Abstract
Diabetic Nephropathy (DN) is believed to be a major microvascular complication of diabetes. The hallmark of DN includes deposition of Extracellular Matrix (ECM) proteins, such as, collagen, laminin and fibronectin in the mesangium and renal tubulo-interstitium of the glomerulus and basement membranes. Such an increased expression of ECM leads to glomerular and tubular basement membranes thickening and increase of mesangial matrix, ultimately resulting in glomerulosclerosis and tubulointerstitial fibrosis. The characteristic morphologic glomerular mesangial lesion has been described as Kimmelstiel–Wilson nodule, and the process at times is referred to as diabetic nodular glomerulosclerosis. Thus, the accumulation of ECM proteins plays a critical role in the development of DN. The relevant mechanism(s) involved in the increased ECM expression and their regulation in the kidney in diabetic state has been extensively investigated and documented in the literature. Nevertheless, there are certain other mechanisms that may yet be conclusively defined. Recent studies demonstrated that some of the new signaling pathways or molecules including, Notch, Wnt, mTOR, TLRs and small GTPase may play a pivotal role in the modulation of ECM regulation and expression in DN. Such modulation could be operational for instance Notch though Notch1/Jagged1 signaling, Wnt by Wnt/β-catenin pathway and mTOR via PI3-K/Akt/mTOR signaling pathways. All these pathways may be critical in the modulation of ECM expression and tubulo-interstitial fibrosis. In addition, TLRs, mainly the TLR2 and TLR4, by TLR2-dependent and TGF-β-dependent conduits, may modulate ECM expression and generate a fibrogenic response. Small GTPase like Rho, Ras and Rab family by targeting relevant genes may also influence the accumulation of ECM proteins and renal fibrosis in hyperglycemic states. This review summarizes the recent information about the role and mechanisms by which these molecules and signaling pathways regulate ECM synthesis and its expression in high glucose ambience in vitro and in vivo states. The understanding of such signaling pathways and the molecules that influence expression, secretion and amassing of ECM may aid in developing strategies for the amelioration of diabetic nephropathy.
Keywords: diabetic nephropathy, extracellular matrix, Notch, Wnt, mTOR, TLRs and small GTPase
INTRODUCTION
Diabetic nephropathy (DN) is one of the leading microvascular complication in patients with diabetes, and is the most prevalent cause of chronic renal failure [1, 2]. Fundamentally, chronic hyperglycemia is regarded as the main metabolic denominator that seems to be responsible for the development of DN. About 70% of the patients with either type 1 or type 2 diabetes develop DN while presenting clinically having a chronic kidney disease (CKD) at the same time [3]. Furthermore, the incidence of DN is increasing, and at present approximately 50% of the patients that progress to end-stage renal disease (ESRD) have DN [4]. With respect to the progression of DN, the renal lesions due to type 1 or type 2 diabetes are indistinguishable [5]. The pathological hallmarks of DN include deposition of extracellular matrix (ECM) in the mesangium and tubulo-interstitium along with thickening of glomerular and tubular basement membranes, ultimately resulting in glomerulosclerosis and tubulo-interstitial fibrosis. The characteristic morphologic glomerular mesangial lesion in DN has been described as Kimmelstiel–Wilson nodule [6, 7]. The ECM glycoproteins that are increased in DN include collagen, laminin, fibronectin and proteoglycans in different renal compartments, and various mechanism(s) related to ECM expression and their regulation in DN has been described, yet others remain to be defined. Elucidation of novel mechanism(s) involved in ECM accumulation may facilitate the development of effective therapeutic strategies of DN. In this communication we have reviewed various mechanisms related to the ECM amassing in DN while emphasizing some of the new signaling pathways or molecules that may be relevant to the matrix pathobiology of DN in vitro and in vivo states. The signaling pathways or molecules discussed here include, such as, Notch, Wnt, mTOR, TLRs and small GTPases following a brief overview of ECM glycoproteins that are relevant to the pathogenesis of DN.
EXTRACELLULAR MATRIX PROTEINS
Aberrant thickening of glomerular basement membranes (GBM) and tubular basement membranes (TBM) as well as excessive amassing of mesangial matrices in DN is a result of chronic hyperglycemia induced metabolic perturbations leading to the imbalance between extracellular matrix (ECM) glycoproteins’ synthesis and their degradation. The ECM components primarily constitute collagen, laminin, fibronectin and proteoglycans. The major perturbations in GBM ECM components include increased expression of collagen IV (α3 and α4 chains), collagen V, collagen VI, laminin and fibronectin, while there is a decreased expression of heparan sulfate proteoglycans [7–9]. Likewise, mesangial matrix changes include elevated expression of collagen I, collagen III, collagen IV (α1 and α2 chains), collagen V, collagen VI, laminin, fibronectin and small-leucine-rich (SLR) proteoglycans [7, 8]. Additionally, the changes in the ECM proteins of the tubulo-interstitial compartment include increased expression of collagen I and SLR proteoglycans, like decorin and biglycans [10] (Table 1). The relevant mechanism(s) or the molecules involved in increased ECM expression and their regulation in kidney in diabetic state has been extensively investigated. To name a few are glucose transporter proteins (GLUTs) [11], protein kinase C (PKC) [12], advanced glycation end-products (AGEs) [13], reactive oxygen species (ROS) [14], Matrix metallo-proteinases (MMPs) [15, 16], microRNA [17], growth factors/cytokines and hormones [18, 19]. The latter two would include transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF), growth hormone-insulin-like growth factor (GH-IGF), connective tissue growth factor (CTGF) and Angiotensin II (Ang II) etc. Recently, increasing evidence indicates that some of the new signaling pathways or molecules also play a pivotal role in the regulation and expression of ECM components in DN, and their relevance to matrix pathobiology is discussed in the following sections of this article.
Table 1.
Increased/Decreased expression of various ECM proteins in different compartments of the kidney in diabetic nephropathy
| GBMs [7–9] | Increased: Collagen IV (α3/α4 chains), collagen V, collagen VI, laminin and fibronectin |
| Decreased: Heparan sulfate proteoglycans | |
| Mesangial matrix [7, 8] | Increased: collagen I, collagen III, collagen IV (α1/α2 chains), collagen V, collagen VI, laminin, fibronectin and small-leucine- rich (SLR) proteoglycans |
| Tubulointerstitial matrix [10] | Increased: Collagen I, decorin and biglycans |
NOTCH AND ECM
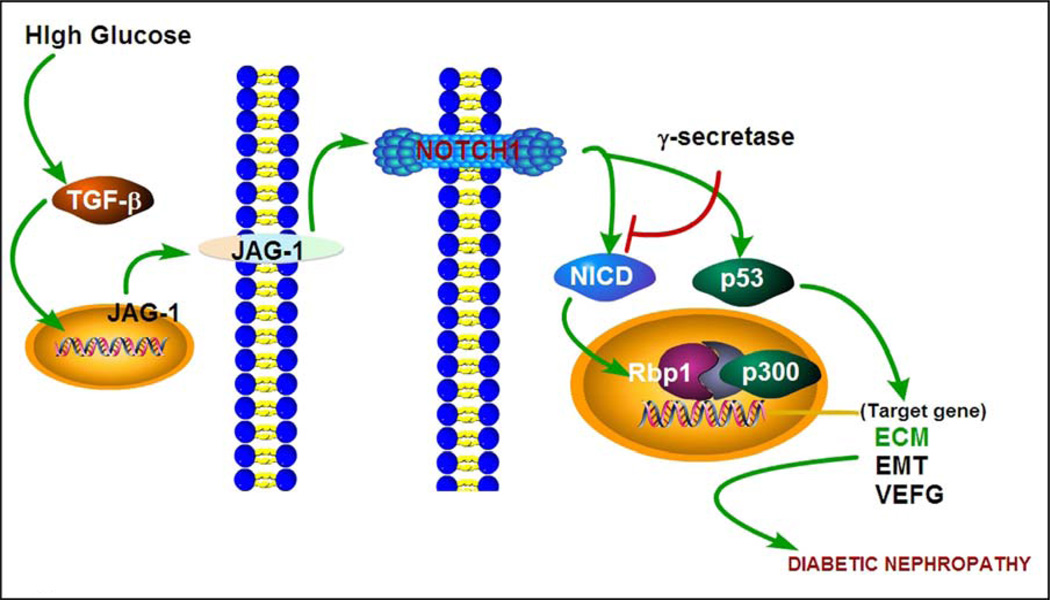
Notch signaling is evolutionary conserved across species, and in mammalian system the main constituents of this pathway include four transmembrane receptors (Notch1 - Notch4), three delta-like ligands (DLL1, DLL3 and DLL4) and another two ligands belonging to Jagged family members (JAG1 and JAG2). In this scenario the ligand:receptor interaction induces conformational changes in the Notch receptors with subsequent proteolytic cleavage and release of Notch intracellular domain (NICD). The released intracellular domain translocates into the nucleus, where it associates with CSL (CBF1/Su(H)/Lag-1) transcription factor complex and triggers gene transcription of canonical Notch target genes, such as, Hes1 (hairy enhancer split-1) and Hey (Hes with YRPW motif protein 1) genes (Figure 1) [20].
Figure 1.
Schematic drawing depicting events related to Notch signaling that are relevant to ECM accumulation in diabetic nephropathy. High glucose increases TGF-β levels and up-regulates JAG-I expression. This induces a conformational change in Notch1 by proteolytic cleavage resulting in the release and translocation of Notch intracellular domain (NICD) into nucleus, where NICD interacts with Rbp1, p300 and CSL, and forms CBF1/Su(H)/Lag-1 transcription factor complex. Conceivably, the complex triggers transcription of target genes, such as, of ECM, EMT and VEGF, and ultimately leading to renal fibrosis in diabetic nephropathy.
There is increasing evidence suggesting that Notch signaling pathway may be involved in renal disease processes associated with fibrosis. In a unilateral ureteral obstruction (UUO) model Morrissey et al. confirmed that there is an increased expression of JAG1 ligand protein, and it is dependent upon modulation by profibrogenic cytokine, TGF-β, suggesting that the JAG1 may contribute to kidney injury and renal interstitial fibrosis [21]. While taking into consideration that Notch/jagged pathway plays an important role in fibrogenesis and subsequent development of kidney disease, it would suggest that Notch blockade may reduce renal fibrosis and kidney damage, as highlighted in several recent publications [22–25]. Notch-Jagged signaling may also induce epithelial-mesenchymal transition (EMT) of tubular epithelial cells with consequential induction of renal fibrosis [26, 27]. In an experimental acute kidney injury model Bielesz et al. observed an increased mRNA expression of Notch1, Jag1 and Hey1, along with elevated expression of Notch1 and Jag1 proteins. While the treatment with Notch inhibitor led to a decreased expression of ECM proteins, such as, fibronectin (Fn1) and collagen (Col1α1, Col3 α1 and Col4 α1). These observations reinforce the concept that Notch1/Jag1 signaling is involved in tubulo-interstitial fibrosis. Furthermore, it has been shown that in cultured murine tubular epithelial cells TGF-β up-regulates the expression of Notch1 and Jag1 (Figure 1). Along these lines Notch signaling has been reported to induce an increase of EMT regulator factor Snail1, suggesting that Notch1/Jag1 pathway probably utilizes EMT in the induction of tubulo-interstitial fibrosis. Interestingly, these studies have also shown that the expression of Notch1 and Jagged1 is significantly increased in tubular epithelial cells (TECs) in patients’ samples with diabetic nephropathy [28].
Similarly, Mariana et al. observed that in various kidney diseases, including the biopsy samples from patients with diabetic kidney disease (DKD), the expression of Notch1 protein is significantly elevated in the tubulo-interstitial compartment, and it is highly correlative with the severity of renal fibrosis [29]. Walsh et al. found that Jagged1, Hes1 and Gremlin mRNA expression increased in the tubulo-interstitium in diabetic nephropathy patients as compared to the control. Likewise, TGF-β treated HK-2 cells in vitro had increased mRNA expression of Jagged1, Hes1 and Gremlin, indicating that Notch1/Jagged1 can be activated as a TGF-β1 downstream signaling pathway in DN [30]. Since TGF-β promotes EMT [31] and induces kidney cells to synthesize ECM proteins via various pathways resulting in glomerulosclerosis and tubulo-interstitial fibrosis in DN [32, 33], it would mean that Notch1/Jagged1 signaling have an important role in TGF-β-mediated EMT and tubulo-interstitial fibrosis [30], and therefore, TGF-β activation of Notch signaling may aid in further sustenance and promotion of fibrosis and in the process of scarring in DN. Similarly, Bonegio et al. found that Notch signaling pathways is reactivated in diabetic nephropathy, and it is associated with increased synthesis of extracellular matrix proteins that ultimately promote the development of tubulo-interstitial fibrosis [34]. Further support for this notion was derived from the fact that treatment with Notch signaling inhibitors leads to amelioration of tubulo-interstitial fibrosis and dampened the progression of diabetic nephropathy [34]. Besides the tubulo-interstitium, Liu et al. discovered that high glucose (HG) also increases the expression of Notch1, Jagged1 and Hes1 along with the profibrogenic cytokine, TGF-β, and ECM protein fibronectin in rat glomerular mesangial cells [35]. The Notch signaling pathway also exerts its effects on podocytes and thus plays an important role in DN development [36]. These authors also described that intracellular domain of Notch1 (ICN1) expression was increased in kidneys of both human and experimental diabetic models. Their in vitro and in vivo studies showed that ICN1 induced apoptosis of podocytes through the activation of p53, and genetic deletion of Notch transcriptional partner (Rbpj) in podocytes or treatment of γ-secretase inhibitor alleviated albuminuria, glomerulosclerosis and apoptosis [37]. Along these lines, Lin et al. also reported that Notch signaling pathway was significantly up-regulated in HG-treated human podocytes and kidneys of diabetic animals, and Notch pathway activation augmented the VEGF expression which consequentially led to down-regulation of nephrin and induced apoptosis in podocytes. Interestingly, following the treatment with γ-secretase inhibitor, the expression of VEGF and nephrin were normalized in the kidneys of streptozotocin-induced diabetes model along with decreased albuminuria [38]. All these above studies by various investigators emphasize that Notch1/Jagged1 signaling pathway and TGF-β-mediated pathway synergistically may be responsible for the progression of DN (Figure 1).
WNT/β-CATENIN AND ECM
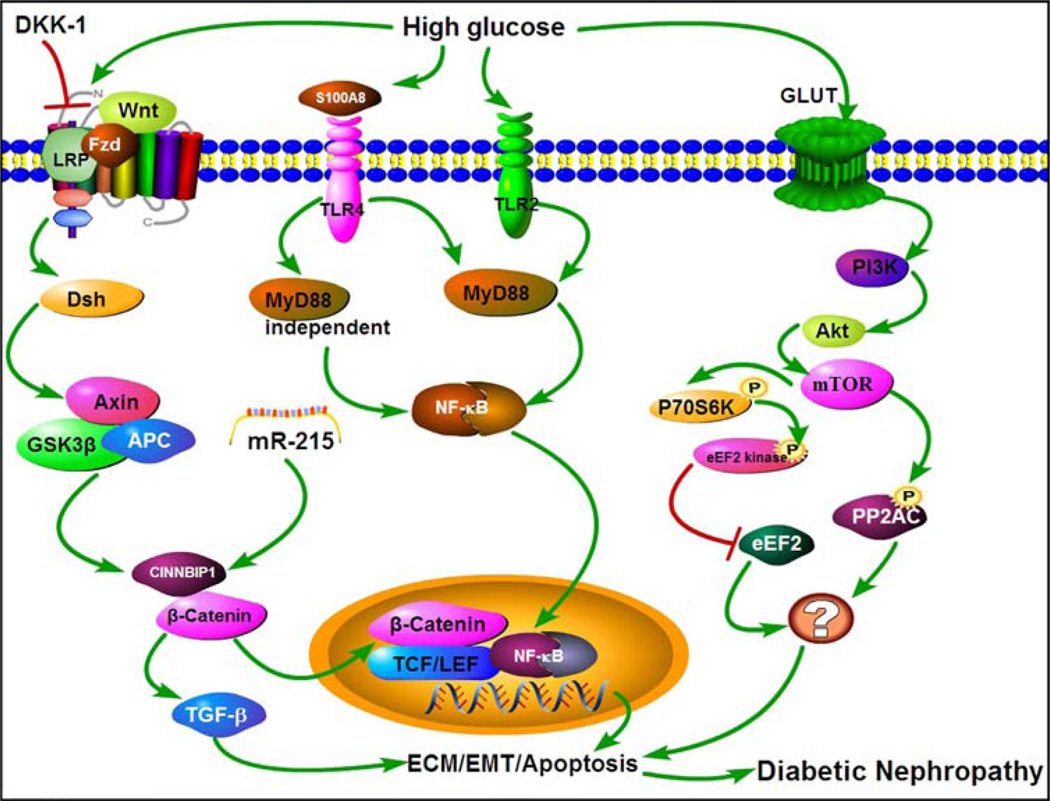
Wnt/β-Catenin is also an evolutionary conserved signal transduction pathway which affects cellular events that modulate various disease processes besides playing a critical role in kidney development (Figure 2). The Wnts family is comprised of 19 Wnt proteins under the umbrella of 12 conserved Wnt subfamilies [39]. The canonical Wnt/β-Catenin pathway has been extensively studied and characterized. In the activation of classical Wnt signaling pathway, the Wnt proteins are secreted into the extracellular space and bind with the extracellular N-domain of transmembrane frizzled (Fzd) receptor and another transmembrane co-receptor lipoprotein related receptor protein (LRP5/LRP6). These interactions send a signal to the phosphoprotein Dishevelled (Dsh) in the cytoplasm while simultaneously inhibiting the activity of glycogen synthase kinase 3 (GSK-3β) and its falling off from the Axin; and as a consequence formation of β-catenin degradation complex is prevented. The complex is mainly made up of adenomatosis polyposis coli (APC), Axin and GSK-3β. In doing so, the β-catenin cannot be phosphorylated and undergo degradation [40, 41]. After achieving certain intracellular threshold concentration, Cytoplasmic β-catenin is translocated into the nucleus, where it serve as a transcriptional co-activator of the transcription factors belonging to the TCF/LEF family, and it then stimulates the target gene expression [42]. Overall, intracellular accumulation of β-catenin is a critical effector in this canonical Wnt/β-catenin signaling that modulates a variety of biological processes, including epithelial-mesenchymal transition (EMT) [43].
Figure 2.
An overview of different signaling pathways activated by high glucose ambience, which leads to an altered expression of various ECM glycoproteins, and of genes relevant to EMT and apoptosis processes. WNT/β-CATENIN signaling: Under high-glucose ambience, Wnt proteins are secreted into the extracellular space and bind with LRP. These interactions send a signal to the phosphoprotein Dishevelled (Dsh) in the cytoplasm, while simultaneously inhibit the activity of glycogen synthase kinase 3 (GSK-3β) followed by its falling off from the Axin complex. Increased cytoplasmic β-catenin is translocated into the nucleus, and it serves as a transcriptional co-activator of various transcription factors which stimulate the expression of the target EMT genes. TLRs signaling: It can be activated by the ligand TLR2, and subsequents events are channeled via MyD88-dependent signaling pathway. Whereas, TLR4 activation leads to channeling of events via MyD88-dependent as well as MyD88-independent pathways. Both pathways lead to the activation of nuclear factor Kappa-B (NF-κB), which in turn increases the expression and secretion of pro-fibrotic and pro-inflammatory cytokines with over-expression of ECM proteins like fibronectin. PI3-K/Akt/mTOR signaling: High glucose activates PI3-K/Akt/mTOR signaling with increase of phospho-p70S6 and phospho-eEF2 kinase activities while reducing phospho-eEF2 (factor) expression. In addition, mTOR pathway activation causes over-activation of PP2Ac. In all these three pathways there is an increased expression of ECM glycoproteins and initiation of events related to the process of EMT with consequential apparent thickening of GBMs and expansion of glomerular mesangium.
Given the involvement Wnt/β-catenin signaling pathways in various biological processes, it is likely that they contribute to the pathogenesis of tubulo-interstitial fibrosis in different renal diseases as well. Various investigations support the idea that Wnt/β-catenin pathway is relevant to the evolution of renal fibrosis since its inhibition reduces ECM expression significantly and thereby renal scarring [44–46]. In agreement with these results, He et al. reported that blocking of Wnt/β-catenin pathway with paricalcitol treatment ameliorates glomerular and tubular related pathologies and reduces the expression of various ECM proteins in a model of adriamycin nephropathy [47]. Surendran et al. also found that in the unilateral ureteral obstruction (UUO) model, there are increased levels of β-catenin, T-cell factor (TCF), fibronectin and α-SMA along with a decrease of secreted frizzled-related protein-4 (sFRP4). Interestingly, the administration of this Wnt signaling inhibitor, i.e., sFRP4, decreased the levels of β-catenin in the renal tubular epithelial and interstitial cells, and down-regulated the expression of fibronectin and α-SMA [48]. Similarly, over-expression of Wnt antagonist Dickkof-1 (DKK-1) has been reported to reduce dermal fibrosis, and this process is likely related to the TGF-β-mediated activation of Wnt signaling which on the other hand may be operative through p38-depedent mechanisms, suggesting thereby an intricately interwoven relationship between TGF-β and Wnt signaling [49].
