Abstract
Metabolomics, the characterization of the set of small molecules in a biological system, is advancing research in multiple areas of islet biology. Measuring a breadth of metabolites simultaneously provides a broad perspective on metabolic changes as the islets respond dynamically to metabolic fuels, hormones, or environmental stressors. As a result, metabolomics has the potential to provide new mechanistic insights into islet physiology and pathophysiology. Here we summarize advances in our understanding of islet physiology and the etiologies of type-1 and type-2 diabetes gained from metabolomics studies.
Keywords: Metabolomics, Lipidomics, Metabolic flux, Metabolism, Diabetes, Islet
1. Introduction
Type 2 diabetes (T2D) mellitus is the most common metabolic disease worldwide, affecting 21 million people in the US alone, and both its incidence and prevalence are on the rise globally. Type 1 diabetes (T1D) is less common, with approximately 3 million patients afflicted in the US [1]. Both T1D and T2D are diseases of insulin deficiency, but with different etiologies. T2D is often associated with excess caloric intake and obesity, which contribute to development of peripheral insulin resistance. Over time, pancreatic β-cells fail to compensate for the increased insulin demand due to loss of key β-cell functions such as glucose-stimulated insulin secretion (GSIS), in concert with a gradual depletion of β-cell mass [2]. In contrast, T1D, which often manifests during childhood, is a result of selective autoimmune destruction of the pancreatic β-cells, leading to insulin deficiency [3]. The mechanisms linking glucose metabolism and insulin secretion in the β-cell are still incompletely understood, as are the mechanisms of β-cell dysfunction and death.
A clearer understanding of β-cell function is essential for ultimate success in creation of surrogate cells for insulin replacement therapy in T1D and for developing better drugs for treatment of T2D. Metabolomics represents an emergent tool for understanding of β-cell biology, and offers a systems level view of metabolism as the cells respond dynamically to glucose stimulation in the healthy state, as well as global changes that occur as β-cells become dysfunctional in the diseased state. Since pancreatic islets are intrinsically difficult and expensive to isolate, and are typically available in limited quantities, pancreatic β-cell lines have been developed as alternative systems for study (reviewed in [4]). The most utilized cell lines for metabolomics studies are the Min6, 832/13 and INS-1E cell lines. Recent studies have compared unbiased GC–MS metabolic profiles from 832/13 cells and primary rat islets [5] as well as from a recently developed human cell line EndoC-βH1 and human islets [6] with the finding that the profiles were quite similar [5,6]. These studies suggest that while not perfect, β-cell lines can serve as reasonable surrogate systems for studies of biochemical features of primary pancreatic β-cells.
A thorough discussion of metabolomics applied to diabetes in general [7,8] or of all current mechanistic models of islet function [9] and dysfunction [2,10] is beyond the scope of this review, and we therefore refer the reader to excellent work elsewhere. Instead, we choose to focus the current review on new insights in islet biology derived from application of static and dynamic metabolic profiling methods, and the implications of this new information for translational applications.
2. General principles and methods
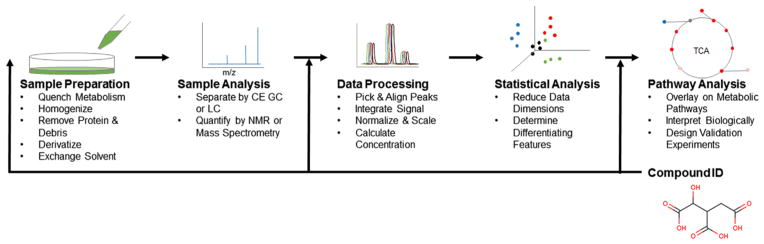
Metabolomics refers to the measurement of the set of small molecules in a biological system, referred to as the metabolome. The primary value of metabolomics is that it measures analytes that integrate gene expression and environmental regulation on a short time scale to identify emergent properties of a system. The global perspective provided by such analyses can be used to generate and test hypotheses. The variety of chemistries represented by the metabolome provides unique processing and analytical challenges. A generalized typical workflow is illustrated in Fig. 1. These methods typically, but not always, involve a cell lysis and metabolite extraction step to quench metabolism and solubilize the metabolome. The extract is then usually purified and/or derivatized, and the solvent is exchanged to improve compatibility with analytical techniques. Methods may then include optional fractionation steps including capillary electrophoresis, gas or liquid chromatography prior to detection of the analytes by mass spectrometry or nuclear magnetic resonance [11,12]. The resulting data is then processed as needed for the particular technique, and data features are selected, aligned, and scaled. Multivariate statistical tools are often applied in these studies to reduce data dimensionality, identify the metabolites mainly responsible for the differences between experimental groups, and to build classification models. Compound identification may happen during experimental design when choosing instrument settings (targeted analysis), after data processing by searching the data for a known set of compounds (targeted data analysis), or after statistical analysis by matching data of interest to metabolite databases (untargeted analysis). Once identified, compounds can be mapped to known metabolic pathways to aid hypothesis generation and biological interpretation [13–15]. Of the 35 studies referenced in this review, 24 used a mass spectrometry method, 5 used an NMR method, and 6 used a combination of both. Metabolomics methods developed specifically for use in β-cell lines include an extraction protocol determined by statistical design prior to GC–MS analysis [16], and an extraction procedure developed for adherent cells compatible with LC–MS [17]. Cell numbers required for each analysis vary by method. For the studies included here, a typical GC–MS analysis uses 1.2–3.4 million cells [5,18], LC–MS 3.4–35 million [19,20], NMR 1.5–180 million [21–23], and lipidomics 9–94 million cells [24–26]. For context, 300–400 medium-to-large sized islets can be isolated from a single rat, with each islet comprised of 1000–1500 cells, yielding around 400,000 cells per animal. Some of the following studies include data collected from 200 to 500 primary islets [5,27], but this is the exception and not the rule. Further advances in instrument sensitivity and analyte detection are needed to fully maximize the potential of metabolomics methods applied to islet biology. Two emergent technologies that have been reported to this end: a 2D capillary LC–MS strategy to improve metabolite coverage and facilitate analysis of small tissue samples [28], and direct MADLI-TOF analysis of single islets [29].
Fig. 1.
A generic metabolomics workflow. Samples are harvested and minimally processed before NMR or MS analysis. Features are selected from the data set, aligned, integrated and scaled before statistical analysis and compound identification. Identified compounds may then be mapped to biological pathways for interpretation. Each step may vary depending on the experimental design and analytical technique.
3. Healthy islet function
The main function of pancreatic islet β-cells is to synthesize and secrete insulin at appropriate rates to control blood glucose within a narrow range. It has been appreciated for many years that the effect of glucose to stimulate insulin secretion is not mediated by a cell surface glucose receptor but rather requires metabolism of the sugar to generate coupling factors that trigger insulin exocytosis [30–32]. The contributions of glycogen synthesis and the pentose phosphate pathway (PPP) to total glucose utilization are thought to be low in β-cells, and >90% of glucose molecules entering β-cells are estimated to engage in glycolysis and subsequent mitochondrial metabolism [33,34]. In agreement with this, unbiased metabolomics analysis of 832/13 cells has demonstrated a significant glucose-induced increment of all glycolytic intermediates measured (glucose-6-phosphate, fructose 1,6-bisphosphate, dihydroxyactone phosphate, 2/3-phosphoglycerate, phosphoenolpyruvate and pyruvate) [18,20]. Furthermore, targeted as well as unbiased metabolomics analyses have revealed a robust increase in TCA intermediates ((iso)citrate, aconitate, α-ketoglutarate, succinate, fumarate and malate) in response to 45–120 min of glucose stimulation [18–20,35,36]. Glucose-mediated changes in metabolite levels reported throughout Section 3 are summarized in Table 1.
Table 1.
