Abstract
Ovarian cancer is the most lethal gynecological cancer among women worldwide. Adverse side effects and acquired resistance to conventional platinum based chemotherapy are major impediments in ovarian cancer treatment, and drive the development of more selective anticancer drugs that target cancer-specific defects. In this study, theaflavin-3, 3′-digallate (TF3), the major theaflavin monomer in black tea, exhibited a potent growth inhibitory effect on the cisplatin-resistant ovarian cancer A2780/CP70 cells (IC50, 23.81 μM), and was less cytotoxic to a normal ovarian IOSE-364 cells (IC50, 59.58 μM) than to the cancer cells. Flow cytometry analysis indicated that TF3 induced preferential apoptosis and G2 cell cycle arrest in A2780/CP70 cells with respect to IOSE-364 cells. TF3 induced apoptosis through both the intrinsic and extrinsic apoptotic pathways, and caused G2 cell cycle arrest via cyclin B1 in A2780/CP70 cells. The p53 protein played an important role in TF3-induced apoptosis and G2 cell cycle arrest. TF3 might upregulate the p53 expression via the Akt/MDM2 pathway. Our findings help elucidate the mechanisms by which TF3 may contribute to the prevention and treatment of platinum-resistant ovarian cancer.
Keywords: theaflavin-3, 3′-digallate, ovarian cancer, apoptosis, cell cycle arrest, p53, Akt
Introduction
Ovarian cancer is the second most common and the most lethal of malignancies arising in the female reproductive system (1). Response rates to first-line platinum-based therapy are >80%, but the overall 5-year survival rate for patients with advanced ovarian cancer is only ~20% because of acquired drug resistance and adverse side effects. Therefore, overcoming drug resistance is the key to successful treatment of ovarian cancer, and the development of novel therapeutic approaches is urgently needed (2).
Apoptosis is an active death process genetically encoded that can be triggered by a wide variety of extra- and intra-cellular stimuli. Recent evidence suggests that the failure of drug-induced apoptosis may be an underlying cause of drug resistance. Molecular mechanisms of failed apoptosis in chemoresistant ovarian cancer cells include p53 mutations, abnormal expression of the Bcl-2 family proteins and P-glycoproteins, and upregulation of other inhibitors of apoptosis that block caspases and stabilize the mitochondrial permeability pore. Triggering apoptosis is one of the potential strategies to overcome chemoresistance (3,4). The cell cycle is a series of events that take place in a cell leading to its division and duplication that produces two daughter cells. This process involves four sequential phases that go from quiescence (G0 phase) to proliferation (G1, S, G2, and M phases) and back to quiescence. A disregulation of the cell cycle components may lead to tumor formation. A number of potential molecular targets for novel anticancer drug discovery have been identified in cell cycle control mechanisms (5,6).
It is a common belief that compounds from fruits, vegetables and other foods will help reduce the risk of various chronic diseases including cancer, and have less side effects (7). Black tea is the most widely consumed beverage worldwide, and accounted for ~80% of the dried tea manufactured annually (8). It was reported that black tea extract possess potently antiproliferative property against a variety of cancer cells in vitro and in vivo (9,10). In particular, the previous prospective cohort study showed that black tea was a main dietary source of flavonols for US women, and its intake was associated with lower risk of ovarian cancer (11).
Theaflavins are major bioactive components in black tea, and contribute importantly to properties of black tea including its color, ‘mouth feel’ and extent of tea cream formation. They possess a benzotropolone skeleton that is formed from co-oxidation of appropriate pairs of catechins during the production of black tea (12). The major theaflavins in black tea are theaflavin (TF1), theaflavin-3-gallate (TF2A), theaflavin-3′-gallate (TF2B) and theaflavin-3, 3′-digallate (TF3), and TF3 (Fig. 1) is usually most abundant among them (13). Theaflavins have been demonstrated to inhibit proliferation and induce apoptosis in a variety of cancer cells including human breast cancer MCF-7 cells, malignant melanoma A375 cells and oral squamous carcinoma HSC-2 cells. In addition, theaflavins are responsible for the inhibition of ROS-potentiated AH109A adhesion and invasion to the cultured rat mesothelial cell monolayer (14–17).
Figure 1.
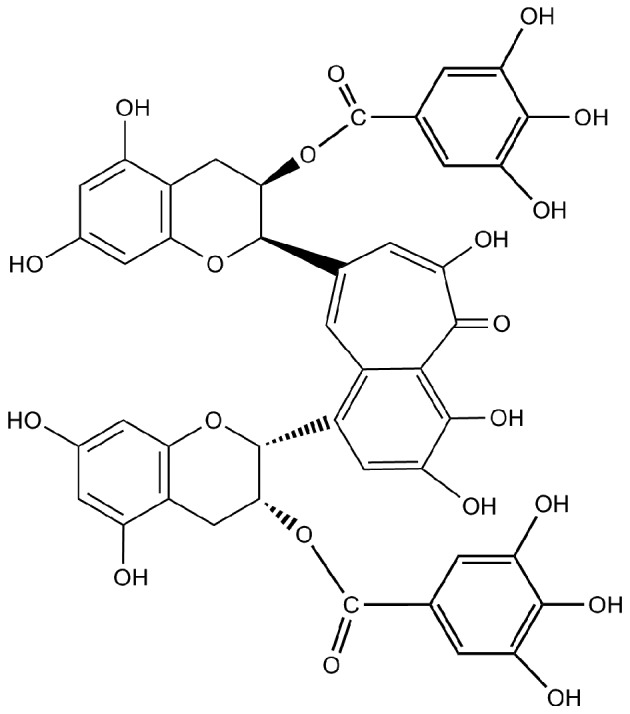
Chemical structure of TF3 used in this study.
Although theaflavins have received considerable attention for their anticancer activity, their effect on the ovarian cancer is still not clear. Therefore, the aim of this study was to investigate the apoptotic and cell cycle arrest effects of TF3 in the platinum-resistant ovarian cancer cell line A2780/CP70 and a normal ovarian surface epithelial cell line IOSE-364. The possible mechanisms underlying these modulations of TF3 on the ovarian cancer cells were also examined.
Materials and methods
Cell culture and reagents
The platinum-resistant human ovarian cancer cell line A2780/CP70 (p53 wild-type) was a generous gift from Dr B. Jiang at West Virginia University. IOSE-364, a normal ovarian surface epithelial cell line, was presented by Dr N. Auersperg at University of British Columbia, Canada. The cells were cultured in RPMI-1640 medium (Sigma) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) at 37°C in a humidified incubator with 5% CO2. Theaflavin-3, 3′-digallate (TF3, >90.0%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The primary antibodies against Bcl-xL, Bad, p21, p53, MDM2 and GAPDH were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The primary antibodies against caspase-8 and -9, Puma, Bax, cyclin B1, phospho-cdc2 (Tyr15), cdc2, DR5, FADD, phospho-Akt (Thr180/Tyr182) and total-Akt were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell viability assay
The cell viability was assessed a using 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Briefly, the cells (1×104 cells per well) were seeded in 96-well plate and incubated overnight. Then various concentrations of TF3 (0–50 μM) were added, and an equal amount of DMSO was used as control. After treatment for 24 h, 20 μl of MTT (5 mg/ml) was added to each well and incubated for an additional 4 h at 37°C in the dark. The medium was discarded, and the formazan crystals formed in the cells were dissolved in 200 μl DMSO. The optical density was measured at 570 nm using a Synergy™ HT Multi-Mode Microplate Reader (BioTek).
Flow cytometric analysis of apoptotic cells
The apoptotic cell death was determined using an Alexa Fluor® 488 Annexin V/ Dead Cell Apoptosis kit (Invitrogen). After exposure to TF3 (0–20 μM) for 24 h, cells were harvested, centrifuged for 10 min at 1500 rpm and then washed twice with PBS. Cells were suspended in binding buffer with Alexa Fluor 488 Annexin V and propidium iodide (PI) for 15 min. The stained cells were analyzed by flow cytometry (FACSCalibur system, BD Biosciences), measuring the fluorescence emission at 530 and 575 nm using 488 nm excitation.
Flow cytometry analysis of the cell cycle
Cells treated with TF3 (0–20 μM) for 24 h were digested with trypsin and harvested by 1500 rpm centrifugation for 10 min. The cell pellet was suspended with 70% ethanol at −20°C overnight, washed with PBS, then incubated with 180 μg/ml RNase A at 37°C for 15 min. After 15 min staining with 50 μg/ml PI in the dark at 37°C, the samples were analyzed by flow cytometry (FACSCalibur system, BD Biosciences). Data were plotted and analyzed by using FCS Software (De Novo Software, Los Angeles, CA, USA).
Caspase-3/7 assay
The caspase-3/7 activities in A2780/CP70 and IOSE-364 cells were detected using a Caspase-Glo 3/7 Assay kit (Promega). Cells were seeded in 96-well plates at 1×104 cells/well, incubated overnight, and treated with TF3 (0–20 μM) for 24 h. After treatment, 100 ml of caspase-3/7 reagent were added to each well, mixed and incubated for 1 h at room temperature. Luminescence was measured using a Synergy™ HT Multi-Mode Microplate Reader (BioTek). Caspase-3/7 activities were normalized by total protein levels, and were expressed as percentage of the untreated control. The total protein levels were measured with a BCA assay kit (Pierce).
Western blotting
A2780/CP70 ovarian cancer cells were seeded in 60-mm dishes at 1×106 cells/dish, incubated overnight, and treated with 0–20 μM TF3 for 24 h. Then cells were harvested with M-PER Mammalian Protein Extraction Reagent (Pierce) supplemented with Halt Protease and Phosphatase Inhibitor (Pierce), and total protein levels were assayed with BCA Protein assay kit (Pierce). Cell lysates were separated by SDS-PAGE and blotted onto nitrocellulose membrane with a Mini-Protean 3 System (Bio-Rad). The membrane was blocked with 5% skim milk, and then incubated with specific primary antibodies and appropriate secondary antibodies conjugated with horseradish peroxidase. The antigen-antibody complex was visualized with the ECL kit (Pierce). Protein bands were quantitated with NIH Image J software and normalized by GAPDH bands for analysis.
Transfection with small interfering RNA (siRNA)
A2780/ CP70 ovarian cancer cells were seeded in 60-mm dishes at 5×105 cells/dish, incubated overnight, and then transfected with p53 siRNA (Santa Cruz) using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's protocol. Cells transfected with control siRNA (Santa Cruz) were used as controls. After a 24-h transfection period, cells were treated with TF3 (0–20 μM) for 24 h. Cell lysates were collected for western blot analysis.
Statistical analysis
In this study, all samples were prepared and analyzed in triplicate. The data are presented as means ± standard deviations (SD). Multiple comparisons were performed by one-way analysis of variance (ANOVA) followed with Student-Newman-Keuls (SNK) test. Statistical differences between two groups were evaluated using the Student's t-test. All statistical analyses of data were performed using Statistical Analysis System (SAS) for windows V8. A p<0.05 was considered statistically significant, and p<0.01 was considered statistically highly significant.
Results
Effect of TF3 on A2780/CP70 and IOSE-364 cell viability
The cytotoxicity of TF3 on A2780/CP70 and IOSE-364 cells was analyzed using the MTT assay. As shown in Fig. 2, TF3 inhibited the growth of both the cell types in a dose-dependent manner. The cell viability with TF3 treatment (0–50 μM) for 24 h ranged from 100 to 11.38% for A2780/CP70 cells, and from 100 to 58.14% for IOSE-364 cells. The IC50 values of TF3 for A2780/CP70 and IOSE-364 cells were estimated to be 23.81 and 59.58 μM, respectively. These results indicated that TF3 had lower cytotoxic effect against normal ovarian IOSE-364 cells than ovarian cancer A2780/CP70 cells.
Figure 2.

Effect of TF3 on cell growth in human ovarian cancer cells A2780/ CP70 and normal ovarian cells IOSE-364. Cells (1×104/well) were seeded in 96-well plates, incubated overnight, and then treated with TF3 for 24 h. Cell viability was determined by MTT assay. Data represent means ± SD from three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
TF3 induces apoptosis in A2780/CP70 cells
To investigate whether TF3 inhibited cell viability by inducing apoptosis in A2780/CP70 and IOSE-364 cells, the cells were treated with TF3 (0–20 μM) for 24 h and subsequently subjected to double staining with Annexin V FITC and propidium iodide (PI) followed by flow cytometry analysis. As shown in Fig. 3A, the treatment of TF3 increased the total percent of apoptotic cells (upper right quadrant + low right quadrant) from 3.28 to 22.47% in A2780/CP70 cells, but could hardly induce apoptosis of IOSE-364 cells in the test range of 0–20 μM. To confirm that TF3 induced apoptosis, caspase-3/7 enzymatic activities were evaluated in both cell lines. As shown in Fig. 3B, treatment with TF3 maximally increased the caspase-3/7 enzymatic activity to 2.19- and 1.20-fold of that in controls for A2780/CP70 and IOSE-364 cells, respectively (p<0.05), indicating that TF3 had more potential to induce apoptosis in the ovarian cancer cells than the normal ovarian cells in this study.
Figure 3.
TF3 induces apoptosis in A2780/CP70 and IOSE-364 cells. (A) Flow cytometry of TF3 treated A2780/CP70 and IOSE-364 cells using a double-staining method with FITC-conjugated Annexin V and PI. The upper left and low left quadrant indicate the percentage of dead and live cells, respectively, and the upper right and low right quadrants indicate the percentage of late and early apoptotic cells, respectively. (B) Caspase-3/7 activity levels with TF3-treatment for 24 h. The caspase-3/7 activity of the control cells after treatment was arbitrarily expressed as 100%. Data represent means ± SD of three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
TF3 induces G2 cell cycle arrest in A2780/CP70 cells
To determine whether the growth inhibitory effect of TF3 is caused by specific perturbation of cell cycle-related events, cell cycle phase distribution of cells treated with TF3 (0–20 μM) for 24 h was analyzed by flow cytometry after propidium iodide staining. As shown in Fig. 4 and Table I, treatment of A2780/CP70 and IOSE-364 cells with TF3 resulted in a significant increase in the proportion of cells at the G2 phase in a concentration-dependent manner (p<0.05). The G2 phase percentage of the A2780/CP70 cells increased by 21.78%, while that of IOSE-364 cells increased by 6.68% after 20 μM TF3 treatment for 24 h. These results suggested that TF3 had more potent capability to induce G2 cell cycle arrest for ovarian cancer cells than the ovarian normal cells.
Figure 4.
TF3 induces cell cycle arrest in A2780/CP70 and IOSE-364 cells. Cells were treated with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h, fixed in 70% ethanol, and stained with propidium iodide. DNA contents were determined by flow cytometry.
Table I.
Cell cycle phase distribution of A2780/CP70 and IOSE-364 cells with TF3 treatment.
| Cell cycle phase distribution | ||||
|---|---|---|---|---|
|
|
||||
| Cell lines | TF3 (μM) | % G1 | % G2 | % S |
| A2780/CP70 | 0 | 42.47±1.33a | 18.00±1.22d | 39.53±2.65a,b |
| 5 | 39.32±2.71a | 25.04±0.79c | 35.64±1.87b | |
| 10 | 26.57±1.26b | 29.08±0.86b | 44.35±3.37a | |
| 20 | 21.81±1.94c | 39.78±1.73a | 38.41±2.83a,b | |
| IOSE-364 | 0 | 32.93±0.85a | 32.91±1.47b | 34.16±1.93a |
| 5 | 31.52±2.23a | 35.35±2.39b | 33.14±2.61a | |
| 10 | 29.55±2.71a | 39.08±1.24a | 31.37±1.06a | |
| 20 | 30.32±1.64a | 39.59±1.67a | 30.08±2.27a | |
Data represent means ± SD from three independent experiments. Values in a column followed by different letters are significantly different (p<0.05).
Effect of TF3 on the intrinsic apoptotic pathway in A2780/ CP70 cells
Apoptosis can be initiated via two canonical pathways: the intrinsic or mitochondrial pathway, and the extrinsic or receptor-mediated pathway. To clarify whether the intrinsic pathway is involved in TF3-induced apoptosis of A2780/CP70 cells, we examined the protein expression of pro-apoptotic Bcl-2 family proteins (Puma, Bax and Bad), anti-apoptotic Bcl-2 family protein Bcl-xL, and caspase-9 by western blotting. Fig. 5 shows TF3 significantly increased the protein levels of Bax, Bad and cleaved caspase-9, reduced protein expressions of Bcl-xL and procaspase-9 (p<0.05), and had no effect on Puma protein expression (p>0.05). These results suggested that TF3 induced apoptosis in A2780/CP70 cells through the intrinsic apoptotic pathway.
Figure 5.

Effect of TF3 on the intrinsic apoptotic pathway in A2780/CP70 cells. Protein lysates were prepared from A2780/CP70 cells after treatment with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h. Puma, Bax, Bad, Bcl-xL and caspase-9 protein levels were analyzed by western blotting. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
Effect of TF3 on the extrinsic apoptotic pathway in A2780/ CP70 cells
Next, we investigated whether TF3 could activate the extrinsic apoptotic pathway in A2780/CP70 cells. As shown in Fig. 6, TF3 significantly increased protein expression of DR5, FADD and cleaved caspase-8, and decreased the protein level of procaspase-8 (p<0.05). These results indicated that the extrinsic pathway was involved in TF3-induced apoptosis of A2780/CP70 cells.
Figure 6.

Effect of TF3 on the extrinsic apoptotic pathway in A2780/CP70 cells. Protein lysates were prepared from A2780/CP70 cells after treatment with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h. DR5, FADD, procaspase-8 and cleaved caspase-8 protein levels were analyzed by western blotting. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. Significant differences among different treatments are indicated by different letters (p<0.05).
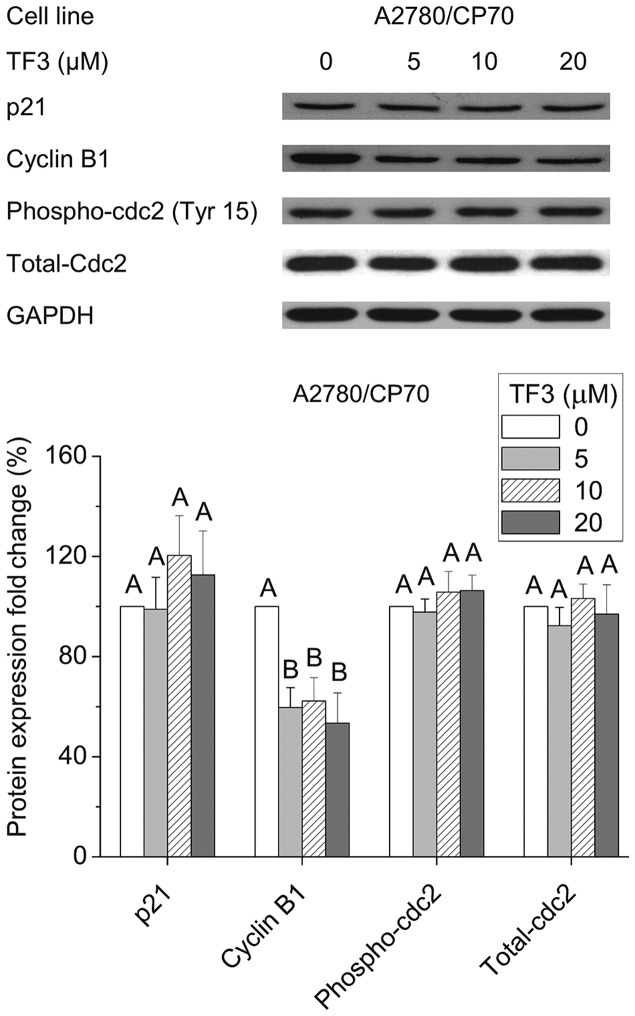
Effect of TF3 on cell cycle G2-related proteins
To investigate the underlying mechanism of the G2 arrest induced by TF3 in A2780/CP70 cells, we evaluated the effect of TF3 on cell cycle regulatory proteins that play important roles in G2 cell cycle progression by western blot analysis. As shown in Fig. 7, TF3 could effectively suppress cyclin B1 expression (p<0.05), but had no effect on the protein levels of p21, phospho-cdc2 (Tyr15) or total-cdc2 (p>0.05). These results implied that the down-regulation of cyclin B1 protein expression may be responsible for the G2 growth arrest induced by TF3 in A2780/CP70 cells.
Figure 7.
Effect of TF3 on cell cycle G2-related proteins in A2780/CP70 cells. Protein lysates were prepared from A2780/CP70 cells after treatment with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h. p21, cyclin B1, phospho-cdc2 and total-cdc2 protein expression levels were analyzed by western blotting. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
Role of p53 in TF3-induced apoptosis and cell cycle arrest
The p53 signaling pathway is a well-known crucial modulator of cell survival, apoptosis and cell cycle arrest (18). To clarify the role of p53 in TF3-induced apoptosis and cell cycle arrest, p53 protein expression was determined by western blotting. Fig. 8A shows that TF3 upregulated the protein expression of p53 in A2780/CP70 cells (p<0.05). To further analyze the role of p53 in TF3-induced cell death, p53 was selectively knocked down by siRNA approach. Fig. 8B shows that p53 protein level was obviously inhibited after treatment with 50 nM p53 siRNA (p<0.01). This p53 depletion led to abrogation of TF3-induced decrease in cyclin B1, procaspase-8 and procaspase-9 protein expression (p<0.05). These results suggested that TF3 induced apoptosis and G2 cell cycle arrest at least partly through p53 in A2780/CP70 cells.
Figure 8.
Role of p53 in TF3-induced apoptosis and G2 cell cycle arrest of A2780/CP70 cells. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. (A) The effects of TF3 on the protein expression of p53 determined by western blotting. Significant differences among the treatments are indicated by different letters (p<0.05). (B) The effects of p53 siRNA (50 nM) on the protein expression of procaspase-8, procaspase-9 and cyclin B1 determined by western blotting. *p<0.05, **p<0.01, compared with respective controls.
Regulation of phospho-Akt, total-Akt and MDM2 protein expression by TF3
Akt is considered as a determinant of cisplatin resistance in ovarian cancer cells (19). Murine double minute 2 (MDM2) is an important negative regulator of p53 that directly inhibits p53 transcriptional activity and enhances p53 degradation via the ubiquitin-proteasome pathway (20). Therefore, we tested the effects of TF3 on the expression of Akt and its downstream effector MDM2. As shown in Fig. 9, TF3 significantly decreased the phosphorylation of Akt without the influence on total Akt protein expression, and reduced the protein level of MDM2 (p<0.05).
Figure 9.
Effect of TF3 on Akt/MDM2 pathway in A2780/CP70 cells. Protein lysates were prepared from A2780/CP70 cells after treatment with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h. phospho-Akt, total-Akt and MDM2 protein expression levels were analyzed by western blotting. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
Discussion
Although cisplatin-centered chemotherapy is the first-line anticancer agent for human ovarian cancer, chemoresistance and adverse side effects remain major hurdles to successful treatment (21). Natural products have recently received renewed focus as a rich repository for drug discovery (22). (−)-Epigallocatechin-3-gallate (EGCG), the most abundant catechin monomer in green tea, was reported to potently suppress ovarian cancer cell growth alone, and potentiate the inhibiting effect of sulforaphane on paclitaxel-resistant ovarian cancer cell lines (23). TF3 is the oxidation product of (−)-epicatechin gallate (ECG) and EGCG, and has been found to possess stronger antioxidant and anti-inflammation activities compared with EGCG (8,24). These findings stimulated our interest in investigating the effect of TF3 on the ovarian cancer cells.
In this study, we found that the IC50 value of TF3 for A2780/CP70 was 23.81 μM, which was around half of the IC50 (59.58 μM) for TF3 in the IOSE-364 cells (Fig. 2), and that (47.9 μM) for cisplatin in the A2780/CP70 cells as reported in our previous study (25). Flow cytometry analysis and caspase-3/7 activity assay suggested that TF3 had more potential to induce apoptosis and G2 cell cycle arrest of A2780/CP70 cells with respect to IOSE-364 cells (Figs. 3 and 4), which provided a possible explanation for the preferential cell growth inhibitory effect by TF3.
A decreased susceptibility of ovarian cancer to apoptosis was strongly associated with drug resistance (26). Agents that target the apoptotic pathway have been shown to sensitize tumor cells to chemotherapy and radiotherapy (27). Apoptosis can be initiated via the intrinsic and extrinsic pathways, which both ultimately activate the same effector caspases (3, 6 and 7) and apoptosis effector molecules. The intrinsic apoptosis pathway is triggered by intracellular signals such as cellular and DNA damage. Key events are the depolarization of the mitochondrial membrane potential and the release of mitochondrial factors such as cytochrome c into the cytosol, which results in activation of initiator caspase-9. Mitochondrial activation is critically controlled by the family of Bcl-2 proteins, which consists of pro- and anti-apoptotic members (28). The extrinsic pathway implicates the activation of death receptors at the plasma membrane level. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) interacts with the death domain-containing receptors DR4/DR5 or Fas, resulting in the recruitment of Fas-associated death domain (FADD), procaspase-8, and procaspase-10 to form the death-inducing signaling complex (DISC) (29). It was reported that the differential induction of apoptosis in cancer versus normal cells involves direct targeting of mitochondria associated with alterations in the balance of Bcl-2 proteins (30). Another explanation is that normal cells are resistant to the tumor necrosis factor related apoptosis-inducing ligand (TRAIL) due to the high TRID levels, whereas tumor lines carrying TRAIL-receptors in most cases can not be protected (31). Theaflavins have been reported to target the Fas/caspase-8 pathway to induce apoptosis in human breast cancer cells, and influence Bcl-2 protein expression in an experimental lung cancer mouse model (32,33). In this study, treatment with TF3 significantly regulated the protein expression of Bcl-2 family proteins (Bax, Bad and Bcl-xL), DR5, FADD, caspase-8 and -9 (Figs. 5 and 6), indicating that TF3 induced apoptosis through both intrinsic and extrinsic pathways in A2780/CP70 cells. Induction of apoptosis might account for the preferential inhibitory effect of TF3 on ovarian cancer A2780/CP70 cells vs. normal ovarian IOSE-364 cells.
Cancer is a disease of inappropriate cell proliferation. It was revealed that many cancer cells have defective G1 checkpoint mechanisms, and depend on G2 checkpoint during cell replication far more than normal cells. These insights have given birth to the idea of cell cycle G2 checkpoint abrogation as a cancer cell specific therapy (34). Our cell cycle analysis showed that TF3 induced more prominent G2 arrest in A2780/ CP70 cells than in IOSE-364 cells (Fig. 4 and Table I), suggesting that in addition to apoptosis, G2 cell cycle arrest was another factor that contributed to the preferential cell growth inhibition. Transition through G1 to S phase is regulated by the cyclin A-Cdk2 and cyclin E-Cdk2 complexes. Inactivation of cyclin B-Cdc2 leads to the G2/M arrest (35). The p21 protein is a potent cyclin-dependent kinase inhibitor, and functions as a regulator of cell cycle progression at G1 and G2 phase (36). A previous study showed that theaflavins induced G2/M arrest by modulating expression of p21, cdc25C and cyclin B in human prostate carcinoma PC-3 cell (37). However, our data showed that TF3 could not influence the p21, phospho-cdc2 (Tyr15) and total-cdc2 protein levels, but significantly decreased cyclin B1 expression (Fig. 7), indicating cyclin B1 played an important role in the TF3-induced G2 arrest of A2780/CP70 cells.
The tumor suppressor protein, p53, transcriptionally activates genes that control cell cycle arrest, apoptosis, and other cellular processes that help to prevent tumor development. p53 stimulates apoptosis by a wide network of signals including the extrinsic pathway such as Killer/DR5 and Fas/APO-1, and intrinsic pathway including proteins Bax, Puma and Noxa (38). In addition, p53 contributes to cell cycle arrest, blocking the cell at the G1, S or G2/M phase by inducing expression of p21 and the consequent inhibition of cyclin D/Cdks, decreasing cyclin B1 transcription and synthesis, or inactivates Cdc2 through Cdc25C (39). It is of particular interest in ovarian cancer in view of the association of platinum sensitivity and p53 pathway alterations. However, wild-type p53 status is often, but not always, associated with cisplatin sensitivity (40).
It is known that A2780/CP70 cell line has a wild-type p53 gene sequence (41). In this study, we found that TF3 significantly increased the p53 protein expression (Fig. 8A). Furthermore, transfection with the p53 siRNA tended to attenuate the reduction of procaspase-8, 9 and cyclin B1 protein expression induced by TF3 (Fig. 8). These results suggested that p53 played an important role in TF3 induced apoptosis and G2 cell cycle arrest of A2780/CP70 cells, which was consistent with the previous reports that p53 promoted theaflavin-induced apoptosis in human breast and prostate cancer cells through mitochondrial death cascade (42,43), and prevented G2/M transition by decreasing intracellular levels of cyclin B1 protein in ovarian cancer Ts-SKOV3 cells (44).
Akt is a serine/threonine kinase that plays a critical role in the malignant transformation of human tumors and their subsequent growth, proliferation, and metastasis. Targeted inhibition of the Akt pathway is a promising strategy for cancer therapy and can also be useful for overcoming chemotherapy resistance (45). The functional link between Akt-mediated chemoresistance and p53 has been found. It was reported that Akt could induce an MDM2-dependent downregulation of p53, and suppression of Akt sensitized chemoresistant cells to cisplatin in a p53-dependent manner (46). Our data showed that TF3 treatment significantly decreased the phosphorylation of Akt and MDM2 protein level, indicating that TF3 might upregulate p53 expression through inactivation of Akt and MDM2 in A2780/CP70 cells. Further in vivo studies in animal models and human patients are necessary to ascertain the anticancer effect of theaflavins against ovarian cancer.
In conclusion, this study demonstrated that TF3 had a potent and preferential cell growth inhibitory effect on the cisplatin-resistant ovarian cancer cell line A2780/CP70 with respect to normal cell line IOSE-364 via apoptosis and G2 cell cycle arrest. TF3 induced apoptosis through both the intrinsic and extrinsic pathways, and caused G2 cell cycle arrest via cyclin B1 in A2780/CP70 cells. These activities were at least partly mediated by p53 upregulation dependent of Akt and MDM2. Based on these results, TF3 would be a promising natural compound for prevention and treatment of platinum-resistant ovarian cancer.
Acknowledgements
We thank Dr Kathy Brundage from the Flow Cytometry Core at West Virginia University for providing technical help on apoptosis and cell cycle analysis. We acknowledge financial support from the West Virginia Higher Education Policy Commission/ Division of Science Research, the National Natural Science Foundation of China (grant no. 31501474), the Natural Science Foundation of Zhejiang Province (grant no. LY15C200007), the Major Project of Hubei Province for Science and Technology Development (grant no. 2013ABC002), and the Agricultural Science and Technology Independent Innovation Foundation in Jiangsu Province [grant no. CX(14)2122]. This study was also supported by NIH grants P20RR016477 from the National Center for Research Resources and P20GM103434 from the National Institute for General Medical Sciences (NIGMS) awarded to the West Virginia IDeA Network of Biomedical Research Excellence.
References
- 1.McLean K, VanDeVen NA, Sorenson DR, Daudi S, Liu JR. The HIV protease inhibitor saquinavir induces endoplasmic reticulum stress, autophagy, and apoptosis in ovarian cancer cells. Gynecol Oncol. 2009;112:623–630. doi: 10.1016/j.ygyno.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Lee RX, Li QQ, Reed E. β-elemene effectively suppresses the growth and survival of both platinum-sensitive and -resistant ovarian tumor cells. Anticancer Res. 2012;32:3103–3113. [PMC free article] [PubMed] [Google Scholar]
- 3.Yang YI, Kim JH, Lee KT, Choi JH. Costunolide induces apoptosis in platinum-resistant human ovarian cancer cells by generating reactive oxygen species. Gynecol Oncol. 2011;123:588–596. doi: 10.1016/j.ygyno.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Wahl H, Tan L, Griffith K, Choi M, Liu JR. Curcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecol Oncol. 2007;105:104–112. doi: 10.1016/j.ygyno.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 5.Buolamwini JK. Cell cycle molecular targets in novel anticancer drug discovery. Curr Pharm Des. 2000;6:379–392. doi: 10.2174/1381612003400948. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Moralli S, Tarrado-Castellarnau M, Miranda A, Cascante M. Targeting cell cycle regulation in cancer therapy. Pharmacol Ther. 2013;138:255–271. doi: 10.1016/j.pharmthera.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Luo H, Rankin GO, Li Z, Depriest L, Chen YC. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011;128:513–519. doi: 10.1016/j.foodchem.2011.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkar A, Bhaduri A. Black tea is a powerful chemopreventor of reactive oxygen and nitrogen species: Comparison with its individual catechin constituents and green tea. Biochem Biophys Res Commun. 2001;284:173–178. doi: 10.1006/bbrc.2001.4944. [DOI] [PubMed] [Google Scholar]
- 9.Koňariková K, Ježovičová M, Keresteš J, Gbelcová H, Ďuračková Z, Žitňanová I. Anticancer effect of black tea extract in human cancer cell lines. Springerplus. 2015;4:127. doi: 10.1186/s40064-015-0871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian Y, Zhu K, Wang Q, Li G, Zhao X. Antimutagenic activity and preventive effect of black tea on buccal mucosa cancer. Oncol Lett. 2013;6:595–599. doi: 10.3892/ol.2013.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassidy A, Huang T, Rice MS, Rimm EB, Tworoger SS. Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am J Clin Nutr. 2014;100:1344–1351. doi: 10.3945/ajcn.114.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin D, Xu Y, Mei X, Meng Q, Gao Y, Li B, Tu Y. Antiobesity and lipid lowering effects of theaflavins on high-fat diet induced obese rats. J Funct Foods. 2013;5:1142–1150. doi: 10.1016/j.jff.2013.03.011. [DOI] [Google Scholar]
- 13.Li B, Vik SB, Tu Y. Theaflavins inhibit the ATP synthase and the respiratory chain without increasing superoxide production. J Nutr Biochem. 2012;23:953–960. doi: 10.1016/j.jnutbio.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharya U, Halder B, Mukhopadhyay S, Giri AK. Role of oxidation-triggered activation of JNK and p38 MAPK in black tea polyphenols induced apoptotic death of A375 cells. Cancer Sci. 2009;100:1971–1978. doi: 10.1111/j.1349-7006.2009.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adhikary A, Mohanty S, Lahiry L, Hossain DM, