Abstract
Introduction
Health sector management is increasingly complex as new health technologies, treatments, and innovative service delivery strategies are developed. Many of these innovations are implemented prematurely, or fail to be implemented at scale, resulting in substantial wasted resources.
Methods
A scoping review was conducted to identify articles that described the scale up process conceptually or that described an instance in which a healthcare innovation was scaled up. We define scale up as the expansion and extension of delivery or access to an innovation for all end users in a jurisdiction who will benefit from it.
Results
Sixty nine articles were eligible for review. Frequently described stages in the innovation process and contextual issues that influence progress through each stage were mapped. 16 stages were identified: 12 deliberation and 4 action stages. Included papers suggest that innovations progress through stages of maturity and the uptake of innovation depends on the innovation aligning with the interests of 3 critical stakeholder groups (innovators, end users and the decision makers) and is also influenced by 3 broader contexts (social and physical environment, the health system, and the regulatory, political and economic environment). The 16 stages form the rows of the Nose to Tail Tool (NTT) grid and the 6 contingency factors form columns. The resulting stage-by-issue grid consists of 72 cells, each populated with cell-specific questions, prompts and considerations from the reviewed literature.
Conclusion
We offer a tool that helps stakeholders identify the stage of maturity of their innovation, helps facilitate deliberative discussions on the key considerations for each major stakeholder group and the major contextual barriers that the innovation faces. We believe the NTT will help to identify potential problems that the innovation will face and facilitates early modification, before large investments are made in a potentially flawed solution.
Keywords: Health innovation, pilot test, implementation, scale up, stakeholders, researchers, end users, decision makers
Introduction
While innovation in drugs, technologies, procedures and healthcare delivery approaches is a major influence on health systems, uncertainty around their benefits and unintended consequences complicates the management of innovation in the healthcare system. While innovation is almost universally attempted the vast majority of health innovation ideas do not progress into viable products, services or changes in healthcare delivery. Few of those that are successfully developed and pilot tested in one locale are implemented effectively or achieve expected outcomes in that initial locale, and even fewer scale up to their full potential, eventually to be institutionalized into common practice: fewer than 5 percent of drug or technology innovations reach scale and are sustained 1. And yet, this low success rate for scaling up a new health intervention takes on average 14 years and 2 billion US dollars per successful effort; the cost of the unsuccessful efforts is unknown and not included. The proportion of successes is simply not known for health service delivery or policy changes, nor is the cost known. Given the relatively low investment in early stage development and evaluation of healthcare delivery innovations, and the absence of a regulatory framework judging the balance of benefits, cost and harm, the success rate may be even lower.
Analyses of unsuccessful efforts to scale up innovations provides insights into why scale up is rare 2. Common challenges include underestimating the resources required for scale up, failure to understand the importance of politics and policy in successful scale up, not considering the conditions needed for scale up early in the process of innovation development and an overemphasis of either the vertical or horizontal spread of innovations as opposed to considering both 2. This relatively simple set of causes is belied by the chaos of the theoretical literature on the same topic. A recent review of models and frameworks for dissemination and implementation found 61 such frameworks 3, 4, many overlapping conceptually, but with no common terminology. A shared terminology would improve communication among and between researchers and implementation groups 3.
Based on the apparent simplicity of the problems inhibiting successful scale up, and doubting the value of yet another theoretical framework, we elected to take a different approach to the problem of improving success in the scale up of innovations 5. In this paper we describe an atheoretical, stage based tool that was developed for stakeholders, who may be developing, testing, implementing, funding or regulating a particular innovation. The tool helps stakeholders identify the stage of maturity of their innovation and helps facilitate deliberative discussions on the key considerations for major stakeholder groups and the major contextual barriers that the innovation faces. The goal is to help innovation teams identify which issues have been successfully managed up to their present stage of development and identify issues that still need to be addressed to move the innovation forward towards scale up. The tool incorporates research papers from several “disciplines”, such as rapid cycle innovation, dissemination and implementation science, knowledge translation and quality improvement that currently study innovation development and deployment. We merge ideas from all of these into an overarching tool that goes from nose (the problem and the initial idea for its solution) to tail (scale up and sustaining the solution) of the innovation process.
The Nose to Tail Tool
The Nose to Tail Tool (NTT) is intended to offer innovation teams, consisting of innovators and the essential stakeholders including end users and decision makers, a guide to: (a) identify what stage in the process their idea/innovation is at; (b) identify key considerations from each stakeholder perspective that should be addressed at the stage that their innovation has reached; and (c) identify contextual barriers at that stage that may be fatal to an innovation’s success and which must be overcome to move forward. The NTT is a comprehensive and consistent way of prompting context and stakeholder aware planning through the entire innovation process. The tool was designed with the belief that if stakeholders of an innovation consider the end stages from the very beginning of the process their innovation is more likely to achieve scale up and institutionalization 2, 6, 7. This tool helps innovation teams identify barriers, both those that can be overcome, and those that cannot, early in the innovation process, giving teams an opportunity to re-design the innovation at an early stage, or the opportunity to cease work on the project before too many resources have been invested.
The tool is aimed at newly developed innovations in healthcare and it is assumed that innovators have already excluded the availability of an existing intervention, either from the health field or products in another field that could potentially address the problem. The tool however can also be used to adapt existing innovations, developed elsewhere or for another situation or problem, into a different context.
Health innovations can be described as discrete innovations, multicomponent interventions and paradigmatic innovations 2. Discrete innovations are simple and well defined such as zinc in early childhood 8, combination therapy ART 9 or the use of new technology for diagnosis and treatment of TB 10. Multicomponent interventions involve several interacting program elements to produce a composite set of innovations that may also be targeted at multiple system levels 2. Examples include multilevel initiatives to decrease childhood obesity 11 or scale up of post abortion care services 12. Paradigmatic innovations require a shift in the way we understand health problems and the potential solutions to address them 2. China’s quality of care reforms for family planning, is an example, which required a systems wide approach, and partnerships between international groups and all levels of governments in China, including those that extend outside of public health 13. Paradigmatic innovations attempt to address the causes of poor health by using a determinants of health approach; they are complex, require a systems level approach and partnerships among key stakeholders from across sectors 2. Given their size and complexity, paradigmatic innovations are more difficult to stage and assess feasibility; however, in many cases they can be broken down into several smaller components similar to individual discrete or multicomponent innovations working together. The NTT may be most usefully applied to simple and multicomponent innovations; including service delivery, diagnostic, product, device or information technology innovations.
Methodology
We conducted a scoping review of the literature to identify whether a pre-existing dialogue or tool existed that could be used to help innovators and decision makers successfully implement and scale up innovations 14. Through this review it was identified that such a tool did not exist.