Changes in metabolite concentrations in β-cell lines after glucose stimulation. The list is not intended to be inclusive, but rather highlight metabolites in major pathways regulated by GSIS.
| Pathway | First-phase | Second-phase | Metabolite | Refs. |
|---|---|---|---|---|
| Glycolysis | ||||
| Up | Up | Glucose-6-phosphate | [5,18,48,60] | |
| Up | Up | Fructose phosphate | [5] | |
| Up | Up | Fructose bisphosphate | [20,60] | |
| Up | Up | Dihydroxyacetone phosphate | [18,19,47,48] | |
| Up | Up | Phosphoglycerate | [5,18,20,47,60] | |
| Up | Up | Phosphoenolpyruvate | [5,20,47] | |
| Up | Up | Pyruvate | [5,18,19,47,48] | |
| Up | Lactate | [47,48] | ||
| TCA cycle | ||||
| Up | Acetyl-CoA | [20,60] | ||
| Up | Up | (iso)Citrate | [5,18–20,47,48,60] | |
| Up | Aconitate | [18,47] | ||
| Up | Up | α-Ketoglutarate | [5,18–20,47,48,60] | |
| Up | Succinyl-CoA | [19,20] | ||
| Up | Up | Succinate | [18,20,47,48] | |
| Up | Up | Fumarate | [5,18,19,47,48] | |
| Up | Up | Malate | [5,18–20,47,48,60] | |
| PPP | ||||
| Up | Up | 6-Phosphogluconolactone | [48] | |
| Up | Up | 6-Phosphogluconate | [18,20,60] | |
| Up | Up | Ribose-5-phosphate | [5,18,20,48,60] | |
| Up | Up | Sedoheptulose phosphate | [20,60] | |
| Up | Up | PRPP | [20] | |
| Amino acids | ||||
| Up | Up | Alanine | [5,18,47,48] | |
| Down | Down | Asparagine | [20,60] | |
| Down | Down | Aspartate | [5,18–20,47,48,60] | |
| Up | Up | Glutamate | [5,19,47] | |
| Inconsistent | Glutamine | [19,20] | ||
| Down | Down | Glycine | [48] | |
| Inconsistent | Hydroxyproline | [47,102] | ||
| Inconsistent | (iso)Leucine | [18,47] | ||
| Inconsistent | Lysine | [18,20] | ||
| Down | Down | Proline | [48] | |
| Up | Sarcosine | [48] | ||
| Inconsistent | Serine | [18,20] | ||
| Lipids | ||||
| Up | Up | Glycerol-3-phosphate | [5,19,20,47,60] | |
| Up | Capric acid (C10:0) | [48] | ||
| Up | Medium-chain fatty acids | [18,48] | ||
| Up | Up | Long-chain fatty acid | [20] | |
| Up | Up | Malonyl-CoA | [19,20,60] | |
| Down | Down | HMG-CoA | [19,20,60] | |
| Down | Long-chain acyl-CoA | [20] | ||
| Up | Up | Farnesyl pyrophosphate | [20] | |
| Nucleotides | ||||
| Down | Down | CDP | [20,60] | |
| Down | Down | CMP | [20] | |
| Down | Down | UDP | [20] | |
| Down | Down | UMP | [20] | |
| Up | Glycinamideribotide | [20,60] | ||
| Up | Up | ZMP | [20,60] | |
| Inconsistent | ATP | [19,20] | ||
| Down | Down | ADP | [20,60] | |
| Down | Down | AMP | [19,20] | |
| Up | GTP | [19] | ||
| Down | Down | GDP | [20,60] | |
| Down | Down | GMP | [20] | |
| Up | Up | GDP-mannose | [20] | |
| Up | Up | NADH | [19,20,60] | |
| Down | Down | NADP | [20] | |
| Up | Up | NADPH | [19] | |
| Other | Up | Up | Phosphocreatine | [20,60] |
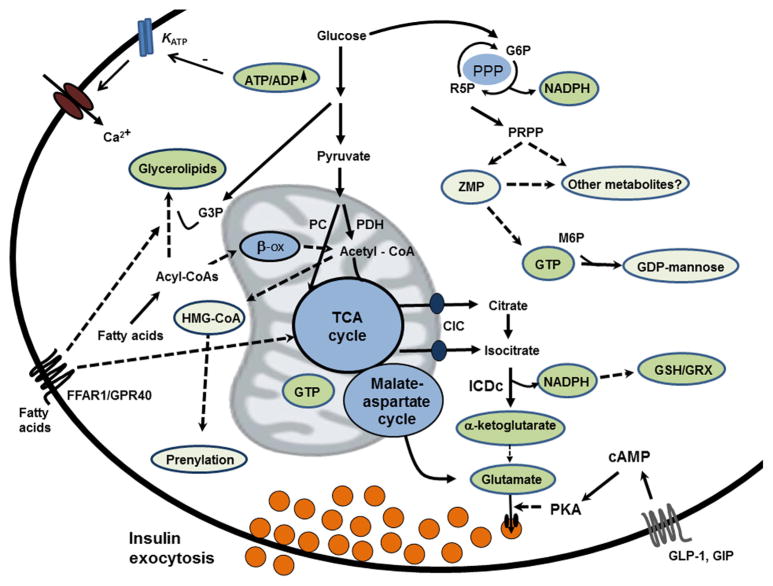
Insulin secretion from primary β-cells is biphasic. The first phase starts within minutes of glucose stimulation, peaks around 10 min, and is followed by a rapid decline of insulin output. The second and quantitatively more important phase of insulin secretion is characterized by a gradually increasing rate of release until a plateau is reached after an additional 25–30 min [32]. One particular model of glucose stimulus/secretion coupling has gained wide acceptance. In this model, glycolytic and mitochondrial metabolism of glucose lead to increases in cytosolic ATP:ADP ratio, resulting in closure of ATP-sensitive K+ (KATP) channels, plasma membrane depolarization, and activation of voltage-dependent Ca2+ channels. The resulting increase in cellular calcium promotes exocytosis of insulin-containing granules [37]. However, significant GSIS also occurs under conditions where closure of KATP channels is prevented, demonstrating an important role for KATP channel-independent pathways for fuel-regulated insulin granule exocytosis [38,39]. The general consensus is that the KATP channel-dependent pathway is particularly important in first phase insulin secretion, and although ATP may participate in the second phase of insulin secretion, it is evident that other factors are involved as well. Suggested stimulus/secretion coupling factors for the second phase include NADPH [40,41], GTP [42,43], glycerolipid/free fatty acid cycle and monoacylglycerol [44,45] and glutamate [46] (Fig. 2).
Fig. 2.
Mechanisms of glucose stimulated insulin secretion (GSIS) and suggested stimulus/secretion coupling factors. The canonical model of GSIS holds that an increase in β-cell glycolytic and mitochondrial metabolism of glucose lead to increases in cytosolic ATP:ADP ratio, resulting in closure of ATP-sensitive K+ (KATP) channels, plasma membrane depolarization, activation of voltage-dependent Ca2+ channels and Ca2+-mediated activation of insulin granule exocytosis. β-cells have high capacity for anaplerotic influx of glucose-derived pyruvate into the TCA cycle via pyruvate carboxylase (PC), which in turn is paired with efflux of citrate and isocitrate via the citrate-isocitrate carrier (CIC), and cytosolic isocitrate dehydrogenase (ICDc)-mediated conversion of isocitrate into α-ketoglutarate under generation of NADPH, a suggested coupling factor for GSIS. NADPH is also generated to lesser degree in the pentose phosphate pathway (PPP), a revived pathway of interest for regulation of GSIS. Other suggested metabolic coupling factors include glutamate, GTP, protein prenylation and two new candidates, ZMP and GDP-mannose identified from metabolomics studies. Fatty acids potentiate GSIS via mechanisms that involve activation of the surface receptor FFAR/GPR40 as well as intracellular fatty acid metabolism, and fatty acid-mediated coupling factor candidates include glycerolipids such as monoacylglycerol. GSIS is also amplified by incretins secreted from the small intestine in response to a meal. A recent metabolomics study has identified a novel pathway involving cytosolic glutamate derived from glucose to a signal for incretin-induced insulin secretion (see text for details). G3P: glycerol-3-phosphate; G6P: glucose -6-phosphate; GRX: glutaredoxin; GSH: reduced glutathione; R5P: ribose-5-phosphate; M6P: mannose-6-phosphate.
3.1. Metabolite changes during the first phase of GSIS
Several labs have explored metabolic changes during the first phase of insulin secretion using metabolomics and have detected significant increases in glycolytic and TCA intermediates within 2–15 min of glucose stimulation in 832/13 cells. The degree and timing of induction varies slightly among the studies [20,47,48]. Furthermore, Lorenz et al. [20] observed a drop in AMP and ADP, resulting in a distinct increment in ATP/ADP ratio within the first 5 min of glucose stimulation which is in agreement with an important role of KATP channels in triggering the first phase of insulin secretion.
Using untargeted GC–MS in 832/13 cells, first phase insulin secretion was also found to be negatively associated with aspartate, lysine and proline and positively with ribose-5-phosphate, 6-phosphoglucono-1,5-lactone, gulonic acid γ-lactone, ribitol, sedoheptulose, N-acetylglucosamine, lactose, sorbitol, fructose, alanine, and 3-aminoisobutyrate [48]. In another GC–MS based study, first phase insulin secretion was found to be negatively correlated with aspartate, (iso)leucine, hydroxyproline, proline and valine, and positively correlated with ribose-5-phosphate, lactate, glycerol-3-phosphate, alanine, cysteine, glutamate, and glycine [47]. Lorenz et al. [20] found an inverse relationship between asparagine, glutamine, lysine, serine, long chain fatty acids, long-chain acyl-CoAs, HMG-CoA, NADP, mono- and diphosphonucleotides and first phase insulin secretion using unbiased LC–MS/MS in glucose-stimulated 832/13 cells. In contrast, this group also observed increases in farnesyl pyrophosphate, NADH, 6-phosphogluconate, sedoheptulose phosphate, phosphoribosyl pyrophosphate, ZMP, glycinamideribotide, and GDP-mannose.
