Abstract
Defects in the biogenesis of or transport through primary cilia affect Hedgehog protein signaling, and many Hedgehog pathway components traffic through or accumulate in cilia. The Hedgehog receptor, Patched, negatively regulates the activity and ciliary accumulation of Smoothened, a seven transmembrane protein that is essential for transducing the Hedgehog signal. We found that this negative regulation of Smoothened required the ciliary localization of Patched, as specified either by its own cytoplasmic tail or by provision of heterologous ciliary localization signals. Surprisingly, given that Hedgehog binding promotes the exit of Patched from the cilium, we observed that an altered form of Patched that is retained in the cilium nevertheless responded to Hedgehog, resulting in Smoothened activation. Our results indicate that, whereas ciliary localization of Patched is essential for suppression of Smoothened activation, the primary event enabling Smoothened activation is binding of Hedgehog to Patched, and Patched ciliary removal is secondary.
Introduction
Hedgehog (Hh) are proteins that function as cell-to-cell signals with embryonic roles in specification of tissue patterning and cell differentiation (1) and postembryonic roles in organ homeostasis and regeneration (2, 3). Additionally, pathway activity can stimulate or suppress growth of various cancers (4–7). During vertebrate Hh signaling, the processed, lipid-modified N-terminal signaling domain of Hh activates the pathway by binding to Patched1 (Ptch1) (8), a member of the Resistance-Nodulation-Division (RND) family of proton-driven 12-transmembrane (TM) transporters, thus relieving Ptch1 suppression of the 7-transmembrane protein Smoothened (Smo), which is structurally related to G protein-coupled receptors. Activation of Smo, in turn, stimulates transcription by inhibiting the constitutive, proteolytic processing of Gli family proteins and switching them to their activated states.
The primary cilium plays a central role in transduction of vertebrate Hh signals (9). ShhN, the signaling domain of mammalian Sonic hedgehog, binds to the shaft of primary cilia which contain Ptch1 and induces Ptch1 removal from the cilium, which is accompanied by ciliary accumulation of Smo (10, 11). Ciliary accumulation of activated Smo then triggers pathway activation through Gli proteins, which also accumulate within the primary cilium prior to ciliary exit and nuclear entry(12). Despite these findings, the mechanisms by which Ptch1 suppresses Smo activity and by which Hh inactivates this inhibitory function of Ptch1 remain unclear. One model is that Ptch1 inhibits the pathway by excluding Smo from the cilium in resting cells and that Hh binding triggers removal of Ptch1 from the cilium, thus enabling Smo to enter and activate signaling (11, 13). However, Smo accumulates in the primary cilium without Hh stimulation in cells in which cytoplasmic dynein 2, the microtubule motor for retrograde ciliary trafficking, is genetically or pharmacologically impaired (14–17), suggesting that Smo may traffic into the cilium even in the presence of active Ptch1. Accumulation of Smo in primary cilia in the absence of Hh-mediated Ptch1 inactivation also occurs in cells with functional impairment of other ciliary transport proteins (18–20), and Smo also accumulates in cilia of cells exposed to pharmacologic agents that directly bind and activate (or in some cases inactivate) Smo (11, 21). Thus, the relationship of the ciliary trafficking of Ptch1 to activation and ciliary accumulation of Smo is unclear. To address these issues we used systematic deletions to show that sequences within the Ptch1 cytoplasmic tail contributed to Ptch1 ciliary localization and that removal of these sequences disrupted the Smo-inhibitory function of Ptch1. Furthermore, replacement of the Ptch1 cytoplasmic tail with heterologous ciliary localization signals (CLS) not only restored ciliary localization but also Hh-responsive regulatory activity of Ptch1. We thus provide evidence that Ptch1 suppression of Hh pathway activity and response to Hh requires localization to the primary cilium. We also found that modification of Ptch1 such that the protein remained in the primary cilium even in the presence of ShhN nevertheless repressed downstream signaling activity in the absence of ligand and this inhibitory activity was relieved by exposure of the cells to ShhN. Therefore, although the removal of Ptch1 from the primary cilium may fine tune pathway activity, our evidence indicates that ciliary removal of Ptch1 is not essential for Hh-induced pathway activation.
Results
Ptch1 C-terminal cytoplasmic tail is necessary for ciliary localization
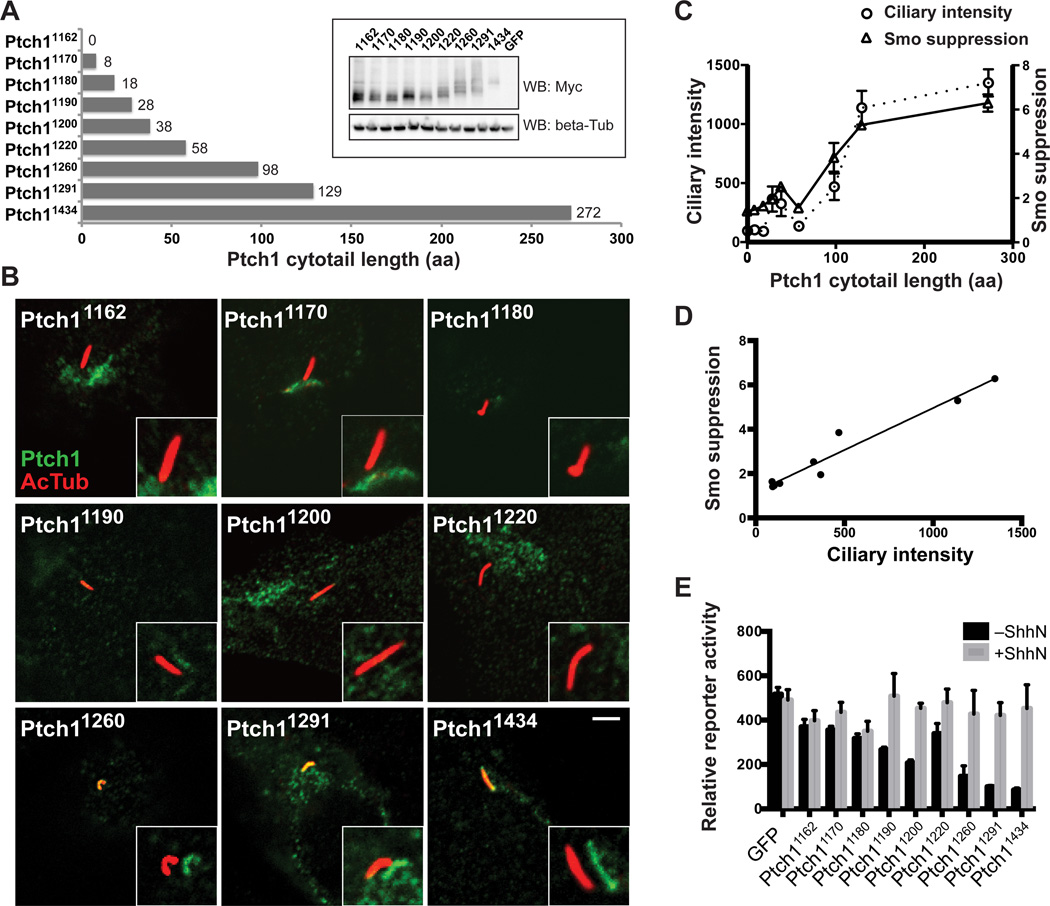
The CLS of Ptch1 has not been clearly defined, and such signals are insufficiently catalogued for reliable identification by sequence comparison. We constructed a series of truncations that progressively remove sequences from an epitope-tagged form of Ptch1, expressed them in Ptch1−/− cells (Fig. 1A), and assessed ciliary localization by laser confocal microscopy (Fig. 1B). In addition, we tested for Smo suppression activity by cotransfecting these Ptch1 variants at low levels (1% w/w DNA) with a Gli-controlled luciferase reporter. We used Ptch1−/− cells to avoid interference by endogenous Ptch1, which is reported to homomultimerize (22). These cells do not express the Patched ortholog, Ptch2; and therefore, Ptch1 is the Hh receptor responsible for Smo regulation and control of Hh pathway activity in these cells (23, 24).
Fig. 1.
Ptch1 cytoplasmic tail is necessary for ciliary localization. (A) Schematic diagram of Ptch1 cytoplasmic tail deletions. Ptch11434 is WT. The inset shows abundance of the indicated Myc-tagged Ptch proteins expressed in HEK293 cells. (B) Ptch1−/− cells transfected with Myc tagged Ptch1 carrying various cytoplasmic tail deletions were fixed and stained with antibodies recognizing acetylated tubulin (primary cilium, red) and Myc (Ptch1 variants, green). The insets show shifted overlays of the red and the green channels. Scale bar, 5 µm. (C) Mean fluorescence intensity of Ptch1 variants in cilia of transfected Ptch1−/− cells and their ability to suppress Smo activity are plotted against the cytoplasmic tail lengths of Ptch1 variants. Ciliary intensity is the mean ± SD of fluorescence from 10–20 cilia. Smo-suppression activity is defined as RGFP/RPtch1, the ratio of Gli reporter activity from Ptch1−/− cells cotransfected with GFP or each individual Ptch1 variant: a higher value of this quotient indicates more effective suppression by a particular Ptch1 variant. Error bars indicate SD, n=3. (D) Smo-suppression activity was plotted as a function of ciliary intensity for each Ptch1 cytoplasmic tail deletion. The Pearson correlation coefficient is 0.97 (r=0.97). (E) Ptch1−/− cells transfected for expression of Myc-tagged Ptch1 variants, Gli reporter, and control SV40-Renilla luciferase were grown to confluency, incubated with or without ShhN, and assayed for reporter activity. Error bars indicate SD, n=3.
Removal of all or most amino-acid sequence C-terminal to the final transmembrane segment (in constructs Ptch11162, Ptch11170, and Ptch11180) fully disrupted Ptch1 ciliary localization (Fig. 1B and C) and inactivated Smo suppression, leading to constitutively high reporter activity (Fig. 1C). The addition of amino acids from amino-acid residue 1180 to the C-terminus progressively restored ciliary localization and Smo suppression (Fig. 1B and C). Furthermore, Smo suppression correlated highly with the ability of these sequences to target Ptch1 to the cilium (Fig. 1D). Restoration of Ptch1 cytoplasmic tail sequences also restored the response to Hh stimulation (Fig. 1E).
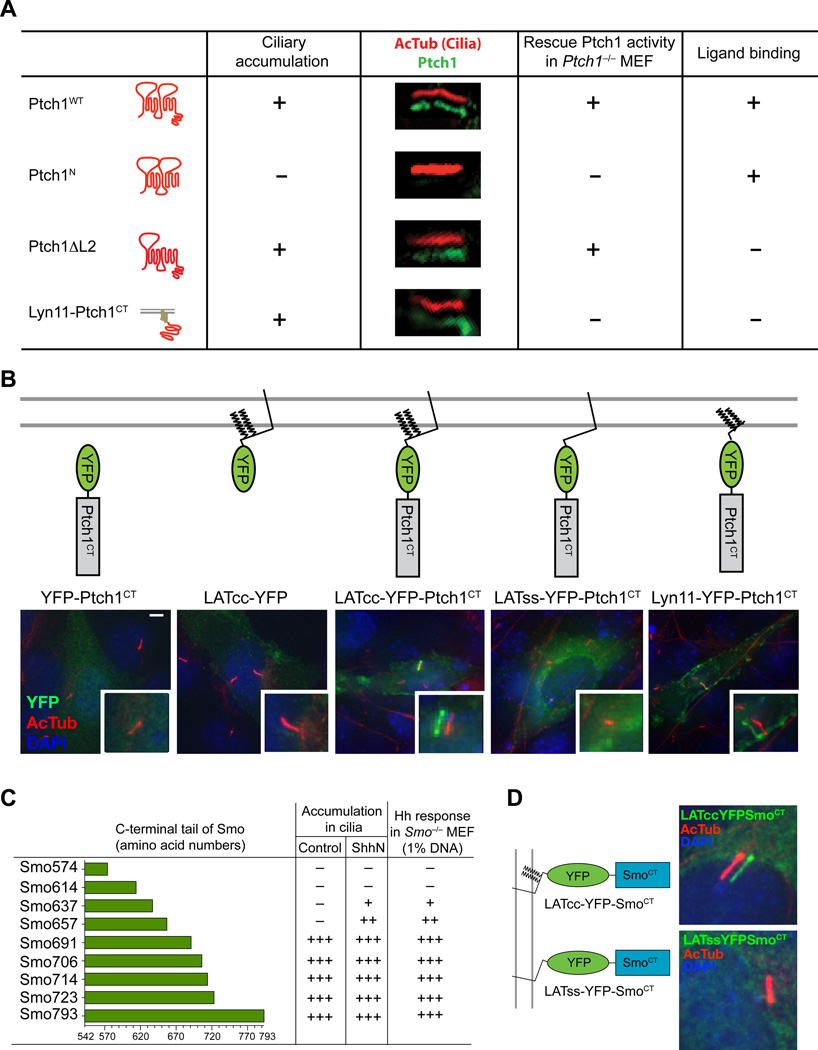
Ptch1 cytoplasmic tail and Smo cytoplasmic tail mediate ciliary localization
Ptch1 with full cytoplasmic tail but lacking a segment of the TM7-TM8 extracellular loop fully suppressed Smo activity but did not respond to Hh (Ptch1ΔL2, Fig. 2A), consistent with loss of Hh binding (25, 26). We found that the Ptch1 C-terminal cytoplasmic tail (Ptch1CT) sufficed to specify ciliary localization when fused to peptides containing tandem acylation signals from the kinase Lyn11 or the adaptor LAT (Fig. 2B), consistent with previous reports that acylated peptides targeted to membrane microdomains can reveal the activity of ciliary localization signals to which they are fused (27, 28).
Fig. 2.
The cytoplasmic tails of Ptch1 and Smo specify ciliary trafficking and Hh responsiveness. (A) Summary of ciliary localization and signaling activity of Ptch1 variants and a Ptch1 cytoplasmic tail fusion protein. The constructs indicated in the first column were transfected at high levels (50% w/w DNA) into Ptch1−/− cells to assess ciliary localization by laser confocal microscopy and cotransfected at low levels (1% w/w DNA) with the Gli reporter to test for signaling activity. (B) NIH 3T3 cells were transfected with YFP-tagged fusion proteins as schematically indicated at the top and fixed and stained with DAPI (nucleus, blue) and antibodies recognizing acetylated tubulin (primary cilium, red) or GFP (fusion protein, green). The lower right insets show shifted overlays. Scale bar, 5 µm. (C) The cytoplasmic tail of Smo is required for ciliary localization. Constructs with indicated cytoplasmic tails of mouse Smo listed in the first column were transfected at high levels (50% w/w DNA) into Smo−/− cells to assess ciliary localization by laser confocal microscopy and cotransfected at low levels (1% w/w DNA) with the Gli reporter to test for signaling activity. Smo793 is wild-type Smo. (D) Smo cytoplasmic tail is sufficient for ciliary localization. NIH 3T3 cells transfected with the indicated constructs were fixed and stained as described in (B). Images and data shown are representative of many cilia and signaling assays from three experiments.
As with Ptch1, truncation of the Smo cytoplasmic tail impaired Smo accumulation in cilia and the ability of Smo to rescue Hh pathway activity in Smo−/− cells (Fig. 2C). Furthermore, the cytoplasmic tail of Smo sufficed to deliver acylated LAT into primary cilia (Fig. 2D). Thus, although the exact motif responsible for ciliary localization is not defined, both Ptch and Smo have CLS that are necessary for the ability of these proteins to traffic into the cilium.
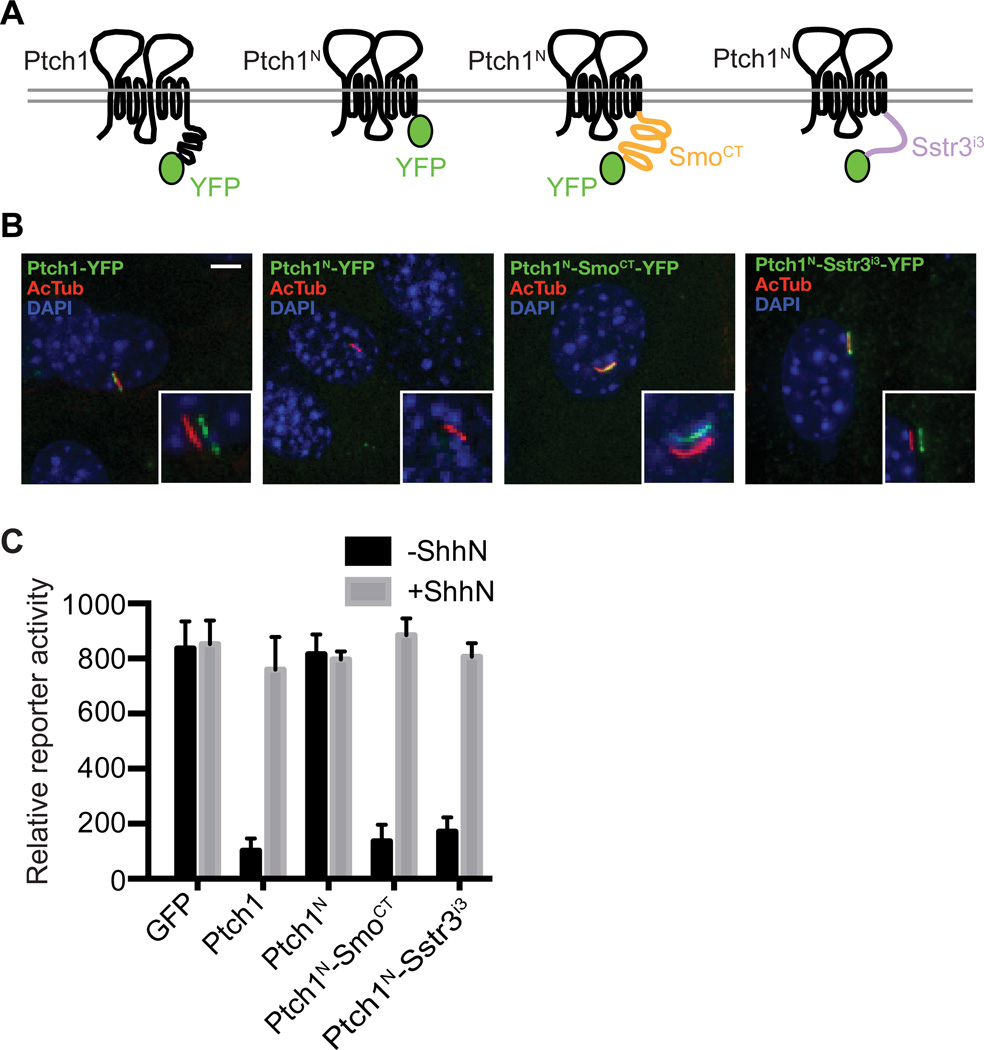
Signaling activity of truncated Ptch1 is restored by heterologous CLSs
We tested the importance of ciliary localization for Ptch1 function by attaching heterologous CLS sequences to Ptch1 lacking the cytoplasmic tail (Ptch1N). We found that the fusion of the Smo cytoplasmic tail (SmoCT) onto Ptch1N (Ptch1N-SmoCT) rescued ciliary localization and regulatory function of Ptch1N and that the Ptch1N-SmoCT localized to the primary cilium in 100% of transfected NIH 3T3 cells (Fig. 3A and B). Importantly, the Ptch1N-SmoCT fusion protein also suppressed Gli reporter activity when introduced into Ptch1−/− cells, and this reporter activity was then induced in response to ShhN (Fig. 3C). We also tested the effect of the third intracellular loop (icl3) of the 7TM protein Sstr3 (Sstr3icl3), which contains a well-defined CLS responsible for Sstr3 ciliary localization (29, 30). Fusion of this CLS to Ptch1N (Ptch1N-Sstr3icl3) also restored both ciliary localization and Hh-responsive regulatory activity (Fig. 3).
Fig. 3.
Exogenous ciliary localization signals rescue the impaired signaling activity of Ptch1N. (A) Schematic diagrams of YFP-tagged Ptch1 fusion proteins. (B) NIH 3T3 cells transfected with the indicated YFP-tagged Ptch1 constructs were fixed and stained with DAPI (nucleus, blue) and antibodies recognizing acetylated tubulin (primary cilium, red) or GFP (fusion protein, green). The insets show shifted overlays. Scale bar, 5 µm. (C) Ptch1−/− cells were transiently transfected for expression of YFP-tagged Ptch1 variants, Gli reporter, and control SV40-Renilla luciferase. Following transfection, cells were grown to confluency, incubated with or without ShhN, and assayed for reporter activity. Error bars indicate SD, n=3.
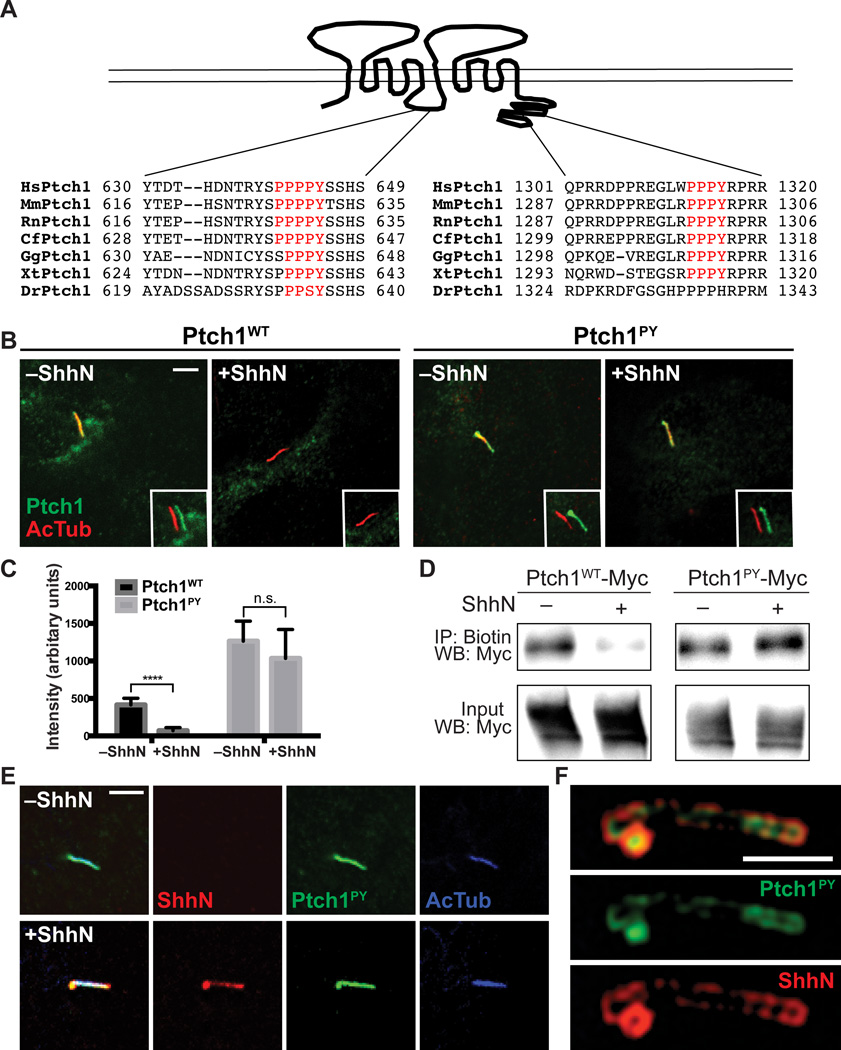
Ptch1 with mutated PPXY motifs (Ptch1PY) accumulates in the primary cilium
We tested the model that Hh-dependent pathway activation depends upon removal of Ptch1 from the cilium. Our analysis focused on two recognition motifs (PPXY) for binding by HECT E3 ubiquitin ligases (31), one within the cytoplasmic tail and one within the large cytoplasmic loop between TM6 and TM7 (Fig. 4A) (22). Ptch1 with mutations in both of these PPXY motifs persisted in the cilium even in the presence of ShhN (Fig. 4B and C). Using a biotin labeling assay with a non-cell-permeable reagent to compare the amount of surface-localized Ptch1 and Ptch1PY after stimulation with ShhN, we found that the amount of Ptch1 on the cell surface was reduced after 1 hour of exposure to ShhN; whereasPtch1PY remained at the cell surface in the presence of ShhN (Fig. 4D).
Fig. 4.
Ptch1PY fails to exit the primary cilium. (A) Schematic diagram of Ptch1 shows conserved PPXY motifs within the large TM6-TM7 cytoplasmic loop and the cytoplasmic tail. (B) Ptch1−/− cells were transfected with Myc-tagged Ptch1WT (wild-type Ptch1) or Ptch1PY. After incubation with or without ShhN for 4 h, cells were fixed and stained for acetylated tubulin (primary cilium, red) and Myc (Ptch1 variants, green). The insets show shifted overlays. Scale bar, 5 µm. (C) Mean fluorescence intensity in cilia of Ptch1WT or Ptch1PY in transfected Ptch1−/− cells, with or without ShhN treatment for 4 h. Each bar shows the mean ± SD of fluorescence from 10–20 cilia and representative images are shown in (B). Unpaired t test was used for statistical analysis. ****p<0.0001, n.s., not significant (p>0.05). (D) Cell surface proteins from HEK293 cells transfected with Myc-tagged Ptch1WT or Ptch1PY were biotinylated, isolated using streptavidin beads, and examined for the presence of Ptch1 by immunoblotting for Myc. IP, immunoprecipitation; WB, Western blot. (E) NIH 3T3 cells were transfected with Myc-tagged Ptch1PY. After incubation with or without mCherry-tagged ShhN for 4 h, cells were fixed and stained with antibodies recognizing dsRed (ShhN, red), acetylated tubulin (primary cilium, blue), or Myc (Ptch1 variants, green) and imaged by confocal microscopy. Scale bar, 5 µm. (F) Cells transfected and stained as in (F) were imaged by structured illumination microscopy (SIM). Scale bar, 2.5 µm. Images shown in (E) and (F) are representative of many cilia.
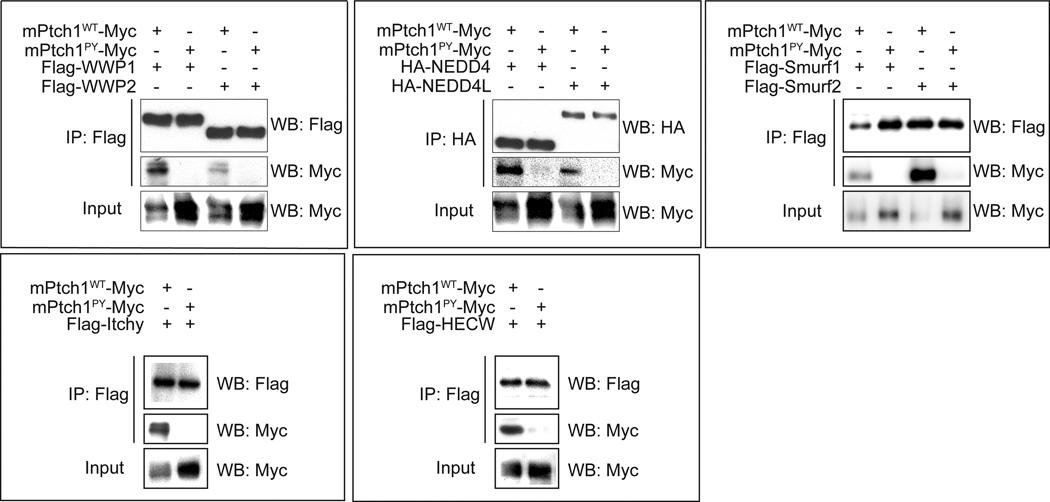
This ciliary persistence was not due to disruption of ShhN binding, because structured illumination microscopy (SIM), which lowers the limit of optical resolution to less than 200 nm (32), showed that ShhN added to cells expressing Ptch1PY localized in a thin layer overlying regions of the cilium with higher concentrations of Ptch1PYprotein (Fig. 4E and F). Consistent with the hypothesis that Ptch1PY persistence on the ciliary membrane is due to loss of interaction with HECT E3 ubiquitin ligases, we found that, in contrast to wild-type Ptch1, Ptch1PY did not coimmunoprecipitate with any of the eight tested HECT E3 ubiquitin ligases (Wwp1, Wwp2, Nedd4, Nedd4L, Itchy, HECW, Smurf1, and Smurf2; Fig. 5), suggesting that the interaction with and ubiquitylation by one or more of these E3 ubiquitin ligases is necessary for trafficking of Ptch1 out of the cilium.
Fig. 5.
PY mutations abolish binding of Ptch1 to eight Nedd4 family HECT E3 ubiquitin ligases. Myc-tagged Ptch1WT or Ptch1PY was cotransfected with individual epitope-tagged Nedd4 family HECT E3 ubiquitin ligases into HEK 293 cells. After cell lysis, E3 ubiquitin ligases were immunoprecipitated with antibodies recognizing the epitope tags and the Myc antibody was used detect coprecipitated Ptch1WT or Ptch1PY. Data are representative of two different experiments.
Direct binding of ShhN to Ptch1, but not ciliary removal of Ptch1, is required for ShhN-induced pathway activation
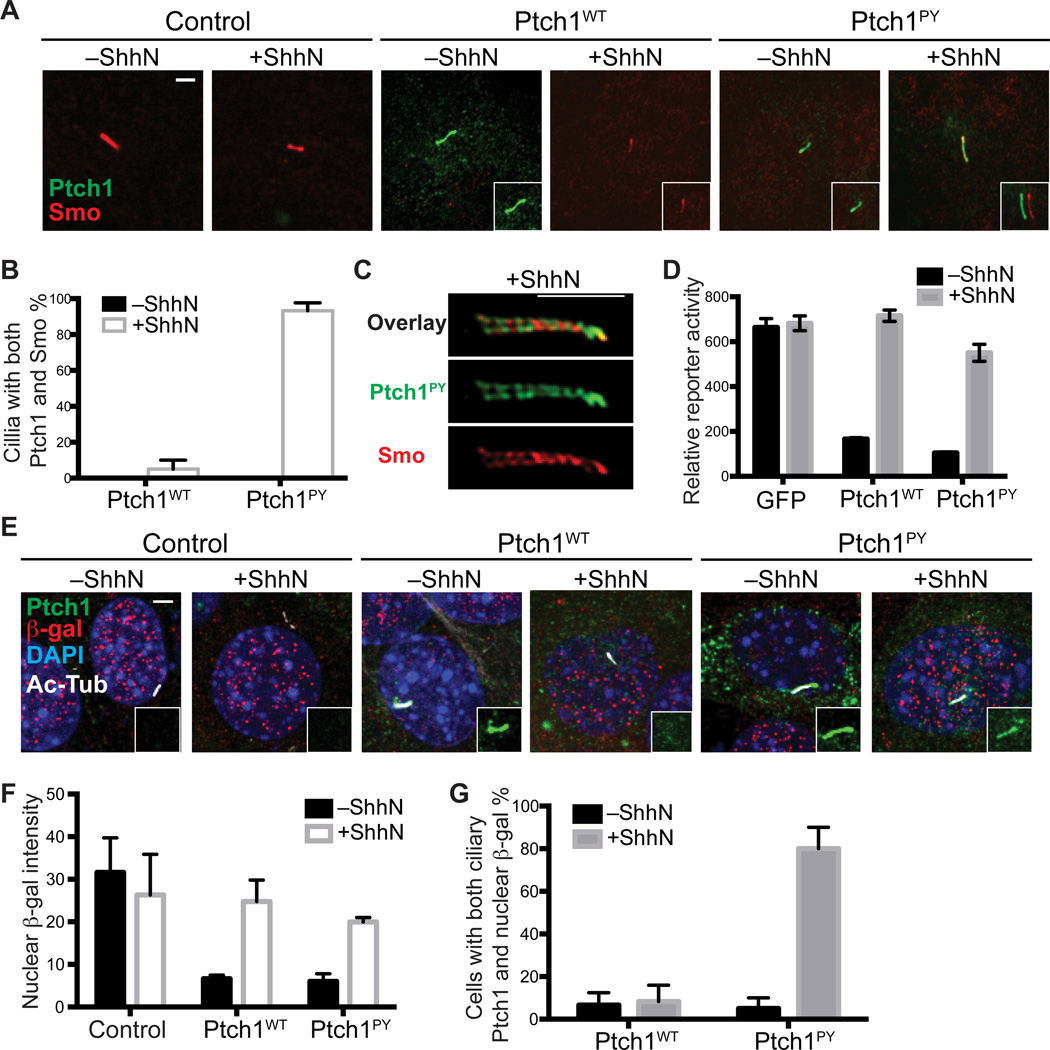
Persistence of Ptch1PY within the cilium provides an opportunity to test the role of Ptch1 ciliary removal upon Hh stimulation in activation of the pathway. We found that Ptch1PY introduced into Ptch1−/− cells blocked the ciliary accumulation of Smo in a manner similar to wild-type Ptch1 (Fig. 6A). Upon addition of ShhN, endogenous Smo accumulated in the primary cilia not only of cells transfected for expression of wild-type Ptch1, but also in the primary cilia of cells transfected for expression of Ptch1PY (Fig. 6A). Furthermore, we confirmed simultaneous localization of Smo within the primary cilium of 95% of ShhN-stimulated cells expressing Ptch1PY (Fig. 6B). Imaging of cilia containing both Ptch1PY and Smo by SIM revealed that they localized in distinct, nonoverlapping but intermingled regions of the primary cilium (Fig. 6C). The functional relevance of these nonoverlapping localizations is unclear, but may relate to the proclivity of Ptch1 and Smo to homomultimerize (22, 33).
Fig. 6.
Ciliary removal of Ptch1 is not required for Shh-induced pathway activation. (A) Ptch1−/− cells were transfected with Myc-tagged Ptch1WT or Ptch1PY. After incubation with or without ShhN for 4 h, cells were fixed and stained with anti-Smo (red) and anti-Myc (Ptch1, green). The insets show shifted overlays. Scale bar, 5 µm. (B) Quantification of percentage of transfected cells with simultaneous ciliary presence of Smo and Ptch1WT or Ptch1PY. Note that in the absence of ShhN, there are no cilia that contain both Ptch1 and Smo (black bars are not visible). (C) Cells transfected and stained as in (A) were imaged by SIM. Scale bar, 2.5 µm. (D) Ptch1−/− cells transiently transfected for expression of Myc-tagged Ptch1 variants, Gli-luciferase reporter, and control SV40-Renilla luciferase were grown to confluency, incubated with or without ShhN, and assayed for reporter activity. Error bars indicate SD, n=3. (E) Ptch1−/− cells transfected with GFP-tagged Ptch1WT or Ptch1PY were incubated with or without ShhN overnight, fixed and stained with anti-β-gal (pathway activity, red), anti-GFP (Ptch1, green), anti-AcTub (primary cilium, white), and DAPI (nucleus, blue). The insets show a separate green-channel view of ciliary Ptch1. The presence of nuclear β-gal indicates pathway activation. Scale bar, 5 µm. (F) Mean fluorescence intensity of β-gal in the nuclei of Ptch1−/− cells and Ptch−/− cells transfected with Ptch1WT or Ptch1PY. Each bar shows the mean ± SD of fluorescence from 10–20 cells. Images are representatives of experiments. (G) Quantification of percentage of transfected, ciliated cells that contain both nuclear β-gal and ciliary Ptch1.
We also examined the ability of Ptch1−/− cells to respond to ShhN stimulation upon cotransfection with a Ptch1PY expression construct and the Hh-sensitive Gli reporter. We found little difference in the ability of wild-type Ptch1 and Ptch1PY to suppress transcriptional activity or in the responsiveness of cells expressing either of these Ptch1 proteins to ShhN stimulation (Fig. 6D). To extend this analysis to the single-cell level and confirm that the transcriptional response occurred in the very cells that show simultaneous Ptch1PY and Smo ciliary localization, we examined expression of nuclear β-galactosidase in ShhN-stimulated Ptch1−/− cells transfected to express Ptch1PY. Ptch1 in Ptch1−/− cells is disrupted by in frame insertion of lacZ with a nuclear localization signal (34): the production of nuclear β-galactosidase thus provides a measure of transcriptional activation of the Ptch1 promoter, a Hh pathway target. As expected, nuclear β-galactosidase was abundant in control Ptch1−/− cells regardless of ShhN stimulation, whereas Ptch1−/− cells transfected with either wild-type Ptch1 or Ptch1PY showed very low nuclear β-galactosidase that increased upon ShhN stimulation (Fig. 6E and G). Moreover, Ptch1−/− cells transfected with Ptch1PY displayed simultaneous presence of ciliary Ptch1PY and nuclear β-galactosidase upon ShhN stimulation (Fig. 6, E and G).
Discussion
Our results indicated that Ptch1 function is modular: N-terminal sequences through the 12th TM domain confer Smo suppression, as well as binding and response to Hh, and ciliary localization conferred by the Ptch1 cytoplasmic tail. Although the ciliary localization specified by the Ptch1 cytoplasmic tail was required for Hh-sensitive Smo regulation, the Ptch1 cytoplasmic tail itself was dispensable if a heterologous CLS was provided instead.
We also found that, although ciliary localization of Ptch1 and direct binding of Hh to Ptch1 was required for Hh-sensitive Smo regulation, Hh-induced ciliary removal of Ptch1 was not necessary for ciliary accumulation of Smo and for downstream pathway activation. This finding is analogous to the finding in Drosophila (where primary cilia are not required for Hh signaling) that the trafficking of Ptc from the plasma membrane, which is normally triggered by Hh stimulation, is neither required for Hh-dependent activation of Smo nor for initiation of downstream signaling events (35).
Although our data showed that Ptch1 ciliary removal is not essential for pathway activation, Hh-induced ciliary removal of Ptch1 may have an important role in tuning the response to Hh signaling during development. Yue et al. (36) demonstrated that Hh-dependent granule neuronal precursor proliferation is reduced by deletion of the Ptch1 PPXY sequences or by inactivation of the HECT E3 ubiquitin ligases that interact with them. Given our finding that Ptch1 suppression of Smo activity required ciliary localization, clearance of Ptch1 from the cilium and its subsequent degradation may result in a higher degree of Hh responsiveness. Hh-induced ciliary clearance of Ptch1 thus may be critical in fine tuning pathway activity and hence the differentiation program induced by the amount of Hh present at a particular position within a field of Hh-responsive cells. Nevertheless, our data indicate that Hh binding to Ptch1 is the critical event for suppression of Ptch1 activity and that ciliary removal, although potentially important, is a secondary event. The mechanism of Ptch1 inactivation by Hh binding awaits molecular elucidation, but may involve stabilization of a particular conformation among several alternatives typically associated with transporter function.
Materials and Methods
Cell culture
HEK 293 cells (Life Technologies), Ptch1−/− MEFs, and stably transfected HEK293-ShhN cells (37) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Omega Scientific) and 1% penicillin/ streptomycin/glutamine. NIH 3T3 cells (ATCC) were maintained in DMEM with 1% penicillin/streptomycin/glutamine and 10% bovine calf serum (HyClone) as previously described (16).
Constructs and Reagents
Mouse full-length Ptch1 and Ptch1 with cytoplasmic tail deletions were fused with either 3×Myc tag or a single YFP tag at the C terminus. Ptch1N-SmoCT-YFP was constructed by directly fusing N-terminus of Ptch1 (aa 1–1169) with the Smo cytoplasmic tail (aa 550–793) followed by a YFP tag. Ptch1N-Sstr3icl3-YFP was constructed by directly fusing Ptch1 (aa 1–1169) with the icl3 of Sstr3 (30) followed by a YFP tag. ShhN-mCherry was constructed by fusing mCherry at the C-terminus of ShhN. Ptch1PY, with two point mutations of Y631F and Y1320F, was constructed by the QuikChange method (Agilent). Full-length Nedd4, Neddd4L, Itchy, Smurf1, and Smurf2 were obtained from Addgene and fused with the HA or FLAG tag at the N-terminus. Full-length WWP1 and WWP2 were obtained from Open Biosystems and fused with the FLAG tag at the N-terminus. Full-length HECW was obtained from DNASU Plasmid Repository and fused with the FLAG tag at the N-terminus. ShhN-conditioned medium was prepared as previously described (37).
Antibodies
Antibodies were used at the following concentrations: rabbit antibody recognizing Smo (16) 1:1,000 dilution of 0.7 µg/µl, mouse monoclonal antibody recognizing acetylated tubulin (Sigma) 1:2,000, rabbit antibody recognizing Myc (Santa Cruz) 1:1,000, mouse antibody recognizing Myc (9E10, Santa Cruz) 1:200, rabbit antibody recognizing GFP (Invitrogen) 1:200, chicken antibody recognizing GFP (Abcam) 1:5,000, rabbit antibody recognizing HA (Invitrogen) 1:1000, rabbit antibody recognizing dsRed (Clontech) 1:1,000, mouse antibody recognizing Flag (M2, Sigma) 1:2,000, rabbit antibody recognizing -β-galactosidase (MP Biomedicals) 1:2,000, HRP-conjugated goat anti-mouse (Promega, W4021) 1:10,000, HRP-conjugated goat anti-rabbit (Jackson Immuno-Research Lab, 111-035-144) 1:10,000. Fluorophore-conjugated secondary antibodies were from Jackson Immuno-Research Laboratories and used at 1:500.
Protein Immunoprecipitation
HEK 293 cells were transfected using FuGENE 6 transfection reagent (Promega). Cells were lysed at 48 h after transfection in a buffer containing 1% NP40, 50 mM Tris-HCl pH 7.5, 150 mM NaCl, and protease inhibitors for 30 min at 4°C. The lysate was clarified by centrifugation at 12,000×g for 20 min at 4°C, and incubated with antibody-coupled Protein A-coated magnetic beads (Invitrogen) for 2 h at 4°C. Beads were washed and proteins were recovered directly in SDS-PAGE sample buffer.
Cell-Surface Biotinylation
HEK 293 cells were washed at 48 h after transfection with PBS, pH 7.4 and incubated for 60 min in PBS containing 1mg/ml Sulfo-NHS-LC-Biotin (Pierce) at 4°C. The reaction was quenched by washing the cells with 100 mM glycine in PBS for 3 times. Cells were then lysed in a buffer containing 1% NP40, 50 mM Tris-HCl pH 7.5, 150 mM NaCl, and protease inhibitors for 30 min. The lysate was clarified by centrifugation at 12,000×g for 20 min at 4°C, and biotinylated proteins were recovered by binding to Streptavidin-Sepharose beads (GE healthcare) for 2 h at 4°C. Beads were washed and proteins were recovered in SDS-PAGE sample buffer.
Immunoblotting
Proteins separated on Criterion TGX SDS-PAGE gels (Bio-Rad) were transferred to polyvinylidene difluoride membranes, blocked in 5% milk in PBS/0.1% Tween-20, and detected with the relevant primary and secondary antibodies.
Gli Reporter Assays
NIH 3T3 cells plated at 5–9 × 104 cells/well of 24-well plates were transfected the next day using FuGENE HD (Promega) with Gli luciferase reporter, control pRL-SV40 Renilla luciferase, and other DNA constructs as indicated. After reaching confluence (~2 days), cells were shifted to 0.5% serum medium and incubated 24 h with conditioned medium containing ShhN.
Immunofluorescence and Quantification of Microscopic Images
Cells were fixed in 4% formaldehyde for 10 min, and then washed 3 times with PBS. Fixed cells were placed in blocking solution (PBS with 1% normal goat serum and 0.1% Triton X-100) for 30 min. Primary antibodies were diluted in blocking solution and used to stain cells for 1 h at room temperature. After 3 washes in PBS, secondary antibodies and DAPI (Invitrogen) were added in blocking solution at a dilution of 1:500 for 1 h at room temperature. The samples were mounted in VECTASHIELD Mounting Medium (Vector Laboratories) for microscopy. Microscopy was either performed on a Leica spinning disc confocal microscope SD6000 or a Zeiss laser scanning confocal microscope LSM710 (with a 63× objective) or an OMX structured illumination microscope. Quantitative analyses were performed using ImageJ, as described previously (21). To assess protein amounts in primary cilia or nucleus, images used for comparison within an experiment were obtained with identical settings on the microscope and then used for quantification without any manipulation. A mask was constructed by manually outlining cilia or nucleus in the image taken in the acetylated-tubulin or DAPI channel, respectively. This mask was then applied to the image taken in the channel for protein of interest, and the fluorescence at the cilium or in the nucleus was measured. Local background correction was performed by moving the mask to measure fluorescence at a representative nearby region, and then subtracting this value from that of ciliary or nuclear fluorescence. All points represent mean fluorescence ± standard deviation from 10–20 individual cilia or nuclei. Statistical analysis was performed using GraphPad Prism software.
Acknowledgments
We thank Applied Precision for OMX imaging, Addgene and DNASU for the plasmids expressing Nedd4, Nedd4L, Itchy, HECW, Smurf1 and Smurf2, and members of the Beachy lab for comments on the manuscript.
Funding: This research was supported in part by funding from the National Institutes of Health to X.Z. and P.A.B and from the ARC to AB and AP. P.A.B. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Author contributions: J.K., X.Z., and P.A.B designed experiments and analyzed data, J.K., E.H., and X.Z. performed experiments, A.B. and A.P. provided data forming the basis for the Ptch1PY mutation, and J.K., X.Z., and P.A.B wrote the paper.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All materials are available upon request.
References and Notes
- 1.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nature reviews. Molecular cell biology. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 2.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 3.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S, Nagle JM, Deshpande V, Boucher Y, Kato T, Chen JK, Willmann JK, Bardeesy N, Beachy PA. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3091–E3100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin K, Lim A, Zhao C, Sahoo D, Pan Y, Spiekerkoetter E, Liao JC, Beachy PA. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer cell. 2014;26:521–533. doi: 10.1016/j.ccell.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochimica et biophysica acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes & development. 2010;24:2001–2012. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nature reviews. Genetics. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 11.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 12.Hui CC, Angers S. Gli proteins in development and disease. Annual review of cell and developmental biology. 2011;27:513–537. doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- 13.Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nature medicine. 2013;19:1410–1422. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 14.Firestone AJ, Weinger JS, Maldonado M, Barlan K, Langston LD, O'Donnell M, Gelfand VI, Kapoor TM, Chen JK. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 2012;484:125–129. doi: 10.1038/nature10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Hsia EY, Kim J, Sever N, Beachy PA, Zheng X. Simultaneous measurement of smoothened entry into and exit from the primary cilium. PloS one. 2014;9:e104070. doi: 10.1371/journal.pone.0104070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ocbina PJ, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:2030–2038. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Developmental cell. 2012;22:940–951. doi: 10.1016/j.devcel.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, Sheffield VC. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS genetics. 2011;7:e1002358. doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Seo S, Bugge K, Stone EM, Sheffield VC. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Human molecular genetics. 2012;21:1945–1953. doi: 10.1093/hmg/dds004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3196–3201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu X, Liu S, Kornberg TB. The C-terminal tail of the Hedgehog receptor Patched regulates both localization and turnover. Genes & development. 2006;20:2539–2551. doi: 10.1101/gad.1461306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 24.Zhulyn O, Nieuwenhuis E, Liu YC, Angers S, Hui CC. Ptch2 shares overlapping functions with Ptch1 in Smo regulation and limb development. Developmental biology. 2015;397:191–202. doi: 10.1016/j.ydbio.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Briscoe J, Chen Y, Jessell TM, Struhl G. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Molecular cell. 2001;7:1279–1291. doi: 10.1016/s1097-2765(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 26.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 27.Tao B, Bu S, Yang Z, Siroky B, Kappes JC, Kispert A, Guay-Woodford LM. Cystin localizes to primary cilia via membrane microdomains and a targeting motif. Journal of the American Society of Nephrology : JASN. 2009;20:2570–2580. doi: 10.1681/ASN.2009020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 29.Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Molecular biology of the cell. 2008;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nature reviews. Molecular cell biology. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 32.Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso MC, Agard DA, Gustafsson MG, Leonhardt H, Sedat JW. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320:1332–1336. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Wu H, Katritch V, Han GW, Huang XP, Liu W, Siu FY, Roth BL, Cherezov V, Stevens RC. Structure of the human smoothened receptor bound to an antitumour agent. Nature. 2013;497:338–343. doi: 10.1038/nature12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 35.Torroja C, Gorfinkiel N, Guerrero I. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development. 2004;131:2395–2408. doi: 10.1242/dev.01102. [DOI] [PubMed] [Google Scholar]
- 36.Yue S, Tang LY, Tang Y, Tang Y, Shen QH, Ding J, Chen Y, Zhang Z, Yu TT, Zhang YE, Cheng SY. Requirement of Smurf-mediated endocytosis of Patched1 in sonic hedgehog signal reception. eLife. 2014;3 doi: 10.7554/eLife.02555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maity T, Fuse N, Beachy PA. Molecular mechanisms of Sonic hedgehog mutant effects in holoprosencephaly. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17026–17031. doi: 10.1073/pnas.0507848102. [DOI] [PMC free article] [PubMed] [Google Scholar]