The initial search terms used for the scoping review were duplicated from Yamey’s article, “Scaling Up Global Health Interventions: A proposed Framework for Success.” 15 The search terms “global health” in text or “international health” in text and “implementation science” in title [ti] and abstract [ab] or “scaling up” in title or “scaling-up” in title were used in PubMed on June 19, 2013. Non-English articles were excluded. This search resulted in 13 full text articles. The titles and abstracts for these articles were reviewed by MZ and 5 articles were selected. Articles were selected if they described a process whereby an innovation was scaled up or if authors conceptually described the scale up process. We were specifically interested in papers that highlighted the process of jurisdiction or organization wide scale up. To broaden the review for global coverage, a second search was done in PubMed excluding the terms “global health” and “international health, leaving “implementation science[tiab] OR scaling up[ti] OR scaling-up[ti]”. This search strategy retrieved 383 full text articles. The titles and abstracts for these articles were reviewed by MZ and 60 additional articles from this second set were deemed relevant for a total of 65 articles. Articles were then read in full and validated by AG and two articles were excluded. Additional articles (n=6) were then included from the reference lists of papers identified in PubMed searches, from key informants in the field, and from the investigator’s own files. In total 69 articles were included. Over half of the articles reviewed (n=35) come from literature based in or describing innovations in the low and middle income country (LMIC) context.
The scoping review was not meant to be an extensive literature review, but rather a means to identify, using the qualitative research concept of saturation, enough relevant studies to describe a coherent set of stages of the innovation process and the major considerations for each stage. Key information from the literature was sorted and charted according to the key issues and themes using a narrative review approach. All articles were printed and read in full with key phrases and sections typed manually into an Excel spreadsheet ( Dataset 1). Information was recorded under the following headings: Authors, Title, Summary and Purpose, Referenced Papers and Theories, Stages Discussed, Methods Used, Research Values, and Details of the Stages Discussed. The information was then collated and summarized paying attention to the frequency in which ideas appeared. The literature was reviewed until saturation was reached. The scoping review was conducted using a realist approach utilizing a heterogeneous collection of studies including primary papers as well as reviews; papers were included if they described a process whereby an innovation was scaled up or conceptually described the scale up process; data extraction and analysis used an iterative approach; and an iterative approach was also used to extract and analyze data 16.
The included articles from the search (n=63) were reviewed by AG to define and map frequently described stages in the innovation process and themes that influence success in these stages. The articles were reviewed again to identify contingency factors and important considerations that should be asked at each stage. These contingency factors were sub-categorized under the two broad themes.
Development of the tool, including stages, definitions and contingency factors was an iterative process. In the first iteration AG reviewed the articles and proposed a number of stages. After discussion with MZ and CT, stages were agreed on, which were then validated against the included articles. We repeated this process for both themes and then contingency factors. Each of the three categorizations (stages, themes and contingency factors) went through three iterations. After these three iterations, we reached the point where the stages and contingency factors were well defined and mutually exclusive. We regarded this process as complete when AG and MZ could use the tool independently to categorize both stage and contingency factors for a large group of innovations in the same way. From these 63 articles we successfully built a grid with series of rows (stages) and columns (contingency factors) resulting in 72 cells, into which content from these articles were added and edited to avoid duplication within each cell. This text was reframed in the form of asking questions to the user of the tool. When we completed the extraction of information from the 63 articles we felt some cells were insufficiently detailed so we then snowballed from the reference lists of the included papers and sought suggestions for other papers from authors and experts. An additional 6 articles were included to increase content in these identified cells. At this point we agreed that each cell had sufficient coverage. Finally, the tool was compared by AG to other frameworks and tools that were described in the literature reviewed to identify similarities and differences.
Results
Includes the initial information collected from the initial 63 articles reviewed which were used to determine the stages (rows) and contingency factors (columns) for the NTT.
Copyright: © 2016 Gupta A et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Development of the NTT
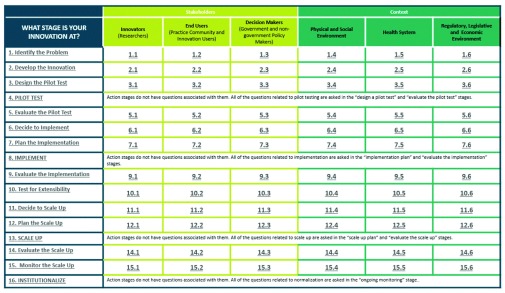
From the primary literature we identified sixteen commonly described stages of innovation development: (1) identify the problem 12, 17– 24, (2) develop the innovation 15, 21, 22, 24– 31; (3) design the pilot test 17, 22, 29– 31; (4) pilot test; (5) evaluate the pilot test 13, 22, 24– 26, 31– 34; (6) decide to implement 12, 15, 17, 19, 21, 22, 28, 35– 41; (7) plan the implementation 15, 17, 21– 25, 27, 28, 33, 35– 40, 42– 49; (8) implement; (9) evaluate the implementation 22– 27, 31, 32, 42, 48, 50– 53; (10) test for extensibility 25, 26, 31, 51, 53, 54; (11) decide to scale up 12, 18, 20, 52, 53, 55– 59; (12) plan the scale up 12, 13, 20, 27, 31, 33, 34, 36, 42, 47, 52, 55– 67; (13) scale up; (14) evaluate the scale up 11, 18, 31, 42, 52, 68; (15) monitor the scale up 17, 36, 56, 68; and (16) institutionalize 12, 13, 42, 47. These sixteen stages are used as the rows of the NTT grid ( Figure 1). Of the sixteen stages 12 are considered deliberation stages (1–3, 5–7, 9–13, 14–16) and 4 are action stages (4,8,13,16). Stages 3, 7, 12 and 15 are considered design and planning stages which prepare innovation teams for the action stages 4, 8, 13 and 16. Stages 5, 9, and 14 are evaluation stages. Stages 6 and 11 are unique in that they are decision stages that encourage innovation teams to consider critically whether they are ready for implementation or scale up.
Figure 1. Image of the NTT Grid.
Stage 10, testing for extensibility, is unique because the concept of the stage was acquired by the scoping review, however nowhere in the literature was it defined. It was typically described as an undertaking that would aid with successful scale up, however given its significance, we defined it as a stage in itself. Extensibility in the NTT defines the stage where innovation teams should “conduct multiple studies in various settings and with variable populations” to ensure that the innovation can produce positive outcomes in contextually different, or heterogeneous, environments 54. If through testing it is shown that the innovation is no longer as effective, innovation teams should try modifying adaptable components of the innovation. Many interventions are complex and can be conceptualized as being composed of core essential components that cannot be altered without harming integrity and adaptable components that can be altered to fit context 25, 31. In our model, it is during the first 5 stages that innovation teams should be preparing for future extensibility; considering the need for future adjustment, adaption or growth. The term “extensibility” is borrowed from the field of software and systems engineering which describes it as “the ability of a system to be extended with new functionality with minimal or no effects on its internal structure” 69 or the “capability to adjust and adopt to a variety of reporting [or environment] demands” 70.
Scale up is most commonly described as the general process of increasing or spreading coverage of health interventions. However, given that the reviewed literature proposes that testing for extensibility is a facilitator of successful scale up, or a pre-requisite, we propose that scale up requires two steps; spread to first similar and second to different settings. This spread can be to more units, patients, facilities or settings that are rather similar to those in which initial implementation took place which we name as expansion, or spread to sites which may be different, which we name as extension. While presently there is no distinguishing use of the term expansion in knowledge translation we propose its use to mean spread to homogenous sites, contrasting nicely with extension, and its attendant meaning of stretching.