Lorenz et al. employed [U-13C]glucose isotopologue analyses to assess flux through metabolic pathways in response to glucose [20]. In these studies, it was observed that the aspartate used for anaplerosis is not derived from exogenously added glucose. It was also demonstrated that rapid esterification of long chain acyl-CoA with de novo synthesized glycerol-3-phosphate occurs during first phase, explaining the rapid fall in long chain acyl-CoAs, and possibly contributing to enhanced closure of KATP channels [49]. Finally, a significant flux of glucose-derived carbon into HMG-CoA was observed. This was accompanied by a rapid depletion of the levels of this metabolite, with a parallel rise in farnesyl pyrophosphate, potentially providing substrate for prenylation of proteins. This observation is in agreement with earlier reports demonstrating that specific small G-proteins (Cdc42 and Rac1) that are essential for the transport and fusion of insulin-containing secretory granules with the plasma membrane and insulin exocytosis are regulated by prenylation [50]. The authors hypothesized that the rapid increase in farnesyl pyrophosphate allows prenylation to occur in a time frame relevant to first phase insulin secretion, and that the dramatic reduction in HMG-CoA may become limiting to flux through this pathway, thus participating in the fall in insulin secretion after the first phase peak. This interesting, but yet unproven idea, deserves further investigation.
3.2. Metabolite changes during second phase of GSIS
As mentioned above, the second phase of insulin secretion correlates with elevated pools of glycolytic and TCA intermediates. Furthermore, the changes in nucleotide levels, PPP intermediates and long chain fatty acids observed in the first phase by unbiased LC–MS/MS are sustained in the second phase [20]. Also, PPP intermediates ribose-5-phophate and 6-phosphogluconate, and the amino acids alanine, (iso)leucine, lysine, serine, and medium and long chain fatty acids associate with the second phase, whereas aspartate, sarcosine, and 3-aminobutyric acid are down-regulated by glucose (unbiased GC–MS; [18,48]). In another study, targeted LC–MS/MS analyses on 832/13 cells demonstrated increases in ATP, GTP, NADH, NADPH, glutamine, glutamate, succinyl-CoA and malonyl-CoA pools after 45 min of glucose stimulation whereas adenosine, AMP, aspartate and HMG-CoA levels decreased [19].
Pancreatic β-cells have high capacity for anaplerotic influx of substrates into the TCA cycle, and it has been estimated that ~40–50% of pyruvate enters β-cell mitochondrial metabolism through pyruvate carboxylase (PC) at stimulatory glucose concentrations [33,34,51,52]. Using NMR-based lux analysis of variously glucose-responsive β-cell lines, including 832/13, our laboratory observed a strong positive correlation between insulin secretion and pyruvate carboxylase flux but no correlation with pyruvate dehydrogenase-catalyzed glucose oxidation [51]. The anaplerotic influx of carbon into the mitochondria is paired with efflux of certain TCA cycle intermediates, particularly malate, citrate and isocitrate [34,53,54]. Using an interdisciplinary approach involving 13C NMR-based metabolic flux analyses and targeted knockdown of specific organic acid transporters and metabolic enzymes, we have shown that the mitochondrial export of citrate and isocitrate and engagement of isocitrate with cytosolic, NADP-dependent isocitrate dehydrogenase (also known as IDH1, or hereafter, ICDc) play a central role in regulation of GSIS [41,55,56]. Furthermore, our group has recently demonstrated that NADPH generated by ICDc enhances exocytosis via signaling through reduced glutathione (GSH). Furthermore, intracellular provision of isocitrate, NADPH, or GSH rescues impaired exocytotic function in β-cells from donors with T2D. Thus, the isocitrate-NADPH-GSH pathway represents an important link between glucose metabolism and insulin exocytosis ([57], submitted for publication).
3.3. Fatty acids and incretins
Free fatty acids play an important role in regulating β-cell function under physiological conditions. Exposure to fatty acid is known to amplify GSIS, with maximal potentiation depending upon both fatty acid metabolism within the β-cell [58] and activation of the G-protein coupled receptor, FFAR1/GPR40 [59]. Extensive LC–MS/MS metabolite profiling and [U-13C]glucose flux analyses of 832/13 cells exposed to 0.5 mM palmitate showed a fatty acid-induced increase in long chain acyl-CoAs that were rapidly esterified with glucose-derived glycerol-3-phosphate to form lysophosphatidic acid, mono- and diacylglycerols, and other glycerolipids [60]. Exposure to fatty acids also caused a surprising increase in glycolytic flux, along with a reduction in the NADH/NAD ratio. Using pulse-chase experiments, the authors demonstrated that increased glycolytic flux was due to enhanced conversion of dihydroxyacetone phosphate to glycerol-3-phosphate (NADH dependent) driven by the fatty acid-mediated increase in glycerol-3-phosphate consumption. Furthermore, increased glucose flux into the TCA cycle was observed as well as increased oxygen consumption. Treatment of cells with an FFAR1/GPR40 antagonist decreased fatty acid-induced glycerolipid formation, attenuated fatty acid-mediated increases in glucose oxidation, and increased mitochondrial fatty acid flux, as evidenced by increased acylcarnitine levels. Conversely, FFAR1/GPR40 activation in the presence of low fatty acid concentrations increased flux into glycerolipids and enhanced glucose oxidation. While, the study convincingly demonstrates extensive remodeling of glucose and lipid metabolism in response to fatty acids, no clear mechanistic link between these changes and insulin granule exocytosis was established. Further studies will be needed to fully define this potential signaling mechanism.
Metabolomics has also provided insight into the mechanisms of incretin-mediated insulin release. Incretins such as GLP-1 and GIP are critical for preventing postprandial hyperglycemia by amplifying GSIS through cAMP signaling [61]. Evidence suggests cAMP enhances insulin secretion through protein kinase A (PKA) dependent and -independent signaling [62] but a complete mechanistic understanding remains elusive. Gheni and coworkers compared incretin-responsive and unresponsive Min6 cells. These cell lines display no differences in cAMP production in response to incretins, and the integrity of downstream signaling targets of cAMP was intact in both lines, indicating that the difference in incretin responsiveness is not due to disruption of the incretin/cAMP signaling pathways. Instead, since incretin-induced insulin secretion is glucose dependent, they hypothesized that the impaired incretin responsiveness results from compromised “metabolism-cAMP coupling.” To test this, the authors applied CE-MS analyses and observed decreased glucose 6-phosphate, fructose 6-phosphate, fructose 1,6-bisphosphate, NADH, glutamate, and aspartate levels in the incretin-unresponsive cell line, suggesting reduced malate-aspartate shuttle activity [63]. The involvement of the malate-apartate shuttle in incretin response was further indicated by use of an array of inhibitor and siRNA manipulations of key shuttle enzymes in incretin sensitive Min6 cells and primary mouse islets. MS analyses on β-cells exposed to [U-13C]-glucose demonstrated increased cytosolic glutamate and GLP-mediated transport of glutamate into insulin granules via cAMP/protein kinase A signaling. Finally, using siRNA techniques in incretin sensitive Min6 cells and islets from knockout animals, the authors demonstrated that vesicular glutamate transporter-1 is essential for incretin-mediated insulin secretion. These studies indicate that cytosolic glutamate derived from glucose through the malate-aspartate shuttle is a signaling factor in incretin-induced insulin secretion [63].
3.4. Healthy β-cells – lessons from metabolomics
Metabolomics has confirmed our current perception that glucose stimulation of β-cells increases glycolysis and TCA cycle flux. The increases in glycolytic and TCA metabolites happen early in first phase and are prolonged in second phase of insulin secretion, and these pathways and their derivative pathways are likely involved in generating stimulus/secretion coupling factors such as α-ketoglutarate and NADPH via ICDc [41] (Fig. 2). As discussed in Sections 3.1 and 3.2, simultaneous measurements of a whole array of metabolites have revealed several new and potentially interesting metabolites that associate with the first and/or second phase of insulin secretion. In most cases, however, only correlations have been reported and it remains to be tested if the observed changes in metabolite levels are directly involved in regulation of insulin secretion.
An interesting candidate is GDP-mannose which increases 14-fold over basal within 8 min of glucose stimulation [20]. Another candidate is ZMP which is an intermediate in purine synthesis pathway. ZMP levels increase 9-fold and reach a plateau at 25 min [20] (Fig. 2). Further studies are needed to elucidate if GDP-mannose, ZMP or their metabolites are involved in regulation of GSIS.
