Abstract
The temporomandibular joint (TMJ) is a synovial joint essential for hinge and sliding movements of the mammalian jaw. Temporomandibular joint disorders (TMD) are dysregulations of the muscles or the TMJ in structure, function, and physiology, and result in pain, limited mandibular mobility, and TMJ noise and clicking. Although approximately 40–70% adults in the USA have at least one sign of TMD, the etiology of TMD remains largely unknown. Here, we highlight recent advances in our understanding of TMD in mouse models.
Keywords: temporomandibular joint disorders, mouse model
Introduction
The temporomandibular joint (TMJ) is one of the most frequently used joints in the human body. The TMJ is a synovial joint essential for hinge and sliding movements of the mammalian jaw. The TMJ consists of the mandibular condyle, the articular disk (or disc), the glenoid fossa, and the capsule (Tanaka et al, 2008). The articular disk separates joint space between the glenoid fossa and the condyle into two distinct parts: the upper and lower articular cavities. The upper and lower articular cavities are bounded by the articular fossa and the articular eminence and by the condyle, respectively (Nozawa-Inoue et al, 2003). TMJ disorders (TMD) are dysregulations of the muscles or the TMJ in structure, function, and physiology (Naidoo, 1995). Approximately 40–70% of adults in the USA have at least one sign of TMD and at least 33% among those have one symptom, such as pain, limited mandibular mobility, and TMJ noise and clicking (Scrivani et al, 2008). However, there are some important questions which have not been answered yet. Why does TMD occur so often? What determines the occurrence and progress of TMD and osteoarthritis (OA) in the TMJ? What causes differences in the TMJ from other synovial joints in the body?
Unique features of the TMJ
Unlike the articular cartilage of the knee, the cartilage of the mandibular condyle is considered a secondary cartilage (independent from the chondroskeleton) and has a different embryonic origin [derived from cranial neural crest (CNC) cells] (Shen and Darendeliler, 2005).
The condyle of the mandible has a lower amount of collagen type I (COLIA1) compared to the other synovial joints (Benjamin and Ralphs, 2004).
In contrast to the articular cartilage of the other joints, the superficial layer of mandibular condylar cartilage does not express collagen type II (COLIIA1) (Hinton et al, 2009).
The articular surfaces are not composed of hyaline cartilage but of fibrous tissue (Hinton et al, 2009).
Animal models including mice and rats are useful to study the development of the TMJ because the process of development/morphogenesis and the molecular mechanism are well conserved in these animal models (Herring, 2003) (Figure 1). In contrast, rodent models (mice and rats) are less useful for a TMD study because of morphological difference in the TMJ among humans and rodents; thereby, rodents do not show typical TMD symptoms (Figure 2).
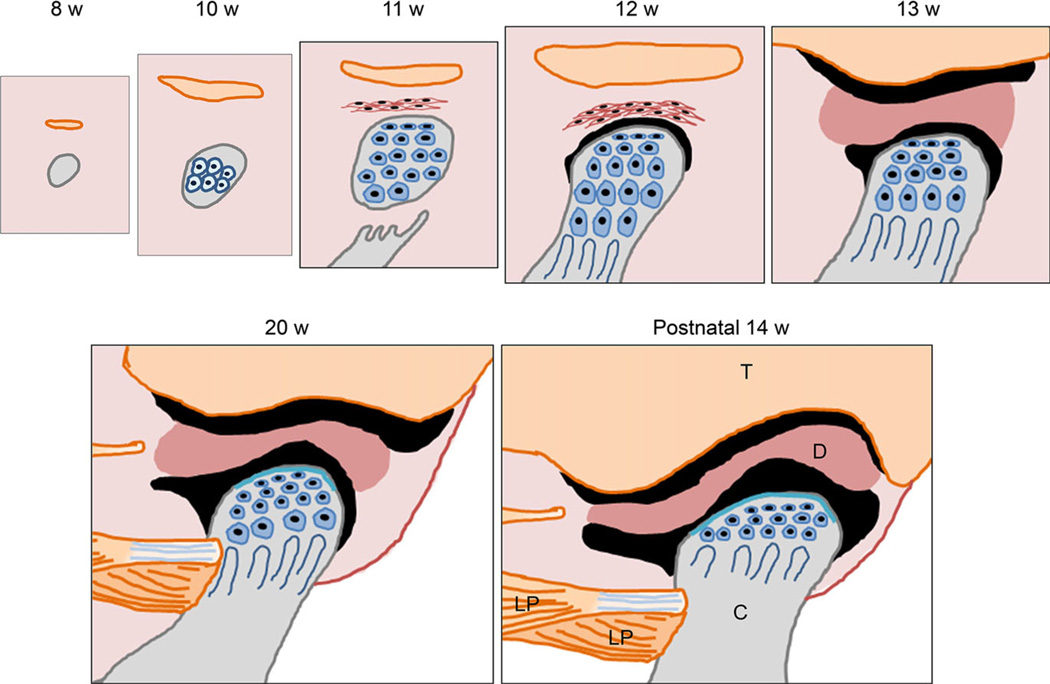
Figure 1.
Time course of temporomandibular joint development in humans. At the 8th week of gestation, mesenchymal condensation forms at the TMJ region. Orange, mesenchymal condensation of the future temporalis bone; gray, mesenchymal condensation of the future mandibular condyle. At the 10th week of gestation, mesenchymal cells differentiate into chondrocytes and start to form the core of cartilage which is the future mandibular condyle. The temporal bone starts intramembranous ossification. During the 11th week of gestation, intramembranous ossification of the ramus of the mandible reaches the base of the future condyle. There are no joint cavities during this stage. The mesenchymal condensation that forms the anlage of the articular disk appears in connective tissue between the anlage of the temporal bone and the mandibular condyle. During the 12th week of gestation, a small space or cleft appears between the anlage of the articular disk and the mandibular condyle that defines the initial formation of the lower articular cavity. During the 13th week of gestation, the organization of the upper articular cavity starts between the temporal bone and the articular disk. At approximately 20th week of gestation, the glenoid fossa of the temporal bone forms, but the articular surface of the temporal bone has a flat surface. At postnatal 14th week, the glenoid fossa and the articular eminence of the temporal bone well form and fit with the shape of the mandibular condyle. T: the temporal bone, D: the articular disk, C: the mandibular condyle, LP: the lateral pterygoid muscle
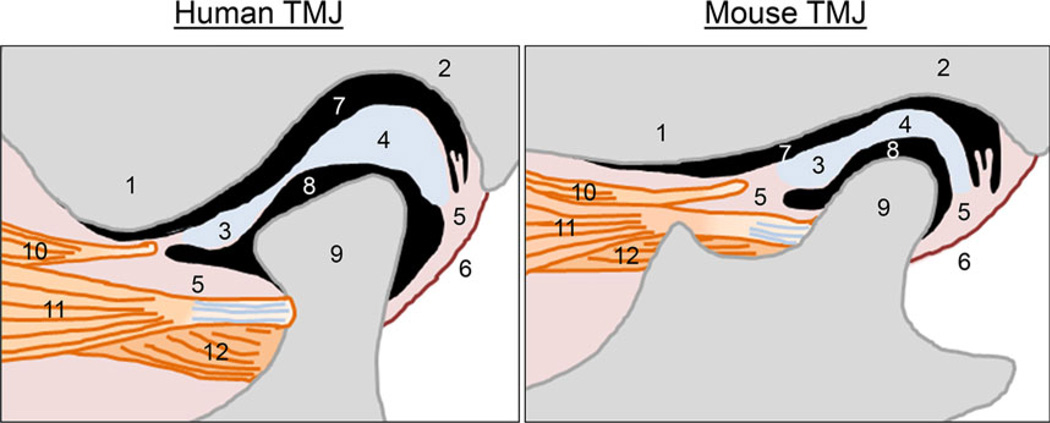
Figure 2.
Comparison of the structure of the temporomandibular joint between humans and mice. TMJ, temporomandibular joint. 1: the articular eminence of the temporal bone, 2: the glenoid fossa of the temporal bone, 3: anterior band of the articular disk, 4: posterior band of the articular disk, 5: connective tissue, 6: the posterior joint capsule, 7: the upper articular cavity, 8: the lower articular cavity, 9: mandibular condyle, 10: a part of upper head of the lateral pterygoid muscle, associated with the articular disk, 11: upper head of the lateral pterygoid muscle, connected with the mandibular condyle, 12: lower head of the lateral pterygoid muscle, connected with the mandibular condyle
Morphological features of the TMJ in rodents compared to humans
The glenoid fossa is shallow and flatted.
There is no articular eminence.
The lateral pterygoid muscle is less in the volume and functional force.
The upper articular cavity forms first, and then, the lower articular cavity forms.
The articular disk rarely becomes fibrous cartilage with aging.
These anatomical factors cause the limitation of usage of rodent models for TMD study. Until now, some specific devises and methods have been developed to induce TMD in mice and rats. In addition, some genetic animal models for OA are used to understand TMJ OA (known as a severe form of TMD) (Hinton, 2014).
Mouse models for temporomandibular joint disorders (TMD)
Extracellular matrix
The growth of the TMJ is regulated through synthesis and degradation of various components of the extracellular matrix (ECM) (Wang and Detamore, 2007). The changes of ECM composition are associated with pathological processes of cartilage degeneration in TMJ OA, accompanied by the upregulated matrix metalloproteases (MMPs) expression (Kanyama et al, 2000). Elevated MMP activity is responsible for ECM degradation in the processes of TMJ OA. Collagen type II (COLIIA1) is the most abundant protein in hyaline cartilage (Ricks et al, 2013). Mice with a disproportionate micromelia (Dmm) mutation, a three-nucleotide deletion of the Col2a1 gene, display mild dwarfism in the heterozygotes (Dmm/+ mice). Dmm/+ mice also show condylar cartilage abnormalities in the TMJ at early ages. The TMJ of Dmm/+ shows fissuring of condylar cartilage as early as 6 months of age (Pace et al, 1997). Mice with deficiency of Col9a1 (Col9a1−/−) or chondrodysplasia (Cho/+) exhibit TMJ OA (Xu et al, 2003; Lam et al, 2007). Col9a1−/− and Cho/+ mice develop OA-like changes in the knee and TMJ from the age of 3 months and show a severe OA-like pathology over 9–12 months (Tanaka et al, 2008). Both chondrocyte clustering and increased production of proteoglycan in the pericellular matrix have been identified as early OA indicators (Tanaka et al, 2008).
A mouse model for progressive ankylosis (ank/ank mice) shows narrower and/or ankylosed upper and lower articular cavities filled with fibrous connective tissue throughout the entire articular cavity after 3 months of age (Huang et al, 2011). The ANKH gene is a human homolog of the murine progressive ankylosis gene, ank, and plays a critical role in the transport of pyrophosphate ions (Timms et al, 2003). ANKH polymorphism seems to be associated with human TMJ closed lock (a permanently displaced disk) (Huang et al, 2011).
Mice with inactivation of Golgi-associated N-sulfotransferase 1 (Ndst1), which catalyzes the constituent sulfation of heparan sulfate proteoglycan glycosaminoglycan chains, exhibit TMJ deformities with a variation of a thicker prechondroblastic cell layer and ectopic ossification with increased proliferation to lack of TMJ (Yasuda et al, 2010).
Transcription factors
The Runx2 (runt-related transcription factor 2; essential for osteoblast differentiation) and Sox9 (sex determining region Y box 9; essential for chondrocyte differentiation) genes are expressed in the mesenchymal condensation that initiates the formation of the mandibular condyles. Mice with deficiency of Runx2 (Runx2−/− mice) exhibit the absence of the mandibular condylar cartilage (Shibata et al, 2004). Mice with the deletion of Sox9 in CNC cells (Sox9F/F;Wnt1-Cre mice) display the mandibular condylar cartilage agenesis, abbreviated mandibular fossa formation, altered articular disk formation with irregular cell shape, and incomplete articular cavity formation (Mori-Akiyama et al, 2003; Wang et al, 2011). Mice with the loss of Trps1, a transcription factor mutated in human trichorhino-phalangeal syndrome, which is characterized by an abnormal development of various organs including the craniofacial skeleton, exhibit an extremely small condylar process, and the complete absence of the articular disk and articular cavities (Michikami et al, 2012). Vitamin D is a prohormone that can be metabolically converted from 25-hydroxyvitamin D by 1α-hydroxylase [1α(OH)ase] to the active form, 1,25-dihydroxyvitamin D [1,25(OH)2D]. 1,25(OH)2D binds to its nuclear receptor and alters transcription activity of target genes. Mice with 1,25(OH)2D deficiency [1α(OH)ase−/− mice] exhibit an erosive TMJ OA phenotype starting at 6 months of age, following DNA damage, cellular senescence, and the production of senescence-associated inflammatory cytokines (Shen et al, 2013).
Indian hedgehog signaling
Indian hedgehog (IHH) ligands express in mouse condylar cartilage at embryonic day 15.5 (E15.5). Mice with loss of Ihh (Ihh−/− mice) exhibit the absence of the articular disk and the lower articular cavity, and severe joint deformities at newborn. These defects in Ihh−/− mice can be partially restored by the loss of Gli3 (a negative regulator of IHH signaling pathway) (Ihh−/−;Gli3−/− mice), indicating that IHH inhibits GLI3 expression and function (Shibukawa et al, 2007). Mice with deficiency of Gli2 display aberrant TMJ development including cellular disorganization of condylar cartilage and no articular disk formation (Purcell et al, 2009). In addition, a conditional deletion of Smo, a receptor for IHH, from chondrocyte progenitors in mice (SmoF/F;Sox9-Cre mice) results in a failure of articular disk separation from the mandibular condyle (Purcell et al, 2009). Thus, IHH signaling plays important roles in disk morphogenesis, condyle initiation, and disk–condyle separation. Interestingly, tamoxifen-induced conditional Ihh knockout mice (IhhF/F;Col2a1-CreER mice) at postnatal days 4, 7, 14, and 56 show disorganization and growth retardation of condylar cartilage, and reduced proliferation in a polymorphic zone of the condyle, and abnormal adhesion of the articular disk with the condylar surface and/or glenoid fossa (Ochiai et al, 2010). Thus, IHH signaling also plays a crucial role in postnatal TMJ maintenance for proper tissue homeostasis. Short stature homeobox 2 (Shox2) is expressed in condylar chondrocytes and the glenoid fossa of the developing TMJ and is essential for the process of TMJ morphogenesis. Shox2-deficient (Shox2−/−) mice exhibit severe defects in a number of developing organs, as well as the TMJ dysplasia and ankylosis (Gu et al, 2008). Mice with overexpression of Shox2 in CNC cells (Wnt1-Cre;pMes-stopShox2 mice) show the dysplasia in the condyle and glenoid fossa after 2 weeks of age, following increased apoptosis and the upregulated expression of MMPs and downregulated expression of IHH signaling molecules, COLIA1 and COLIIA1 (Li et al, 2014a). Mice with the replacement of the mouse Shox2 gene with the human SHOX gene (Shox2SHOX-KI/KI mice) show increased apoptosis in the articular disk, following the decreased expression of COLIA1 and aggrecan, accompanied by increased MMP activities (Li et al, 2014b). Senescence-accelerated 8 (Samp8) mice (a spontaneous mouse line) develop early-onset OA-like changes after 4 months of age, following reduced Ihh and other IHH signaling mediators expression in chondrocytes and Col10 in the hypertrophic chondrocyte of the condylar cartilage (Ishizuka et al, 2014). This degeneration of condylar cartilage is accelerated by malocclusion in mutant mice, following increasing apoptosis and decreasing cell proliferation (Ishizuka et al, 2014). Primary cilium is known to be a sensor for mechanical stresses in many organs and regulates hedgehog signaling (Kinumatsu et al, 2011). Mice with deficiency of kinesin family member 3A (Kif3a) (Kif3aF/F;Col2a1-Cre mice), which encodes ciliary transport protein, exhibit narrow and flat condyles which are often fused with the articular disk and display an irregular surface (Kinumatsu et al, 2011).
Transforming growth factor beta (TGFβ) superfamily signaling
Mice with transgene of TGFβ1 point mutation in bone, a mouse model for Camurati–Engelmann disease (CED), show high levels of active TGFβ1 in bone and result in increased apoptosis and MMPs expression in the hypertrophic zone of the condylar cartilage after 4 months of age (Jiao et al, 2014). CED mice exhibit abnormal bone remodeling in the condylar subchondral bone such as microstructure and increased activities of osteoclasts and osteoblasts without coupling, and cartilage degradation in the condylar cartilage. Bone morphogenetic protein receptor type I (BMPR1A) is expressed in the developing condyle, glenoid fossa, and interzone mesenchymal cells that are all derived from CNC cells (Gu et al, 2014). Mice with the deletion of Bmpr1a (aka Alk2) in CNC cells (Bmpr1aF/F;Wnt1-Cre mice) display defective TMJ development, including a failure of articular disk separation from the condyle, and persistence of interzone cells between the glenoid fossa and the articular disk-like structure (Gu et al, 2014). In contrast, augmented BMPR1A signaling in CNC cells (caBmpr1a;Wnt1-Cre transgenic mice) inhibits osteogenesis in the glenoid fossa and induces ectopic primary cartilage formation (normally secondary cartilage formation in the developing condyle) in the condylar primordium (Gu et al, 2014).
Epithelial growth factor signaling
MIG6 (aka ERRFI1) acts as a negative regulator of epidermal growth factor receptor (EGFR) family members. MIG6 is essential for maintaining the integrity of postnatal synovial joints, and loss of Mig6 (Mig6−/−) leads to the formation of large osteophytes along with the degradation of articular cartilage and subchondral cyst formation in the joints, including the knee, ankle, and TMJ in mice at approximately 1 month of age (Zhang et al, 2005). The onset of the OA-like phenotype in Mig6−/− mice likely involves mechanical stress on the joints in life (Staal et al, 2014). Osteophyte formation, which appears at the edge of the meniscus as newly formed cartilage rich in proteoglycans and collagens, is the most profound pathological change observed in the knee joints of Mig6F/F;Col2a1-Cre mice, followed by chondrocyte maturation, hypertrophy, and mineralization (Staal et al, 2014). The formation of osteophytes is likely the result of inappropriate cell proliferation in the absence of Mig6. EGFR signaling is overactivated in chondrocytes of the articular cartilage and in the osteophyte of Mig6F/F;Col2a1-Cre knee joints (Staal et al, 2014). Interestingly, Mig6F/F;Col2a1-Cre mice exhibit aggressive OA-like phenotype only in the knee joints (rare in the TMJ and the ankle), suggesting that the other cell types (Col2a1-negative cells) may be responsible for the OA-like phenotype in the TMJ.
WNT/β-catenin signaling
β-Catenin (CTNNB1) is critical for the induction of TMJ cartilage degeneration (Wang et al, 2014). The conditional constitutive activation of the Ctnnb1 gene in TMJ cartilage (Ctnnb1(ex3);Col2-CreER) leads to an OA-like phenotype in mice after 1 month of age (Wang et al, 2014). The deletion of Mmp13 (matrix metallopeptidase 13; aka collagenase 3) or Adamts5 (a disintegrin and metalloproteinase with thrombospondin motif 5), which are the key enzymes for cartilage degradation, partially restores the OA-like phenotype in Ctnnb1(ex3);Col2-CreER mice in the cartilage thickness and area (Wang et al, 2014).
Fibroblast growth factor signaling
Mice with a knock-in mutation of Fgfr3, which significantly enhances the affinity of fibroblast growth factor (FGF) receptor type III (FGFRIII) to FGF ligands, (Fgfr3P244R mice) display early degenerative changes of condylar articular cartilage, abnormal development of the articular eminence/glenoid fossa, and fusion of the articular disk in the TMJ at postnatal day 21, following reduced cell proliferation, diminished Sox9 and Col10 expression, and a compromised trabecular bone network underlying the cartilage (Yasuda et al, 2012). The Sprouty genes, Spry1 and Spry2, encode intracellular inhibitors of receptor tyrosine kinase signaling pathways, including FGF signaling. Spry1 and Spry2 are highly expressed in muscles attached to the TMJ. Mice with combined inactivation of Spry1 and Spry2 (Spry1−/−;Spry2−/− mice) exhibit overgrowth of lateral pterygoid and temporal muscles and regression of the developing mandibular fossa, and smaller condylar cartilage compared to controls (Purcell et al, 2012).
Parathyroid hormone-related peptide signaling
Parathyroid hormone (PTH) and parathyroid hormone-related peptide (PTHrP) regulate calcium homeostasis; PTHrP further regulates growth and development. Mice with constitutively active PTH/PTHrP receptor expression in bone (Col1-caPPR mice) show abnormal growth and disruption of postnatal TMJ. The condylar cartilage is mostly composed of immature chondrocytes and fibroblastic cells, with only an occasional island of hypertrophic chondrocytes (Tsutsui et al, 2008).
Discoidin signaling
Disordered cell matrix interactions play a central role in the pathogenesis of OA (Lam et al, 2007). Mice with the deletion of discoidin domain receptor tyrosine kinase 1 (Ddr1−/− mice) exhibit a high incidence of OA in the TMJ beginning at as early as 9 weeks of age with typical signs of OA, including surface fissures, loss of proteoglycans, chondrocyte cluster formation, upregulated Col1a1 expression, and atypical collagen fibril arrangements (Schminke et al, 2014). Loss of Ddr1 causes major changes in ECM components. Ddr1−/− mice exhibit a greater relative bone mineral density of the subchondral bone. Ddr1−/− chondrocytes from the TMJ exhibit decreased expression of Col2a1, Col3a1, Col9a1, Aggrecan (Acan), and Sox9, and increased expression of Nidogen-2 (Nid2) and Runx2. Moreover, loss of the superficial cartilage layer, deep surface fissures, and destroyed condylar surface are observed in Ddr1−/− mice. Interestingly, expression of hedgehog interacting protein (HHIP), which is overexpressed in human OA and in cartilage from other OA mouse models, is increased in Ddr1−/− mice (Schminke et al, 2014). In addition, vascular endothelial growth factor A (VEGF-A), which is associated with the wingless in Drosophila (WNT) pathway in OA pathogenesis and angiogenesis, and MMP13 are increased in Ddr1−/− chondrocytes, followed by the degradation of collagens (Schminke et al, 2014). Thus, the loss of Ddr1 influences OA pathogenesis through the increased expression of Mmp13, ColIa1, Runx2, etc., and results in the change of ECM components.
Conclusion
There are limited numbers of studies using mouse genetic models for TMD and TMJ OA. The molecular mechanism of TMD and TMJ OA is presumably different from the pathogenesis of knee joint OA because of differences in the structure and origin of cells that give rise to TMJ structures. The further characterization of CNC cells (a source of TMJ structures) in the TMJ will facilitate the understanding of normal development of the TMJ and its dysfunction (TMD and TMJ OA). Recent studies suggest that mechanical stress is a key factor to induce and progress TMD with a combination of some intent with genetics.
Acknowledgments
This study was supported by start-up fund to J. Iwata.
Footnotes
Author contributions
J. Iwata made substantial contributions to the conception and design of the article, drafted and revised the article critically for important intellectual content, and involved in the final approval of the version to be published. A. Suzuki revised it for intellectual content and was involved in the final approval of the version to be published. All authors read and approved the final manuscript.
Conflict of interest
None declared.
References
- Benjamin M, Ralphs JR. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- Gu S, Wei N, Yu L, Fei J, Chen Y. Shox2-deficiency leads to dysplasia and ankylosis of the temporomandibular joint in mice. Mech Dev. 2008;125:729–742. doi: 10.1016/j.mod.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Wu W, Liu C, et al. BMPRIA mediated signaling is essential for temporomandibular joint development in mice. PLoS ONE. 2014;9:e101000. doi: 10.1371/journal.pone.0101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW. TMJ anatomy and animal models. J Musculoskelet Neuronal Interact. 2003;3:391–394. discussion 406-7. [PMC free article] [PubMed] [Google Scholar]
- Hinton RJ. Genes that regulate morphogenesis and growth of the temporomandibular joint: a review. Dev Dyn. 2014;243:864–874. doi: 10.1002/dvdy.24130. [DOI] [PubMed] [Google Scholar]
- Hinton RJ, Serrano M, So S. Differential gene expression in the perichondrium and cartilage of the neonatal mouse temporomandibular joint. Orthod Craniofac Res. 2009;12:168–177. doi: 10.1111/j.1601-6343.2009.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Takahashi K, Sakata T, et al. Increased risk of temporomandibular joint closed lock: a case–control study of ANKH polymorphisms. PLoS ONE. 2011;6:e25503. doi: 10.1371/journal.pone.0025503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka Y, Shibukawa Y, Nagayama M, et al. TMJ degeneration in SAMP8 mice is accompanied by deranged Ihh signaling. J Dent Res. 2014;93:281–287. doi: 10.1177/0022034513519649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Zhang M, Niu L, et al. Overexpressed TGF-beta in subchondral bone leads to mandibular condyle degradation. J Dent Res. 2014;93:140–147. doi: 10.1177/0022034513513034. [DOI] [PubMed] [Google Scholar]
- Kanyama M, Kuboki T, Kojima S, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids of patients with temporomandibular joint osteoarthritis. J Orofac Pain. 2000;14:20–30. [PubMed] [Google Scholar]
- Kinumatsu T, Shibukawa Y, Yasuda T, et al. TMJ development and growth require primary cilia function. J Dent Res. 2011;90:988–994. doi: 10.1177/0022034511409407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam NP, Li Y, Waldman AB, et al. Age-dependent increase of discoidin domain receptor 2 and matrix metalloproteinase 13 expression in temporomandibular joint cartilage of type IX and type XI collagen-deficient mice. Arch Oral Biol. 2007;52:579–584. doi: 10.1016/j.archoralbio.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liang W, Ye H, Weng X, Liu F, Liu X. Overexpression of Shox2 leads to congenital dysplasia of the temporomandibular joint in mice. Int J Mol Sci. 2014a;15:13135–13150. doi: 10.3390/ijms150813135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu H, Gu S, et al. Replacing Shox2 with human SHOX leads to congenital disc degeneration of the temporomandibular joint in mice. Cell Tissue Res. 2014b;355:345–354. doi: 10.1007/s00441-013-1743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikami I, Fukushi T, Honma S, et al. Trps1 is necessary for normal temporomandibular joint development. Cell Tissue Res. 2012;348:131–140. doi: 10.1007/s00441-012-1372-1. [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci USA. 2003;100:9360–9365. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo LC. Disorders of the temporomandibular joint: a review of anatomical concepts. J Dent Assoc S Afr. 1995;50:461–466. [PubMed] [Google Scholar]
- Nozawa-Inoue K, Amizuka N, Ikeda N, Suzuki A, Kawano Y, Maeda T. Synovial membrane in the temporomandibular joint–its morphology, function and development. Arch Histol Cytol. 2003;66:289–306. doi: 10.1679/aohc.66.289. [DOI] [PubMed] [Google Scholar]
- Ochiai T, Shibukawa Y, Nagayama M, et al. Indian hedgehog roles in post-natal TMJ development and organization. J Dent Res. 2010;89:349–354. doi: 10.1177/0022034510363078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace JM, Li Y, Seegmiller RE, Teuscher C, Taylor BA, Olsen BR. Disproportionate micromelia (Dmm) in mice caused by a mutation in the C-propeptide coding region of Col2a1. Dev Dyn. 1997;208:25–33. doi: 10.1002/(SICI)1097-0177(199701)208:1<25::AID-AJA3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Purcell P, Joo BW, Hu JK, et al. Temporomandibular joint formation requires two distinct hedgehog-dependent steps. Proc Natl Acad Sci USA. 2009;106:18297–18302. doi: 10.1073/pnas.0908836106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell P, Jheon A, Vivero MP, Rahimi H, Joo A, Klein OD. Spry1 and spry2 are essential for development of the temporomandibular joint. J Dent Res. 2012;91:387–393. doi: 10.1177/0022034512438401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricks ML, Farrell JT, Falk DJ, et al. Osteoarthritis in temporomandibular joint of Col2a1 mutant mice. Arch Oral Biol. 2013;58:1092–1099. doi: 10.1016/j.archoralbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schminke B, Muhammad H, Bode C, et al. A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell Mol Life Sci. 2014;71:1081–1096. doi: 10.1007/s00018-013-1436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med. 2008;359:2693–2705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- Shen G, Darendeliler MA. The adaptive remodeling of condylar cartilage—a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84:691–699. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- Shen M, Luo Y, Niu Y, et al. 1,25(OH)2D deficiency induces temporomandibular joint osteoarthritis via secretion of senescence-associated inflammatory cytokines. Bone. 2013;55:400–409. doi: 10.1016/j.bone.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Shibata S, Suda N, Yoda S, et al. Runx2-deficient mice lack mandibular condylar cartilage and have deformed Meckel’s cartilage. Anat Embryol. 2004;208:273–280. doi: 10.1007/s00429-004-0393-2. [DOI] [PubMed] [Google Scholar]
- Shibukawa Y, Young B, Wu C, et al. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev Dyn. 2007;236:426–434. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- Staal B, Williams BO, Beier F, Vande Woude GF, Zhang YW. Cartilage-specific deletion of Mig-6 results in osteoarthritis-like disorder with excessive articular chondrocyte proliferation. Proc Natl Acad Sci USA. 2014;111:2590–2595. doi: 10.1073/pnas.1400744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- Timms AE, Zhang Y, Bradbury L, Wordsworth BP, Brown MA. Investigation of the role of ANKH in ankylosing spondylitis. Arthritis Rheum. 2003;48:2898–2902. doi: 10.1002/art.11258. [DOI] [PubMed] [Google Scholar]
- Tsutsui TW, Riminucci M, Holmbeck K, Bianco P, Robey PG. Development of craniofacial structures in transgenic mice with constitutively active PTH/PTHrP receptor. Bone. 2008;42:321–331. doi: 10.1016/j.bone.2007.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Detamore MS. Tissue engineering the mandibular condyle. Tissue Eng. 2007;13:1955–1971. doi: 10.1089/ten.2006.0152. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu C, Rohr J, et al. Tissue interaction is required for glenoid fossa development during temporomandibular joint formation. Dev Dyn. 2011;240:2466–2473. doi: 10.1002/dvdy.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li S, Xie W, et al. Activation of beta-catenin signalling leads to temporomandibular joint defects. Eur Cells Mater. 2014;28:223–235. doi: 10.22203/ecm.v028a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Flahiff CM, Waldman BA, et al. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho) Arthritis Rheum. 2003;48:2509–2518. doi: 10.1002/art.11233. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Mundy C, Kinumatsu T, et al. Sulfotransferase Ndst1 is needed for mandibular and TMJ development. J Dent Res. 2010;89:1111–1116. doi: 10.1177/0022034510373766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Nah HD, Laurita J, et al. Muenke syndrome mutation, Fgf R3P(2)(4)(4)R, causes TMJ defects. J Dent Res. 2012;91:683–689. doi: 10.1177/0022034512449170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Su Y, Lanning N, et al. Targeted disruption of Mig-6 in the mouse genome leads to early onset degenerative joint disease. Proc Natl Acad Sci USA. 2005;102:11740–11745. doi: 10.1073/pnas.0505171102. [DOI] [PMC free article] [PubMed] [Google Scholar]