In addition to the literature suggesting that innovations progress through several stages on route to scale up and institutionalization, it also suggests that the ability to progress forward is contingent on several factors. It is dependent on the characteristics of the innovation itself 21, 23– 25, 29– 31, 33, 34, 36– 38, 40, 42, 53, 55, 57, 62, 68, 71, 72 and the interests of the key stakeholders including: a) innovators (researchers) who are involved in developing the innovation 11, 15, 20, 22– 25, 27– 34, 37, 41– 44, 48, 52, 53, 56; b) end users (the practice community and innovation users) from the health system unit (i.e. organization, clinic, hospital, community, province etc.) or patients 11– 13, 18, 20– 25, 27, 29– 31, 33– 39, 41– 43, 47, 48, 53, 56, 62, 65, 72; and c) decision makers (government and non-government policy makers) who have policy jurisdiction within the health system unit 12, 13, 15, 18– 20, 25, 28, 32, 34, 36, 37, 40, 42, 43, 48, 53, 61, 62, 67, 72. It is also dependent on the broader context including the social and physical environment 11, 15, 17– 19, 22– 25, 28, 31, 34, 40, 53, 56, 58, 67, the health system unit where the innovation will be integrated (i.e. organization, clinic, hospital, community, province etc.) 11, 15, 17, 22– 25, 27, 28, 31, 33, 34, 37, 39, 43, 47, 48, 53, 56– 58, 67, and the regulatory, political and economic environment 12, 13, 15, 17– 19, 22, 24, 25, 28, 34, 39, 40, 43, 48, 52, 53, 55, 57, 58, 61, 67, 68, 72– 74. These contingency factors are grouped into two themes including stakeholders and context factors, and are used as the headings of the columns of the NTT grid ( Figure 1 and Box 1).
Box 1. Definitions for the main collaborator groups and contingency factor themes used in the NTT.
COLUMNS:
Researchers (Innovators): Individuals involved in developing the innovation.
Practice Community and Innovation Users (End Users): Individuals from the “health system unit” who will use the innovation (whether that is a health professional, administrator or patient).
Government and non-government Policy Makers (Decision Makers): Individuals with policy jurisdiction with the end “health system unit”.
Physical and Social Environment: The broader physical and social environment where the innovation will be implemented/scaled up.
Health System (unit): Where the innovation will be integrated (organization, hospital, community, system etc.).
Regulatory, Legislative and Economic Environment: The broader political and economic landscape.
ROWS:
Pilot Test: Small scale preliminary study conducted in order to evaluate questions such as the general characteristics of the innovation, cost, and potential impact and capacity to improve health outcomes.
Implement: Is the process of putting an innovation into practice in such a way that it meets the necessary standards to achieve the innovations desired outcomes within specific settings. The implementation phase in the tool assumes that the innovation will be implemented in a single setting, or among sites that are contextually homogeneous.
Scale Up: Describes an increase in the coverage of health innovations, to both populations that are contextually similar (expand) and diverse (extend), that have been tested in order to benefit more people at a large, national, or international scale.
Institutionalize: The sustainable integration of the innovation into existing health systems as a part of their regular service delivery.
Using the Nose to Tail Tool
The NTT is designed such that the user’s first task is to determine which of the sixteen stages best represents the current level of maturity of the innovation. The characteristics of the innovation, along with evaluation results, for each stage are described as a standard against which users can gauge where their innovation is in the process. Figure 2 provides an example of the staging guideline used to determine if the user is at stage 6 ( decide to implement).
Figure 2. Example of the types of questions asked at stage 6 ( decide to implement) to help users of the NTT determine if they are at this stage.
We suggest that all stakeholders (innovators, end users, and decision makers) deliberate together to determine which stage they are at; and that they should start by reviewing the definitions from stage 1 and move forward from there, stopping when they feel they have reached a stage that is not yet completed. This first incomplete stage they reach is the stage their innovation is at.
The second task is for the users to move across the grid for that row, and review, column by column, the specific considerations for that stage, based on the 6 themes represented in the columns (3 stakeholder perspectives and 3 context factors). The questions, prompts and considerations in each cell are extracted from the reviewed literature. Users should review the questions and prompts to identify potential gaps in their innovation process and to bring attention to factors that may not have been considered sufficiently. Discussion among different stakeholders facilitates consensus on what response or development is required to move forward. Figure 3 provides an example of the types of questions decision makers should consider at stage 6 ( decide to implement stage). Of note, in the NTT, action stages (4, 8, 13, and 16) do not have any questions associated with them, as the relevant questions are all considered in the planning (3, 7, and 12) or evaluation stages (5, 9, and 14) preceding and proceeding the action. Evaluation suggestions are embedded throughout the innovation process and formally included after each of the main action stages, highlighting the recognition in the literature of the importance of implementing and scaling up innovations with demonstrated effectiveness and discouraging forward movement, or promoting redevelopment, of those that do not achieve the desired results.
Figure 3. Example of the types of questions the NTT asks decision makers to consider at stage 6 ( decide to implement).
The tool is meant to be used by innovation teams collectively and as such, the questions associated with each stakeholder perspective are directed to that specific stakeholder group (for example, questions under end users are directed to and should be answered primarily by end users who are involved in the innovation development process). However, users of the tool are encouraged to think of the innovation process from all of the stakeholder perspectives and thus consider the questions in each of the columns, as opposed to their own column only, particularly if your team does not have key players from each of these three groups. The questions asked under the context themes are directed to all three stakeholder groups’ collectively.
Although the NTT model appears to follow a simple, single linear path, it is anticipated that not all innovations follow this trajectory. The NTT is built such that users may enter the model at any stage, may go forwards or backwards through the stages, or may skip stages all together. The NTT is meant to be a guide to thoughtful deliberation of the innovation process and not a rigid doctrine.
The NTT grid is available at: http://nosetotailtool.org/. To access this tool, please follow the instructions available at: http://nosetotailtool.org/consent/. In brief, following registration users are provided with the password required to access the tool automatically via email.
Comparison of the NTT to other frameworks and tools
We compared the NTT to each existing framework or implementation tool found in our search to ensure that the NTT was not duplicative. In our review we found 11 papers describing 7 different frameworks/models which defined one or more stages to the process of innovation, along with contextual factors. None of the frameworks reviewed described more than 7 of the NTT stages (stages 7–14 in Yamey’s Framework for Success in Scaling Up 15) while others described as few as 3 stages (stages 7–9 in the QIF 23). Individual non-framework papers touched on one or more stages, with 4 papers describing stage 16, institutionalization 12, 13, 42, 47, a stage not covered in any of the frameworks reviewed. With respect to the context columns, only one pre-existing framework (CFIR 25) considered all of the contextual themes considered in the NTT. Although many frameworks described the importance of working with collaborators, only one (QIF 23) developed their framework to be used by all three of the stakeholder groups (decision makers, end users, and innovators). We concluded that the NTT tool offers a more comprehensive and thus potentially useful approach to innovation in healthcare. The Supplementary material provides a descriptive comparison of the NTT in contrast to the five frameworks and two tools we found. A summary of the comparisons can be found in Table 1.