As highlighted earlier, metabolomics studies seem to demonstrate a global increase in intermediates of the PPP in response to glucose stimulation of β-cells, including 6-phosphogluconate, ribose-5-phosphate, sedoheptulose 7-phosphate, and phosphoribosyl pyrophosphate (PRPP) [18,20,47]. Since flux through the PPP is low relative to glycolytic flux in β-cells, it has been suggested that it contributes very little to overall NADPH production, as compared to other NADPH producing enzymes such as ICDc [53]. However, recent studies involving siRNA-mediated or pharmacologic suppression of the rate-limiting enzyme of the PPP, glucose-6-phosphate dehydrogenase (G6PDH), have reported a modest impairment of GSIS, although direct measurements of NADPH were not reported in these studies [47,64]. In contrast, no effect on GSIS was observed by another group when siRNA was used to suppress G6PDH, or 6-aminonicotinamide to inhibit the second step of the PPP pathway catalyzed by 6-phosphogluconate dehydrogenase (6PGDH) [18]. The reasons behind the conflicting results are not clear, and further studies are needed to clarify the role of the PPP in GSIS. One possibility is that the PPP may provide substrates for nucleotide biosynthesis and salvage pathways via ribose-5-phosphate and PRPP. Given the wide-ranging changes in nucleotides pools with glucose [20,42] and intriguing glucose-induced increases in intermediates such as ZMP, it is possible that the PPP plays a regulatory role in GSIS independent of NADPH. Changes in these metabolites were not monitored in any of the foregoing studies on manipulation of the PPP, and could explain the discrepant results between groups.
4. β-Cell dysfunction and T2D
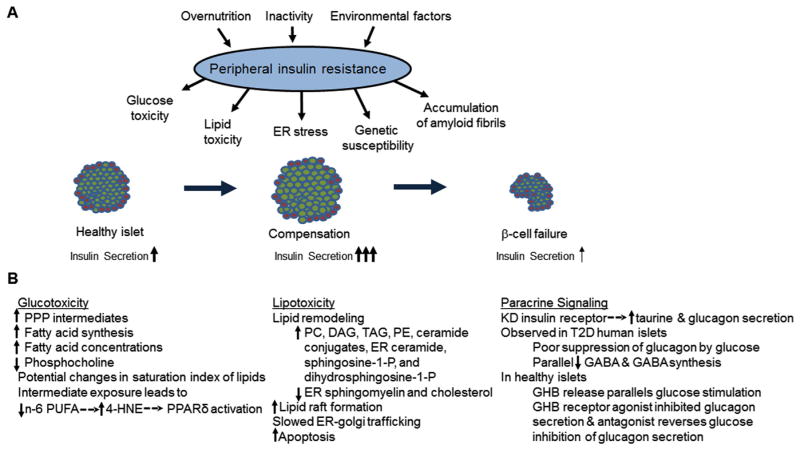
Obesity is strongly associated with peripheral insulin resistance, which induces compensatory increases in insulin secretion and β-cell mass. T2D is characterized by the failure of β-cell compensation in response to this stress and reductions in β-cell function and mass (Fig. 3A). The current mechanistic model of β-cell failure includes varying contributions by genetic susceptibility, ER stress due to insulin synthesis demands, the accumulation of amyloid fibrils in the cell membrane, and metabolic stress from glucose and fatty acid overload [2]. The majority of metabolomics literature in this area focuses on identifying metabolites that may mediate metabolic stress induced by overnutrition.
Fig. 3.
Metabolic disruption in models of T2D. Peripheral insulin resistance generates metabolic demand in the β-cell which compensates by increasing cell mass and insulin secretion. T2D is characterized by the failure of β-cell compensation in response to this stress and reductions in β-cell function and mass (A). Biological processes observed to change in metabolomics studies of glucotoxicity, lipotoxicity, or α-cell paracrine signaling dysfunction models of T2D are summarized in (B). 4-HNE: 4-hydroxynonenal; DAG: diacylglycerol; ER: endoplasmic reticulum; GABA: γ-aminobutyric acid; GHB: γ-hydroxybutyric acid; KD: knock-down; PC: glycerophosphocholines; PE: glycerophosphoethanolamine; PPP: pentose phosphate pathway; PUFA: polyunsaturated fatty acid; TAG: triacylglycerol; T2D: type-2 diabetes.
4.1. Glucotoxicity
Prolonged exposure to high blood glucose concentrations leads to blunted GSIS in response to normally stimulatory glucose levels, and β-cell apoptosis [65–67]. Chronic hyperglycemia causes changes in gene expression including reduced insulin gene expression and altered signaling pathways [68]. Multiple contributors to β-cell dysfunction have been proposed, including oxidative stress [69,70], ER stress [70], altered glucose metabolism [71] or impaired exocytosis mechanisms [72–75]. Several GC–MS-based metabolomics studies have been reported for insulinoma cell models of glucotoxicity, three of which were combined with 13C-tracer and NMR experiments [21,22,36,64,76]. Despite similarities in methods used, significant variation in metabolite coverage remains. Changes reported in the metabolome in models of T2D are summarized in Fig. 3B, and responses to prolonged exposure to high glucose media are summarized in Table 2.
Table 2.
Changes in metabolite concentrations in β-cell lines after exposure to glucotoxic conditions.
| Pathway | Metabolite | Refs. | |
|---|---|---|---|
| Glycolysis | |||
| Up | Glucose | [21,64] | |
| Up | Glucose-6P | [36,64] | |
| Up | Pyruvate | [36,64] | |
| Up | Lactate | [64] | |
| TCA | |||
| Up | Citrate | [36,64] | |
| Up | Isocitrate | [76] | |
| Up | α-Ketoglutarate | [64] | |
| Up | Succinate | [64] | |
| Up | Fumarate | [36,76] | |
| Up | Malate | [36,64,76] | |
| PPP | |||
| Up | Gluconic acid | [64] | |
| Up | Glucono-δ-lactone | [64] | |
| Up | 6-Phosphogluconic acid | [64] | |
| Up | Ribose 5-phosphate | [36,64] | |
| Amino acids | |||
| Down | Glutamine | [36] | |
| Down | Serine | [36] | |
| Down | Ornithine | [36,64] | |
| Up | Glycine | [21] | |
| Down | Aspartate | [21] | |
| Inconsistent | Alanine | [21,36] | |
| Up | Hydroxyproline | [36] | |
| Up | GABA | [21,64] | |
| Lipids | |||
| Down | Glycerophosphocholine phosphocholine | [22] | |
| Down | Phosphocholine | [21] | |
| Inconsistent | Creatine/creatinine | [21] | |
| Up | Myristic acid (C14:0) | [21] | |
| Down | Arachidonic acid (C20:4n6) | [21] | |
| Down | EPA (C20:5n3) | [21] | |
| Down | cis-8,11,14-eicosatrienoic acid (C20:3n6) | [21] | |
| Up | Total monounsaturated fatty acids | [21] | |
| Down | Total polyunsaturated fatty acids | [21] | |
| Up | Total fatty acids | [22] | |
| Other | |||
| Up | Carbamylaspartic acid | [64] | |
| Up | Sorbitol | [64] | |
| Up | NAD | [21] | |
Glycolysis and TCA cycle intermediates accumulate when cells are exposed to chronic hyperglycemia. However, the elevated concentrations of these intermediates are rapidly lowered in response to lowering of the extracellular glucose concentration. Chronic exposure of β-cells to high levels of pyruvate does not have the same toxic effects as hyperglycemia, suggesting that unique metabolic fates of glucose rather than “bioenergetic overload” induced by provision of a bolus of mitochondrial substrate is driving glucotoxicity [76]. Similar to glucotoxicity, inhibition of 6PGDH inhibits insulin gene expression, GSIS and causes PPP intermediates to accumulate. Both glucotoxic conditions and 6PGDH inhibition have a similar effect to activate ERK1/2, and an ERK inhibitor attenuates glucose-induced PPP intermediate accumulation and GSIS inhibition, supporting the hypothesis that metabolites derived from the PPP activate ERK1/2 and contribute to the decline in β-cell function [64]. Finally, chronic exposure to hyperglycemia caused glutamine concentrations and C4-glutamate labeling from U-13C-glucose to decrease, while GABA concentrations increased. These changes may indicate changes in flux through the GABA shunt pathway and glutamate dehydrogenase which have both been implicated in β-cell function [21]. While this study did not investigate the mechanism of these changes, the results partly align with another report that employed the GABA transaminase inhibitor gabaculine and succinic semialdehyde supplementation of the GABA shunt to link GABA metabolism to GSIS in rat islets [77].
Glucotoxic conditions increase expression of lipogenic genes, activate fatty acid synthesis (eg. increased palmitate labeling in the presence of [U-13C]glucose) and elevate total fatty acid concentrations [22]. A parallel decrease in phosphocholine concentrations has also been observed [21,22]. One study reported a glucose-induced reduction in percent composition of low abundance polyunsaturated fatty acids (PUFAs) [21], whereas another study found no change in percent composition for high-abundance fatty acids over a variety of chain lengths and saturation levels [22]. As a result, the extent to which glucotoxicity affects membrane phospholipid composition and functional properties of islet cell membranes remains to be determined.
Lipidomics analysis of INS-1E cells pre-exposed to intermediate periods of hyperglycemia (16 h at 16 mM glucose), demonstrated decreased PUFA content, including arachidonic acid and linoleic acid, higher monounsaturated fatty acid content and higher concentrations of 4-hydroxy-2E-nonenal (4-HNE) a product of arachidonic acid oxidation via a nonenzymatic route [25]. Relative abundance of n-3 PUFAs increased while n-6 PUFAs decreased. These changes occurred in parallel with a compensatory increase in insulin secretion, increased phosphorylation and activation of calcium-dependent phospholipase A2 (cPLA2), and accumulation of reactive oxygen species. Supplementation of 4-HNE activated PPARδ and GSIS in a dose dependent manner, and these effects were inhibited by the antioxidant N-acetylcysteine and PPARδ antagonist GSK0660. PPARδ activity potentiated by 4-HNE may therefore have an amplifying effect on insulin secretion during β-cell adaptation to intermediate hyperglycemic exposure.
4.2. Lipotoxicity
Prolonged exposure to fatty acids in the presence of intermediate glucose can also impair β-cell function [78]. A lipidomic profile of Min6 cells treated with 0.4 mM palmitate in the presence of 6 mM glucose for 48 h found increases in total phosphatidylcholine, di-, and triacylglycerol content with enhanced incorporation of palmitate, but no significant changes in total cholesterol esters or cholesterol saturation index. Phosphatidylethanolamine pools were 50% higher in response to palmitate. In the sphingolipid class, pools of glucosylceramide, lactosylceramide, and trihexosylceramide increased with palmitate, while ceramide and sphingomyelin pools were unchanged. Unsaturated and long chain glucosylceramide variants also accumulated with palmitate treatment [79]. A more detailed analysis of lipid species in the subcellular fractions of the same Min6 model identified a significant rise in the ceramide pools in the ER and lysosome fractions, an increase in the glycosylceramide pools of the ER and plasma membrane, and a decrease in sphingomyelin and free cholesterol content in the ER [24]. An independent laboratory found sphingosine-1-phosphate and dihydrosphingosine-1-phosphate accumulated upon chronic palmitate exposure in INS-1 cells [26] (Fig. 3B).
Palmitate-induced changes in the lipid profile were not replicated by thapsigargin or tunicamycin, agents that trigger ER stress, indicating that these changes are not the result of ER stress-induced lipid remodeling. The functional implications of changes in ER lipid profile were also investigated. Sphingomyelin and cholesterol are co-regulated by palmitate and colocalize in lipid rafts. Palmitate promoted ER lipid raft formation in Min6 cells and islets [24], slowed ER-to-Golgi protein trafficking [24,26], and enhanced apoptosis [24].
Specific enzymatic machinery was interrogated for a direct role in lipid profile changes and inhibited ER and β-cell function. Malonyl-CoA decarboxylase overexpression in lipid-exposed insulinoma cells or islets from Zucker diabetic fatty (ZDF rats) prevents triacylglycerol accumulation, but does not reverse impaired GSIS in these models. The mechanism of GSIS inhibition is therefore more complex than “overstorage” of triacylglycerols [23]. Sphingolipid metabolism may play a more direct role. Inhibition of serine palmitoyltransferase 1, the enzyme that catalyzes the first step of ceramide synthesis, inhibits induction of CHOP and activation of caspase 3 in response to palmitate, implicating ceramide synthesis in lipotoxic ER stress and apoptosis [79]. Conversely, glucosylceramide synthase overexpression, which increases the conversion of ceramide to glucosylceramide, protected Min6 cells from ER stress and apoptosis. However, glucosylceramide accumulation was not observed in primary mouse islets when exposed to lipotoxic conditions as reported for Min6 cells, casting some doubt on the importance of this pathway in development of islet dysfunction [24]. Expression of sphingosine kinase 1 (SphK1), an ER localized enzyme, is induced by palmitate, and SphK1 overexpression potentiates dihydrosphingosine-1-phosphate accumulation while attenuating accumulation of multiple ceramides. Pharmacological inhibition of SphK1 increases caspase 3/7 activation in response to palmitate exposure. SphK1 overexpression also partly protects against the palmitate induced ER-to-Golgi trafficking defect and apoptosis [26]. Sphingomyelin synthase 1 (SMS1) overexpression attenuated palmitate-induced apoptosis, while sphingomyelinase Smpd4 potentiated it [24]. These findings, among others, point to palmitate-induced changes in sphingolipid metabolism that contribute to defects in ER-to-Golgi protein trafficking, and support the hypothesis that protein overload in the ER contributes to induction of ER stress and apoptosis under lipotoxic conditions.
The ob/ob mouse, a model of metabolic syndrome and obesity, was cross-bred with a PPARγ2−/− mouse to produce the POKO mouse. POKO mice are less obese than ob/ob mice, but become more hyperglycemic and hyperinsulinaemic than the ob/ob mice at 4 weeks of age [80]. In contrast, mice with islet specific knockout of PPARγ did not become hyperglycemic [81], indicating that the POKO mouse phenotype is not due to loss of PPARγ in islets. Lipidomic analysis of islets isolated from POKO and PPARγ2 KO mice at 4 weeks of age showed no dysregulation of lipid content [82], whereas at 16 weeks diacylglycerides and short chain tri-acylglycerides were decreased in the knockout strains compared to wild-type. This was associated with decreased levels of ethanol-amine plasmalogen (36:2), and increased levels of phosphatidylethanolamine (36:2) and ceramides (20:0 and 22:0) in islets of POKO mice compared to PPARγ2 knockouts -and wild type mice [80]. These findings implicate similar lipids in development of β-cell dysfunction in living mice as in the in vitro models of lipotoxicity described above.
4.3. Transcription factor ARNT/HIF-1β
Gene expression profiling of diabetic human islets revealed down regulation of transcription factor ARNT/HIF-1β and its targets, and siRNA knockdown of ARNT/1β expression in Min6 cells negatively affected β-cell function [83]. Metabolomic analysis of 832/13 cells treated with siRNA duplex targeting ARNT/HIF-1β demonstrated impaired glucose metabolism [84]. This study represents an interesting workflow from an observation in humans to analysis of its consequences in a model system by metabolomics. Further work will be necessary to determine if ARNT/HIF-1β regulates GSIS in a physiological context.
4.4. Paracrine signaling and islet α-cells
The glucagon secreting islet α-cell is also involved in the pathogenesis of diabetes since glucagon raises blood glucose levels and is suppressed by insulin [85–87]. Abnormal glucagon secretion may contribute to poor glucose control in diabetic patients [88–90]. An α-cell model of deficient insulin sensing, IRKD, was produced by lentiviral knockdown of the insulin receptor in the αTCl-6 cell line, and the metabolome was analyzed by CE-TOF–MS after glucose stimulation [91]. PCA and cluster analysis demonstrated global differences in metabolism. Specifically, CDP-choline, choline, taurine, and hypotaurine levels were increased at all glucose concentrations in the IRKD cells compared to controls. This de-repression of taurine concentrations by insulin was correlated with higher rates of taurine uptake, and enhanced expression of cysteine sulfinic acid decarboxylase which produces hypotaurine. IRKD cells secreted abnormally high levels of glucagon in response to glucose stimulation and this effect was potentiated by the provision of taurine. The significance of these findings for regulation of glucagon secretion in vivo is yet to be determined.
Paracrine signaling between α-cells and β-cells was also investigated directly in healthy and diabetic human islets [27]. Of the six diabetic donors, three had low basal secretion of both insulin and glucagon, and glucagon secretion failed to be suppressed by glucose (T2D-αNGR). These islets were analyzed by GC–MS for amino acid content and 13C enrichment after stimulation with [U-13C]glucose. Normal islets displayed a decrease in aspartate and GABA pools and increased 13C enrichment in alanine, aspartate, GABA and glutamate in response to glucose stimulation. The T2D-αNGR islets accumulated alanine, glutamate, glutamine, glycine and serine while GABA levels dropped 80% at basal and stimulatory glucose compared to normal islets. GABA 13C enrichment, GABA/glutamate, and glutamate decarboxylase expression were also significantly lower in the T2D-αNGR islets. The GABA shunt was therefore severely impaired in this group and may be mediating glucagon suppression by glucose. Further studies found the γ-hydroxybutyrate (GHB) loop enzymes were expressed in normal islets, and that both GHB content and release increased with glucose stimulation. The GHB mimicking agonist 3-CPA inhibited amino acid-stimulated glucagon secretion, whereas the GHB receptor antagonist reversed glucose inhibition of glucagon secretion. Hence, survey of a relatively small metabolite panel was used to uncover a glucose-derived metabolite, GHB, that appears capable of paracrine regulation of glucagon secretion.
Islets may also receive paracrine signals from peripancreatic adipose tissue [92]. Metabolomics was employed to study differences in the secretomes of peripancreatic and epididymal adipose tissue and their effects on gene expression in islets from a model of diet-induced obesity [93]. This complex design offers an example of future application of metabolomics to systems biology circuits.
4.5. Evaluation of T2D drug targets
One strong impetus for applying metabolomics to islet biology is the potential for identification of novel drug targets. Acetyl-CoA carboxylase (ACC) was a proposed target for the development of new diabetes treatments because its inhibition in peripheral tissues has beneficial effects such as increasing insulin sensitivity [94]. However, Ronnebaum and coworkers found that chronic ACC1 suppression via siRNA or administration of the inhibitor 5-(tetra-decyloxy)-2-furoic acid (TOFA) impairs GSIS. siACC1 also impaired glucose oxidation and glucokinase expression, and caused a reduction in levels of pyruvate, α-ketoglutarate, and malate and acetylcarnitine in 832/13 cells [95]. Metabolic profiling therefore supported the conclusion that ACC inhibition is likely to inhibit glucose rather than lipid metabolism, and diminish β-cell function in the long term.
Metabolomics was also used to evaluate FFAR1/GPR40 antagonists and agonists including phase II candidate fasiglifam (TAK-875), as discussed in Section 3.3 [60].
5. β-Cell death and T1D
Type 1 diabetes is a chronic autoimmune disease in which the immune system selectively destroys islet β-cells, leading to insulin deficiency. Evidence suggests both genetic susceptibility and an environmental trigger are required for progression to type 1 diabetes [3]. A metabolomics experiment could detect effects due to either of these factors.
5.1. Progression to T1D
A prospective clinical study in children at higher genetic risk of developing T1D identified metabolic perturbations that precede the development of autoantibodies [96]. Efforts to validate these findings in a model system such as the non-obese diabetic (NOD) mouse model of T1D are needed to demonstrate the disease specificity of the metabolic phenotype, and to provide a model for understanding the significance of predictive metabolic profiles at a mechanistic level. In this vein, a recent study in NOD mice found plasma lipid metabolite phenotypes similar to those observed during progression to T1D in children [97]. After validation of the model, an independent study stratified 8- and 19-wk old NOD mice into low and high risk groups by lipid profile and the presence of autoantibodies. Metabolite analysis by GC × GC–TOF in isolated islets from each group found increased concentrations of glucose-6-phosphate, glutamate, aspartate, valine, leucine, and isoleucine and other metabolites in the high risk group that correlated with enhanced insulin secretion in response to glucose and upregulated expression of genes in the TCA cycle and glycolysis/gluconeogenesis independent of the presence of insulin autoantibodies. There are therefore islet specific metabolic disturbances in NOD mice that are progressing to diabetes, prior to the onset of disease [97]. How this might relate to susceptibility of islets to autoimmune damage remains to be investigated.
5.2. Cytokine toxicity
The toxic effects of cytokines on the β-cell are well known [98], but some debate remains surrounding the mechanism of cell death: apoptosis, necrosis or autophagy. In order to find ways to protect β-cells from destruction, it is necessary to understand the mechanism of β-cell death. An LC–MS/MS metabolomics strategy was used to compare the metabolic phenotype of 832/13 cells treated with pro-inflammatory cytokines IL-1β and γ-IFN or the apoptosis-inducing agent camptothecin. The two treatments induced distinct global metabolic responses. For example, citrulline, a product of the iNOS reaction, accumulates upon IL-1β and γ-IFN treatment but not after camptothecin treatment. This data supports the idea that necrosis is an important mechanism of cell death in response to cytokine exposure in the β-cell [99].
5.3. Cytotherapy
One major goal of islet biology is to identify methods for protecting β-cells in the context of transplantation therapy for T1D. To identify markers for screening islet quality prior to transplantation and monitoring transplant success, Tian and coworkers investigated how INS-1 cells respond to hypoxia over time, using a 1H-NMR profiling approach. A decrease in creatine and an increase in taurine were observed over 24 h of hypoxic growth conditions. Choline and phosphocholine concentrations peaked during the first 2–6 h of hypoxic exposure, while glycerophosphocholine levels dipped. These observations correlate well with cell viability, indicating these markers could be exploited to monitor β-cell health in a noninvasive, nondestructive fashion via imaging techniques [100].
Finally, mesenchymal stromal cells isolated from islets were characterized by NMR metabolomics and other techniques to suggest that these cells have similar characteristics to bone-marrow derived mesenchymal stromal cells investigated for use in islet transplantation. One point of departure was identified. The islet-derived cells are more effective at suppressing lymphocyte proliferation. Thus donor banks of islet-derived mesenchymal stromal cells may be a more useful feeder-layer for ex vivo islet culture or to provide a more suitable islet microenvironment after transplantation [101].
6. Conclusions and future directions
Metabolomics has been increasingly applied for understanding of metabolic functions of the pancreatic islet over the past several years. Static profiling studies of β-cells have revealed unanticipated findings, such as the emergence of the PPP as an interesting glucose-regulated pathway deserving of further investigation for its potential mechanistic connection to GSIS. Metabolic flux analysis has also been revealing, for example in identification of the link between pyruvate carboxylase-catalyzed anaplerotic metabolism of glucose and GSIS. Lipidomics analyses have implicated sphingolipid metabolism in development of β-cell dysfunction, and have led to supportive cause and effect experiments involving manipulation of key enzymes of ceramide metabolism. Although many key findings have come from studies of β-cell lines due in part to the practical limitations of pooling hundreds of islets for each sample, a subset of studies have validated key findings in islets, suggesting the legitimacy of the approach. The next phase of application of metabolomics to islet biology will be greatly encouraged by continued improvement in sensitivity and quantitative reproducibility of available methods. This will allow more of the work to be conducted in primary islet preparations from normal and diabetic subjects, thereby facilitating discoveries with high translational relevance.
Acknowledgments
Work from our laboratory summarized in this report was supported by K01-GM109320-02 (to JRG) and DK 42583 (to CBN).
Abbreviations
- 6PGDH
6-phosphogluconate dehydrogenase
- KATP
ATP-sensitive K+ channel
- ER
endoplasmic reticulum
- G6PDH
glucose-6-phosphate dehydrogenase
- GC
gas chromatography
- GHB
γ-hydroxybutyric acid
- GSIS
glucose-stimulated insulin secretion
- ICDc
cytosolic NADP-dependent isocitrate dehydrogenase
- LC
liquid chromatography
- MS
mass spectrometry
- NMR
nuclear magnetic resonance
- PPP
pentose phosphate pathway
- PUFA
polyunsaturated fatty acid
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TCA
tricarboxylic acid cycle
- TOF
time-of-flight mass spectrometry
- ZMP
5-aminoimidazole-4-carboxamide ribonucleotide
References
- 1.Polonsky KS. The past 200 years in diabetes. N Engl J Med. 2012;367(14):1332–1340. doi: 10.1056/NEJMra1110560. [DOI] [PubMed] [Google Scholar]
- 2.Muoio DM, Newgard CB. Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(3):193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 3.Van Belle TL, Coppieters KT, Von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91(1):79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 4.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol. 2004;228(1–2):121–128. doi: 10.1016/j.mce.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Spégel P, et al. Unique and shared metabolic regulation in clonal β-cells and primary islets derived from rat revealed by metabolomics analysis. Endocrinology. 2015 doi: 10.1210/en.2014-1391. p. en.2014-1391. [DOI] [PubMed] [Google Scholar]
- 6.Andersson LE, et al. Characterization of stimulus-secretion coupling in the human pancreatic EndoC-betaH1 beta cell line. PLoS ONE. 2015;10(3):e0120879. doi: 10.1371/journal.pone.0120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oresic M. Metabolomics in the studies of islet autoimmunity and type 1 diabetes. Rev Diabet Stud. 2012;9(4):236–247. doi: 10.1900/RDS.2012.9.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suhre K. Metabolic profiling in diabetes. J Endocrinol. 2014;221(3):R75–R85. doi: 10.1530/JOE-14-0024. [DOI] [PubMed] [Google Scholar]
- 9.Keane K, Newsholme P. Metabolic regulation of insulin secretion. Pancreat Beta Cell. 2014;95:1–33. doi: 10.1016/B978-0-12-800174-5.00001-6. [DOI] [PubMed] [Google Scholar]
- 10.La Torre D. Immunobiology of beta-cell destruction. Adv Exp Med Biol. 2012;771:194–218. doi: 10.1007/978-1-4614-5441-0_16. [DOI] [PubMed] [Google Scholar]
- 11.Bouatra S, et al. The human urine metabolome. PLoS ONE. 2013;8(9):e73076. doi: 10.1371/journal.pone.0073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gika HG, et al. Current practice of liquid chromatography–mass spectrometry in metabolomics and metabonomics. J Pharm Biomed Anal. 2014;87:12–25. doi: 10.1016/j.jpba.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Cho K, et al. After the feature presentation: technologies bridging untargeted metabolomics and biology. Curr Opin Biotechnol. 2014;28:143–148. doi: 10.1016/j.copbio.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso A, Marsal S, Julia A. Analytical methods in untargeted metabolomics: state of the art in 2015. Front Bioeng Biotechnol. 2015;3:23. doi: 10.3389/fbioe.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu ZJ, et al. Liquid chromatography quadrupole time-of-flight mass spectrometry characterization of metabolites guided by the METLIN database. Nat Protoc. 2013;8(3):451–460. doi: 10.1038/nprot.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danielsson APH, et al. Development and optimization of a metabolomic method for analysis of adherent cell cultures. Anal Biochem. 2010;404(1):30–39. doi: 10.1016/j.ab.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz MA, Burant CF, Kennedy RT. Reducing time and increasing sensitivity in sample preparation for adherent mammalian cell metabolomics. Anal Chem. 2011;83(9):3406–3414. doi: 10.1021/ac103313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang M, Joseph JW. Metabolomic analysis of pancreatic beta-cell insulin release in response to glucose. Islets. 2012;4(3):210–222. doi: 10.4161/isl.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guay C, et al. A role for cytosolic isocitrate dehydrogenase as a negative regulator of glucose signaling for insulin secretion in pancreatic beta-cells. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0077097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenz MA, et al. Metabolome response to glucose in the beta-cell line INS-1 832/13. J Biol Chem. 2013;288(15):10923–10935. doi: 10.1074/jbc.M112.414961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace M, Whelan H, Brennan L. Metabolomic analysis of pancreatic beta cells following exposure to high glucose. Biochim Biophys Acta: Gen Subj. 2013;1830(3):2583–2590. doi: 10.1016/j.bbagen.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Nyblom HK, et al. Glucose-induced de novo synthesis of fatty acyls causes proportional increases in INS-1E cellular lipids. NMR Biomed. 2008;21(4):357–365. doi: 10.1002/nbm.1197. [DOI] [PubMed] [Google Scholar]
- 23.Boucher A, et al. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem. 2004;279(26):27263–27271. doi: 10.1074/jbc.M401167200. [DOI] [PubMed] [Google Scholar]
- 24.Boslem E, et al. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic beta-cells. J Biol Chem. 2013;288(37):26569–26582. doi: 10.1074/jbc.M113.489310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen G, et al. Role of lipid peroxidation and PPAR-delta in amplifying glucose-stimulated insulin secretion. Diabetes. 2011;60(11):2830–2842. doi: 10.2337/db11-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veret J, et al. Role of palmitate-induced sphingoid base-l-phosphate biosynthesis in INS-1 beta-cell survival. Biochim Biophys Acta: Mol Cell Biol Lipids. 2013;1831(2):251–262. doi: 10.1016/j.bbalip.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Li C, et al. Regulation of glucagon secretion in normal and diabetic human islets by gamma-hydroxybutyrate and glycine. J Biol Chem. 2013;288(6):3938–3951. doi: 10.1074/jbc.M112.385682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards JL, et al. Effect of decreasing column inner diameter and use of offline two-dimensional chromatography on metabolite detection in complex mixtures. J Chromatogr A. 2007;1172(2):127–134. doi: 10.1016/j.chroma.2007.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards JL, Kennedy RT. Metabolomic analysis of eukaryotic tissue and prokaryotes using negative mode MALDI time-of-flight mass spectrometry. Anal Chem. 2005;77(7):2201–2209. doi: 10.1021/ac048323r. [DOI] [PubMed] [Google Scholar]
- 30.Newgard CB, Matschinsky FM. The Endocrine Pancreas and Regulation of Metabolism. Oxford University Press; 2000. Substrate control of insulin release; pp. 125–151. [Google Scholar]
- 31.Henquin J-C. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in beta-cells. Diabetes Res Clin Pract. 2011;93:S27–S31. doi: 10.1016/S0168-8227(11)70010-9. [DOI] [PubMed] [Google Scholar]
- 32.Straub SG, Sharp GWG. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. 2002;18(6):451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 33.Schuit F, et al. Metabolic fate of glucose in purified islet cells – glucose-regulated anaplerosis in beta cells. J Biol Chem. 1997;272(30):18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald MJ. Estimates of glycolysis, pyruvate (de)carboxylation, pentose-phosphate pathway, and methyl succinate metabolism in incapacitated pancreatic-islets. Arch Biochem Biophys. 1993;305(2):205–214. doi: 10.1006/abbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 35.Jensen MV, et al. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells – preservation of glucose-stimulated insulin secretion. J Biol Chem. 2006;281(31):22342–22351. doi: 10.1074/jbc.M604350200. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez C, et al. Metabolomic and proteomic analysis of a clonal insulin-producing beta-cell line (INS-1 832/13) J Proteome Res. 2008;7(1):400–411. doi: 10.1021/pr070547d. [DOI] [PubMed] [Google Scholar]
- 37.Ashcroft FM, Rorsman P. ATP-sensitive K+ channels – a link between beta-cell metabolism and insulin-secretion. Biochem Soc Trans. 1990;18(1):109–111. doi: 10.1042/bst0180109. [DOI] [PubMed] [Google Scholar]
- 38.Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse beta-cells. J Clin Invest. 1992;89(4):1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato Y, et al. Dual functional-role of membrane depolarization Ca2+ influx in rat pancreatic beta-cell. Diabetes. 1992;41(4):438–443. doi: 10.2337/diab.41.4.438. [DOI] [PubMed] [Google Scholar]
- 40.Ivarsson R, et al. Redox control of exocytosis – regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54(7):2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 41.Ronnebaum SM, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281(41):30593–30602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 42.Detimary P, VandenBerghe G, Henquin JC. Concentration dependence and time course of the effects of glucose on adenine and guanine nucleotides in mouse pancreatic islets. J Biol Chem. 1996;271(34):20559–20565. doi: 10.1074/jbc.271.34.20559. [DOI] [PubMed] [Google Scholar]
- 43.Kibbey RG, et al. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5(4):253–264. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prentki M, Madiraju SRM. Glycerolipid/free fatty acid cycle and islet beta-cell function in health, obesity and diabetes. Mol Cell Endocrinol. 2012;353(1–2):88–100. doi: 10.1016/j.mce.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Zhao S, et al. Alpha/beta-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 2014;19(6):993–1007. doi: 10.1016/j.cmet.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402(6762):685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 47.Spegel P, et al. Time-resolved metabolomics analysis of beta-cells implicates the pentose phosphate pathway in the control of insulin release. Biochem J. 2013;450:595–605. doi: 10.1042/BJ20121349. [DOI] [PubMed] [Google Scholar]
- 48.Huang M, Joseph JW. Assessment of the metabolic pathways associated with glucose-stimulated biphasic insulin secretion. Endocrinology. 2014;155(5):1653–1666. doi: 10.1210/en.2013-1805. [DOI] [PubMed] [Google Scholar]
- 49.Branstrom R, et al. Long-chain CoA esters activate human pancreatic beta-cell K-ATP channels: potential role in Type 2 diabetes. Diabetologia. 2004;47(2):277–283. doi: 10.1007/s00125-003-1299-x. [DOI] [PubMed] [Google Scholar]
- 50.Kowluru A, et al. Protein farnesylation-dependent Raf/extracellular signal-related kinase signaling links to cytoskeletal remodeling to facilitate glucose-induced insulin secretion in pancreatic beta-cells. Diabetes. 2010;59(4):967–977. doi: 10.2337/db09-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu DH, et al. C-13 NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proc Natl Acad Sci USA. 2002;99(5):2708–2713. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan A, Ling ZC, Landau BR. Quantifying the carboxylation of pyruvate in pancreatic islets. J Biol Chem. 1996;271(5):2539–2542. doi: 10.1074/jbc.271.5.2539. [DOI] [PubMed] [Google Scholar]
- 53.Jensen MV, et al. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol-Endocrinol Metab. 2008;295(6):E1287–E1297. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huypens P, et al. The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia. 2011;54(1):135–145. doi: 10.1007/s00125-010-1923-5. [DOI] [PubMed] [Google Scholar]
- 55.Joseph JW, et al. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem. 2006;281(47):35624–35632. doi: 10.1074/jbc.M602606200. [DOI] [PubMed] [Google Scholar]
- 56.Joseph JW, et al. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem. 2007;282(43):31592–31600. doi: 10.1074/jbc.M706080200. [DOI] [PubMed] [Google Scholar]
- 57.Dai X, et al. An isocitrate-NADPH-GSH pathway controls insulin exocytosis and rescues function in b-cells from patients with type 2 diabetes. submitted for review. [Google Scholar]
- 58.Nolan CJ, et al. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55:S16–S23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- 59.Latour MG, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56(4):1087–1094. doi: 10.2337/db06-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Azzouny M, et al. Increased glucose metabolism and glycerolipid formation by fatty acids and GPR40 receptor signaling underlies the fatty acid potentiation of insulin secretion. J Biol Chem. 2014;289(19):13575–13588. doi: 10.1074/jbc.M113.531970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 62.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85(4):1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 63.Gheni G, et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 2014;9(2):661–673. doi: 10.1016/j.celrep.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goehring I, et al. Identification of an intracellular metabolic signature impairing beta cell function in the rat beta cell line INS-1E and human islets. Diabetologia. 2011;54(10):2584–2594. doi: 10.1007/s00125-011-2249-7. [DOI] [PubMed] [Google Scholar]
- 65.Leahy JL, et al. Chronic hyperglycemia is associated with impaired glucose influence on insulin-secretion – a study in normal rats using chronic in vivo glucose infusions. J Clin Invest. 1986;77(3):908–915. doi: 10.1172/JCI112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson RP. Type-II diabetes, glucose non-sense, and islet desensitization. Diabetes. 1989;38(12):1501–1505. doi: 10.2337/diab.38.12.1501. [DOI] [PubMed] [Google Scholar]
- 67.Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes – a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143(2):339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 68.Evans-Molina C, et al. Glucose regulation of insulin gene transcription and pre-mRNA processing in human islets. Diabetes. 2007;56(3):827–835. doi: 10.2337/db06-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Asfari M, et al. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell-lines. Endocrinology. 1992;130(1):167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 70.Marchetti P, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50(12):2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 71.Andreozzi F, et al. Activation of the hexosamine pathway leads to phosphorylation of insulin receptor substrate-1 on Ser(307) and Ser(612) and impairs the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin insulin biosynthetic pathway in RIN pancreatic beta-cells. Endocrinology. 2004;145(6):2845–2857. doi: 10.1210/en.2003-0939. [DOI] [PubMed] [Google Scholar]
- 72.Robertson RP, et al. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52(3):581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 73.Tajiri Y, Moller C, Grill V. Long term effects of aminoguanidine on insulin release and biosynthesis: evidence that the formation of advanced glycosylation end products inhibits B cell function. Endocrinology. 1997;138(1):273–280. doi: 10.1210/endo.138.1.4851. [DOI] [PubMed] [Google Scholar]
- 74.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29(3):351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanaka Y, et al. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci USA. 2002;99(19):12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gohring I, et al. Chronic high glucose and pyruvate levels differentially affect mitochondrial bioenergetics and fuel-stimulated insulin secretion from clonal INS-1 832/13 cells. J Biol Chem. 2014;289(6):3786–3798. doi: 10.1074/jbc.M113.507335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pizarro-Delgado J, et al. Glucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in beta-cells. Biochem J. 2010;431:381–389. doi: 10.1042/BJ20100714. [DOI] [PubMed] [Google Scholar]
- 78.Jacqueminet S, et al. Inhibition of insulin gene expression by long-term exposure of pancreatic beta cells to palmitate is dependent on the presence of a stimulatory glucose concentration. Metab – Clin Exp. 2000;49(4):532–536. doi: 10.1016/s0026-0495(00)80021-9. [DOI] [PubMed] [Google Scholar]
- 79.Boslem E, et al. A lipidomic screen of palmitate-treated MIN6 beta-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem J. 2011;435:267–276. doi: 10.1042/BJ20101867. [DOI] [PubMed] [Google Scholar]
- 80.Medina-Gomez G, et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3(4) doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosen ED, et al. Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol. 2003;23(20):7222–7229. doi: 10.1128/MCB.23.20.7222-7229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medina-Gomez G, et al. Adaptation and failure of pancreatic beta cells in murine models with different degrees of metabolic syndrome. Dis Models Mech. 2009;2(11–12):582–592. doi: 10.1242/dmm.003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gunton JE, et al. Loss of ARNT/HIF1 beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122(3):337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 84.Pillai R, et al. Aryl hydrocarbon receptor nuclear translocator/hypoxia-inducible factor-1 beta plays a critical role in maintaining glucose-stimulated anaplerosis and insulin release from pancreatic beta-cells. J Biol Chem. 2011;286(2):1014–1024. doi: 10.1074/jbc.M110.149062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bollyky J, Greenbaum CJ. Editorial: the role of glucagon in postprandial hyperglycemia – the jury’s still out. J Clin Endocrinol Metab. 2007;92(8):2879–2881. doi: 10.1210/jc.2007-1312. [DOI] [PubMed] [Google Scholar]
- 86.Diao JY, et al. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem. 2005;280(39):33487–33496. doi: 10.1074/jbc.M506276200. [DOI] [PubMed] [Google Scholar]
- 87.Kawamori D, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9(4):350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA. 2010;107(37):16009–16012. doi: 10.1073/pnas.1006639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siafarikas A, et al. Early loss of the glucagon response to hypoglycemia in adolescents with type 1 diabetes. Diabetes Care. 2012;35(8):1757–1762. doi: 10.2337/dc11-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Porksen S, et al. Meal-stimulated glucagon release is associated with post-prandial blood glucose level and does not interfere with glycemic control in children and adolescents with new-onset type 1 diabetes. J Clin Endocrinol Metab. 2007;92(8):2910–2916. doi: 10.1210/jc.2007-0244. [DOI] [PubMed] [Google Scholar]
- 91.Bessho M, et al. Possible contribution of taurine to distorted glucagon secretion in intra-islet insulin deficiency: a metabolome analysis using a novel alpha-cell model of insulin-deficient diabetes. PLoS ONE. 2014;9(11):e113254. doi: 10.1371/journal.pone.0113254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palau N, et al. Role of IGFBP-3 in the regulation of beta-cell mass during obesity: adipose tissue/beta-cell cross talk. Endocrinology. 2012;153(1):177–187. doi: 10.1210/en.2011-0181. [DOI] [PubMed] [Google Scholar]
- 93.Malpique R, et al. Integrative analysis reveals novel pathways mediating the interaction between adipose tissue and pancreatic islets in obesity in rats. Diabetologia. 2014;57(6):1219–1231. doi: 10.1007/s00125-014-3205-0. [DOI] [PubMed] [Google Scholar]
- 94.Tong L, Harwood HJ., Jr Acetyl-coenzyme A carboxylases: versatile targets for drug discovery. J Cell Biochem. 2006;99(6):1476–1488. doi: 10.1002/jcb.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ronnebaum SM, et al. Chronic suppression of Acetyl-CoA carboxylase 1 in beta-cells impairs insulin secretion via inhibition of glucose rather than lipid metabolism. J Biol Chem. 2008;283(21):14248–14256. doi: 10.1074/jbc.M800119200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oresic M, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med. 2008;205(13):2975–2984. doi: 10.1084/jem.20081800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sysi-Aho M, et al. Metabolic regulation in progression to autoimmune diabetes. PLoS Comput Biol. 2011;7(10) doi: 10.1371/journal.pcbi.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pukel C, Baquerizo H, Rabinovitch A. Destruction of rat islet cell monolayers by cytokines – synergystic interactions of interferon-gamma, tumor necrosis-factor, lymphotoxin, and interleukin-1. Diabetes. 1988;37(1):133–136. doi: 10.2337/diab.37.1.133. [DOI] [PubMed] [Google Scholar]
- 99.Collier JJ, et al. Pancreatic beta-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian L, et al. Changes in metabolic markers in insulin-producing beta-cells during hypoxia-induced cell death as studied by NMR metabolomics. J Proteome Res. 2013;12(8):3738–3745. doi: 10.1021/pr400315e. [DOI] [PubMed] [Google Scholar]
- 101.Kim J, et al. Biologic and immunomodulatory properties of mesenchymal stromal cells derived from human pancreatic islets. Cytotherapy. 2012;14(8):925–935. doi: 10.3109/14653249.2012.684376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spegel P, et al. Metabolomic analyses reveal profound differences in glycolytic and tricarboxylic acid cycle metabolism in glucose-responsive and -unresponsive clonal beta-cell lines. Biochem J. 2011;435:277–284. doi: 10.1042/BJ20100655. [DOI] [PubMed] [Google Scholar]