50.85% (SD = 31.12); median = 63.20%. Of EX participants who attended at least 1 class, average completion of mood monitoring forms was 41.7% (SD = 21.2) over the course of the intervention. Participants brought their life values and activities form an average of 28.7% (SD = 18.5) of the time and 84.6% of participants completed at least one behavioral contract form.
Treatment Acceptability
For the 12 EX participants who completed the questionnaire, average treatment acceptability ratings for the total scale were high at 3 (M = 4.7, SD = 0.4) and 6 months (M = 4.6, SD = 0.6; Table 2). Ratings for the group classes at 3 (M = 4.7, SD = 0.4) and 6 months (M = 4.5, SD = 0.6) were comparable to ratings for the ability of the group to facilitate exercise goals at 3 (M = 4.8, SD = 0.5) and 6 months (M = 4.7, SD = 0.6).
Table 2.
Behavioral activation and exercise condition treatment acceptability ratings
| How satisfied are you with… | 3-Months Mean (SD) |
6-Months Mean (SD) |
|---|---|---|
| …the class’ ability to begin on time | 4.9 (0.3) | 4.5 (0.9) |
| …the variety of topics discussed in class |
4.6 (0.8) | 4.4 (0.9) |
| …the information provided by the class |
4.8 (0.6) | 4.8 (0.6) |
| …the helpfulness of your class leader | 4.9 (0.3) | 4.9 (0.3) |
| …your ability to discuss your experience with physical activity |
4.8 (0.5) | 4.6 (0.8) |
| …the length of the class | 4.7 (0.7) | 4.7 (0.8) |
| …the convenience of location | 4.6 (0.8) | 4.5 (1.0) |
| …class activities | 4.8 (0.4) | 4.8 (0.6) |
| …the interest level of the class discussions |
4.5 (0.9) | 4.3 (0.9) |
| …the usefulness of the information provided by the class leader |
4.8 (0.4) | 4.8 (0.6) |
| … your ability to find the class | 4.9 (0.3) | 4.8 (0.5) |
| … your ability to contact your class leader |
4.8 (0.4) | 4.7 (0.7) |
| … the cleanliness of the room | 4.8 (0.5) | 4.6 (0.7) |
| … your physical activity goal | 4.8 (0.4) | 4.6 (0.7) |
| … the respect provided by your class leader |
4.8 (0.6) | 4.9 (0.3) |
| … our ability to attend classes | 4.3 (1.0) | 4.1 (1.1) |
| … the availability of class materials | 4.6 (0.7) | 4.8 (0.6) |
| … the safety precautions taken during class |
4.8 (0.4) | 4.8 (0.9) |
| …the ability of the leader to provide interesting information |
4.9 (0.3) | 4.8 (0.6) |
| … the amount of time spent monitoring mood |
4.5 (1.0) | 4.3 (1.3) |
| … the amount of time spent monitoring activities |
4.7 (0.5) | 4.3 (1.3) |
| …the Behavioral Contract form | 4.3 (1.1) | 4.0 (1.3) |
| … the Activity monitoring form | 4.2 (1.1) | 4.0 (1.3) |
| … the Life Areas, Values and Activities form |
4.0 (1.1) | 4.0 (1.3) |
| … the increase in your understanding of the relationship between type 2 diabetes and physical activity |
4.8 (0.4) | 4.8 (0.4) |
| … monitoring your physical activity | 4.8 (0.6) | 4.7 (0.7) |
| …your plan to increase physical activity |
4.8 (0.6) | 4.6 (0.7) |
| … physical activity strategies that you can continue to use |
5.0 (0.0) | 4.7 (0.7) |
| …information on types of physical activity |
4.9 (0.3) | 4.8 (0.6) |
| …new information | 4.9 (0.3) | 4.7 (0.8) |
| …help to reach your physical activity goals |
4.8 (0.4) | 4.5 (0.7) |
Note. Items rated on a 1–5 scale where 1 = extremely dissatisfied and 5 = extremely satisfied.
Adverse Events
Thirty-nine adverse events occurred: 32 in the EX condition and 7 in the EUC condition. Whether the adverse event was related to study participation did not differ by condition (X2 = 2.10, p = 0.55).
Exercise Enjoyment
The Time × Condition interaction was significant for exercise enjoyment, F(2, 48) = 5.64, p =0.006, such that EX condition participants reported increased enjoyment over time, F(2, 25) = 15.30 (p < 0.001; MΔ = 18.3, SD = 14.7 at 3-months; M Δ = 8.8, SD = 14.6 at 6 months), while EUC condition participants reported no change (p = 0.98; MΔ = −0.7, SD = 19.6 at 3 months; M Δ = −4.4, SD = 21.0 at 6 months).
Concurrent Treatment
At baseline, 13 out of 15 participants in the EX condition and 13 out of 14 participants in the EUC condition were taking a medication to manage T2DM. For diabetes-related medications, 13 participants in the EUC condition compared to 8 in the EX condition started a new medication or increased the dose of a diabetes related medication; 3 EUC condition participants and 2 EX condition participants discontinued a diabetes-related medication. Antidepressant medication changes were identical between conditions. One participant in the EUC condition began psychotherapy by the 3-month follow-up. No participants discontinued psychotherapy.
OUTCOMES
The Time × Condition interaction was not significant for HbA1c, depressive symptoms (BDI-II, HRSD), or physical activity (self-reported or accelerometer minutes; Table 3). However, BDI-II and HRSD scores decreased over time (MΔ = −7.3; F[2, 49.91] = 13.67, p < 0.001 and MΔ = −6.6; F[2, 49.56] = 13.74, p < 0.001, respectively) and self-reported physical activity increased significantly over time (MΔ = 162.2 weekly minutes; F[2, 48.32] = 6.34, p = 0.004).
Table 3.
Outcomes by condition and over time
| Baseline |
3-months |
6-months |
Time × condition |
||||||
|---|---|---|---|---|---|---|---|---|---|
| EUC M (SD) |
EX M (SD) | EUC M (SD) |
EX M (SD) | EUC M (SD) |
EX M (SD) | F | p | Cohen’s d (95% CI) |
|
| Beck Depression Inventory-II |
21.6 (4.7) | 18.5 (8.2) | 16.5 (7.2) | 15.1 (13.8) | 12.3 (4.1) | 12.5 (14.8) | 1.02 | 0.37 | −0.49 (−1.21, 0.26) |
| Hamilton Depression Rating Scale |
17.4 (4.3) | 15.7 (4.6) | 9.1 (6.3) | 13.4 (7.1) | 9.2 (5.7) | 10.6 (6.1) | 2.55 | 0.09 | −0.66 (−1.41, 0.09) |
| Glycosylated hemoglobin (%) |
7.9 (0.6) | 7.9 (0.8) | 7.8 (0.9) | 7.8 (1.1) | 7.7 (0.8) | 7.8 (0.9) | 0.25 | 0.78 | −0.24 (−0.97, 0.50) |
| Self-reported MVPA minutes/day |
105.0 (129.8) |
139.3 (139.5) |
377.1 (445.6) |
355.4 (412.8) |
300.0 (188.0) |
274.3 (312.0) |
0.12 | 0.88 | 0.44 (−0.30, 1.17) |
| Accelerometer MVPA minutes/day |
13.2 (12.1) | 12.6 (12.7) | 17.8 (14.9) | 17.0 (9.7) | 13.8 (11.9) | 15.3 (10.5) | 0.96 | 0.39 | −0.17 (−0.89, 0.57) |
| Body mass index (kg/m2) |
34.7 (6.0) | 34.5 (5.4) | 34.5 (5.5) | 34.6 (6.1) | 33.4 (5.2) | 34.3 (6.0) | 0.10 | 0.90 | −0.17 (−0.90, 0.56) |
| Exercise enjoyment | 78.6 (21.4) | 75.1 (19.5) | 77.9 (11.7) | 97.4 (15.8) | 77.6 (22.6) | 83.9 (15.4) | 5.64 | 0.01 | −0.47 (−1.20, 0.28) |
| Exercise self-efficacy | 49.0 (18.8) | 41.3 (12.3) | 41.4 (19.5) | 41.6 (8.6) | 42.6 (13.7) | 43.6 (17.1) | 0.66 | 0.52 | −0.56 (−1.28, 0.20) |
| Family social support | 22.0 (5.3) | 15.5 (7.1) | 22.8 (4.1) | 17.3 (8.1) | 19.2 (9.0) | 19.2 (7.3) | 3.39 | 0.04 | −1.01 (−1.78, 0.24) |
| Friend social support | 18.8 (6.3) | 18.8 (6.9) | 19.9 (6.5) | 16.8 (7.3) | 16.5 (8.4) | 18.4 (7.0) | 1.93 | 0.16 | −0.29 (−1.01, 0.45) |
| Significant other social support |
23.7 (4.6) | 18.4 (8.1) | 22.6 (6.5) | 20.0 (7.5) | 19.1 (10.6) | 21.3 (7.4) | 3.31 | 0.05 | −1.12 (−1.87, 0.31) |
| Family participation-exercise |
16.4 (8.1) | 15.0 (6.1) | 19.5 (8.8) | 24.6 (8.6) | 16.7 (8.4) | 20.8 (8.0) | 2.75 | 0.07 | −0.76 (−1.5, 0.00) |
| Friend participation-exercise |
16.9 (10.7) | 15.8 (6.5) | 17.5 (8.3) | 17.6 (9.3) | 15.0 (7.1) | 17.6 (8.1) | 0.23 | 0.79 | −0.43 (−1.16, 0.32) |
| Family rewards & punishment-exercise |
3.6 (1.3) | 3.1 (0.5) | 3.5 (1.0) | 3.5 (0.8) | 3.3 (0.9) | 2.9 (1.4) | 0.53 | 0.59 | −0.09 (−0.82, 0.64) |
| BADS activation | 10.6 (6.8) | 12.0 (7.9) | 15.5 (5.2) | 19.2 (6.3) | 14.3 (10.1) | 16.1 (6.9) | 0.31 | 0.74 | −0.07 (−0.80, 0.66) |
| BADS avoidance/ rumination |
27.8 (7.9) | 34.8 (10.8) | 28.5 (8.1) | 35.5 (11.9) | 37.7 (10.6) | 34.7 (13.0) | 4.46 | 0.02 | 1.05 (0.25, 1.80) |
| BADS social impairment | 19.8 (6.4) | 21.5 (7.3) | 22.5 (6.4) | 22.4 (8.0) | 23.8 (5.6) | 22.1 (7.7) | 1.05 | 0.36 | 0.49 (−.26, 1.22) |
Note. EUC = Enhanced usual care condition; EX = behavioral activation and exercise condition; MVPA = Moderate/vigorous physical activity; BADS = Behavioral Activation for Depression Scale; CI = confidence interval. Cohen’s d reflects the effect size for the time × condition interaction. For these calculations, time reflected change from baseline to the 6-month assessment.
The Time × Condition interaction predicted BADS avoidance/rumination, F(2, 49) = 4.46, p = 0.02, such that the EX condition maintained goal directedness in the face of cognitive and emotional challenges, F(2, 25) = 0.003, p =0.997 (MΔ = −0.1, SD = 7.1), while it decreased in the EUC condition, F(2, 25) = 5.24, p = 0.01 (MΔ = 7.5, SD = 9.5). Significant Time × Condition interactions were found for general social support from family, F(2, 48) = 3.39, p = 0.04, and a significant other, F(2, 48) = 3.31, p =0.045; the simple effects were not significant, likely due to the small sample size. An examination of the means suggests that family and significant other general social support increased for the EX participants (family: MΔ = 3.7, SD = 5.5; significant other MΔ = 2.9, SD = 5.5), while it declined for EUC participants (family: MΔ = −2.7, SD =9.1; significant other MΔ = −4.7, SD = 9.5). Exercise social support from family participation and BADS activation increased over time in both conditions, F(2, 48) = 9.50, p < 0.001; F(2, 48) = 9.41, p < 0.001, respectively.
POST-HOC ANALYSES: EX CONDITION PERCENT ATTENDANCE TO EXERCISE CLASSES
Higher attendance at group exercise classes was associated with decreases in HbA1c, r(12) = −0.35, BMI, r(12) = −0.42, self-reported physical activity minutes, r(11) = −0.30, and BADS social impairment, r(12) = −03.6, and increases in exercise enjoyment, r(12) = 0.23, friend exercise participation, r(12) = 0.21, and family, r(12) = 0.30, friend, r(12) = 0.33, and significant other general social support, r(12) = 0.38, from baseline to 6 months. All other correlations were r < 0.20.
Discussion
Results suggest that the behavioral activation and exercise intervention is acceptable, as indicated by high treatment acceptability ratings, adequate class adherence, and high participant retention. Retention was comparable to the 80.0% retention rate reported by a study that combined depression treatment with exercise for adults with T2DM and depression (Piette et al., 2011). Our retention is on the higher end of the 78.9 to 93% range reported by studies that used structured exercise for adults with T2DM (Balducci et al., 2008; Church et al., 2010; Dobrosielski et al., 2013; Hordern et al., 2009; Kadoglou et al., 2012), but lower than the 100% retention reported by the study that used structured exercise for adults with depression (Pilu et al., 2007). The intervention significantly improved exercise enjoyment and maintaining goal directedness despite emotional or cognitive challenges and effect sizes suggested greater improvements in exercise social support for women who received the intervention; these are all constructs targeted by behavioral activation.
Despite the benefits, recruitment of females with poorly controlled diabetes and major depression was challenging, which weakens the feasibility of a larger trial or a large-scale clinical program targeting this population. Our recruitment rate is comparable to the 6.1% recruitment rate reported by the only RCT that combined CBT and exercise for individuals with T2DM (Piette et al., 2011). Recruitment rates were 6.6% and 10.8% for studies that used structured exercise for adults with T2DM and used comparable recruitment strategies to the current study (Balducci et al., 2008; Church et al., 2010; Dobrosielski et al., 2013). The one exercise intervention study that reported a high recruitment rate (87.7%) identified eligible patients from outpatient clinics and then recruited only from that pool (Balducci et al.). Similarly, in the current study, 85% of participants deemed eligible after screening were successfully recruited. The only other study that used at least 6 months of structured exercise for adults with depression recruited from a registry of eligible participants; their 71.4% recruitment rate is lower than the 85% rate observed in the current study (Pilu et al., 2007).
Patients with comorbid depression and inadequately controlled diabetes may have limited motivation for a structured exercise program, despite being aware of the benefits of such a program. General exercise barriers and symptoms of major depression, like anhedonia and low energy, may prevent patients with comorbid T2DM and depression from signing up for exercise programs, particularly given the pre-enrollment steps required in this study (i.e., screening, baseline, exercise stress test). Exercise barriers are not unique to adults with T2DM, but may be compounded by depression. Depressive symptoms have been associated with less ability to restructure plans for exercise, less use of relapse prevention behaviors, greater perception of barriers relative to benefits of exercise and lower self-efficacy for exercise in a sample of primary care patients with T2DM (Vickers et al., 2006). Pain is another barrier to exercise, and depression in adults with T2DM is associated with greater likelihood of experiencing pain (Bair et al., 2010; Sacco, Bykowski, & Mayhew, 2013). Further research is needed to determine how to market exercise programs in a manner that motivates patients in the community to sign up for exercise classes despite the barriers they report. Recruiting participants directly from diabetes clinics and providing the exercise intervention through the clinic, perhaps at times adjacent to clinic visits, which has been recommended for improving diabetes management (Sigal & Kenny, 2010), may provide a promising approach.
Study inclusion criteria likely restricted adequate recruitment of participants as the vast majority of individuals showing initial interest in the study were excluded prior to completing the in-person screening and baseline visits. One of the most common reasons for exclusion was not meeting criteria for major depressive disorder. Allowing sedentary adults with T2DM who do not have depression to participate in the behavioral activation exercise intervention might have improved recruitment. Since the behavioral activation strategies, such as connecting exercise to life values and use of behavioral contracts, address common exercise barriers like low enjoyment and poor social support rather than depressive symptoms, the strategies would be appropriate for adults with minimal or no depression. Several trials that investigated the use of behavioral activation as an adjunct treatment in adults with either mild or no depressive symptoms found that behavioral activation facilitated positive health behavior change for problematic alcohol consumption (Reynolds, MacPherson, Tull, Baruch, & Lejuez, 2011), smoking cessation (MacPherson et al., 2010), unprotected sex and methamphetamine use (Mimiaga et al., 2012), and low vision rehabilitation (Rovner et al., 2014), and reduced depressive symptoms or prevented depression (MacPherson et al.; Mimiaga et al.; Rovner et al.).
Another common reason for exclusion was having adequate or extremely poor glycemic control. Eliminating the inadequately controlled diabetes requirement might have improved recruitment. The most recent prevalence estimate of inadequately controlled glycemic control from 1999–2000 was 65% among U.S. adults with type 2 diabetes, which increased 10% from the 1988–1994 estimate (Koro, Bowlin, Bourgeois, & Fedder, 2004). The high prevalence suggests that, at some point, an adult with diabetes will have inadequate glycemic control and thus could benefit from developing a regular exercise habit to prevent worsening glycemic control.
Adherence to the exercise intervention classes was associated with small improvements in glycemic control, BMI, exercise enjoyment, general social support and friend social support for exercise and social impairment. Surprisingly, self-reported physical activity was negatively associated with attendance, although this association was not confirmed with the objective physical activity data. Participants in the behavioral activation and exercise condition were required to check their blood glucose before and after exercise to ensure that they were in a safe range for exercise. The additional blood glucose monitoring may have facilitated improvements in glycemic control, though the impact is likely small (Malanda et al., 2012). Pairing exercise with a nutrition and/or weight loss intervention may be necessary to promote proper glycemic control in this population. Weight loss interventions may also be more appealing than interventions focused only on exercise; thus including a weight loss component might increase interest in the intervention.
Strengths of this study include the use of different types of exercise in the intervention, diagnosing major depressive disorder via structured interviews, the use of blinded raters for the assessments, high retention rate and use of intent-to-treat analyses. Limitations include the small sample size, the inability to generalize to men and the selective sample that is likely not representative of women with depression and diabetes. Participants may have been highly motivated to begin an exercise program. The sample size may have precluded our ability to detect significant differences, and also increases the imprecision of any effect, hence conclusions about the efficacy of the intervention are not warranted (Leon, Davis, & Kraemer, 2011).
In spite of recruitment challenges with this population, integrating behavioral activation content into an exercise intervention was feasible and acceptable to participants. Focusing more broadly on sedentary adults with T2DM regardless of their depressive symptomatology may increase participation and thus impact. Restructuring the intervention to maximize flexibility by incorporating technology and minimizing in-person visits may also increase participation from adults in the community and adherence to the intervention. Delivery of behavioral activation for depression has been expanded to include web-based (Davidson et al., 2014) and mobile app technologies (Ly, Carlbring, & Anderson, 2012). Further research is needed to determine how to activate patients with T2DM to join structured or home-based exercise programs, given the benefits exercise poses to T2DM.
Acknowledgments
The project described was supported by grant R34 MH086678-01 to Dr. Schneider from the National Institute of Mental Health. Its contents are solely the responsibility of the authors and do not necessarily represent the views of the National Institute of Mental Health. Support provided by NIH grants KL2TR000160, U01HL105268 (MEW), 5K23HL109620 (MCW), K23HL107391 (AB). Dr. Appelhans receives NIH funding and support from Hillshire Brands Foundation. We would like to thank our participants for their time and effort, as well as the Edward M. Kennedy Health Center of Worcester, MA for assisting with recruitment.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: A systematic review and meta-analysis. Diabetic Medicine. 2006;23(11):1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=2009346394&site=eds-live&scope=site. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=2001081562&site=eds-live&scope=site. [DOI] [PubMed] [Google Scholar]
- Bair M, Brizendine E, Ackermann R, Shen C, Kroenke K, Marrero D. Prevalence of pain and association with quality of life, depression and glycemic control in patients with diabetes. Diabetic Medicine. 2010;27(5):578–584. doi: 10.1111/j.1464-5491.2010.02971.x. [DOI] [PubMed] [Google Scholar]
- Balducci S, Zanuso S, Massarini M, Corigliano G, Nicolucci A, Missori S, Fallucca F. The Italian diabetes and exercise study (IDES): Design and methods for a prospective Italian multicentre trial of intensive lifestyle intervention in people with type 2 diabetes and the metabolic syndrome. Nutrition, Metabolism and Cardiovascular Diseases. 2008;18:585–595. doi: 10.1016/j.numecd.2007.07.006. http://dx.doi.org/10.1016/j.numecd.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus: An abridged Cochrane review. Diabetic Medicine. 2014;31(7):773–786. doi: 10.1111/dme.12452. http://dx.doi.org/10.1111/dme.12452. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the beck scale for suicide ideation. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. 2nd ed Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Boulé N, Kenny G, Haddad E, Wells G, Sigal R. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia. 2003;46(8):1071–1081. doi: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (CDC) National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. p. 201. [Google Scholar]
- Chartier IS, Provencher MD. Behavioural activation for depression: Efficacy, effectiveness and dissemination. Journal of Affective Disorders. 2013;145(3):292–299. doi: 10.1016/j.jad.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Thompson AM, Priest EL, Rodarte RQ, Saunders T, Blair SN. Exercise without weight loss does not reduce C-reactive protein: The INFLAME study. Medicine and Science in Sports and Exercise. 2010;42(4):708–716. doi: 10.1249/MSS.0b013e3181c03a43. http://dx.doi.org/10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. General Hospital Psychiatry. 2003;25(4):246–252. doi: 10.1016/s0163-8343(03)00055-0. [DOI] [PubMed] [Google Scholar]
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, Mead GE. Exercise for depression. The Cochrane Database of Systematic Reviews. 2013;9 doi: 10.1002/14651858.CD004366.pub6. http://dx.doi.org/10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TM, Yuen EK, Felton JW, Mccauley J, Gros K, Stauffacher, Ruggiero KJ. Feasibility assessment of a brief, web-based behavioral activation intervention for adolescents with depressed mood. International Journal of Psychiatry in Medicine. 2014;48(1):69–82. doi: 10.2190/PM.48.1.f. http://dx.doi.org/10.2190/PM.48.1.f. [DOI] [PubMed] [Google Scholar]
- Dobrosielski DA, Barone Gibbs B, Chaudhari S, Ouyang P, Silber HA, Stewart KJ. Effect of exercise on abdominal fat loss in men and women with and without type 2 diabetes. BMJ Open. 2013;3(11) doi: 10.1136/bmjopen-2013-003897. http://dx.doi.org/10.1136/bmjopen-2013-003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru E. Re-examination of the psychometric characteristics of the multidimensional scale of perceived social support among Turkish university students. Social Behavior And Personality. 2007;35(4):443–452. http://dx.doi.org/10.2224/sbp.2007.35.4.443. [Google Scholar]
- Dutton GR, Johnson J, Whitehead D, Bodenlos JS, Brantley PJ. Barriers to physical activity among predominantly low-income African-American patients with type 2 diabetes. Diabetes Care. 2005;28(5):1209–1210. doi: 10.2337/diacare.28.5.1209. [DOI] [PubMed] [Google Scholar]
- Elobeid MA, Padilla MA, McVie T, Thomas O, Brock DW, Musser B, Allison DB. Missing data in randomized clinical trials for weight loss: Scope of the problem, state of the field, and performance of statistical methods. PLOS One. 2009;4(8):e6624. doi: 10.1371/journal.pone.0006624. http://dx.doi.org/10.1371/journal.pone.0006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, inc. accelerometer. Medicine & Science in Sports & Exercise. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=1999049433&site=eds-live&scope=site. [DOI] [PubMed] [Google Scholar]
- Gleeson-Kreig JM. Self-monitoring of physical activity: Effects on self-efficacy and behavior in people with type 2 diabetes. The Diabetes Educator. 2006;32(1):69–77. doi: 10.1177/0145721705284285. http://dx.doi.org/10.1177/0145721705284285. [DOI] [PubMed] [Google Scholar]
- Grace SL, Barry-Bianchi S, Stewart DE, Rukholm E, Nolan RP. Physical activity behavior, motivational readiness and self-efficacy among ontarians with cardiovascular disease and diabetes. Journal of Behavioral Medicine. 2007;30(1):21–29. doi: 10.1007/s10865-006-9080-5. [DOI] [PubMed] [Google Scholar]
- Groot M, Doyle T, Kushnick M, Shubrook J, Merrill J, Rabideau E, Schwartz F. Can lifestyle interventions do more than reduce diabetes risk? treating depression in adults with type 2 diabetes with exercise and cognitive behavioral therapy. Current Diabetes Reports. 2012;12(2):157–166. doi: 10.1007/s11892-012-0261-z. http://dx.doi.org/10.1007/s11892-012-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill JF. Interpreting the magnitudes of correlation coefficients. American Psychologist. 2003;58(1):78–79. doi: 10.1037/0003-066x.58.1.78. http://dx.doi.org/10.1037/0003-066X.58.1.78. [DOI] [PubMed] [Google Scholar]
- Hordern MD, Coombes JS, Cooney LM, Jeffriess L, Prins JB, Marwick TH. Effects of exercise intervention on myocardial function in type 2 diabetes. Heart (British Cardiac Society) 2009;95(16):1343–1349. doi: 10.1136/hrt.2009.165571. http://dx.doi.org/10.1136/hrt.2009.165571. [DOI] [PubMed] [Google Scholar]
- Kadoglou N, Vrabas I, Kapelouzou A, Lampropoulos S, Sailer N, Kostakis A, Angelopoulou N. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Medical Science Monitor. 2012;18(5):290–295. doi: 10.12659/MSM.882734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR. The behavioral activation for depression scale (BADS): Psychometric properties and factor structure. Journal of Psychopathology and Behavioral Assessment. 2007;29(3):191–202. http://dx.doi.org/10.1007/s10862-006-9038-5. [Google Scholar]
- Kanter JW, Rusch LC, Busch AM, Sedivy SK. Validation of the behavioral activation for depression scale (BADS) in a community sample with elevated depressive symptoms. Journal of Psycholopathology and Behavioral Assessment. 2009;31(1):36–42. [Google Scholar]
- Kendzierski D, DeCarlo KJ. Physical activity enjoyment scale: Two validation studies. Journal of Sport & Exercise Psychology. 1991;13(1):50. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=edb&AN=20710083&site=eds-live&scope=site. [Google Scholar]
- Kokoszka A. Depression in diabetes self-rating scale: a screening tool. Experimental & Clinical Diabetology. 2008;8(1):43–47. [Google Scholar]
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: A preliminary report. Diabetes Care. 2004;27(1):17–20. doi: 10.2337/diacare.27.1.17. [DOI] [PubMed] [Google Scholar]
- Krogh J, Nordentoft M, Sterne JAC, Lawlor DA. The effect of exercise in clinically depressed adults: Systematic review and meta-analysis of randomized controlled trials. Journal of Clinical Psychiatry. 2011;72(4):529–538. doi: 10.4088/JCP.08r04913blu. http://dx.doi.org/10.4088/JCP.08r04913blu. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chapa D, Kao CW, Jones D, Kapustin J, Smith J, Friedmann E. Depression, quality of life, and glycemic control in individuals with type 2 diabetes. Journal of the American Academy of Nurse Practitioners. 2009;21(4):214–224. doi: 10.1111/j.1745-7599.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL. Ten year revision of the brief behavioral activation treatment for depression: Revised treatment manual. Behavior Modification. 2011;35(2):111–161. doi: 10.1177/0145445510390929. http://dx.doi.org/10.1177/0145445510390929. [DOI] [PubMed] [Google Scholar]
- Leon A, Davis L, Kraemer H. The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research. 2011;45(5):626–629. doi: 10.1016/j.jpsychires.2010.10.008. http://dx.doi.org/10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC. Implications of clinical trial design on sample size requirements. Schizophrenia Bulletin. 2008;34(4):664–669. doi: 10.1093/schbul/sbn035. http://dx.doi.org/10.1093/schbul/sbn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, Young B. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- Littell RC, Stroup WW, Milliken GA, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd ed SAS Institute; Cary, NC: 2006. [Google Scholar]
- Livingston JD, Boyd JE. Correlates and consequences of internalized stigma for people living with mental illness: A systematic review and meta-analysis. Social Science & Medicine. 2010;71:2150–2161. doi: 10.1016/j.socscimed.2010.09.030. http://dx.doi.org/10.1016/j.socscimed.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE, Nix BD, Freedland KE, Rubin EH, McGill JB, Hirsch IB. Sertraline for prevention of depression recurrence in diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Archives of General Psychiatry. 2006;63(5):521–529. doi: 10.1001/archpsyc.63.5.521. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=16651509&site=eds-live&scope=site. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS. Cognitive behavior therapy for depression in type 2 diabetes mellitus. Annals of Internal Medicine. 1998;129(8):613–621. doi: 10.7326/0003-4819-129-8-199810150-00005. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=1229211&site=ehost-live&scope=site. [DOI] [PubMed] [Google Scholar]
- Ly KH, Carlbring P, Anderson G. Behavioral activation-based guided self-help treatment administered through a smartphone application: Study protocol for a randomized controlled trial. Trials. 2012;13(1):1–6. doi: 10.1186/1745-6215-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, Lejuez CW. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. Journal of Consulting and Clinical Psychology. 2010;78(1):55–61. doi: 10.1037/a0017939. http://dx.doi.org/10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanda UL, Welschen L, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database of Systematic Reviews. 2012;(1) doi: 10.1002/14651858.CD005060.pub3. http://dx.doi.org/10.1002/14651858.CD005060.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga MJ, Reisner SL, Pantalone DW, O'cleirigh C, Mayer KH, Safren SA. A pilot trial of integrated behavioral activation and sexual risk reduction counseling for HIV-uninfected men who have sex with men abusing crystal methamphetamine. AIDS Patient Care and STDs. 2012;26(11):681–693. doi: 10.1089/apc.2012.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30(2):203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- Paddock LE, Veloski J, Chatterton ML, Gevirtz FO, Nash DB. Development and validation of a questionnaire to evaluate patient satisfaction with diabetes disease management. Diabetes Care. 2000;23(7):951–956. doi: 10.2337/diacare.23.7.951. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=2000054856&site=eds-live&scope=site. [DOI] [PubMed] [Google Scholar]
- Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Willett WC, Hu FB. Increased mortality risk in women with depression and diabetes mellitus. Archives of General Psychiatry. 2011;68(1):42–50. doi: 10.1001/archgenpsychiatry.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada MO, Rivera MJ. Psychometric properties of a scale designed to measure perceived social support in Chilean patients with type 2 diabetes. Universitas Psychologica. 2011;10(1):189–196. [Google Scholar]
- Park H, Nguyen T, Park H. Validation of multidimensional scale of perceived social support in middle-aged Korean women with diabetes. Asia Pacific Journal Of Social Work & Development. 2012;22(3):202. http://dx.doi.org/10.1080/02185385.2012.691719. [Google Scholar]
- Piette JD, Richardson C, Himle J, Duffy S, Torres T, Vogel M, Valenstein M. A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Medical Care. 2011;49(7):641–648. doi: 10.1097/MLR.0b013e318215d0c9. http://dx.doi.org/10.1097/MLR.0b013e318215d0c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilu A, Sorba M, Hardoy MC, Floris AL, Mannu F, Seruis ML, Carta MG. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: Preliminary results. Clinical Practice and Epidemiology in Mental Health. 2007;3 doi: 10.1186/1745-0179-3-8. http://dx.doi.org/10.1186/1745-0179-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikoff R, Brez S, Brunet S. Are exercise social-cognitive factors and behaviours different for adults with diabetes? A randomized community sample. Psychology, Health & Medicine. 2003;8(4):465–471. doi: 10.1080/1354850310001604577. [DOI] [PubMed] [Google Scholar]
- Resnick B, Jenkins LS. Testing the reliability and validity of the self-efficacy for exercise scale. Nursing Research. 2000;49(3):154–159. doi: 10.1097/00006199-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Reynolds WM, Kobak KA. Reliability and validity of the Hamilton Depression Inventory: A paper-and-pencil version of the Hamilton Depression Rating Scale clinical interview. Psychological Assessment. 1995;7:472–483. [Google Scholar]
- Reynolds EK, MacPherson L, Tull MT, Baruch DE, Lejuez CW. Integration of the brief behavioral activation treatment for depression (BATD) into a college orientation program: Depression and alcohol outcomes. Journal of Counseling Psychology. 2011;58(4):555–564. doi: 10.1037/a0024634. http://dx.doi.org/10.1037/a0024634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roupa Z, Koulouri A, Sotiropoulou P, Makrinika E, Marneras X, Lahana I, Gourni M. Anxiety and depression in patients with type 2 diabetes mellitus, depending on sex and body mass index. Health Science Journal. 2009;3(1):32–40. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=2010238524&site=eds-live&scope=site. [Google Scholar]
- Rovner BW, Casten RJ, Hegel MT, Massof RW, Leiby BE, Ho AC, Tasman WS. Low vision depression prevention trial in age-related macular degeneration: A randomized clinical trial. Ophthalmology. 2014;121(11):2204–2211. doi: 10.1016/j.ophtha.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P. Update to random allocation of treatments to blocks. Stata Technical Bulletin. 2000;9(50):36–37. [Google Scholar]
- Sacco WP, Bykowski CA, Mayhew LL. Pain and functional impairment as mediators of the link between medical symptoms and depression in type 2 diabetes. International Journal of Behavioral Medicine. 2013;20(1):22–29. doi: 10.1007/s12529-011-9210-5. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Preventive Medicine. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. http://dx.doi.org/10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- Schneider KL, Pagoto SL, Handschin B, Panza E, Bakke S, Liu Q, Ma Y. Design and methods for a pilot randomized clinical trial involving exercise and behavioral activation to treat comorbid type 2 diabetes and major depressive disorder. Mental Health and Physical Activity. 2011;4:13–21. doi: 10.1016/j.mhpa.2011.04.001. http://dx.doi.org/10.1016/j.mhpa.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenkovich K, Brown M, Svrakic D, Lustman P. Depression in type 2 diabetes mellitus: Prevalence, impact, and treatment. Drugs. 2015;75(6):577–587. doi: 10.1007/s40265-015-0347-4. http://dx.doi.org/10.1007/s40265-015-0347-4. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, Kenny GP. New evidence for the value of supervised exercise training in type 2 diabetes mellitus. Archives of Internal Medicine. 2010;170(20):1790–1791. doi: 10.1001/archinternmed.2010.376. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9) Arthritis care & research. 2011;63(S11):S454–S466. doi: 10.1002/acr.20556. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Gibbon M, Skodol AE, Williams JBW, First MB. DSM-IV-TR casebook: A learning companion to the Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, D.C.: 2002. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2002-00943-000&site=eds-live&scope=site. [Google Scholar]
- Stanley MA, Beck JG, Zebb BJ. Psychometric properties of the MSPSS in older adults. Aging & Mental Health. 1998;2(3):186–193. http://dx.doi.org/10.1080/13607869856669. [Google Scholar]
- Steinhardt MA, Dishman RK. Reliability and validity of expected outcomes and barriers for habitual physical activity. Journal of Occupational Medicine. 1989;31:536–546. doi: 10.1097/00043764-198906000-00011. [DOI] [PubMed] [Google Scholar]
- Thomas D, Elliott E, Naughton G. Exercise for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2006;3(3) doi: 10.1002/14651858.CD002968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Reading J, Shephard R. Revision of the physical activity readiness questionnaire (PAR-Q) Canadian Journal of Sport Sciences. 1992;17(4):338–345. [PubMed] [Google Scholar]
- Trajković G, Starčević V, Latas M, Leštarević M, Ille T, Bukumirić Z, Marinković J. Reliability of the Hamilton Rating Scale for Depression: A meta-analysis over a period of 49 years. Psychiatry Research. 2011;189(1):1–9. doi: 10.1016/j.psychres.2010.12.007. http://dx.doi.org/10.1016/j.psychres.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Vickers KS, Nies MA, Patten CA, Dierkhising R, Smith SA. Patients with diabetes and depression may need additional support for exercise. American Journal of Health Behavior. 2006;30(4):353–362. doi: 10.5555/ajhb.2006.30.4.353. [DOI] [PubMed] [Google Scholar]
- Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. Journal of Personality Assessment. 1990;55(3):610–617. doi: 10.1080/00223891.1990.9674095. Retrieved from http://ezproxy.rosalindfranklin.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=6392221&site=eds-live&scope=site. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Revista Brasileira De Psiquiatria. 2013;35(4):416–431. doi: 10.1590/1516-4446-2012-1048. http://dx.doi.org/10.1590/1516-4446-2012-1048. [DOI] [PubMed] [Google Scholar]
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. Journal Of Personality Assessment. 1988;52(1):30–41. doi: 10.1080/00223891.1990.9674095. http://dx.doi.org/10.1207/s15327752jpa5201_2. [DOI] [PubMed] [Google Scholar]