Table 1. An overview comparison of the NTT in contrast to five frameworks and two tools found in the scoping review.
| Staging | Context Considerations | Focus | Intended Audience of
Framework or Tool |
Tool | |
|---|---|---|---|---|---|
| NTT | 1–16 | Social and Physical Environment;
Health System; Regulatory, Legislative and Economic Environment |
Pilot Testing
Implementation Scale Up |
Innovators Decision
Makers End Users |
Yes |
| PARIHS* | NTT 5–9 | Health System | Implementation | Innovators | Yes |
| CFIR | NTT 2–9 | Social and Physical Environment;
Health System; Regulatory, Legislative and Economic Environment |
Pilot Testing
Implementation |
Innovators | No |
| T0-T4 | NTT 1–9 | Social Environment; Health System;
Economic Environment |
Pilot Testing
Implementation |
Innovators | No |
| QIF | NTT 7–9 | Social and Physical Environment;
Health System |
Implementation | Innovators Decision
Makers End Users |
Yes |
|
Framework for
Success in Scaling Up |
NTT 7–14 | Health System; Regulatory and
Legislative Environment |
Implementation
Scale Up |
Innovators | No |
| AIDED | NTT 10–14 | Social and Physical Environment;
Health System; Regulatory, Legislative and Economic Environment |
Scale Up | Innovators | No |
|
Conceptual Model of
EBP Implementation |
NTT 1–2,
4, 6–9, 15 |
Social Environment; Health System;
Regulatory, Legislative and Economic Environment |
Implementation | Innovators | No |
PARIHS – Promoting Action on Research Implementation in Health Services (Stetler)
*PARIHS was orginally developed by Kitson et al. in 1998 and was revised by Stetler in 2011. This review looked at Stetler’s revised version.
CFIR – Consilidated Framework for Implementation Research (Damschroder)
T0 – T4 - Glasgow’s 5 Key phases in moving Research to Practice/Policy (Glasgow)
QIF – Quality Implementation Framework (Meyers)
Framework for Success in Scaling Up (Yamey)
AIDED (Perez-Escamillla)
Conceptual Model of Evidenced-Based Practice Implementaiton (Aarons)
Discussion
A collaborative and deliberative decision making tool
A striking, yet common theme in much of the literature was the need for improved collaborations among key stakeholders, which we identified as innovators, end users and decision makers. Deliberation is more than just a discussion of issues; it is collective “problem solving” that allows “individuals with different backgrounds, interests and values to listen, understand, potentially persuade and ultimately come to more reasoned and informed decisions” 75.
The NTT has the potential to start the process of closing the gap between research and policy. Despite 40 years of attempting to translate research into evidenced based policy, barriers continue to persist 76. Ellen et al. conducted a qualitative study to identify barriers and facilitators for implementing supports for evidenced informed decision making and highlights three main areas: facilitating pull efforts, establishing a climate for research use, and linkage and exchange 77. Pull efforts include implementing technical infrastructures that allow “easy access” to research through physical tools; and linkage and exchange efforts which ensure that “decision makers have the necessary skills and connections to acquire, assess, adapt and apply the necessary evidence” to decision making 77. From these findings however, it is evident that the authors assume that problems identified by decision makers have existing solutions or answers and the challenge is simply in finding them. We challenge that this is often not the case, and that the healthcare system is too complex to simply “join [found] solutions to problems” 76. There is a process required for solving healthcare problems and that requires all stakeholders to work collaboratively from the onset and not just at the point of implementation or scale up. The NTT proposes that decision makers, end users and innovators be involved from the very beginning, the point of identifying problems in the healthcare system and remain involved throughout the development of the innovation. This allows mutual exchange of information throughout the process; it allows decision makers to discuss with innovators at the onset whether they feel that the problem at hand is a priority that needs to be solved and therefore will have support for it; it allows decision makers and end users to provide input into the design of the innovation, how it’s pilot tested, and can highlight which outcomes are important for them to support moving forward with the project. It ensures that decision makers, end users and innovators share common goals throughout the process. The NTT facilitates the potential to co-create and co-produce knowledge, developing a bridge between research and policy, which allows for a more democratic and useful knowledge exchange 76.
The NTT also emphasizes the importance of collaborative decision making with end users. The field of co-creation and design is an evolving field that was born from the merging of user-centred design (“user as subject”) and the participatory approach (“user as partner”) 78. Co-design and creation can broadly be defined as the creativity of designers (innovators) and people not trained in design (end users and decision makers) working together in the development process 78. Healthcare innovators need to start embracing the attitudes that have led to success in the private business sector; “we believe the key ingredient of innovation is to provide a compelling experience to all participants based on network effects for value creation… a platform of innovation for convergence of expertise/ideas, collaboration among participating organizations, and co-creation of the shared value with customers should be the core of co-innovation” 79. Integrating users in the early stages of the development process can have impacts with positive, long range consequences” 78. Integration and collaboration throughout the innovation process at all key moments of decision making is believed to be the missing ingredient needed for sustainable solutions.
Comprehensiveness of the NTT
The NTT covers the innovation process from problem identification through to institutionalization, where the innovation becomes integrated into common practice. It was intentionally designed to provide a single tool covering the entire process of innovation, from the beginning to end, hence the name, Nose to Tail. The NTT prompts consideration of the most important contextual barriers, categorized in broad domains: social and physical environment, regulatory and economic considerations and health system context. Although several of the models take into consideration some of these contextual domains, only one other, the CFIR 25, takes into consideration all of these, and none groups them in a way that optimizes discussion among stakeholders, as the NTT does. This comprehensiveness is intended to mimic the real world process of innovation, and allow users to assess success or delay in innovations at any stage of their lifecycle; only a comprehensive view from beginning to end allows all successes and failures to be identified. This has a practical value as it improves the continuity of the discussions between stakeholders on a given innovation.
As a tool, the NTT is intended for iterative use, by the deliberating stakeholders, alone and together, at or before each stage of the process. The NTT creates a grid which connects the stages of innovation to the relevant contextual issues, and at each stage identifies the specific concerns that might arise at that stage in relation to each of the contextual domains. This allows a stage specific, and thus more focussed, discussion on what barriers may require adaptations to the innovation, rather than a broad and generalized discussion of barriers, unconnected to the current stage of development of the innovation.
The NTT is designed to be deliberative and thus preventative, focussing discussion between multiple stakeholders, including health innovators, decision makers and end users on potential barriers to scale up as they come into view, allowing for innovations to be sequentially adapted before meeting these problems in the “real world” setting. The use of the tool is meant to practically support an innovation from the stages of development through to sustainment, or alternatively to propose the appropriate discarding of unadaptable, unacceptable, or ineffective innovations, whereby one or more stakeholder group finds an insurmountable barrier to supporting further development or implementation of the innovation.
In addition to being comprehensive in covering the entire process of innovation, the NTT is also all-inclusive in that it can support health innovation development in any healthcare system setting, whether it be in a LMIC or high income country (HIC). Over half of the literature reviewed comes from the LMIC setting. Examples of the entire innovation process, from idea to institutionalization, are more likely to be seen in LMICs given their distinctive characteristics that provide powerful incentives, or “gaps” that drive innovation 80. These gaps include: (1) a performance gap that requires higher volumes of satisfactory performing innovations for lower prices boosting development low-cost innovations of acceptable quality; (2) an infrastructure gap that provides a “clean slate” where building and implementing from scratch eliminates the need to overcome existing infrastructure barriers; (3) a sustainability gap that emphasizes development of “green” solutions that will not deplete existing natural resources or cause further damage in settings with large populations; (4) a regulatory gap that reduces policy barriers on implementation and scale up of innovations; (5) and a preference gap where unique preferences from different populations promote creativity in design 80, 81. In the context of health care, overwhelming need adds motive to create effective solutions that are scalable 80. These gaps describe why reverse innovation (RI) is possible; the process of first identifying or fostering a successful innovation in the LIC that addresses an unmet need in a HIC 80, 81. The NTT is consistent with RI, highlighting that lessons around innovation development can be learned from LMICs and applied to HICs; even if expenditures are different, facilitators and barriers are common in all health systems.
The role of implementation theory in the innovation process
The NTT attempts to address the need raised by Colquhoun for a shared and overarching approach that could promote effective communication between all stakeholders 3. It does so, not by creating a common language for discussing behavioural, organizational or other social sciences theories among researchers in the knowledge translation, dissemination and implementation communities, but by creating a simple scaffolding for deliberative discussion among stakeholders involved in developing, implementing and scaling up a given innovation. Its intent is practical, not theoretical 5, 82.
Although the argument has been made that a shift towards theoretically driven implementation interventions is necessary 83, the choice to be atheoretical was purposeful. The ICEBeRG authors assert that through the use of explicit behavioural theories that produce quantifiable results in implementation research, researchers will be better able to identify predictors of success that are common across different contexts; and should thus use these predictors to design interventions which may be more widely applicable 83. We argue that the plethora of overlapping and contradicting theories makes it difficult to judge the applicability of a piece of empirical evidence in supporting one theory over another; and from a practical viewpoint argue that the usefulness of the theoretical enterprise is undermined by the challenge in designing interventions which closely match only one among several overlapping theories 82. Oxman states that is time for us to “work collaboratively, based on common sense (sound practical judgment that is independent of specialized knowledge or training), sound logic and rigorous evidence to help people make informed choices about health care 5. The NTT is an attempt to organize published opinion and empirical experience for this purpose.
The NTT tool could also, if the reader prefers, be considered to be an implicit, mid-range “theory” 83, 84 along the following rather common sense lines:
-
1.
Innovations progress through stages on route to scale up or failure to scale.
-
2.
Progress through these stages is contingent on overcoming hurdles through adaptation of the innovation.
-
3.
The hurdles are related to
a) features of the innovation itself;
b) the broader context in which the innovation is being implemented or scaled; and
c) support for development and scale up of the innovation from all stakeholders.
This “theory” gives rise to an intervention hypothesis: the process of developing a shared understanding of the different stakeholders’ perspectives through discussion improves adaptation and progress of an innovation through the stages outlined in the NTT (or leads to appropriate abandonment at an early stage).
Limitations
We have derived each of the NTT stages and the contents of each cell’s prompts and issues for consideration from the literature that we reviewed. In that sense, this tool is evidence based rather than theoretical. Admittedly, many of the included papers are themselves not empirical; and even those that are descriptions of instances of innovation, at one or more stages, are not necessarily methodologically excellent, neither in qualitative nor quantitative terms. So in this sense, the NTT is taking as its raw material, a set of opinions and described experiences, and organizing these into patterns for ease of use. We find the simplicity and coherence of the grid to be attractive.
This scoping review which led to the development of the NTT used “scale up” as the central term. It was selected given the widespread agreement that many effective interventions exist to address many of the health problems but fail to be effectively implemented or scaled to sustainment. While further searching, especially using a less specific and wider set of terms e.g., “dissemination” would undoubtedly increase the number of retrieved articles enormously, it is not clear to us that this wider search would improve on the framework of stages and domains which we have assembled and for this reason we elected to move forward with our smaller range of papers now, but leave open the door for further inputs and we do not exclude the possibility of a systematic review in the future.
The NTT is not attempting to replace any of the competing theoretical frameworks, which will presumably strengthen over time, as evidence for one or another framework, or aspects of a framework, accumulates from empirical studies of innovation processes 83. The NTT could be used to collect data on a large number of innovations, and the descriptive and analytic epidemiology of these instances of innovation could contribute to an empirical evidence base for these theories.
Summary and next steps
Using a brief, sensitive and specific search strategy we have identified and abstracted information from 69 published papers describing empirical instances of scale up or descriptions of frameworks for understanding or planning parts of scale up. This scoping review is only the first stage of data gathering for this tool, and was not intended to be comprehensive, but has nevertheless given us sufficient information to compose a grid of 16 distinct and well defined stages and 6 distinct and well defined contextual domains relevant to the progress of an innovation in healthcare from problem identification to sustained solution. This relatively small number of included papers allowed us to reach initial conceptual saturation, which we defined for each cell (the intersection of a stage and contextual domain) as having one or more relevant issues for deliberation between stakeholders. The number of papers contributing to each cell in the table was obviously often much smaller than that supporting most of the stages or domains and so it seems that increasing the deliberation material for each cell warrants further searching, We propose a new approach for obtaining information on issues that should be considered for each cell, namely crowdsourcing, to contribute this added detail. Crowdsourcing can be simply defined as the posting of a problem online whereby a large number of individuals have the opportunity to offer solutions to the problem 85. Alongside publication of this paper, we have set up a website ( nosetotailtool.org) containing the grid, with a straightforward process by which any member of the stakeholder communities can provide comments and feedback on any of the stages, domains or individual cells which we will use to improve the tool. We seek readers’ comments, preferably with specific citations to the literature or brief factual descriptions of their experiences. We will never quote you without permission, never use your information or email details other than to contact you to discuss your comment or request permission to quote you, we will delete your personal information every 24 months, and will at all times store your details in encrypted format.
At this time, we believe the NTT 1.0 is a minimally viable product (MVP) 29 (positioned in stage 2 of the NTT, “Develop the Innovation”) that will evolve over time and we strongly believe that the underlying evidence base will strengthen over time. An MVP is an early prototype of the innovation that is typically deployed to as subset of possible customers, such as early adopters that are more likely to give feedback and able to grasp the innovation vision and hypothesis 29. It is the version of the innovation which allows the team to collect validated learning about from users before large investments are made in its development 29.
In addition to crowdsourcing we are currently conducting “hypothesis testing” 29 using our prototype with healthcare innovation teams within Ontario, Canada and preliminary feedback has been positive. From this testing we have seen that the stages in the tool seem to match the users (innovators) perceptions of stages they have gone through and that the questions asked at each stage expose assumptions requiring further deliberation.
In addition to this, we are investigating some new uses to the NTT (in our terminology, extensions): we are working with healthcare funders to assess the value of the tool in innovation portfolio analysis, whereby the tool could provide an overview of the progress of a portfolio of innovations for which they are currently funding; and, although the tool already emphasizes the importance of evaluation at each stage, we are working on a review to determine what patterns or sequences of evaluation designs best support advancement of the innovation at each stage.
Conclusion
There is a mismatch between good science and the complexity of health systems. Even if you have a good idea and a good innovation that is supported by empirical science that is simply not enough; the health system is complex and good innovations alone will not necessarily be scaled in real world settings. Successful development, implementation and scale up of health innovations is a multi-stage process that requires appraisal at every stage and it is a team effort that requires true collaborations from all stakeholders at every stage. It is essential to be constantly aware of what stage the innovation is at and to identify what contextual barriers require overcoming before moving forward in the process. At present, innovations are commonly rushed through stages and even skip essential stages all together; innovations are implemented or scaled up prematurely without evaluations to verify that they are mature enough to advance forward.
The NTT tool is an atheoretical, stage based and context aware tool that helps innovators, decision makers and end users identify in a deliberative and potentially collaborative fashion, what they have done to get the innovation to its current stage and identify what needs to be done to move it forward successfully. The NTT tool is meant to be a guide to iterative deliberation through the innovation process. This tool emphasizes the need to identify barriers early and repeatedly at each stage in the innovation process. The tool may suggest a need to go back to earlier stages and re-design the innovation, or in some cases to abandon the project all together.
The NTT tool is a comprehensive and consistent way of thinking of the entire innovation process. We believe that if the end goal of widespread jurisdictional scale up and sustainment of appropriately chosen and carefully adapted innovations is kept in mind from the beginning, success is more likely.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2016 Gupta A et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
F1000Research: Dataset 1. NTT scoping review. Includes the initial information collected from the initial 63 articles reviewed which were used to determine the stages (rows) and contingency factors (columns) for the NTT, 10.5256/f1000research.8145.d115651 86
Acknowledgements
We would like to acknowledge contributions to the initial conceptualization of this work and comments from Amanda Terry and Sandra Regan and comments from members of the Scale-Up Committee of a CIHR funded Community-Based Primary Healthcare Research Team, “Patient-Centred Innovations for Persons with Multimorbidity (PACE in MM)", and team members of the INnovations Strengthening PrImary Healthcare through REsearch (INSPIRE-PHC) program. We would also like to acknowledge Maureen Kennedy for her work in developing the search strategy for this review.
Funding Statement
This research was conducted under the Knowledge Translation and Exchange project, INSPIRE-PHC Program, supported by a grant from the Government of Ontario [#06547]. The views expressed are those of the authors and do not necessarily reflect those of the funder.
[version 1; referees: 3 approved
Supplementary material
A descriptive comparison of the NTT in contrast to the five frameworks and two tools found in the scoping review.
References
- 1. National Institute of Health: Clinical and translational science.2014. Reference Source [Google Scholar]
- 2. Edwards N: Scaling-up health innovations and interventions in public health: A brief review of the current state-of-the-science.2014;1–45. Reference Source [Google Scholar]
- 3. Colquhoun H, Leeman J, Michie S, et al. : Towards a common terminology: a simplified framework of interventions to promote and integrate evidence into health practices, systems, and policies. Implement Sci. 2014;9:51. 10.1186/1748-5908-9-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tabak RG, Khoong EC, Chambers DA, et al. : Bridging research and practice: models for dissemination and implementation research. Am J Prev Med. 2012;43(3):337–350. 10.1016/j.amepre.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oxman AD, Fretheim A, Flottorp S: The OFF theory of research utilization. J Clin Epidemiol. 2005;58(2):113–116; discussion 117–20. 10.1016/j.jclinepi.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 6. Canadian Institutes for Health Reserach: Phase I – eHealth innovations partnership program – long description.2014. Reference Source [Google Scholar]
- 7. Nilsen P: Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10:53. 10.1186/s13012-015-0242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larson CP, Saha UR, Nazrul H: Impact monitoring of the national scale up of zinc treatment for childhood diarrhea in Bangladesh: repeat ecologic surveys. PLoS Med. 2009;6(11):e1000175. 10.1371/journal.pmed.1000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harries AD, Makombe SD, Schouten EJ, et al. : How operational research influenced the scale up of antiretroviral therapy in Malawi. Health Care Manag Sci. 2012;15(3):197–205. 10.1007/s10729-011-9187-2 [DOI] [PubMed] [Google Scholar]
- 10. Meyer-Rath G, Schnippel K, Long L, et al. : The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLoS One. 2012;7(5):e36966. 10.1371/journal.pone.0036966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Silva-Sanigorski AM, Bolton K, Haby M, et al. : Scaling up community-based obesity prevention in Australia: background and evaluation design of the Health Promoting Communities: Being Active Eating Well initiative. BMC Public Health. 2010;10:65. 10.1186/1471-2458-10-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Billings DL, Crane BB, Benson J, et al. : Scaling-up a public health innovation: a comparative study of post-abortion care in Bolivia and Mexico. Soc Sci Med. 2007;64(11):2210–2222. 10.1016/j.socscimed.2007.02.026 [DOI] [PubMed] [Google Scholar]
- 13. Kaufman J, Erli Z, Zhenming X: Quality of care in China: scaling up a pilot project into a national reform program. Stud Fam Plann. 2006;37(1):17–28. 10.1111/j.1728-4465.2006.00080.x [DOI] [PubMed] [Google Scholar]
- 14. Arksey H, O'Malley L: Scoping studies: towards a methodological framework. Int J Soc Res Meth. 2005;8(1):19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 15. Yamey G: Scaling up global health interventions: a proposed framework for success. PLoS Med. 2011;8(6):e1001049. 10.1371/journal.pmed.1001049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pawson R, Greenhalgh T, Harvey G, et al. : Realist synthesis: an introduction.2004. Reference Source [Google Scholar]
- 17. Aarons GA, Hurlburt M, Horwitz SM: Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health. 2011;38(1):4–23. 10.1007/s10488-010-0327-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bezanson K, Isenman P: Scaling up nutrition: a framework for action. Food Nutr Bull. 2010;31(1):178–186. [DOI] [PubMed] [Google Scholar]
- 19. Buse K, Lalji N, Mayhew SH, et al. : Political feasibility of scaling-up five evidence-informed HIV interventions in Pakistan: a policy analysis. Sex Transm Infect. 2009;85(Suppl 2):ii37–42. 10.1136/sti.2008.034058 [DOI] [PubMed] [Google Scholar]
- 20. Chamberlain P, Roberts R, Jones H, et al. : Three collaborative models for scaling up evidence-based practices. Adm Policy Ment Health. 2012;39(4):278–290. 10.1007/s10488-011-0349-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fassier JB, Durand MJ, Loisel P: 2nd place, PREMUS best paper competition: implementing return-to-work interventions for workers with low-back pain--a conceptual framework to identify barriers and facilitators. Scand J Work Environ Health. 2011;37(2):99–108. 10.5271/sjweh.3138 [DOI] [PubMed] [Google Scholar]
- 22. Glasgow RE, Vinson C, Chambers D, et al. : National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274–1281. 10.2105/AJPH.2012.300755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyers DC, Durlak JA, Wandersman A: The quality implementation framework: a synthesis of critical steps in the implementation process. Am J Community Psychol. 2012;50(3–4):462–480. 10.1007/s10464-012-9522-x [DOI] [PubMed] [Google Scholar]
