Abstract
Infectious diseases often emerge from interactions among multiple species and across nested levels of biological organization. Threats as diverse as Ebola virus, human malaria, and bat white-nose syndrome illustrate the need for a mechanistic understanding of the ecological interactions underlying emerging infections. We describe how recent advances in community ecology can be adopted to address contemporary challenges in disease research. These analytical tools can identify the factors governing complex assemblages of multiple hosts, parasites, and vectors, and reveal how processes link across scales from individual hosts to regions. They can also determine the drivers of heterogeneities among individuals, species, and regions to aid targeting of control strategies. We provide examples where these principles have enhanced disease management and illustrate how they can be further extended.
Despite notable successes (1, 2), infectious diseases remain a leading source of human morbidity and mortality (3) and continue to threaten wildlife conservation and food production (4–6). A common factor underlying emerging diseases is the involvement of multiple host, vector, or parasite species in complex ecological communities. Nearly 70% of emerging human infectious diseases have wildlife hosts or vectors (7, 8), while several human parasites have spilled over to cause morbidity and mortality in wildlife, such as measles in mountain gorillas and tuberculosis in Asian elephants (9) (Fig. 1). The use of multiple hosts by parasites complicates control efforts that target particular hosts for management; for example, Schistosoma japonicum, the primary cause of human schistosomiasis in Asia, can infect 120 different species of mammals (10). Similarly, more than 20 species of triatomine bugs can transmit Trypanosoma cruzi, which causes Chagas disease in South America, such that efforts to control the dominant vector species alone may be inadequate to achieve elimination (11). Such threats continue to grow in importance as global travel and human activities increase contact with novel sources of parasites and aid their spread across the globe (6).
Fig. 1. The community ecology of infectious disease.
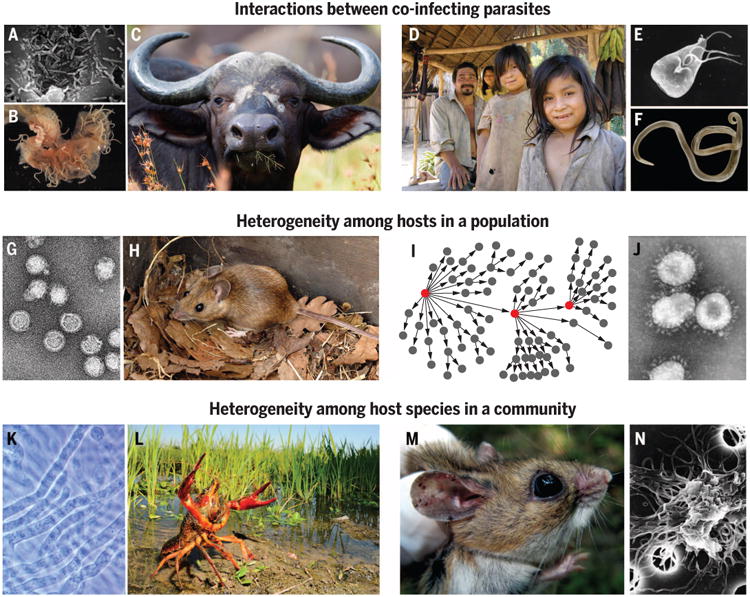
(A to C) Co-infection by nematodes (A) increases host mortality due to bovine TB (B) among African buffalo (C) (63). (D to F) Tsimane villagers in Bolivia (D) reveal negative correlations between Giardia lamblia (E) and Ascaris lumbricoides (F), where deworming increased Giardia (99). (G and H) For tick-borne encephalitis (G), 93% of transmission events involve large-bodied, male yellow-necked mice (H), which constitute <20% of the population (53). (I and J) For humans, disproportionate contact among individuals (I) led to “superspreading events” for SARS (J) (50). (K to N) Among-species heterogeneities can alter community-wide transmission. Crayfish plague (K) introduced to Europe with highly susceptible red swamp crayfish (L) led to native crayfish declines; biodiversity losses tend to promote interactions between ticks and white-footed mice (M), which are highly competent hosts for Borrelia burgdorferi (N) and influence production of infected ticks that transmit Lyme borreliosis (65). [Image credits: [(A), (E), (I), (J)] CDC, (B) R. Grencis, (C) Y. Krishnappa, (D) A. Pisor, (F) F Dubs, (G) (100) (H) V. Dostál, (K) T. Vrålstad, (L) F Pupin, (M) J. Brunner, (N) NIH]
Alongside the multihost nature of many infections, interactions among co-infecting parasites can alter host pathology, parasite transmission, and virulence evolution (12–14). Parasites that disrupt immune function (Fig. 1), such as HIV, have facilitated the reemergence of drug-resistant forms of tuberculosis (15); co-infection with parasitic worms (helminths) such as hookworm can exacerbate malaria (16). Interactions between several parasite species have been similarly implicated in coral reef diseases, epidemics in plants, and marine mammal die-offs (17–19). Because many host-parasite interactions are intimately embedded within communities of organisms, management efforts are sometimes thwarted by “ecological surprises” (20). Recent examples include the unexpected amplification of MERS (Middle East respiratory syndrome) coronavirus in internationally traded camels, and increased contact between badgers and cattle after implementation of badger culling, ultimately leading to increased rather than decreased transmission of bovine tuberculosis in the United Kingdom (21–23). Managing the challenges of emerging infectious diseases thus requires a clear understanding of the full ecological context of infection and transmission.
Our ability to understand and control infectious diseases has much to gain from the discipline of community ecology, which has developed a range of analytical tools for addressing complexity, species interactions, and multilevel scaling (Fig. 2). These tools can be adopted to improve our understanding and management of infectious diseases, both by quantifying environmental and biological factors governing the structure of complex communities of multiple hosts, vectors, and parasites, and also by identifying the effect and source(s) of heterogeneity among individual hosts, host species, and geographic locations. These tools further offer insight into interactions and feedbacks across multiple scales of organization, from within hosts to across regions. We examine how the application of tools and concepts from community ecology can help public health efforts to manage infectious disease threats.
Fig. 2. Ecological hierarchies applied to host-parasite interactions and analogous processes in community ecology.
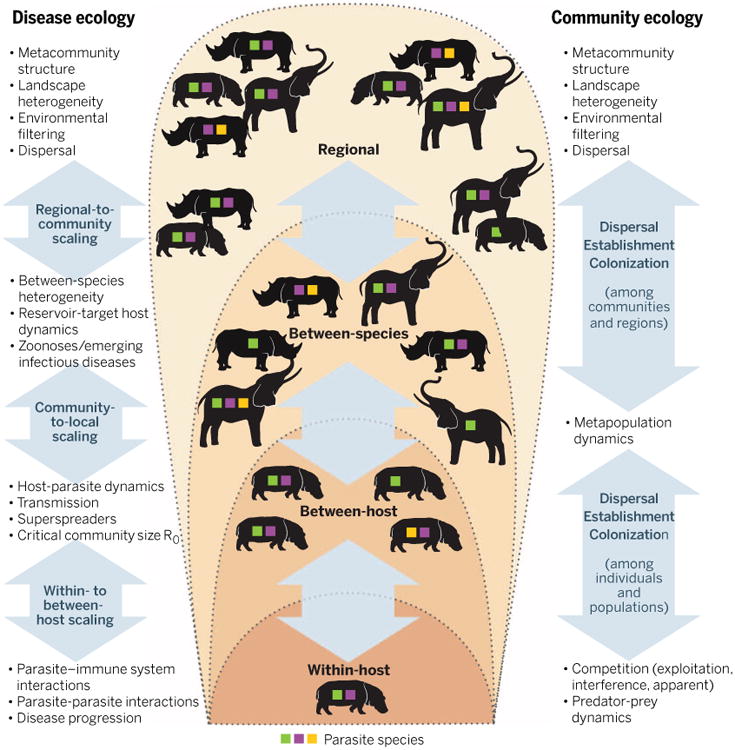
The range of scales includes within-host (“parasite infracommunity,” often dominated by parasite-parasite and parasite-immune system interactions); between-host (“parasite component community,” population biology); among species (“parasite supracommunity,” community ecology); and across regions (macroecology and disease biogeography). The different colored squares represent different parasite species; the text at the right and left highlights the relevant processes from community ecology and disease ecology, respectively. The potential importance for interactions and feedback across these scales represents an essential research frontier in the field of disease community ecology.
Community ecology as a framework to understand infectious diseases
Community ecology offers a mechanistic bridge between processes unfolding at the fine scale of individuals and populations and the ecological and evolutionary drivers of species distributions at coarser scales. Whereas some principles from community ecology have been applied to various host-parasite systems [e.g., (24–27)], the “community ecology of disease” remains in its relative infancy, with most studies focusing on interactions between a single host and parasite species, often at a single scale. Data availability and quality are increasing rapidly, partly through advances in sequencing technology, underscoring both the need for and the opportunity to implement new methods to study infection dynamics in complex natural systems.
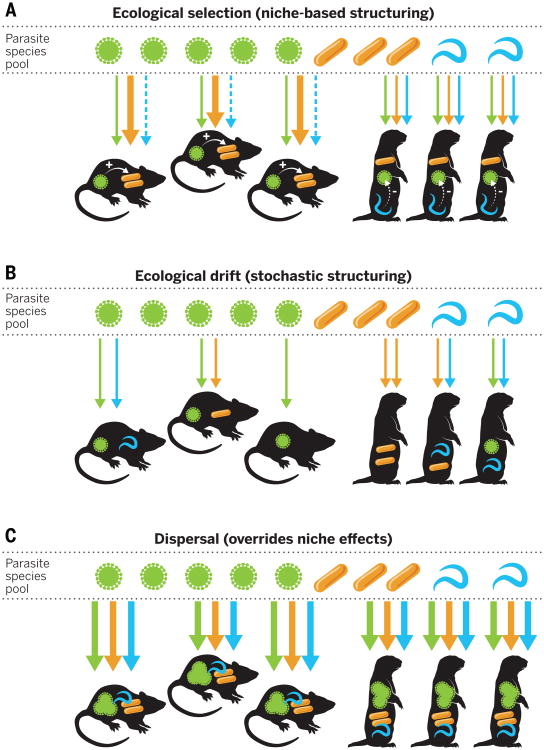
Community ecology theory tells us that, in parallel to the processes underlying population genetics theory (i.e., gene flow, selection, drift, and mutation), the diversity, abundance, and composition of species within a community can be understood in terms of dispersal, ecological selection, ecological drift, and speciation (28). After dispersal from the regional species pool, a species' success within a habitat is filtered by both niche-based and stochastic processes (29, 30). Within this framework, what needs to be understood is the degree to which community structure is built predictably from niche-based effects associated with interactions among species and the environment, or whether it arises through stochastic processes such as historical legacy, demographic stochasticity, and environmental fluctuations (Fig. 3).
Fig. 3. Parasite community assembly depends on a combination of ecological selection, ecological drift, and dispersal.
(A) After input via dispersal (indicated as arrows from the parasite regional pool), parasite establishment depends on ecological selection: different species (mice versus prairie dogs) select for different parasites according to genetics, behavior, immune status, and other host properties (including vaccination status or drug presence). Dashed arrows indicate failed infection. Deterministic, within-host parasite interactions (indicated by + and − signs) are an additional niche-based influence on parasite communities; positive parasite interactions (facilitation) are indicated by solid arrows; negative interactions are indicated by dashed arrows. (B) Parasite community assembly is also influenced by ecological drift (stochasticity), particularly when colonizing populations are small or the outcome of parasite interactions depends on their order of arrival (“priority effects”). As a result, parasite communities can appear random with respect to host species or type, even if strongly affected by species interactions. (C) High rates of dispersal can swamp niche effects and overwhelm stochasticity, resulting in more similar parasite communities across hosts, regardless of host species. For simplicity, no feedback loops are shown from the individual hosts back to the parasite pool, although understanding such feedbacks represents an important research priority (Fig. 2).
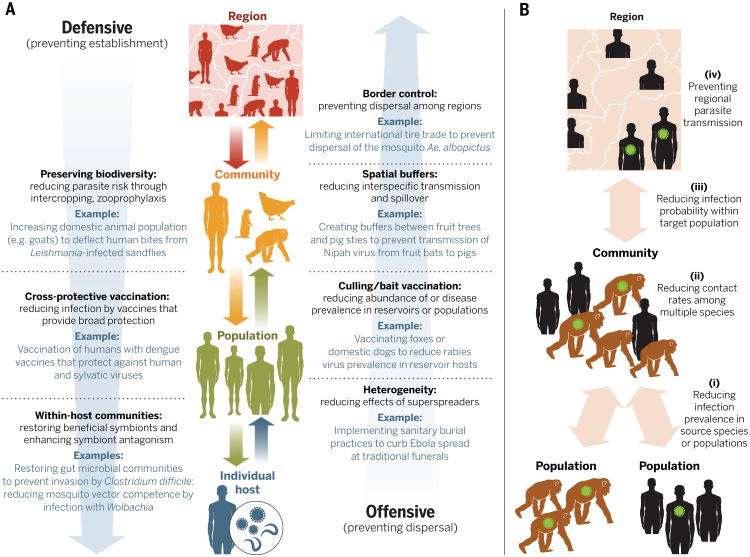
Within their niche, parasites are affected by host condition, immune responses, the abiotic environment, and interactions with co-infecting symbionts or associated free-living organisms. If the assembly of parasite communities is predominantly deterministic, then the richness and composition of parasite species will vary according to measurable characteristics of the host and the environment, and will therefore be predictable (Fig. 3). However, stochastic events and dispersal will also influence parasite colonization-extinction dynamics. In some systems, for instance, the outcome of parasite interactions depends strongly on the order of arrival within the host (14, 31). For example, long-term sampling of wild field vole (Microtus agrestis) populations revealed that infection with the protozoan Babesia microti reduced the probability that a host subsequently became infected with the bacteria Bartonella spp.; however, if Bartonella established first, then B. microti was only 25% as likely to invade (14). Similarly, high propagule dispersal by parasites can overcome niche effects related to host susceptibility (32). For instance, although humans are dead-end hosts with no onward transmission for many zoonotic infections, high exposure to such parasites can have serious consequences for public health, such as West Nile encephalitis and late-stage Lyme disease. Quantifying the relative contributions of niche-based and dispersal-based processes in determining parasite community structure and individual infection risk offers an ecological foundation for guiding resource investment into either defensive strategies, which focus on altering niches to inhibit parasite establishment, or offensive strategies, which focus on limiting dispersal (Fig. 4).
Fig. 4. How community ecology can inform infectious disease management.
(A) Using community ecology–based management strategies for infectious disease. Levels of ecological organization are shown in the middle, and colored arrows indicate the ecological processes that connect these levels. Parasite dispersal connects scales going up through the hierarchy; parasite establishment connects scales moving down the hierarchy. Blue arrows indicate the relative importance of offensive strategies (preventing parasite dispersal) and defensive strategies (preventing parasite establishment), with darker shades reflecting greater importance. (B) Management strategies focused on reducing spillover from wildlife to humans (zoonosis) and from humans to wildlife (anthronosis or reverse zoonosis). Probability of spillover and subsequent spread of infection can be reduced through four major strategies: (i) Control may focus on reducing disease prevalence in reservoir hosts; for instance, vaccine baits have been successfully used to eliminate rabies from several European countries (80). (ii) Contact rates can be reduced between humans and wild animals (8); for example, limiting the proximity between humans and wildlife can reduce spillover of human illnesses such as measles, tuberculosis, and MRSA to wildlife. (iii) Zoonotic risk can be reduced by lowering the probability of infection when contact is unavoidable or unpredictable. For instance, some human dengue vaccine candidates provide cross-protection against sylvatic dengue viruses, which naturally circulate in nonhuman primates (85). (iv) When spillover does occur, regional control strategies—including isolation of infected populations, dispatching of medical personnel and aid, and enhanced border control—can be used to prevent disease transmission across borders.
Approaches for understanding multilevel infection processes
Parasite metacommunities and assembly theory
Metacommunity theory provides a valuable toolkit for understanding the relative importance of niche-based effects and dispersal-based effects in regulating the structure of parasite communities (24, 33). By recognizing that landscapes support a series of ecological communities connected through dispersal, metacommunity theory links interactions across local and regional scales (32). For parasites, this framework is relevant to communities of parasites dispersing among host individuals or across disjunct landscapes. Although rarely applied to parasite communities, metacommunity-based approaches offer the potential to explore the interactive roles of evolutionary history, dispersal limitation, host community composition, and the abiotic environment in driving parasite distributions (34) (Fig. 2). In a long-term study of 65 parasite species from 15 species of desert rodents, for instance, Dallas and Presley (35) found that parasite community structure was driven by niche effects associated with the “patch quality” of host species, including host traits such as body size, longevity, and abundance, rather than by characteristics related to dispersal opportunities, such as host diet breadth, home range size, or evolutionary history. In a study of plant parasites, Parker et al. (36) recently showed that spillover risk in field experiments could be predicted by knowing the abundance of the host and its phylogenetic relationships with other hosts in the community. In contrast to free-living communities, parasite metacommunities do incur some unique analytical challenges, including the potential for infections to sicken or kill individual hosts and thereby alter the availability of habitat “patches” for dispersal (26). Likewise, because parasites also interact with each other, the co-assembly of host and parasite communities needs to be examined concurrently (37), and an extra nested scale (i.e., for the within-host dynamics) often needs to be included in analyses (Fig. 2).
Tools from network theory can be additionally valuable for understanding how interactions between entire host and parasite communities vary over space and time (38). For instance, Griffiths et al. (39) used network approaches to show that co-infecting parasites of humans were organized into dense clusters around distinct locations in the body (e.g., organs) and tended to interact with each other via shared resources within the host, rather than via the immune system. Similar approaches have been applied across other scales of organization–for example, to define contact pathways for transmission among individual hosts (40, 41) and to identify the role of parasites in structuring ecological food webs (42). Although the focus of network approaches thus far has often been on the patterns of links among species, emerging tools allow for more explicit examination of interaction strengths, which will help to forecast dynamic changes in the system (43).
Infection heterogeneity and traits-based approaches
Community ecology emphasizes the importance of understanding individual and species-level functional traits, thereby offering greater mechanistic and predictive power relative to simple taxonomic classifications (44, 45). Predicting the specific identities of species within an assemblage is made difficult by stochastic factors such as historical legacy, whereas the composition and frequency of functional traits may be more deterministic (46). Thus, although hosts and parasites are typically defined in taxonomic terms, it may be more useful to classify them in terms of functional traits that influence performance. For parasites, such traits include transmission mode, site of infection, and resource use; for hosts, they include body size, dispersal ability, and immune competence. For instance, Han et al. (47) identified “trait profiles” of known reservoir species and used these to forecast candidate rodents likely to act as reservoirs for future zoonotic infections. Their analysis revealed the importance of “fast-paced” species that reproduce early and often; by contrast, taxonomic labels did a relatively poor job of classifying reservoir host status.
Trait-based analyses align with the long-standing recognition in disease ecology of the disproportionate influence of superspreader individuals, amplification or reservoir host species, or “hotspot” locations in driving transmission (22, 48, 49). Superspreading events have been recorded for both wildlife and human diseases, including typhoid fever, HIV-1, SARS, and tuberculosis (22, 50, 51), and can sometimes be linked to measurable variation in traits such as host immunity, behavior, age, diet, and sex (52–54). For example, Perkins et al. (53) found that large-bodied, sexually active male mice contribute 93% of potential transmission events for tick-borne encephalitis virus, despite representing only ∼20% of the host population (Fig. 1). Methods to partition the contributions of particular hosts, species, or locations to parasite transmission are beginning to be developed (48, 55). For example, Rudge et al. (10) quantified host species contributions to the number of cases generated (R0) of S. japonicum in China, for which more than 120 host species have been identified. They showed that bovids maintain infection in marshlands, whereas rodents are the main source of transmission in hilly areas, which suggests that different control strategies are needed in the two habitats. The key challenge for management is to identify how much of this heterogeneity is linked to measurable traits, and is therefore predictable (niche-based), or whether it arises stochastically through unpredictable temporal or spatial heterogeneity in exposure (56).
Moving across scales
A core principle of community ecology is the importance of scale in affecting the strength and form of species interactions not only with each other but also with the environment (57) (Fig. 2). Research in disease ecology often falls into one of three distinct levels: (i) within-host, which is concerned with interactions with the host immune system and other parasites (13, 58); (ii) between-host, which is focused on parasite spread through host populations (59, 60) or, less often, through host communities; or (iii) on regional or biogeographical scales, which use comparative methods from macroecology to explore the drivers of parasite distributions and diversity (61).
Studies focused on one scale often ignore, or treat as phenomenological black boxes, the dynamics occurring at higher and lower scales; in reality, it appears that dynamic interactions occur in both directions (41, 57). For instance, interactions among co-infecting parasites within hosts can cause individual variation in susceptibility, infectiousness, behavior, and survival (14, 62, 63), potentially with counterintuitive consequences for transmission at the population level (64). African buffalo co-infected with gastrointestinal nematodes and bovine tuberculosis (bTB) exhibit increased mortality (Fig. 1), such that treating animals to reduce their worm burdens improves individual survival but, by enabling infected hosts to live longer, is predicted to increase population-level spread of bTB (63). Reciprocally, variation in host community composition within a region can affect infection risk and spread at the individual and population levels (10, 55). For vector-borne infections such as Lyme disease, wildlife species vary considerably in their tendency to amplify the bacterium responsible and transmit it to suitable tick vectors, such that regional variation in host species diversity is hypothesized to be a major determinant of local infection risk for humans (65) (Box 1). However, such cross-scale processes are hard to infer from observational data alone, and experimental perturbations are often needed to definitively assess how processes at one scale affect those at another (66). In parallel with the rich legacy of system manipulations from community ecology (67), disease ecologists have increasingly used experimental approaches involving natural systems—for example, through antiparasite drug treatments (68), hormone manipulation (69), nutrient supplementation (70), and diversity manipulations (71, 72). Although these experiments have often focused on single host–single parasite systems, implementing such experiments in more complex natural communities and at larger scales is increasingly important for testing hypotheses about parasite transmission, impact, and control.
Box 1. The role of simple theory in disease ecology and its extension to complex communities.
The pioneering work of Anderson and May (60, 101) formalized our understanding of parasite dynamics by highlighting the importance of the basic reproductive number (R0) as a measure of whether a parasite will spread through a population (R0 > 1) or die out (R0 < 1).The fundamental principles of these basic models—initially developed for single host–single parasite systems—can provide insight into infection dynamics in more complex ecological systems. For example, parasites often face a diverse community of potential host species that differ in abundance, susceptibility, and infectiousness. Simple extensions of basic disease ecology theory can determine the conditions under which one host species amplifies or dilutes infection risk for other species in the community. For directly transmitted parasites, or even those transmitted via infective stages in the environment, theory shows that the parasite's overall basic reproductive number among the available host community (R0,TOT) can simply be proportional to the sum of the R0 for each host species alone, provided there is equal mixing within and between host species (although other relationships between the individual-level and community-level R0 values may occur if mixing is not equal) (10, 102). Hence, there is a clear connection between this more complex scenario and the classical single-host theory.
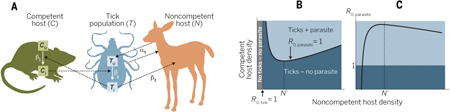
This theory can be extended further for vector-borne parasites, which become complicated to model when hosts differ in their relative competencies for the parasite and the vector. For example, tick-borne parasites may involve a mammalian host species that is parasite-competent but cannot support tick reproduction, as well as another mammalian species that is noncompetent for the parasite but essential for tick reproduction, as shown in the figure. Here, there are three possible outcomes: (i) tick and parasite exclusion, (ii) tick persistence but parasite exclusion, and (iii) tick and parasite persistence, depending on different combinations of the R0 values for the parasite (R0, parasite) and the tick (R0,tick). Ultimately, this results in outcomes that are nonlinearly related to the density of the noncompetent host; initial increases in noncompetent host abundance (N) can cause vector amplification, leading to increased parasite R0, whereas high N dilutes transmission through “wasted” bites on the noncompetent host (103-105).
The figure shows a model of a tick-borne parasite system with two host species, showing potential for both amplification and dilution within the same system. (A) Schematic diagram of the model, where one host species (C) is parasite-competent but cannot support tick reproduction, and the other (N) is noncompetent but essential for tick reproduction. This system can be described by the following equations [modified from (103–105)]:
where T is the total number of ticks (TU in the figure is the number of uninfected ticks; TI is the number of infected ticks), CP is parasite prevalence within C (CU in the figure is the number of uninfected hosts; CI is the number of infected hosts), β1 is the tick → C transmission rate of the parasite, β2 is the C → tick transmission rate of the parasite, β3 is the tick → N biting rate, δC and δT are the respective mortality rates of competent hosts and ticks, aT is the tick reproduction rate, and sT is the strength of tick density dependence. (B) Phase plot of competent host (C) and noncompetent host (N) densities, showing the three regions of dynamical outcome separated by the boundaries of R0,tick = 1 and R0, parasite = 1, where R0,tick = αTβ3N/δT and R0, parasite = [Cβ1β2(αtβ3N − δt)]/[STβ3Nδc(δT + β3N)]. (C) R0,parasite as a function of noncompetent host density, showing that low host densities facilitate parasite transmission due to vector amplification, whereas high host densities reduce parasite transmission through wasted tick bites. The vertical line marked N′ (given by the value of N at which shows the noncompetent host density at which the effect on the parasite switches from amplification to dilution.
How community ecology can help manage infectious diseases
We suggest that disease control strategies would benefit by incorporating community ecology theory and approaches to explicitly account for the joint influences of dispersal and environmental filters. Specifically, the “offensive” versus “defensive” concept developed for invasive species can be applied to disease management [Fig. 4; see also (73)]. Offensive strategies allocate resources to limit the dispersal of an invader from established sites, whereas defensive efforts reduce the vulnerability of uninvaded habitats to colonization (74). Although this concept parallels existing epidemiological emphasis on prevention versus control, its successful application requires deeper insights into whether a parasite community is dispersal-limited, niche-based, or random in its assembly (Fig. 3). This approach can be used to strengthen current methods of infectious disease management across the gamut of multihost parasites, multisymbiont communities, and infection heterogeneities across scales (Fig. 2).
Managing multihost parasites
A current pressing question is how ongoing changes in biodiversity will affect the spread and severity of infectious diseases (66, 75). When diverse communities also support species that interfere with transmission, such as the presence of low-susceptibility hosts, predators, or symbionts, community structure can be manipulated defensively to manage infections by limiting niche suitability (37). For example, zooprophylaxis (in which livestock are used as bait to divert blood-feeding arthropod vectors away from people) has been proposed as a control strategy for vector-borne diseases for more than a century, but has had limited success in some settings because increased livestock density can also increase vector abundance. However, recent models on malaria and zoonotic cutaneous leishmaniasis indicate that carefully chosen livestock densities coupled with insecticide treatment can effectively reduce parasite transmission to humans (76, 77). Similar approaches, such as intercropping and crop rotation, have been used successfully to reduce plant pests and parasites in agricultural systems (78). Although evidence for such dilution effects continues to grow (79), the degree to which biodiversity will regulate infection by a particular parasite depends on the degree to which host assembly is deterministic, whether the parasite is niche- or dispersal-limited, and how increases in richness affect host and vector abundance (66).
Managing host communities is also crucial to mitigating the risk of spillover events from animal reservoirs to humans. To minimize spillover, there are several potential offensive and defensive approaches (Fig. 4), the choice of which will depend on the specific biology of the hosts and vectors involved. The first option is to reduce infection in reservoir hosts. For instance, vaccine baits have successfully eliminated rabies from several European countries through their protective effects on nonhuman hosts (80). The second approach is to limit contacts between wildlife and humans—for example, by reducing bushmeat consumption and its potential to introduce novel infections (81). In West Africa, increasing the use of alternative protein sources such as marine fish could relieve pressure on the bushmeat trade (82). Such approaches require tight coordination among many parties, including medical scientists, anthropologists, and governments. Similarly, the use of transmission barriers can help to limit contact between wildlife reservoirs and domestic animals (83, 84). The third approach is to reduce the probability of infection when contact is unavoidable or unpredictable. Ongoing yet unpredictable spillovers of dengue viruses from nonhuman primates, for instance, complicate the control of human disease in Southeast Asia and Africa. One approach to control such infections is through the implementation of cross-reactive vaccines, which are currently under development (85). When vaccines are not yet available, as was the case for Ebola virus during most of the 2014–2015 epidemic, reducing human-human transmission through contact tracing and subsequent quarantine and treatment can help to limit epidemic spread (86).
Managing symbiont communities
Interactions among co-infecting parasites or symbionts can also be used as niche-based management tools (Fig. 4). For example, treating patients suffering from lymphatic filariasis with the antibiotic doxycycline eliminates essential symbiotic bacteria required by filarial worms, ultimately leading to worm sterility and death (87). Restoration or augmentation of the microbial community within the host can also provide protection against parasite invasion. For example, transferring human-microbial communities by fecal transplants often leads to clinical resolution of intestinal pathology associated with Clostridium difficile infection (88). Finally, interactions among co-infecting parasites, parasite strains, or other symbionts can be manipulated to reduce the spread of disease-causing organisms. Long-lived parasites, such as helminths, may exacerbate disease caused by co-infecting parasites, leading to calls to incorporate deworming to improve management of HIV, malaria, and TB (16, 89). In other cases, antagonistic interactions between parasites or other symbionts may be used to benefit the host. For instance, trials are under development to reduce the vector competence of mosquitoes by infecting them with the bacterium Wolbachia, which inhibits dengue virus and filarial worm survival and transmission through a combination of immune activation, competition for cellular components, and shortened mosquito lifespan (90, 91). These examples emphasize the importance of understanding and predicting the outcome of multiple infections, for which community ecology approaches focused on parasite traits and resource use have already offered added insights (92).
Heterogeneity and scale
The disproportionate roles of particular locations, particular host species, and particular individual hosts in driving epidemics or epizootics raise the tantalizing promise of highly efficient targeted control and treatment (22, 48, 50). In the Serengeti, for example, where rabies can infect up to 12 carnivore species, domestic dogs are responsible for more than 70% of transmission events to humans (93). Annual vaccination of 60% of dogs is projected to control the virus, a target that is logistically and economically feasible (94). During the recent Ebola epidemic in West Africa, close contact between deceased patients and family or friends during traditional burials functioned as superspreading events (95), and implementation of “sanitary burials” that reduced such contacts helped to curb the epidemic. Thus, targeting superspreading hosts or events is feasible when transmission heterogeneities are deterministic and can be linked to measurable traits or characteristics.
Ultimately, the efficacy of offensive and defensive approaches will depend on whether the scale of application is local or regional, the transmission and dispersal characteristics of the parasite involved, and the point in the epidemic when the intervention is initiated (73). Defensive, niche-based management strategies, ranging from vaccination and prophylaxis to ecological competition by probiotic symbionts, are more likely to be effective when parasite dispersal is high, for parasites with high or unpredictable propagule pressure, and for epidemics already under way (Fig. 4). In contrast, offensive strategies that focus on reducing dispersal are more likely to succeed at community and regional scales than at individual and population scales, because parasite dispersal between individuals within a host population is often harder to control than dispersal between sites. For instance, established populations of the Asian tiger mosquito Aedes albopictus, recently linked to a large outbreak of the viral disease chikungunya on the Indian Ocean island La Réunion (96), are almost impossible to eliminate; however, because most introductions of this vector have occurred through the shipment of used tires, focused efforts to limit this trade offer the best potential for containing future spread of the vector (96).
Outlook
The disciplines of epidemiology and community ecology have developed largely independently of one another. Nonetheless, the multispecies nature of many contemporary disease threats demands a community-scale approach to complement more traditional biomedical treatments. The proposed synthesis of “disease community ecology” offers a theoretical framework and the analytical tools to move beyond the historical emphasis on particular host-parasite interactions and consider the full suite of species that influence infection dynamics. We have emphasized approaches from community ecology that can advance our ability to manage infections by (i) identifying the factors that govern the structure and dynamics of communities composed of multiple hosts, vectors, and symbionts; (ii) isolating the drivers of heterogeneity; and (iii) understanding how processes and patterns link across multiple scales of biological organization. For many emerging infections, complete eradication is unlikely to be successful, but a broader understanding of the ecological communities in which host-parasite interactions are embedded will facilitate more effective management.
Transforming this broader understanding into practical disease management requires tight integration of surveillance, community ecology analysis, and public health implementation (97) (Fig. 4). Ongoing technological advances are rapidly over-coming previous barriers in data quality and quantity, highlighting emerging opportunities to incorporate approaches from community ecology into existing disease research and to evaluate the factors driving the structure and dynamics of natural disease systems. Combining analyses of these high-resolution data with modeling approaches and large-scale manipulations of host-parasite interactions—similar to the foundational experiments from community ecology (67)—offers excellent opportunities for developing a deeper understanding of the processes underlying disease emergence and control. To date, there have been some practical successes that follow this broad approach. For example, following the observation of five dead howler monkeys—a key host for yellow fever virus—a collaborative effort between the U.S. Agency for International Development (USAID) PREDICT program and the Bolivian government led to rapid implementation of human vaccination and mosquito control in the affected area (98). Similarly, increased use of buffer zones between fruit trees and livestock housing has been effectively used in Malaysia to reduce Nipah virus transmission into pigs and the risks of human outbreaks (83), while electrified fences in Kruger National Park have helped limit contact between bovine TB-infected wildlife and cattle in surrounding areas (84). Such scenarios demonstrate how a broader appreciation for the epidemiological links among humans, domestic animals, and wildlife can promote disease community ecology as a discipline and result in more effective control of disease risk in ecological communities.
Supplementary Material
Acknowledgments
For discussions and feedback helpful in shaping the manuscript, we thank S. Altizer, D. Calhoun, G. Devevey, I. Doron, S. Haas, K. Hoang, B. Hoye, M. Joseph, J. Koprivnikar, T. McDevitt-Galles, J. Mihaljevic, A. Pedersen, O. Petchey, A. Pierce, D. Preston, Y. Springer, W. Stutz, L. Tao, S. White, and members of the Macroecology of Infectious Disease Research Coordination Network (funded by NSF/NIH/USDA DEB 131223). Supported by NSF grant DEB-1149308 and NIH grant R01GM109499 (P.T.J.J.), NSF grant DEB-1257160 and NIH grant R01GM109501 (J.C.d.R.), and UK Natural Environment Research Council grants NE/G006830/1 and NE/I024038/1 (A.F.).
Footnotes
References and Notes
- 1.Jasny B, Roberts L, Enserink M, Smith O. What works [introduction to Global Health special issue] Science. 2014;345:1256–1257. doi: 10.1126/science.345.6202.1256. [DOI] [PubMed] [Google Scholar]
- 2.Sepúlveda J, Murray C. The state of global health in 2014. Science. 2014;345:1275–1278. doi: 10.1126/science.1257099. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. The Top 10 Causes of Death. 2014 www.who.int/mediacentre/factsheets/fs310/en.
- 4.Fisher MC, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purse BV, et al. Climate change and the recent emergence of bluetongue in Europe. Nat Rev Microbiol. 2005;3:171–181. doi: 10.1038/nrmicro1090. [DOI] [PubMed] [Google Scholar]
- 6.Smith KF, et al. Global rise in human infectious disease outbreaks. J R Soc Interface. 2014;11:20140950. doi: 10.1098/rsif.2014.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Smith JO, et al. Epidemic dynamics at the human-animal interface. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messenger AM, Barnes AN, Gray GC. Reverse zoonotic disease transmission (zooanthroponosis): A systematic review of seldom-documented human biological threats to animals. PLOS ONE. 2014;9:e89055. doi: 10.1371/journal.pone.0089055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudge JW, et al. Identifying host species driving transmission of schistosomiasis japonica, a multihost parasite system, in China. Proc Natl Acad Sci USA. 2013;110:11457–11462. doi: 10.1073/pnas.1221509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinovich JE, et al. Ecological patterns of blood-feeding by kissing-bugs (Hemiptera: Reduviidae: Triatominae) Mem Inst Oswaldo Cruz. 2011;106:479–494. doi: 10.1590/S0074-02762011000400016. [DOI] [PubMed] [Google Scholar]
- 12.Alizon S, de Roode JC, Michalakis Y. Multiple infections and the evolution of virulence. Ecol Lett. 2013;16:556–567. doi: 10.1111/ele.12076. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen AB, Fenton A. Emphasizing the ecology in parasite community ecology. Trends Ecol Evol. 2007;22:133–139. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Telfer S, et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan CK, Ernst JD. HIV and tuberculosis: A deadly human syndemic. Clin Microbiol Rev. 2011;24:351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Druilhe P, Tall A, Sokhna C. Worms can worsen malaria: Towards a new means to roll back malaria? Trends Parasitol. 2005;21:359–362. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Gibson AK, et al. Polyparasitism is associated with increased disease severity in Toxoplasma gondii-infected marine sentinel species. PLOS Negl Trop Dis. 2011;5:e1142. doi: 10.1371/journal.pntd.0001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voss JD, Mills DK, Myers JL, Remily ER, Richardson LL. Black band disease microbial community variation on corals in three regions of the wider Caribbean. Microb Ecol. 2007;54:730–739. doi: 10.1007/s00248-007-9234-1. [DOI] [PubMed] [Google Scholar]
- 19.Susi H, Barrès B, Vale PF, Laine AL. Co-infection alters population dynamics of infectious disease. Nat Commun. 2015;6:5975. doi: 10.1038/ncomms6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doak DF, et al. Understanding and predicting ecological dynamics: Are major surprises inevitable? Ecology. 2008;89:952–961. doi: 10.1890/07-0965.1. [DOI] [PubMed] [Google Scholar]
- 21.Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus: Transmission and phylogenetic evolution. Trends Microbiol. 2014;22:573–579. doi: 10.1016/j.tim.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson PJ, Perkins SE, Cattadori IM. In: Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. Ostfeld RS, Keesing F, Eviner VT, editors. Princeton Univ Press; Princeton, NJ: 2008. pp. 347–367. [Google Scholar]
- 23.Woodroffe R, et al. Culling and cattle controls influence tuberculosis risk for badgers. Proc Natl Acad Sci USA. 2006;103:14713–14717. doi: 10.1073/pnas.0606251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihaljevic JR. Linking metacommunity theory and symbiont evolutionary ecology. Trends Ecol Evol. 2012;27:323–329. doi: 10.1016/j.tree.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Rynkiewicz EC, Pedersen AB, Fenton A. An ecosystem approach to understanding and managing within-host parasite community dynamics. Trends Parasitol. 2015;31:212–221. doi: 10.1016/j.pt.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Seabloom EW, et al. The community ecology of pathogens: Coinfection, coexistence and community composition. Ecol Lett. 2015;18:401–415. doi: 10.1111/ele.12418. [DOI] [PubMed] [Google Scholar]
- 27.Holt RD, Dobson A. In: Disease Ecology: Community Structure and Pathogen Dynamics. Collinge SK, Ray C, editors. Oxford Univ Press; Oxford: 2006. pp. 6–27. [Google Scholar]
- 28.Vellend M. Conceptual synthesis in community ecology. Q Rev Biol. 2010;85:183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- 29.Adler PB, HilleRisLambers J, Levine JM. A niche for neutrality. Ecol Lett. 2007;10:95–104. doi: 10.1111/j.1461-0248.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 30.Chase JM. Drought mediates the importance of stochastic community assembly. Proc Natl Acad Sci USA. 2007;104:17430–17434. doi: 10.1073/pnas.0704350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292:1099–1102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- 32.Leibold MA, et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol Lett. 2004;7:601–613. doi: 10.1111/j.1461-0248.2004.00608.x. [DOI] [Google Scholar]
- 33.Presley SJ, Higgins CL, Willig MR. A comprehensive framework for the evaluation of metacommunity structure. Oikos. 2010;119:908–917. doi: 10.1111/j.1600-0706.2010.18544.x. [DOI] [Google Scholar]
- 34.Suzán G, et al. Metacommunity and phylogenetic structure determine wildlife and zoonotic infectious disease patterns in time and space. Ecol Evol. 2015;5:865–873. doi: 10.1002/ece3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dallas T, Presley SJ. Relative importance of host environment, transmission potential and host phylogeny to the structure of parasite metacommunities. Oikos. 2014;123:866–874. doi: 10.1111/oik.00707. [DOI] [Google Scholar]
- 36.Parker IM, et al. Phylogenetic structure and host abundance drive disease pressure in communities. Nature. 2015;520:542–544. doi: 10.1038/nature14372. [DOI] [PubMed] [Google Scholar]
- 37.Johnson PTJ, Preston DL, Hoverman JT, LaFonte BE. Host and parasite diversity jointly control disease risk in complex communities. Proc Natl Acad Sci USA. 2013;110:16916–16921. doi: 10.1073/pnas.1310557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poulin R. Network analysis shining light on parasite ecology and diversity. Trends Parasitol. 2010;26:492–498. doi: 10.1016/j.pt.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths EC, Pedersen AB, Fenton A, Petchey OL. Analysis of a summary network of co-infection in humans reveals that parasites interact most via shared resources. Proc R Soc B. 2014;281:20132286. doi: 10.1098/rspb.2013.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis S, Abbasi B, Shah S, Telfer S, Begon M. Spatial analyses of wildlife contact networks. J R Soc Interface. 2014;12:20141004. doi: 10.1098/rsif.2014.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tompkins DM, Dunn AM, Smith MJ, Telfer S. Wildlife diseases: From individuals to ecosystems. J Anim Ecol. 2011;80:19–38. doi: 10.1111/j.1365-2656.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- 42.Cirtwill AR, Stouffer DB. Concomitant predation on parasites is highly variable but constrains the ways in which parasites contribute to food web structure. J Anim Ecol. 2015;84:734–744. doi: 10.1111/1365-2656.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poisot T, Canard E, Mouillot D, Mouquet N, Gravel D. The dissimilarity of species interaction networks. Ecol Lett. 2012;15:1353–1361. doi: 10.1111/ele.12002. [DOI] [PubMed] [Google Scholar]
- 44.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol Evol. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Webb CT, Hoeting JA, Ames GM, Pyne MI, LeRoy Poff N. A structured and dynamic framework to advance traits-based theory and prediction in ecology. Ecol Lett. 2010;13:267–283. doi: 10.1111/j.1461-0248.2010.01444.x. [DOI] [PubMed] [Google Scholar]
- 46.Weiher E, et al. Advances, challenges and a developing synthesis of ecological community assembly theory. Philos Trans R Soc B. 2011;366:2403–2413. doi: 10.1098/rstb.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han BA, Schmidt JP, Bowden SE, Drake JM. Rodent reservoirs of future zoonotic diseases. Proc Natl Acad Sci USA. 2015;112:7039–7044. doi: 10.1073/pnas.1501598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paull SH, et al. From superspreaders to disease hotspots: Linking transmission across hosts and space. Front Ecol Environ. 2012;10:75–82. doi: 10.1890/110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Streicker DG, Fenton A, Pedersen AB. Differential sources of host species heterogeneity influence the transmission and control of multihost parasites. Ecol Lett. 2013;16:975–984. doi: 10.1111/ele.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen Z, et al. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10:256–260. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graham AL, et al. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science. 2010;330:662–665. doi: 10.1126/science.1194878. [DOI] [PubMed] [Google Scholar]
- 53.Perkins SE, Cattadori IM, Tagliapietra V, Rizzoli AP, Hudson PJ. Empirical evidence for key hosts in persistence of a tick-borne disease. Int J Parasitol. 2003;33:909–917. doi: 10.1016/S0020-7519(03)00128-0. [DOI] [PubMed] [Google Scholar]
- 54.Stutz WE, Lau OL, Bolnick DI. Contrasting patterns of phenotype-dependent parasitism within and among populations of threespine stickleback. Am Nat. 2014;183:810–825. doi: 10.1086/676005. [DOI] [PubMed] [Google Scholar]
- 55.Streicker DG, Fenton A, Pedersen AB. Differential sources of host species heterogeneity influence the transmission and control of multihost parasites. Ecol Lett. 2013;16:975–984. doi: 10.1111/ele.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calabrese JM, Brunner JL, Ostfeld RS. Partitioning the aggregation of parasites on hosts into intrinsic and extrinsic components via an extended Poisson-gamma mixture model. PLOS ONE. 2011;6:e29215. doi: 10.1371/journal.pone.0029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chesson P. Scale transition theory: Its aims, motivations and predictions. Ecol Complex. 2012;10:52–68. doi: 10.1016/j.ecocom.2011.11.002. [DOI] [Google Scholar]
- 58.Fenton A, Perkins SE. Applying predator-prey theory to modelling immune-mediated, within-host interspecific parasite interactions. Parasitology. 2010;137:1027–1038. doi: 10.1017/S0031182009991788. [DOI] [PubMed] [Google Scholar]
- 59.Anderson RM, May RM. Regulation and stability of hostparasite population interactions. I. Regulatory processes. J Anim Ecol. 1978;47:219–247. doi: 10.2307/3933. [DOI] [Google Scholar]
- 60.Anderson RM, May RM. The population dynamics of microparasites and their invertebrate hosts. Philos Trans R Soc London Ser B. 1981;291:451–524. doi: 10.1098/rstb.1981.0005. [DOI] [Google Scholar]
- 61.Dunn RR, Davies TJ, Harris NC, Gavin MC. Global drivers of human pathogen richness and prevalence. Proc R Soc B. 2010;277:2587–2595. doi: 10.1098/rspb.2010.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knowles SCL, et al. Stability of within-host-parasite communities in a wild mammal system. Proc R Soc B. 2013;280:20130598. doi: 10.1098/rspb.2013.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ezenwa VO, Jolles AE. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science. 2015;347:175–177. doi: 10.1126/science.1261714. [DOI] [PubMed] [Google Scholar]
- 64.Fenton A. Dances with worms: The ecological and evolutionary impacts of deworming on coinfecting pathogens. Parasitology. 2013;140:1119–1132. doi: 10.1017/S0031182013000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ostfeld RS, Keesing F. Effects of host diversity on infectious disease. Annu Rev Ecol Evol Syst. 2012;43:157–182. doi: 10.1146/annurev-ecolsys-102710-145022. [DOI] [Google Scholar]
- 66.Johnson PTJ, Ostfeld RS, Keesing F. Frontiers in research on biodiversity and disease. Ecol Lett. 2015 doi: 10.1111/ele.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schindler DW. Replication versus realism: The need for ecosystem-scale experiments. Ecosystems. 1998;1:323–334. doi: 10.1007/s100219900026. [DOI] [Google Scholar]
- 68.Pedersen AB, Fenton A. The role of antiparasite treatment experiments in assessing the impact of parasites on wildlife. Trends Parasitol. 2015;31:200–211. doi: 10.1016/j.pt.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 69.Grear DA, Perkins SE, Hudson PJ. Does elevated testosterone result in increased exposure and transmission of parasites? Ecol Lett. 2009;12:528–537. doi: 10.1111/j.1461-0248.2009.01306.x. [DOI] [PubMed] [Google Scholar]
- 70.Pedersen AB, Greives TJ. The interaction of parasites and resources cause crashes in a wild mouse population. J Anim Ecol. 2008;77:370–377. doi: 10.1111/j.1365-2656.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 71.Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD. Biodiversity decreases disease through predictable changes in host community competence. Nature. 2013;494:230–233. doi: 10.1038/nature11883. [DOI] [PubMed] [Google Scholar]
- 72.Suzán G, et al. Experimental evidence for reduced rodent diversity causing increased hantavirus prevalence. PLOS ONE. 2009;4:e5461. doi: 10.1371/journal.pone.0005461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langwig KE, et al. Context-dependent conservation responses to emerging wildlife diseases. Front Ecol Environ. 2015;13:195–202. doi: 10.1890/140241. [DOI] [Google Scholar]
- 74.Drury KLS, Rothlisberger JD. Offense and defense in landscape-level invasion control. Oikos. 2008;117:182–190. doi: 10.1111/j.2007.0030-1299.16081.x. [DOI] [Google Scholar]
- 75.Keesing F, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franco AO, Gomes MGM, Rowland M, Coleman PG, Davies CR. Controlling malaria using livestock-based interventions: A one health approach. PLOS ONE. 2014;9:e101699. doi: 10.1371/journal.pone.0101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaabi B, Ahmed SB. Assessing the effect of zooprophylaxis on zoonotic cutaneous leishmaniasis transmission: A system dynamics approach. Biosystems. 2013;114:253–260. doi: 10.1016/j.biosystems.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Zhu Y, et al. Genetic diversity and disease control in rice. Nature. 2000;406:718–722. doi: 10.1038/35021046. [DOI] [PubMed] [Google Scholar]
- 79.Civitello DJ, et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc Natl Acad Sci USA. 2015;112:8667–8671. doi: 10.1016/j.biosystems.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mähl P, et al. Twenty year experience of the oral rabies vaccine SAG2 in wildlife: A global review. Vet Res. 2014;45:77. doi: 10.1186/s13567-014-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolfe ND, et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc Natl Acad Sci USA. 2005;102:7994–7999. doi: 10.1073/pnas.0501734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brashares JS, et al. Bushmeat hunting, wildlife declines, and fish supply in West Africa. Science. 2004;306:1180–1183. doi: 10.1126/science.1102425. [DOI] [PubMed] [Google Scholar]
- 83.Daszak P, Plowright R, Epstein J, Pulliam J, Abdul Rahman S, Field H, Smith C, Olival K, Luby S, Halpin K, Hyatt AD, Cunningham AA. In: Disease Ecology: Community Structure and Pathogen Dynamics. Collinge SK, Ray C, editors. Oxford Univ Press; Oxford: 2006. pp. 186–201. [Google Scholar]
- 84.Renwick AR, White PCL, Bengis RG. Bovine tuberculosis in southern African wildlife: A multi-species host-pathogen system. Epidemiol Infect. 2007;135:529–540. doi: 10.1017/S0950268806007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Durbin AP, et al. Emergence potential of sylvatic dengue virus type 4 in the urban transmission cycle is restrained by vaccination and homotypic immunity. Virology. 2013;439:34–41. doi: 10.1016/j.virol.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drake JM, et al. Ebola cases and health system demand in Liberia. PLOS Biol. 2015;13:e1002056. doi: 10.1371/journal.pbio.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- 88.van Nood E, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 89.Harms G, Feldmeier H. HIV infection and tropical parasitic diseases - deleterious interactions in both directions? Trop Med Int Health. 2002;7:479–488. doi: 10.1046/j.1365-3156.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- 90.Hoffmann AA, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 91.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Graham AL. Ecological rules governing helminthmicroparasite coinfection. Proc Natl Acad Sci USA. 2008;105:566–570. doi: 10.1073/pnas.0707221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lembo T, et al. Exploring reservoir dynamics: A case study of rabies in the Serengeti ecosystem. J Appl Ecol. 2008;45:1246–1257. doi: 10.1111/j.1365-2664.2008.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hampson K, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLOS Biol. 2009;7:e53. doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pandey A, et al. Strategies for containing Ebola in West Africa. Science. 2014;346:991–995. doi: 10.1126/science.1260612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scholte EJ, Schaffner F. In: Emerging Pests and Vector- Borne Diseases in Europe. Takken W, Knols BGJ, editors. Wageningen Academic; Wageningen, Netherlands: 2007. pp. 241–260. [Google Scholar]
- 97.Morse SS, et al. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.One Health Institute; 2014. PREDICT success: Yellow fever discovered in Bolivian howler monkeys. www.vetmed.ucdavis.edu/ohi/predict/news/bolivia-success-howler-monkeys.cfm. [Google Scholar]
- 99.Blackwell AD, Martin M, Kaplan H, Gurven M. Antagonism between two intestinal parasites in humans: The importance of co-infection for infection risk and recovery dynamics. Proc R Soc B. 2013;280:20131671. doi: 10.1098/rspb.2013.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stiasny K, et al. Characterization of a structural intermediate of flavivirus membrane fusion. PLOS Pathog. 2007;3:e20. doi: 10.1371/journal.ppat.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anderson RM, May RM. Population biology of infectious diseases: Part I. Nature. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- 102.Dobson A. Population dynamics of pathogens with multiple host species. Am Nat. 2004;164(suppl. 5):S64–S78. doi: 10.1086/424681. [DOI] [PubMed] [Google Scholar]
- 103.Gilbert L, Norman R, Laurenson KM, Reid HW, Hudson PJ. Disease persistence and apparent competition in a three-host community: An empirical and analytical study of large-scale, wild populations. J Anim Ecol. 2001;70:1053–1061. doi: 10.1046/j.0021-8790.2001.00558.x. [DOI] [Google Scholar]
- 104.Norman R, Bowers RG, Begon M, Hudson PJ. Persistence of tick-borne virus in the presence of multiple host species: Tick reservoirs and parasite mediated competition. J Theor Biol. 1999;200:111–118. doi: 10.1006/jtbi.1999.0982. [DOI] [PubMed] [Google Scholar]
- 105.Wood CL, Lafferty KD. Biodiversity and disease: A synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol. 2013;28:239–247. doi: 10.1016/j.tree.2012.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.