Much of the research has recognized that Wnt/β-Catenin signaling plays a key role for the expression of ECM proteins, the question that needs to be addressed is the relevance of this pathway to the pathogenesis of DN. High glucose-induced ECM accumulation and increased synthesis of matrix proteins in the mesangial cells has been described to be related to impaired Wnt/β-catenin signaling; on the other hand, modulation of Wnt/β-catenin pathway via transfection with stable β-catenin, Wnt4 and Wnt5a constructs can reduce ECM expression in mesangial cells [50, 51]. Other mechanisms by which the Wnt/β-catenin signaling pathway could affect renal pathobiology may be by induction of apoptosis in mesangial cells under HG ambience, and studies have demonstrated a correlation between mesangial cell apoptosis and increased concomitant aberrant ECM accumulation. Under high glucose ambience Wnt/β-catenin pathway is down-regulated along with reduced expression of Wnt4, Wnt5a and β-catenin, but an increase of GSK-3β activity and mesangial cell apoptosis. In this scenario, inhibition of the GSK-3β activation or increase of intracellular β-catenin levels have been observed to be associated with reduced mesangial cell apoptosis [52, 53]. Such a notion has been reinforced in other studies where relevance of Wnt/β-catenin signaling in progression of DN with caspases-dependent mesangial cell apoptosis has been described [54, 55]. In addition, Lin et al. discovered that Simvastatin restores HG induced impaired Wnt signaling and thereby reduce apoptosis in mesangial cells [56]. Likewise, these authors demonstrated that Dickkopf-1 (DKK-1) can inhibit Wnt signaling and reduce β-catenin levels leading to an increase in HG-induced ECM synthesis in mesangial cells and promotion of cellular apoptosis [57]. They also demonstrated that inhibition of DKK-1 attenuates the accumulation of fibrotic matrix protein in diabetic models. Moreover, Mu et al. demonstrated that miR-215 via targeting catenin-β interacting protein1 (CTNNBIP1) and activation of Wnt/β-catenin pathway increases TGF-β-mediated fibronectin matrix production in mesangial cells under HG ambiance [58]. In another study, Zhou et al reported that the expression of Wnt proteins and β-catenin are increased in kidneys of both type 1 and 2 experimental diabetic models; and also HG stimulated Wnt signaling and increased fibronectin matrix de novo synthesis in cultured human renal proximal tubular epithelial cells [59]. They also reported that the treatment with LDL-receptor-related protein 6 (LRP6) blocking antibody dampened Wnt signaling and significantly reduced renal ECM accumulation and fibrosis in diabetic mice. Also, Rooney et al. showed that β-catenin levels are increased in models of UUO and type 1 experimental diabetic mice. Besides, microarray analyses of renal biopsies in diabetic patients revealed elevation of Wnt related genes which correlated with renal fibrosis and loss of kidney functions [60,61]. Taken together, the above findings from various studies suggest that aberrations in Wnt signaling pathway are impaired in some instances while accentuated in others; ultimately leading to excessive mesangial cell apoptosis or renal tubulointerstitial fibrosis, and thus modulating the development and progression of diabetic nephropathy.
Additionally in DN, epithelial-mesenchymal transition (EMT) has been reported in the pathogenesis of tubulo-interstitial fibrosis that has certain degree of correlation to the Wnt/β-catenin signaling. Recently, Liu reviewed the involvement of Wnt/β-Catenin in EMT in tubular epithelial and glomerular cells in states of renal fibrosis. Upon activation of Wnt/β-catenin pathway, there is a promotion in the expression of β-catenin mediated genes (snail, twist, etc), which can inhibit the expression of E-cadherin, while increase in the expression of matrix protein fibronectin and mesenchymal markers like vimentin and α-SMA [62]. Interestingly, treatment with troglitazone, a peroxisome proliferator-activated receptor-γ (PPARγ) agonist, conceivably suppresses β-catenin and thus ameliorates HG-induced EMT in renal proximal tubule cells [63]. Another study demonstrated that connective tissue growth factor (CTGF) through the up-regulation of Wnt/β-catenin pathway induces EMT in HK-2 cells and thus modulates renal fibrosis [64].
Wnt/β-catenin signaling has also been described to be associated with podocyte dysfunction and albuminuria in DN. Dai et al. [65] discovered that an up-regulation of Wnt1 and active β-catenin in podocytes in human proteinuric kidney diseases, such as, diabetic nephropathy. In addition, blockade of Wnt signaling with Dickkopf-1 or podocyte-specific knockout of β-catenin ameliorated podocyte lesions and protected against development of albuminuria in adriamycin-induced kidney injury. Further investigations demonstrated that the Wnt/β-catenin signaling-related genes, such as Wnt1, Wnt2B, Wnt4, Wnt6, Wnt16 were significant increased in glomeruli of biopsies kidney of DKD patients and kidneys of mice with diabetes [66]. Podocyte injury is considered to cause glomerular albuminuria at early onset of DN. Li et al also noted that podocyte injury at early stage of diabetes accompanied with a activation of transient receptor potential cation channel 6 (TRPC6) and upregulation of Wnt/β-catenin, whereas blockade of Wnt/β-catenin signaling with Dickkopf related protein 1 (Dkk1) ameliorated podocyte injury in DN [67]. Overall, one can conclude from the above studies that Wnt/β-catenin signaling pathway may be also critical to the EMT process that is a precursor of growth factor cytokines-mediated ECM accumulation in DN, in addition to its key role in the progression of DN (Figure 2).
mTOR AND ECM
The mammalian target of rapamycin (mTOR) is an atypical serine/threonine kinase and it includes two major complexes, mTORC1 and mTORC2 [68]. In mTORC1 pathway following its activation through PI3-K/Akt/mTOR signaling phosphorylation of 4E binding protein1 (4E-BP1) and 70-kDa ribosomal protein S6 kinase (p70S6K) occurs and thus the protein synthesis is regulated, which ultimately affects cell growth and proliferation. 4E-BP1 is a negative regulatory factor for mRNA translation, and it binds to eukaryotic initiation factor 4E (eIF-4E) to inhibit initiation phases of translation. Upon a given stimulus and activation of mTOR, the mTOR induced phosphorylation of 4E-BP1 makes it inactive resulting in the dissociation of 4E-BP1 and eIF-4E. The free eIF-4E associates with eIF-4G and eIF-4A and forms eIF4F initiation complex which then caps the mRNAs. Secondly, phospho-p70S6K via mTOR modulation can also regulate elongation phases of translation. p70S6K activation leads to phosphorylation of eukaryotic elongation factor 2 kinase (eEF2 kinase) that results in decrease of eEF2 kinase activation. The down-regulation of eEF2 kinase activation leads to reduce of phosphorylation of eukaryotic elongation factor 2 and this factor’s subsequent activation [9, 69].
Recent evidence suggests that PI3-K/Akt/mTOR signaling pathways are critical in the modulation of ECM expression and tubulo-interstitial fibrosis in vivo and in vitro in DN. The involvement of various pathways including mTOR signaling pathway activation in the pathogenesis of DN has been discussed in a recent endocrinology/metabolism review [70]. Interestingly, PI3-K/Akt/mTOR signaling pathway has been considered in the mRNA translation that plays a pivotal role in ECM proteins synthesis in DN [71]. In this regard, Mariappan et al. have shown that under HG ambience, laminin-β1 synthesis increased in renal proximal epithelial cells, and such an increase was reported to be related to the activation of PI3-K/Akt/mTOR signaling pathways with boosting of RNA translation rather than transcription that ultimately led to an increased matrix protein synthesis in the progression of diabetic nephropathy [72]. In another recent study, Lieberthal et al. reported that mTOR pathway activation led to an increased expression of ECM with consequential GBM and TBM thickening and mesangial matrix production in DN [73]. These changes in the glomerular and tubular matrices were considerably reduced following rapamycin treatment. Similarly, Lloberas et al. found that compared with non-diabetic rats, the expression of phosphorylated Akt and mTOR and accumulation of ECM was much greater in diabetic rats, and treatment with mTOR blocker sirolimus (SRL) significantly reduced the phosphorylated Akt and mTOR expression and ECM accumulation [74]. Likewise, Mori et al. described that p70S6-kinase, a downstream factor of mTOR, had an increased activation in diabetic mice, while inhibition of mTOR signaling by rapamycin significantly attenuated mesangial matrix expansion and improved deranged renal functional parameters [75]. In addition, Sakaguchi et al. demonstrated that in STZ-induced diabetes mice, the mTOR pathway activation results in renal hypertrophy, and in vitro overexpression of p70S6kinase in tubular cells leads to rapamycin-inhibitable cellular hypertrophy [76]. In addition, other investigators noted that rapamycin treatment, besides reducing the thickening of glomerular basement membranes, the levels of TGF-β, VEGF, MCP-1 and PCNA are reduced during the early stages of diabetic kidney disease in rats, while the blood glucose levels were unaffected [77, 78]. However, HG can activate PI3-K/Akt/mTOR signaling with increase of phospho-p70S6 and phospho-eEF2 kinase activities while reducing phospho-eEF2 (factor) expression in proximal tubular epithelial cells [79]. Subsequently this promotes elongation phase of mRNA translation and contribute to the enhanced expression of ECM laminin-β1, and all these changes were normalized following rapamycin treatment in diabetic mice. Using losartan, an angiotensin II receptor antagonist, Mavroeidi et al. demonstrated that PI3-K/Akt/mTOR signaling is involved in the pathogenesis of diabetic nephropathy, and its treatment decreases both mTOR and pAKT protein levels [80]. Another study described that sirolimus and rosiglitazone, used in combination, can also reduce ECM related changes in the kidney and decrease albuminuria via the dampening of the mTORC1 signaling pathway and over-activated catalytic protein phosphatase 2A (PP2Ac) in diabetic rats [81]. Interestingly, another protein known as “tuberin” by activation of mTOR signaling has been reported to play a pivotal role in ECM protein accumulation in renal tubular cells of diabetic patients [82].
On the other hand, studies have shown that mTOR activation in podocyte is related to the progression of DN in humans and mice [83]. Gödel et al described that mTOR signaling pathway was activated in podocytes of patients with DN and diabetic mouse kidney. In the latter case signaling was associated with glomerular hypertrophy and hyperfiltration in DN at early stages of the disease. Interestingly, gene-dose reduction of mTOR complex 1 (mTORC1) in mouse podocytes was found to be associated with prevention of glomerulosclerosis and dampened the progression of diabetic nephropathy [84]. The role of mTORC1 in the pathogenesis of diabetic nephropathy is also supported in studies by Inoki et al. [85]. They noted that mTORC1is hyperactive in the podocytes in diabetic mouse and that apparently may be responsible for podocyte loss, GBM thickening and proteinuria. In a nutshell, the above review of various literature reports strongly suggest that PI3-K/Akt/mTOR signaling plays an essential role in ECM proteins synthesis and the progression of DN (Figure 2). Thus targeting this signaling pathway may serve an opportunity as a new interventional strategy in the amelioration of DN.
TLRs AND ECM
TLRs (Toll-like receptors) are a group of proteins that play a critical role in the regulation of innate immune system of the body. These receptors (TLR1 –TLR13) recognize pathogen-associated molecular patterns (PAMPs), but in addition they are also instrumental in the identification of damage-associated molecular patterns (DAMPs), and thus likely to participate various disease processes that affect kidney [86]. Among the various mammalian TLR 10 members have been described in humans, namely TLR1 - TLR10; whereas, in mice up to 13 members have been described. They are involved in a wide variety of disease processes affecting kidney [87]. Recent evidence indicate that TLRs, mainly TLR2 and TLR4, are intimately involved in the processes of inflammation and fibrogenesis that are seen in the progression of DN [88]. In such a diabetic milieu, kidney cells release endogenous ligands that conceivably activate TLR2 and TLR4. Upon activation by the ligands, TLR2 through MyD88-dependent signaling pathway whereas TLR4 through MyD88-dependent and MyD88-independent pathways cause activation of nuclear factor Kappa-B (NF-κB) (Figure 2). This would subsequently increase the expression and secretion of pro-fibrogenic and pro-inflammatory cytokines resulting in inflammation and fibrosis, leading to exacerbation of DN [89, 90]. There are certain documented studies that confirm that TLR2 is responsible for the progression of DN. Ma et al. observed that STZ treated TLR2−/− mice in comparison with control diabetic wild mice are significantly protected against the development of DN, and have less albuminuria, inflammation and attenuated expression of TGF-β and ECM protein fibronectin, and reduced deposition of collagen in the interstitial compartment and decreased myofibroblast activation and expression of α-SMA. Likewise, in vitro studies support this notion that TLR2-dependent pathway directly modulates ECM expression and generates a fibrogenic response in primary podocytes isolated from WT mice and TLR2−/− mice and exposed to HG ambience [91]. In addition, high serum lipopolysaccharide (LPS) activity has been shown to be involved in the progression of DN. Recently Saurus et al. found that LPS activates the expression of 3-phosphoinositide-dependent kinase-1 (PDK1), which leads to podocyte apoptosis by the TLR signaling pathway [92]. Furthermore, Devaraj et al. described that the kidneys in STZ-induced diabetic wild mice had an increased expression of ECM laminin and TGF-β and excessive albuminuria, decreased kidney nephrin and podocin expression and podocyte number compared to TLR2−/− mice having STZ-induced diabetes [93]. Like TLR2, emerging evidence has shown that TLR4 also plays a vital role in renal fibrogenesis via the modulation of inflammatory (TNF-α) and growth factor (TGF-β) cytokines [94]. Pulskens et al. found that in the model of UUO, TLR4-deficient mice have less renal fibrosis than wild-type mice; and in vitro, TLR4-deficient renal tubular epithelial cells compared to wild type cells have reduced TGF-β-induced collagen expression [95]. Thus, they concluded that TLR4 may exert its effect in a TGF-β-dependent manner to promote renal fibrosis. Recent evidence has also indicated that TLR4 could be involved in fibrogenesis that is seen in DN. Ma et al. demonstrated that TLR4−/− diabetic mice versus diabetic wild-type mice have significantly reduced deposition of collagen, fibronectin matrix and TGF-β expression and activation of myofibroblast, which is accompanied with lesser degree of albuminuria, inflammation, glomerular hypertrophy and podocytes and tubular injury [96]. Similarly, Kuwabara et al. reported that administration of high fat diet to mice with STZ induced diabetes leads to an activation of TLR4 and S100A8 ligand resulting in the exacerbation of hyperlipidemia induced DN [97]. They further demonstrated that TLR4−/− mice had reduced mesangial expansion and accumulation of ECM in glomeruli compared with the diabetic wild-type mice. In addition, Jialal et al. also demonstrated that the STZ-induced mice have significantly increased macrophage and TLR4 expression which is associated with increase in the expression of MyD88, interferon regulatory factor-3 (IRF-3), tumor necrosis factor-α (TNF-α), interlukin-6 (IL-6), monocyte chemotactic factor-1(MCP-1), and activity of NF-κB, and fibrosis markers, such as, collagen IV, and TGF-β. Conversely, the podocyte numbers and podocin expression were reduced. However, all these changes were significantly reversed in the STZ-TLR4 KO mice [98]. Other investigators also reported that HG ambience induces activation and expression of TLR4 in mouse mesangial and tubular cells and concluded that these receptors may contribute to the ECM production and progression of DN [90, 99]. In addition, TLR4 may also promote tubulo-interstitial inflammation in DN via NF-κB signaling pathway [100]. Interestingly, recent study reported that db/db mice treated with GIT27 ({S,R} −3-phenyl-4, 5-dihydro-5-isoxasole acetic acid) an inhibitor for TLR4 and TLR2/6-mediated signaling pathway in macrophages, markedly decreased TGF-β, Coll IV and NF-κB expression which was accompanied with reduced excretion rate of proteinuria and pro-inflammatory cytokine expression in the diabetic mouse kidney [101].
SMALL GTPase AND ECM
Small GTPases constitute mainly the five members, including Ras, Rho, Rab, Sar1/Arf and Ran [102, 103]. Recent studies have demonstrated that small GTPases like Ras, Rho and Rab family influence accumulation of ECM proteins and cause renal fibrosis in hyperglycemic states.
Ras and ECM
Ras family mainly consists of four members: H-ras, K-ras, N-ras and others, such as, the Raps (1A, 1B, 2A and 2B), R-Ras, Ral proteins and Rheb. Rap1 is a member of the Raps, and it includes two subtypes Rap1A and Rap1B [104]. There are literature reports which indicate the association of Rap1 with ECM synthesis and fibrosis. Rufanova et al. demonstrated that in cultured mesangial cells, Rap1 with ET-1 stimulation through ET-1-Pyk2-p130Cas/BCAR3- Rap1 pathway modulates cell adhesion, cell spreading and ECM synthesis [105]. Huang et al. observed that transfection of Rap1GAP (Rap1GTPase activating protein) or dominant-negative Rap1 (Rap1N17) reduced the activation of Rap1 and significantly attenuated the Prostaglandin E2 (PGE2) proliferative effects and activation of fibroblasts, which then led to inhibition of fibrosis, while transfection of constitutively active Rap1 (Rap1V12) yielded opposite results [106].
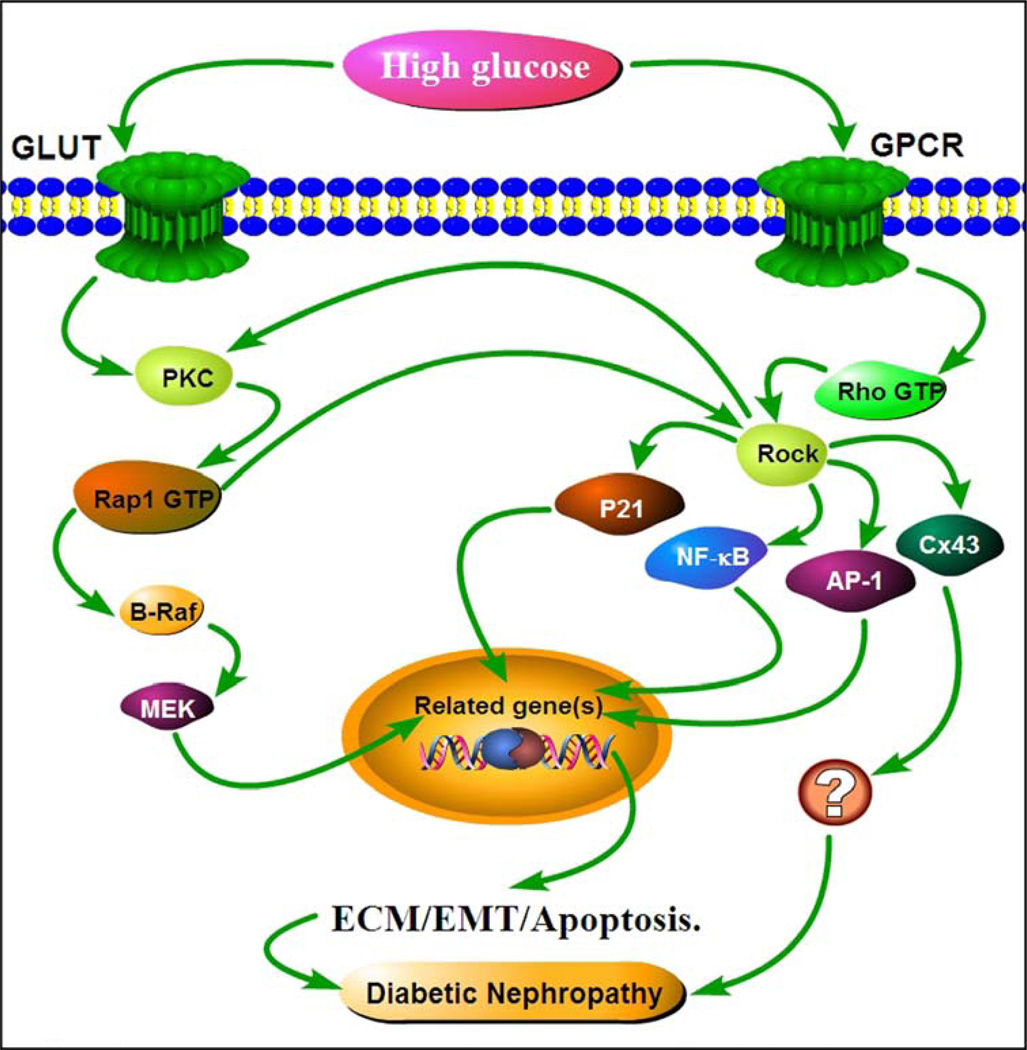
The Ras family also plays an essential role in ECM accumulation and pathogenesis of DN. Our past research work indicates that Rap1 expression is increased in both embryonic and newborn kidneys of experimental diabetic mice, as originally assessed by suppression subtractive hybridization techniques [107, 108]. In subsequent studies, we also showed that Rap1 is increased in diabetic mice and in mesangial cells under HG ambience with up-regulation of fibronectin synthesis. The Rap1b downstream effects were mediated through a novel PKC-Rap1-B-Raf signaling pathway that ultimately modulated HG-induced ECM synthesis [109] (Figure 3). Our follow-up studies indicated that over-expression of Rap1b in kidneys of STZ-induced Rats could ameliorates tubular damage, reduce ECM protein expression and slow the progression of DN by modulating C/EBP-β binding to the promoter region of PGC-1α and the interaction between PGC-1α and catalase modulated mitochondrial dynamics [110]. Furthermore, overexpression of Rap1b can reverse the HG induced mitochondrial dysfunction, ROS production and ECM expression in renal tubular cells [111]. On the other hand, Lin et al. noted that Ras induces generation of superoxide and subsequent activation of ERK and nuclear c-Jun, leading to TGF-β1-induced ECM accumulation in mesangial cells under high glucose milieu [112]. Additionally, it has been shown that advanced glycation end products (AGEs) can activate Ki-Ras and ERK by PI3-kinase-dependent manner in mesangial cells, which then leads to excessive ECM protein synthesis [113]. The activation ERK and PI3-kinase could be achieved by the treatment with AGEs’ receptor agonist, S100, while these effects were negated by inhibition of PI3-kinase and administration of ROS scavengers, suggesting a potential role Ras family of GTPases in the pathogenesis of DN. In addition, a recent study has shown that the Ras GTPase-activating-like protein (IQGAP1) plays a key role in the cellular biology of cytoskeleton of the glomerular podocytes. Zhou et al. demonstrated that IQGAP1 expression in podocytes is reduced in renal biopsies of patients with DN [114]. Their in vitro studies also indicated that the expression of IQGAP1 was also down-regulated in podocytes under HG via ERK-dependent pathway since ERK1/2 activation inhibitor, PD98059, could attenuate the down-regulated response.
Figure 3.
Schematics depicting potential mechanism(s) by which high glucose induces ECM glycoprotein synthesis via the activation of small GTPases, Ras or Rho, in diabetic nephropathy. Under high glucose conditions, PKC is activated which induces Rap1-GDP transition to Rap1-GTP, the activated form of small GTPase. This up-regulates the expression of ECM related genes in various cells of the kidney via B-Raf /MEK pathway. On the other hand, high glucose by activation of Rho/Rock signaling induces over-expression of transcription factor(s), such as NF-κB, AP-1 and p21, which are associated with increased fibronectin matrix protein synthesis and genes relevant to the process of EMT, ultimately leading to the progression of DN.
Rho and ECM
Rho GTPase is a family member of small GTPase which mainly include three classes of proteins: Rho, Rac and Cdc42. The Rho family itself is made up of RhoA, RhoB, and RhoC [115]. The Rho family downstream effector is Rho kinase (ROCK), which has two isoforms: ROCK1 and ROCK2 [116, 117].
Quite a bit of work has been reported in the literature that suggests a link between Rho/Rock signaling and ECM accumulation in DN and increased synthesis of matrix proteins in vitro cell culture systems. In diabetic state, Rho/Rock signaling pathway is activated in renal cells and it is associated with increased expression ECM proteins and tubulo-interstitial fibrosis [118, 119]. In line with these observations, Peng et al. reported that HG induced the activation of Rho/Rock pathway, an increased activity of transcription factor AP-1 and increased fibronectin expression, and these changes could be prevented by Rho-kinase inhibition with fasudil, as reflected by attenuation in glomerular matrix accumulation, GBM thickening in a model of STZ-induced diabetes in rats [120, 121]. Ma et al. also showed that inhibition of RhoA activation with Rho-siRNA in human mesangial cells reduced the expression of fibronectin, connective tissue growth factor (CTGF) and TNF-α, meaning that the inhibition in the synthesis of ECM may be also related to the status of growth factors and inflammatory cytokines [122]. Xie et al. demonstrated that Rho/Rock modulation of NF-κB increased TGF-β1 and ICAM-1 expression, which resulted in increased fibronectin matrix protein synthesis in cultured human mesangial cells. Inhibition of ROCK with fasudil reduced the activation of Rho/Rock, along with reduced NF-κB translocation and fibronectin accumulation in rats with STZ-induced diabetes [123]. Fasudil treatment not only inhibited the ROCK activity but also reduces the process of epithelial-myofibroblast transdifferentiation or epithelial-mesenchymal transition of human renal HK-2 tubular epithelial cells subjected to HG ambience [124] (Figure 3).
In other experimental animal models of diabetes, e.g., db/db mice, Kolavennu et al. discovered that activation of Rho and ROCK is increased, and their inhibition with fasudil or simvastatin reduced mesangial expansion and decreased urinary albumin excretion [125]. Likewise, ROCK inhibition with high dosages of fasudil or administration of olmesartan, angiotensin II receptor blocker, over a long period was found to ameliorate glomerulosclerosis and tubulo-interstitial fibrosis in Otsuka Long-Evans Tokushima fatty (OLETF) rats, a model of insulin-resistant diabetes [126]. There are certain other drugs that target the Rho/Rock signaling pathway and yield reno-protection from DN. For instance, Benidipine, a calcium channel blocker, through inhibiting the activation of Rho-kinase reduces EMT and renal tubulo-interstitial fibrosis in type1 diabetic animal models [127]. Berberine (BBR), an ancient Chinese medicine with anti-inflammatory properties, has also been shown to inhibit RhoA/Rock pathway by down-regulating the activation of NF-κB, along with reduced expression of TGF-β1 and fibronectin matrix in glomerular mesangial cells treated with high glucose and in kidneys of rats with diabetes [128]. Several other studies support that fasudil by inhibiting Rho/Rho-kinase signaling pathway ameliorates diabetic injury to the kidney by modulating the expression of pro-fibrogenic cytokines, TGF-β and CTGF, as well as that of NAD(P)H oxidase 4 (NOX4), which as a consequence would lead to reduced tubulo-interstitial fibrosis and glomerulosclerosis [129, 130]. Here it is worth mentioning that TGF-β suppresses the expression of microRNA-29 and that is accompanied with up-regulation of ECM proteins in diabetic states. Whereas the treatment with Rho-kinase inhibitor, fasudil, decreased ECM deposition while at the same time restoring the microRNA-29 expression [131]. Another molecular complex, i.e., sphingosine-1-phosphate (S1P) and its receptor S1P2, has been found to promote EMT in renal tubular epithelial cells by activation of Rho-kinase, followed by the activation of the process of EMT that ultimately would contribute to increased synthesis of ECM proteins and expression of α-SMA [132]. These phenotypic expressions are reversed with the treatment of Rho kinase inhibitor or SIP2 receptor blockade, thus suggesting that the Rho/Rock signaling plays a critical pathogenetic role in the progression of fibrosis and DN (Figure 3).
In addition, previously our laboratories also demonstrated that 3-hydroxy-3-methylglutaryl CoA reductase inhibitor, statin, inhibits HG induced proliferation of mesangial cells and ECM protein synthesis that is modulated via Rho GTPase/P21 signaling pathway [133]. Another molecule, Connexin43 (Cx43), has been found in kidneys with DN, and interestingly it can regulate NF-κB activation in mesangial cells subjected to HG ambience. Chen et al. found that activated RhoA/ROCK signaling induces Cx34 degradation in HG treated mesangial cells depending on the F-actin regulation [134]. Furthermore, evidence suggests that mitochondrial dysfunction plays a critical role in the pathogenesis of DN, which can cause ROS production and ECM protein synthesis [110]. In this regard, Danesh et al. demonstrated that ROCK1 plays an unexpected role in regulating mitochondrial dysfunctions by Drp1, a mitochondrial dynamic protein, which also participates in ECM protein synthesis in DN [135].
Rab34 and ECM
Rab34 belongs to Rab family of proteins, which participate in various stages of vesiculo-tubular transport [136]. The effector of Rab34 is a cytosolic diacylglycerol (DAG)-binding protein known as munc13-2, which apparently responds to hyperglycemic stimuli in various cell types of the kidney and translocates into the Golgi apparatus for further trafficking and secretion of proteins [137]. In mesangial cells, Goldenberg et al. confirmed that increased secretion of fibronectin is due to the interaction between Rab34 and munc13-2 under HG ambience; and secretion of the ECM proteins can be abolished by the treatment of munc13-2 siRNA, suggesting a potential role of GTPase in pathogenesis of DN [138]. Basically, the above discussion of this section underscores the importance of RAS family of proteins in the pathobiology of kidney in diabetic nephropathy (DN).
CONCLUSION
At present, treatment of DN is mainly dependent upon instituting strict glycemic control and modulation of renin-angiotensin-aldosterone system (RAAS); however, absolute amelioration of DN has not been achieved so far. There are other therapeutic agents that have been used in sporadic studies for reno-protection in diabetic states. They include inhibitors of AGEs, growth factor cytokines, protein kinase C, sodium glucose transporters and etc. but their use again has yielded limited success, and therefore it seems that large-scale clinical studies may be required to assess their appropriate efficacies [139]. Since the DN phenotype is characterized by increased synthesis of ECM proteins, it is conceivable that the molecules which inhibit their overexpression may be worth the exploration to identify precise therapeutic targets. Herein, we reviewed some of the new signaling pathways or molecules, such as, Notch (Figure 1), Wnt, TLRs,mTOR signaling pathway(s) (Figure 2), and small GTPase Molecular signal(Figure 3) that are believed to play a role in expression and regulation of ECM proteins in DN. Conceivably, understanding of the pathobiology of these signaling pathways or the biology of these molecules may aid in developing future interventional strategies for the amelioration of diabetic nephropathy.
Acknowledgments
Supported by grants from the Creative Research Group Fund of the National Foundation Committee of Natural Sciences of China (81100541, 81370832 and 81270812), the Doctoral Fund of Ministry of Education of China (20110162110012), the Furong Scholars Fund from Hunan Province Education Department, and USA NIH grant (DK60635).
Abbreviations
- NICD
notch intracellular domain
- TLR2
Toll-like receptor 2
- TLR4
Toll-like receptor 4
- TIRAP
TIR-domain-containing adaptor protein
- MyD88
myeloid differentiation factor 88
- TRAM
TRIF-related adaptor molecule
- TRIF
TIR-domain-containing adaptor-inducing interferon-β
- NF-κB
nuclear factor kappa B
- LRP
low density lipoprotein receptor-related protein
- FZ
Frizzled
- Dvl
dishevelled
- GSK3β
glycogen synthase kinase 3β
- APC
adenomatous polyposis coli
- TCF
T cell factor
- LEF
lymphoid enhancing factor
- GPCR
G-protein-coupled receptors
- Rock
Rho kinase
- PI3K
phosphatidylinositol 3-kinase
- Akt
protein kinase B
- mTOR
mammalian target of rapamycin
- p70S6K
70-kDa ribosomal protein S6 kinase
- 4EBP1
4E binding protein1
- PKC
protein kinase C
- HG
high glucose
- ECM
extracellular matrix
- IRF-3
interferon regulatory factor-3
- MCP-1
monocyte chemotactic factor-1
Footnotes
Conflict of Interest: The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp. Biol. Med. (Maywood) 2008;233(1):4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 2.Murphy M, Crean J, Brazil DP, Sadlier D, Martin F, Godson C. Regulation and consequences of differential gene expression in diabetic kidney disease. Biochem. Soc. Trans. 2008;36(Pt 5):941–945. doi: 10.1042/BST0360941. [DOI] [PubMed] [Google Scholar]
- 3.Haider DG, Peric S, Friedl A, Fuhrmann V, Wolzt M, Horl WH, Soleiman A. Kidney biopsy in patients with diabetes mellitus. Clin. Nephrol. 2011;76(3):180–185. doi: 10.5414/cn106955. [DOI] [PubMed] [Google Scholar]
- 4.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin. Nephrol. 2007;27(2):195–207. doi: 10.1016/j.semnephrol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur. J. Clin. Invest. 2004;34(12):785–796. doi: 10.1111/j.1365-2362.2004.01429.x. [DOI] [PubMed] [Google Scholar]
- 7.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc.Nephrol. 2003;14(5):1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 8.Kolset SO, Reinholt FP, Jenssen T. Diabetic nephropathy and extracellular matrix. J. Histochem. Cytochem. 2012;60(12):976–986. doi: 10.1369/0022155412465073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariappan MM. Signaling mechanisms in the regulation of renal matrix metabolism in diabetes. Exp. Diabetes Res. 2012;2012:749812. doi: 10.1155/2012/749812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokes MB, Holler S, Cui Y, Hudkins KL, Eitner F, Fogo A, Alpers CE. Expression of decorin, biglycan, and collagen type I in human renal fibrosing disease. Kidney Int. 2000;57(2):487–498. doi: 10.1046/j.1523-1755.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 11.Heilig CW, Deb DK, Abdul A, Riaz H, James LR, Salameh J, Nahman NS., Jr GLUT1 regulation of the pro-sclerotic mediators of diabetic nephropathy. Am. J. Nephrol. 2013;38(1):39–49. doi: 10.1159/000351989. [DOI] [PubMed] [Google Scholar]
- 12.Miller CG, Pozzi A, Zent R, Schwarzbauer JE. Effects of high glucose on integrin activity and fibronectin matrix assembly by mesangial cells. Mol. Biol. Cell. 2014;25(16):2342–2350. doi: 10.1091/mbc.E14-03-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busch M, Franke S, Ruster C, Wolf G. Advanced glycation end-products and the kidney. Eur. J. Clin. Invest. 2010;40(8):742–755. doi: 10.1111/j.1365-2362.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 14.Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Curr. Med. Chem. 2010;17(34):4256–4269. doi: 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thrailkill KM, Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine. 2009;35(1):1–10. doi: 10.1007/s12020-008-9114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Xiao L, Xiao P, Yang S, Chen G, Liu F, Kanwar YS, Sun L. A glimpse of matrix metalloproteinases in diabetic nephropathy. Curr. Med. Chem. 2014;21(28):3244–3260. doi: 10.2174/0929867321666140716092052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato M, Natarajan R. Diabetic nephropathy--emerging epigenetic mechanisms. Nat. Rev. Nephrol. 2014;10(9):517–530. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiarelli F, Gaspari S, Marcovecchio ML. Role of growth factors in diabetic kidney disease. Horm. Metab. Res. 2009;41(8):585–593. doi: 10.1055/s-0029-1220752. [DOI] [PubMed] [Google Scholar]
- 19.Davis LK, Rodgers BD, Kelley KM. Angiotensin II- and glucose-stimulated extracellular matrix production: mediation by the insulin-like growth factor (IGF) axis in a murine mesangial cell line. Endocrine. 2008;33(1):32–39. doi: 10.1007/s12020-008-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrissey J, Guo G, Moridaira K, Fitzgerald M, McCracken R, Tolley T, Klahr S. Transforming growth factor-beta induces renal epithelial jagged-1 expression in fibrotic disease. J. Am. Soc. Nephrol. 2002;13(6):1499–1508. doi: 10.1097/01.asn.0000017905.77985.4a. [DOI] [PubMed] [Google Scholar]
- 22.Chuang PY, Menon MC, He JC. Molecular targets for treatment of kidney fibrosis. J. Mol. Med. (Berl) 2013;91(5):549–559. doi: 10.1007/s00109-012-0983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S, Sirin Y, Susztak K. The story of Notch and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2011;20(1):56–61. doi: 10.1097/MNH.0b013e3283414c88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kretzler M, Allred L. Notch inhibition reverses kidney failure. Nat. Med. 2008;14(3):246–247. doi: 10.1038/nm0308-246. [DOI] [PubMed] [Google Scholar]
- 25.Leask A. Targeting the jagged/notch pathway: a new treatment for fibrosis? J. Cell. Commun. Signal. 2010;4(4):197–198. doi: 10.1007/s12079-010-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirin Y, Susztak K. Notch in the kidney: development and disease. J. Pathol. 2012;226(2):394–403. doi: 10.1002/path.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO. J. 2004;23(5):1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH;Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Invest. 2010;120(11):4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murea M, Park JK, Sharma S, Kato H, Gruenwald A, Niranjan T, Si H, Thomas DB, Pullman JM, Melamed ML, Susztak K. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int. 2010;78(5):514–522. doi: 10.1038/ki.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh DW, Roxburgh SA, McGettigan P, Berthier CC, Higgins DG, Kretzler M, Cohen CD, Mezzano S, Brazil DP, Martin F. Co-regulation of Gremlin and Notch signalling in diabetic nephropathy. Biochim. Biophys. Acta. 2008;1782(1):10–21. doi: 10.1016/j.bbadis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Hills CE, Squires PE. TGF-β-Induced Epithelial-to-Mesenchymal Transition and Therapeutic intervention in Diabetic Nephropathy. Am. J. Nephrol. 2010;31:68–74. doi: 10.1159/000256659. [DOI] [PubMed] [Google Scholar]
- 32.Loeffler I, Wolf G. Transforming growth factor-beta and the progression of renal disease. Nephrol. Dial. Transplant. 2014;29(Suppl 1):i37–i45. doi: 10.1093/ndt/gft267. [DOI] [PubMed] [Google Scholar]
- 33.Choi ME. Mechanism of transforming growth factor-beta1 signaling: Role of the mitogen-activated protein kinase. Kidney Int. Suppl. 2000;77:S53–S58. [PubMed] [Google Scholar]
- 34.Bonegio R, Susztak K. Notch signaling in diabetic nephropathy. Exp. Cell Res. 2012;318(9):986–992. doi: 10.1016/j.yexcr.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Gao C, Chen G, Li X, Li J, Wan Q, Xu Y. Notch Signaling Molecules Activate TGF- beta in Rat Mesangial Cells under High Glucose Conditions. J. Diabetes Res. 2013;2013:979702. doi: 10.1155/2013/979702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn SH, Susztak K. Getting a Notch Closer to Understanding Diabetic Kidney Disease. Diabetes. 2010;59(8):1865–1867. doi: 10.2337/db10-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat. Med. 2008;14(3):290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 38.Lin CL, Wang FS, Hsu YC, Chen CN, Tseng MJ, Saleem MA, Chang PJ, Wang JY. Modulation of Notch-1 Signaling Alleviates Vascular Endothelial Growth Factor - Mediated Diabetic Nephropathy. Diabetes. 2010;59(8):1915–1925. doi: 10.2337/db09-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Mi K, Dolan PJ, Johnson GV. The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. J. Biol. Chem. 2006;281(8):4787–4794. doi: 10.1074/jbc.M508657200. [DOI] [PubMed] [Google Scholar]
- 41.Maarouf OH, Ikeda Y, Humphreys BD. Wnt signaling in kidney tubulointerstitium during disease. Histol. Histopathol. 2015;30(2):163–171. doi: 10.14670/HH-30.163. [DOI] [PubMed] [Google Scholar]
- 42.Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp. Mol. Med. 2006;38(1):1–10. doi: 10.1038/emm.2006.1. [DOI] [PubMed] [Google Scholar]
- 43.Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr. Biol. 2005;15(2):R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 44.Hwang I, Seo EY, Ha H. Wnt/beta-catenin signaling: a novel target for therapeutic intervention of fibrotic kidney disease. Arch. Pharm. Res. 2009;32(12):1653–1662. doi: 10.1007/s12272-009-2200-3. [DOI] [PubMed] [Google Scholar]
- 45.Cisternas P, Vio CP, Inestrosa NC. Role of Wnt signaling in tissue fibrosis, lessons from skeletal muscle and kidney. Curr. Mol. Med. 2014;14(4):510–522. doi: 10.2174/1566524014666140414210346. [DOI] [PubMed] [Google Scholar]
- 46.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 2009;20(4):765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 2011;22(1):90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surendran K, Schiavi S, Hruska KA. Wnt-Dependent β-Catenin Signaling Is Activated after Unilateral Ureteral Obstruction, and Recombinant Secreted Frizzled-Related Protein 4 Alters the Progression of Renal Fibrosis. J. Am. Soc. Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 49.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat. Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL. Sustained Wnt/beta-catenin signaling rescues high glucose induction of transforming growth factor-beta1-mediated renal fibrosis. Am. J. Med. Sci. 2012;344(5):374–382. doi: 10.1097/MAJ.0b013e31824369c5. [DOI] [PubMed] [Google Scholar]
- 51.Xiao L, Wang M, Yang S, Liu F, Sun L. A glimpse of the pathogenetic mechanisms of Wnt/beta-catenin signaling in diabetic nephropathy. Biomed. Res. Int. 2013;2013:987064. doi: 10.1155/2013/987064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J. Am. Soc. Nephrol. 2006;17(10):2812–2820. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- 53.Pulkkinen K, Murugan S, Vainio S. Wnt signaling in kidney development and disease. Organogenesis. 2008;4(2):55–59. doi: 10.4161/org.4.2.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin CL, Wang JY, Ko JY, Surendran K, Huang YT, Kuo YH, Wang FS. Superoxide destabilization of beta-catenin augments apoptosis of high-glucose-stressed mesangial cells. Endocrinology. 2008;149(6):2934–2942. doi: 10.1210/en.2007-1372. [DOI] [PubMed] [Google Scholar]
- 55.Mishra R, Emancipator SN, Kern T, Simonson MS. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int. 2005;67(1):82–93. doi: 10.1111/j.1523-1755.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 56.Lin CL, Cheng H, Tung CW, Huang WJ, Chang PJ, Yang JT, Wang JY. Simvastatin reverses high glucose-induced apoptosis of mesangial cells via modulation of Wnt signaling pathway. Am. J. Nephrol. 2008;28(2):290–297. doi: 10.1159/000111142. [DOI] [PubMed] [Google Scholar]
- 57.Lin CL, Wang JY, Ko JY, Huang YT, Kuo YH, Wang FS. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J. Am. Soc. Nephrol. 2010;21(1):124–135. doi: 10.1681/ASN.2008101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mu J, Pang Q, Guo YH, Chen JG, Zeng W, Huang YJ, Zhang J, Feng B. Functional implications of microRNA-215 in TGF-beta1-induced phenotypic transition of mesangial cells by targeting CTNNBIP1. PLoS One. 2013;8(3):e58622. doi: 10.1371/journal.pone.0058622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou T, He X, Cheng R, Zhang B, Zhang RR, Chen Y, Takahashi Y, Murray AR, Lee K, Gao G, Ma JX. Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia. 2012;55(1):255–266. doi: 10.1007/s00125-011-2314-2. [DOI] [PubMed] [Google Scholar]
- 60.Rooney B, O'Donovan H, Gaffney A, Browne M, Faherty N, Curran SP, Sadlier D, Godson C, Brazil DP, Crean J. CTGF/CCN2 activates canonical Wnt signalling in mesangial cells through LRP6: implications for the pathogenesis of diabetic nephropathy. FEBS. Lett. 2011;585(3):531–538. doi: 10.1016/j.febslet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J. Pathol. 2013;229(2):221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010;21(2):212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee YJ, Han HJ. Troglitazone ameliorates high glucose-induced EMT and dysfunction of SGLTs through PI3K/Akt, GSK-3beta, Snail1, and beta-catenin in renal proximal tubule cells. Am. J. Physiol .Renal. Physiol. 2010;298(5):F1263–F1275. doi: 10.1152/ajprenal.00475.2009. [DOI] [PubMed] [Google Scholar]
- 64.Yang Z, Sun L, Nie H, Liu H, Liu G, Guan G. Connective tissue growth factor induces tubular epithelial to mesenchymal transition through the activation of canonical Wnt signaling in vitro. Ren. Fail. 2015;37(1):129–135. doi: 10.3109/0886022X.2014.967699. [DOI] [PubMed] [Google Scholar]
- 65.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/β-catenin signaling promotes podocyte dysfunction and abuminuria. J. Am. Soc. Nephrol. 2009;20(9):1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato H, Gruenwald A, Suh JH, Miner JH, Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB, Susztak K. Wnt/β-catenin Pathway in Podocytes Integrates Cell Adhesion, Differentiation, and Survival. J. Biol. Chem. 2011;286(29):26003–26015. doi: 10.1074/jbc.M111.223164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z, Xu J, Xu P, Liu S, Yang Z. Wnt/β-catenin signalling pathway mediates high glucose induced cell injury through activation of TRPC6 in podocytes. Cell Prolif. 2013;46(1):76–85. doi: 10.1111/cpr.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 2009;21(2):209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 70.Brosius FC, Khoury CC, Buller CL, Chen S. Abnormalities in signaling pathways in diabetic nephropathy. Expert. Rev. Endocrinol. Metab. 2010;5(1):51–64. doi: 10.1586/eem.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kasinath BS, Mariappan MM, Sataranatarajan K, Lee MJ, Feliers D. mRNA translation: unexplored territory in renal science. J. Am. Soc. Nephrol. 2006;17(12):3281–3292. doi: 10.1681/ASN.2006050488. [DOI] [PubMed] [Google Scholar]
- 72.Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes. 2007;56(2):476–485. doi: 10.2337/db05-1334. [DOI] [PubMed] [Google Scholar]
- 73.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J. Am. Soc. Nephrol. 2009;20(12):2493–2502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 74.Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyo JM. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease. J. Am. Soc. Nephrol. 2006;17(5):1395–1404. doi: 10.1681/ASN.2005050549. [DOI] [PubMed] [Google Scholar]
- 75.Mori H, Inoki K, Masutani K, Wakabayashi Y, Komai K, Nakagawa R, Guan KL, Yoshimura A. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem. Biophys. Res. Commun. 2009;384(4):471–475. doi: 10.1016/j.bbrc.2009.04.136. [DOI] [PubMed] [Google Scholar]
- 76.Sakaguchi M, Isono M, Isshiki K, Sugimoto T, Koya D, Kashiwagi A. Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in the early diabetic mice. Biochem. Biophys. Res. Commun. 2006;340(1):296–301. doi: 10.1016/j.bbrc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, Chen Y, Chen J. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am. J. Nephrol. 2007;27(5):495–502. doi: 10.1159/000106782. [DOI] [PubMed] [Google Scholar]
- 78.Grahammer F, Wanner N, Huber TB. mTOR controls kidney epithelia in health and disease. Nephrol. Dial. Transplant. 2014;29(Suppl 1):i9–i18. doi: 10.1093/ndt/gft491. [DOI] [PubMed] [Google Scholar]
- 79.Sataranatarajan K, Mariappan MM, Lee MJ, Feliers D, Choudhury GG, Barnes JL, Kasinath BS. Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am. J. Pathol. 2007;171(6):1733–1742. doi: 10.2353/ajpath.2007.070412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mavroeidi V, Petrakis I, Stylianou K, Katsarou T, Giannakakis K, Perakis K, Vardaki E, Stratigis S, Ganotakis E, Papavasiliou S, Daphnis E. Losartan affects glomerular AKT and mTOR phosphorylation in an experimental model of type 1 diabetic nephropathy. J. Histochem. Cytochem. 2013;61(6):433–443. doi: 10.1369/0022155413482925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flaquer M, Lloberas N, Franquesa M, Torras J, Vidal A, Rosa JL, Herrero-Fresneda I, Grinyo JM, Cruzado JM. The combination of sirolimus and rosiglitazone produces a renoprotective effect on diabetic kidney disease in rats. Life Sci. 2010;87(5–6):147–153. doi: 10.1016/j.lfs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Habib SL. Alterations in tubular epithelial cells in diabetic nephropathy. J. Nephrol. 2013;26(5):865–869. doi: 10.5301/jn.5000287. [DOI] [PubMed] [Google Scholar]
- 83.Lu MK, Gong XG, Guan KL. mTOR in podocyte function Is rapamycin good for diabetic nephropathy? Cell Cycle. 2011;10(20):3415–3416. doi: 10.4161/cc.10.20.17686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 2011;121(6):2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 2011;121(6):2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 87.Robson MG. Toll-like receptors and renal disease. Nephron Exp. Nephrol. 2009;113(1):e1–e7. doi: 10.1159/000228077. [DOI] [PubMed] [Google Scholar]
- 88.Lin M, Tang SC. Toll-like receptors: sensing and reacting to diabetic injury in the kidney. Nephrol. Dial. Transplant. 2014;29(4):746–754. doi: 10.1093/ndt/gft446. [DOI] [PubMed] [Google Scholar]
- 89.Mudaliar H, Pollock C, Panchapakesan U. Role of Toll-like receptors in diabetic nephropathy. Clin. Sci. (Lond) 2014;126(10):685–694. doi: 10.1042/CS20130267. [DOI] [PubMed] [Google Scholar]
- 90.Tang SC, Yiu WH, Lin M, Lai KN. Diabetic nephropathy and proximal tubular damage. J. Ren. Nutr. 2015;25(2):230–233. doi: 10.1053/j.jrn.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 91.Ma J, Wu H, Zhao CY, Panchapakesan U, Pollock C, Chadban SJ. Requirement for TLR2 in the development of albuminuria, inflammation and fibrosis in experimental diabetic nephropathy. Int. J. Clin. Exp. Pathol. 2014;7(2):481–495. [PMC free article] [PubMed] [Google Scholar]
- 92.Saurus P, Kuusela S, Lehtonen E, Hyvönen ME, Ristola M, Fogarty CL, Tienari J, Lassenius MI, Forsblom C, Lehto M, Saleem MA, Groop PH, Holthöfer H, Lehtonen S. Podocyte apoptosis is prevented by blocking the Toll-like receptor pathway. Cell Death Dis. 2015;6:e1752. doi: 10.1038/cddis.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, Jialal I. Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler. Thromb. Vasc. Biol. 2011;31(8):1796–1804. doi: 10.1161/ATVBAHA.111.228924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell MT, Hile KL, Zhang H, Asanuma H, Vanderbrink BA, Rink RR, Meldrum KK. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J. Surg. Res. 2011;168(1):e61–e69. doi: 10.1016/j.jss.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pulskens WP, Rampanelli E, Teske GJ, Butter LM, Claessen N, Luirink IK, van der Poll T, Florquin S, Leemans JC. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J. Am. Soc. Nephrol. 2010;21(8):1299–1308. doi: 10.1681/ASN.2009070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma J, Chadban SJ, Zhao CY, Chen X, Kwan T, Panchapakesan U, Pollock CA, Wu H. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS. One. 2014;9(5):e97985. doi: 10.1371/journal.pone.0097985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Ogawa Y, Imamaki H, Kawanishi T, Ishii A, Koga K, Mori KP, Kato Y, Sugawara A, Nakao K. Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by Toll-like receptor 4 in mice. Diabetologia. 2012;55(8):2256–2266. doi: 10.1007/s00125-012-2578-1. [DOI] [PubMed] [Google Scholar]
- 98.Jialal I, Major AM, Devaraj SJ. Global Toll-like receptor 4 knockout results in decreased renal inflammation, fibrosis and podocytopathy. J. Diabetes Complications. 2014;28(6):755–761. doi: 10.1016/j.jdiacomp.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 99.Kaur H, Chien A, Jialal I. Hyperglycemia induces Toll like receptor 4 expression and activity in mouse mesangial cells: relevance to diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2012;303(8):F1145–F1150. doi: 10.1152/ajprenal.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J. Am. Soc. Nephrol. 2012;23(1):86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cha JJ, Hyun YY, Lee MH, Kim JE, Nam DH, Song HK, Kang YS, Lee JE, Kim HW, Han JY, Cha DR. Renal protective effects of toll-like receptor 4 signaling blockade in type 2 diabetic mice. Endocrinology. 2013;154(6):2144–2155. doi: 10.1210/en.2012-2080. [DOI] [PubMed] [Google Scholar]
- 102.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 103.Carmena A. A big new job for small GTPases. Small GTPases. 2012;3(3):159–162. doi: 10.4161/sgtp.19631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reuther GW, Der CJ. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr. Opin. Cell. Biol. 2000;12(2):157–165. doi: 10.1016/s0955-0674(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 105.Rufanova VA, Alexanian A, Wakatsuki T, Lerner A, Sorokin A. Pyk2 mediates endothelin-1 signaling via p130Cas/BCAR3 cascade and regulates human glomerular mesangial cell adhesion and spreading. J. Cell Physiol. 2009;219(1):45–56. doi: 10.1002/jcp.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am. J. Respir. Cell Mol. Biol. 2008;39(4):482–489. doi: 10.1165/rcmb.2008-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin S, Chugh S, Pan X, Wallner EI, Wada J, Kanwar YS. Identification of up-regulated Ras-like GTPase, Rap1b, by suppression subtractive hybridization. Kidney Int. 2001;60(6):2129–2141. doi: 10.1046/j.1523-1755.2001.00061.x. [DOI] [PubMed] [Google Scholar]
- 108.Wallner EI, Wada J, Lin S, Pan X, Reddy JK, Chugh SS, Kanwar YS. Renal gene expression in embryonic and newborn diabetic mice. Exp. Nephrol. 2002;10(2):130–138. doi: 10.1159/000049908. [DOI] [PubMed] [Google Scholar]
- 109.Lin S, Sahai A, Chugh SS, Pan X, Wallner EI, Danesh FR, Lomasney JW, Kanwar YS. High glucose stimulates synthesis of fibronectin via a novel protein kinase C, Rap1b, and B-Raf signaling pathway. J. Biol. Chem. 2002;277(44):41725–41735. doi: 10.1074/jbc.M203957200. [DOI] [PubMed] [Google Scholar]
- 110.Xiao L, Zhu X, Yang S, Liu F, Zhou Z, Zhan M, Xie P, Zhang D, Li J, Song P, Kanwar YS, Sun L. Rap1 ameliorates renal tubular injury in diabetic nephropathy. Diabetes. 2014;63(4):1366–1380. doi: 10.2337/db13-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun L, Xie P, Wada J, Kashihara N, Liu FY, Zhao Y, Kumar D, Chugh SS, Danesh FR, Kanwar YS. Rap1b GTPase ameliorates glucose-induced mitochondrial dysfunction. J. Am. Soc. Nephrol. 2008;19(12):2293–2301. doi: 10.1681/ASN.2008030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin CL, Wang FS, Kuo YR, Huang YT, Huang HC, Sun YC, Kuo YH. Ras modulation of superoxide activates ERK-dependent fibronectin expression in diabetes-induced renal injuries. Kidney Int. 2006;69(9):1593–1600. doi: 10.1038/sj.ki.5000329. [DOI] [PubMed] [Google Scholar]
- 113.Xu D, Kyriakis JM. Phosphatidylinositol 3'-kinase-dependent activation of renal mesangial cell Ki-Ras and ERK by advanced glycation end products. J. Biol. Chem. 2003;278(41):39349–39355. doi: 10.1074/jbc.M302771200. [DOI] [PubMed] [Google Scholar]
- 114.Zhou H, Yao C, Bian A, Qian J, Zhao X, Zhao Y, Wang W, Xing C. The Ras GTPase-activating-like protein IQGAP1 is downregulated in human diabetic nephropathy and associated with ERK1/2 pathway activation. Mol. Cell Biochem. 2014;391(1–2):21–25. doi: 10.1007/s11010-014-1982-x. [DOI] [PubMed] [Google Scholar]
- 115.Komers R. Rho kinase inhibition in diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 2011;20(1):77–83. doi: 10.1097/MNH.0b013e32834131f8. [DOI] [PubMed] [Google Scholar]
- 116.David M, Petit D, Bertoglio J. Cell cycle regulation of Rho signaling pathways. Cell Cycle. 2012;11(16):3003–3010. doi: 10.4161/cc.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 2007;50(1):17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Komers R. Rho kinase inhibition in diabetic kidney disease. Br. J. Clin. Pharmacol. 2013;76(4):551–559. doi: 10.1111/bcp.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bach LA. Rho kinase inhibition: a new approach for treating diabetic nephropathy? Diabetes. 2008;57(3):532–533. doi: 10.2337/db07-1768. [DOI] [PubMed] [Google Scholar]
- 120.Peng F, Wu D, Gao B, Ingram AJ, Zhang B, Chorneyko K, McKenzie R, Krepinsky JC. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes. 2008;57(6):1683–1692. doi: 10.2337/db07-1149. [DOI] [PubMed] [Google Scholar]
- 121.Ma DW, Wang QY, Ma XY, Li J, Guan QH, Fu Y. The effect of fasudil via Rho/ROCK signaling pathway on the inflammation and fibrosis in human mesangial cells in high glucose medium. Zhonghua Nei Ke Za Zhi. 2011;50(7):580–584. [PubMed] [Google Scholar]
- 122.Ma DW, Wang QY, Chen QG, Wu D, Wang J, Hou LL. Effects of inhibiting RhoA by Stealth RNA on high glucose-induced RhoA/ROCK signaling pathway in human mesangial cells. Zhonghua Yi Xue Za Zhi. 2011;91(20):1417–1421. [PubMed] [Google Scholar]
- 123.Xie X, Peng J, Chang X, Huang K, Huang J, Wang S, Shen X, Liu P, Huang H. Activation of RhoA/ROCK regulates NF-kappaB signaling pathway in experimental diabetic nephropathy. Mol. Cell Endocrinol. 2013;369(1–2):86–97. doi: 10.1016/j.mce.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 124.Gu L, Gao Q, Ni L, Wang M, Shen F. Fasudil inhibits epithelial-myofibroblast transdifferentiation of human renal tubular epithelial HK-2 cells induced by high glucose. Chem. Pharm. Bull. (Tokyo) 2013;61(7):688–694. doi: 10.1248/cpb.c13-00066. [DOI] [PubMed] [Google Scholar]
- 125.Kolavennu V, Zeng L, Peng H, Wang Y, Danesh FR. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes. 2008;57(3):714–723. doi: 10.2337/db07-1241. [DOI] [PubMed] [Google Scholar]
- 126.Kikuchi Y, Yamada M, Imakiire T, Kushiyama T, Higashi K, Hyodo N, Yamamoto K, Oda T, Suzuki S, Miura S. A Rho-kinase inhibitor, fasudil, prevents development of diabetes and nephropathy in insulin-resistant diabetic rats. J. Endocrinol. 2007;192(3):595–603. doi: 10.1677/JOE-06-0045. [DOI] [PubMed] [Google Scholar]
- 127.Wu G, Xu M, Xu K, Hu Y. Benidipine protects kidney through inhibiting ROCK1 activity and reducing the epithelium-mesenchymal transdifferentiation in type 1 diabetic rats. J. Diabetes Res. 2013;2013:174526. doi: 10.1155/2013/174526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xie X, Chang X, Chen L, Huang K, Huang J, Wang S, Shen X, Liu P, Huang H. Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting activation of RhoA/ROCK signaling. Mol. Cell Endocrinol. 2013;381(1–2):56–65. doi: 10.1016/j.mce.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 129.Gojo A, Utsunomiya K, Taniguchi K, Yokota T, Ishizawa S, Kanazawa Y, Kurata H, Tajima N. The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2007;568(1–3):242–247. doi: 10.1016/j.ejphar.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 130.Wang S, Denichilo M, Brubaker C, Hirschberg R. Connective tissue growth factor in tubulointerstitial injury of diabetic nephropathy. Kidney Int. 2001;60(1):96–105. doi: 10.1046/j.1523-1755.2001.00776.x. [DOI] [PubMed] [Google Scholar]
- 131.Wang B, Komers R, Carew R, Winbanks CE, Xu B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K, Gregorevic P, Cooper ME, Kantharidis P. Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 2012;23(2):252–265. doi: 10.1681/ASN.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ishizawa S, Takahashi-Fujigasaki J, Kanazawa Y, Matoba K, Kawanami D, Yokota T, Iwamoto T, Tajima N, Manome Y, Utsunomiya K. Sphingosine-1-phosphate induces differentiation of cultured renal tubular epithelial cells under Rho kinase activation via the S1P2 receptor. Clin. Exp. Nephrol. 2014;18(6):844–852. doi: 10.1007/s10157-014-0933-x. [DOI] [PubMed] [Google Scholar]
- 133.Danesh FR, Sadeghi MM, Amro N, Philips C, Zeng L, Lin S, Sahai A, Kanwar YS. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/ p21 signaling pathway: Implications for diabetic nephropathy. Proc. Natl. Acad. Sci. U. S. A. 2002;99(12):8301–8305. doi: 10.1073/pnas.122228799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie X, Chen C, Huang K, Wang S, Hao J, Huang J, Huang H. RhoA/rho kinase signaling reduces connexin43 expression in high glucose-treated glomerular mesangial cells with zonula occludens-1 involvement. Exp. Cell Res. 2014;327(2):276–286. doi: 10.1016/j.yexcr.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 135.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15(2):186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zerial M, McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2(2):107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 137.Speight P, Silverman M. Diacylglycerol-activated Hmunc13 serves as an effector of the GTPase Rab34. Traffic. 2005;6(10):858–865. doi: 10.1111/j.1600-0854.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 138.Goldenberg NM, Silverman M. Rab34 and its effector munc13-2 constitute a new pathway modulating protein secretion in the cellular response to hyperglycemia. Am. J. Physiol. Cell Physiol. 2009;297(4):C1053–C1058. doi: 10.1152/ajpcell.00286.2009. [DOI] [PubMed] [Google Scholar]
- 139.Turgut F, Bolton WK. Potential new therapeutic agents for diabetic kidney disease. Am. J. Kidney Dis. 2010;55(5):928–940. doi: 10.1053/j.ajkd.2009.11.021. [DOI] [PubMed] [Google Scholar]