- 24. Stetler CB, Damschroder LJ, Helfrich CD, et al. : A Guide for applying a revised version of the PARIHS framework for implementation. Implement Sci. 2011;6:99. 10.1186/1748-5908-6-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Damschroder LJ, Hagedorn HJ: A guiding framework and approach for implementation research in substance use disorders treatment. Psychol Addict Behav. 2011;25(2):194–205. 10.1037/a0022284 [DOI] [PubMed] [Google Scholar]
- 26. Hartzler B, Lash SJ, Roll JM: Contingency management in substance abuse treatment: a structured review of the evidence for its transportability. Drug Alcohol Depend. 2012;122(1–2):1–10. 10.1016/j.drugalcdep.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meyers DC, Katz J, Chien V, et al. : Practical implementation science: developing and piloting the quality implementation tool. Am J Community Psychol. 2012;50(3–4):481–496. 10.1007/s10464-012-9521-y [DOI] [PubMed] [Google Scholar]
- 28. Yamey G: What are the barriers to scaling up health interventions in low and middle income countries? A qualitative study of academic leaders in implementation science. Global Health. 2012;8:11. 10.1186/1744-8603-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blank S: Why the lean start-up changes everything. Harvard Bus Rev. 2013. Reference Source [Google Scholar]
- 30. Brown T, Wyatt J: Design thinking for social innovation. Stanford Soc Innov Rev. 2010. Reference Source [Google Scholar]
- 31. Parry GJ, Carson-Stevens A, Luff DF, et al. : Recommendations for evaluation of health care improvement initiatives. Acad Pediatr. 2013;13(6 Suppl):S23–S30. 10.1016/j.acap.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 32. Kerner JF: Integrating research, practice, and policy: what we see depends on where we stand. J Public Health Manag Pract. 2008;14(2):193–198. 10.1097/01.PHH.0000311899.11197.db [DOI] [PubMed] [Google Scholar]
- 33. Nyonator FK, Awoonor-Williams JK, Phillips JF, et al. : The Ghana community-based health planning and services initiative for scaling up service delivery innovation. Health Policy Plan. 2005;20(1):25–34. 10.1093/heapol/czi003 [DOI] [PubMed] [Google Scholar]
- 34. Pérez-Escamilla R, Curry L, Minhas D, et al. : Scaling up of breastfeeding promotion programs in low- and middle-income countries: the "breastfeeding gear" model. Adv Nutr. 2012;3(6):790–800. 10.3945/an.112.002873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chambers DA: The interactive systems framework for dissemination and implementation: enhancing the opportunity for implementation science. Am J Community Psychol. 2012;50(3–4):282–284. 10.1007/s10464-012-9528-4 [DOI] [PubMed] [Google Scholar]
- 36. Larson CP, Koehlmoos TP, Sack DA: Scaling Up of Zinc for Young Children (SUZY) Project Team. Scaling up zinc treatment of childhood diarrhoea in Bangladesh: theoretical and practical considerations guiding the SUZY Project. Health Policy Plan. 2012;27(2):102–114. 10.1093/heapol/czr015 [DOI] [PubMed] [Google Scholar]
- 37. Leon N, Schneider H, Daviaud E: Applying a framework for assessing the health system challenges to scaling up mHealth in South Africa. BMC Med Inform Decis Mak. 2012;12:123. 10.1186/1472-6947-12-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prata N, Passano P, Sreenivas A, et al. : Maternal mortality in developing countries: challenges in scaling-up priority interventions. Womens Health (Lond Engl). 2010;6(2):311–327. 10.2217/whe.10.8 [DOI] [PubMed] [Google Scholar]
- 39. Rinaldi M, Miller L, Perkins R: Implementing the individual placement and support (IPS) approach for people with mental health conditions in England. Int Rev Psychiatry. 2010;22(2):163–172. 10.3109/09540261003720456 [DOI] [PubMed] [Google Scholar]
- 40. Tkatchenko-Schmidt E, Renton A, Gevorgyan R, et al. : Prevention of HIV/AIDS among injecting drug users in Russia: opportunities and barriers to scaling-up of harm reduction programmes. Health Policy. 2008;85(2):162–171. 10.1016/j.healthpol.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 41. Fixsen DL, Blase KA, Naoom SF, et al. : Core implementation components. Res Social Work Prac. 2009;19(5):531–540. 10.1177/1049731509335549 [DOI] [Google Scholar]
- 42. Hanson K, Nathan R, Marchant T, et al. : Vouchers for scaling up insecticide-treated nets in Tanzania: methods for monitoring and evaluation of a national health system intervention. BMC Public Health. 2008;8(Suppl 1):205. 10.1186/1471-2458-8-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harvey G, Fitzgerald L, Fielden S, et al. : The NIHR Collaborations for Leadership in Applied Health Research and Care (CLAHRC) for Greater Manchester: combining empirical, theoretical and experiential evidence to design and evaluate a large-scale implementation strategy. Implement Sci. 2011;6:96. 10.1186/1748-5908-6-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huicho L, Dávila M, Campos M, et al. : Scaling up integrated management of childhood illness to the national level: achievements and challenges in Peru. Health Policy Plan. 2005;20(1):14–24. 10.1093/heapol/czi002 [DOI] [PubMed] [Google Scholar]
- 45. Knapp H, Anaya HD, Feld JE, et al. : Launching nurse-initiated HIV rapid testing in Veterans Affairs primary care: a comprehensive overview of a self-sustaining implementation. Int J STD AIDS. 2011;22(12):734–737. 10.1258/ijsa.2009.009252 [DOI] [PubMed] [Google Scholar]
- 46. Nilsen P, Ståhl C, Roback K, et al. : Never the twain shall meet?--a comparison of implementation science and policy implementation research. Implement Sci. 2013;8:63. 10.1186/1748-5908-8-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pérez D, Lefèvre P, Castro M, et al. : Process-oriented fidelity research assists in evaluation, adjustment and scaling-up of community-based interventions. Health Policy Plan. 2011;26(5):413–422. 10.1093/heapol/czq077 [DOI] [PubMed] [Google Scholar]
- 48. Powell BJ, McMillen JC, Proctor EK, et al. : A compilation of strategies for implementing clinical innovations in health and mental health. Med Care Res Rev. 2012;69(2):123–157. 10.1177/1077558711430690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Richards DA, Bower P, Pagel C, et al. : Delivering stepped care: an analysis of implementation in routine practice. Implement Sci. 2012;7:3. 10.1186/1748-5908-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Breitenstein SM, Gross D, Garvey CA, et al. : Implementation fidelity in community-based interventions. Res Nurs Health. 2010;33(2):164–173. 10.1002/nur.20373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Glasgow RE, Chambers D: Developing robust, sustainable, implementation systems using rigorous, rapid and relevant science. Clin Transl Sci. 2012;5(1):48–55. 10.1111/j.1752-8062.2011.00383.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gupta R, Irwin A, Raviglione MC, et al. : Scaling-up treatment for HIV/AIDS: lessons learned from multidrug-resistant tuberculosis. Lancet. 2004;363(9405):320–324. 10.1016/S0140-6736(03)15394-9 [DOI] [PubMed] [Google Scholar]
- 53. Milat AJ, King L, Bauman AE, et al. : The concept of scalability: increasing the scale and potential adoption of health promotion interventions into policy and practice. Health Promot Int. 2012;28(3):285–298. 10.1093/heapro/dar097 [DOI] [PubMed] [Google Scholar]
- 54. McDonald SK, Keesler VA, Kauffman NJ, et al. : Scaling-up exemplary interventions. Educ Res. 2006;35(3):15–24. 10.3102/0013189X035003015 [DOI] [Google Scholar]
- 55. Cleary SM: Commentary: Trade-offs in scaling up HIV treatment in South Africa. Health Policy Plan. 2010;25(2):99–101. 10.1093/heapol/czp068 [DOI] [PubMed] [Google Scholar]
- 56. Gilson L, Schneider H: Commentary: Managing scaling up: what are the key issues? Health Policy Plan. 2010;25(2):97–98. 10.1093/heapol/czp067 [DOI] [PubMed] [Google Scholar]
- 57. Gloyd S, Montoya P, Floriano F, et al. : Scaling up antenatal syphilis screening in Mozambique: transforming policy to action. Sex Transm Dis. 2007;34(7 Suppl):S31–6. 10.1097/01.olq.0000264586.49616.72 [DOI] [PubMed] [Google Scholar]
- 58. Hanson K, Ranson MK, Oliveira-Cruz V, et al. : Expanding access to priority health interventions: A framework for understanding the constraints to scaling-up. J Int Dev. 2003;15(1):1–14. 10.1002/jid.963 [DOI] [Google Scholar]
- 59. Mangham LJ, Hanson K: Scaling up in international health: what are the key issues? Health Policy Plan. 2010;25(2):85–96. 10.1093/heapol/czp066 [DOI] [PubMed] [Google Scholar]
- 60. Blanchard JF, Bhattacharjee P, Kumaran S, et al. : Concepts and strategies for scaling up focused prevention for sex workers in India. Sex Transm Infect. 2008;84(Suppl 2):ii19–23. 10.1136/sti.2008.033134 [DOI] [PubMed] [Google Scholar]
- 61. Hanson K, Cleary S, Schneider H, et al. : Scaling up health policies and services in low- and middle-income settings. BMC Health Serv Res. 2010;10(Suppl 1):I1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harries AD, Zachariah R, Jahn A, et al. : Scaling up antiretroviral therapy in Malawi-implications for managing other chronic diseases in resource-limited countries. J Acquir Immune Defic Syndr. 2009;52(Suppl 1):S14–6. 10.1097/QAI.0b013e3181bbc99e [DOI] [PubMed] [Google Scholar]
- 63. Lister S: 'Scaling-up' in emergencies: British NGOs after Hurricane Mitch. Disasters. 2001;25(1):36–47. 10.1111/1467-7717.00160 [DOI] [PubMed] [Google Scholar]
- 64. Norton WE, McCannon CJ, Schall MW, et al. : A stakeholder-driven agenda for advancing the science and practice of scale-up and spread in health. Implement Sci. 2012;7:118. 10.1186/1748-5908-7-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Quelapio MI, Mira NR, Orillaza-Chi RB, et al. : Responding to the multidrug-resistant tuberculosis crisis: mainstreaming programmatic management to the Philippine National Tuberculosis Programme. Int J Tuberc Lung Dis. 2010;14(6):751–757. [PubMed] [Google Scholar]
- 66. Tansella M, Thornicroft G: Implementation science: understanding the translation of evidence into practice. Br J Psychiatry. 2009;195(4):283–285. 10.1192/bjp.bp.109.065565 [DOI] [PubMed] [Google Scholar]
- 67. Subramanian S, Naimoli J, Matsubayashi T, et al. : Do we have the right models for scaling up health services to achieve the Millennium Development Goals? BMC Health Serv Res. 2011;11:336. 10.1186/1472-6963-11-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kumaranayake L: The economics of scaling up: cost estimation for HIV/AIDS interventions. AIDS. 2008;22(Suppl 1):S23–33. 10.1097/01.aids.0000327620.47103.1d [DOI] [PubMed] [Google Scholar]
- 69. Johansson N, Lofgren A: Designing for extensibility: An action research study of maximizing extensibility by means of design principles.[Bachelor of Applied Information Technology]. University of Gothenburg;2009. Reference Source [Google Scholar]
- 70. Debrecency R, Felden C, Ochocki B, et al. : XBRL for interactive data: Engineering the information value chain.Berlin: Springer;2009. 10.1007/978-3-642-01437-6 [DOI] [Google Scholar]
- 71. Knapp H, Anaya HD, Goetz MB: Attributes of an independently self-sustaining implementation: nurse-administered HIV rapid testing in VA primary care. Qual Manag Health Care. 2010;19(4):292–297. 10.1097/QMH.0b013e3181fa06f8 [DOI] [PubMed] [Google Scholar]
- 72. Mushi HP, Mullei K, Macha J, et al. : The challenges of achieving high training coverage for IMCI: case studies from Kenya and Tanzania. Health Policy Plan. 2011;26(5):395–404. 10.1093/heapol/czq068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lewis S: Can a learning-disabled nation learn healthcare lessons from abroad? Healthc Policy. 2007;3(2):19–28. 10.12927/hcpol.2007.19388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chambers DA, Glasgow RE, Stange KC: The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci. 2013;8:117. 10.1186/1748-5908-8-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Abelson J, Forest PG, Eyles J, et al. : Deliberations about deliberative methods: issues in the design and evaluation of public participation processes. Soc Sci Med. 2003;57(2):239–251. 10.1016/S0277-9536(02)00343-X [DOI] [PubMed] [Google Scholar]
- 76. Oliver K, Lorenc T, Innvaer S: New directions in evidence-based policy research: a critical analysis of the literature. Health Res Policy Syst. 2014;12:34. 10.1186/1478-4505-12-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ellen ME, Léon G, Bouchard G, et al. : Barriers, facilitators and views about next steps to implementing supports for evidence-informed decision-making in health systems: a qualitative study. Implement Sci. 2014;9:179. 10.1186/s13012-014-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sanders EBN, Strappers PJ: Co-creation and the new landscapes of design. CoDesign. 2008;4(1):5–18. Reference Source [Google Scholar]
- 79. Lee SM, Olson DL, Trimi S: Co-innovation: Convergenomics, collaboration, and co-creation for organizational values. Management Decision. 2012;50(5):817–831. 10.1108/00251741211227528 [DOI] [Google Scholar]
- 80. DePasse JW, Lee PT: A model for ‘reverse innovation’ in health care. Global Health. 2013;9:40. 10.1186/1744-8603-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Govindarajan V, Trimble C: Reverse innovation: Create far from home, win everywhere. Harvard Business Review. 2010. [Google Scholar]
- 82. Bhattacharyya O, Reeves S, Garfinkel S, et al. : Designing theoretically-informed implementation interventions: fine in theory, but evidence of effectiveness in practice is needed. Implement Sci. 2006;1:5. 10.1186/1748-5908-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Improved Clinical Effectiveness through Behavioural Research Group (ICEBeRG): Designing theoretically-informed implementation interventions. Implement Sci. 2006;1:4. 10.1186/1748-5908-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Reeves S, Albert M, Kuper A, et al. : Why use theories in qualitative research? BMJ. 2008;337:a949. 10.1136/bmj.a949 [DOI] [PubMed] [Google Scholar]
- 85. Brabham D: Crowdsourcing as a model for problem solving: An introduction and cases. Convergence. 2008;14(1):75–90. 10.1177/1354856507084420 [DOI] [Google Scholar]
- 86. Gupta A, Thorpe C, Bhattacharyya O, et al. : Dataset 1 in: Promoting development and uptake of health innovations: The Nose to Tail Tool. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed]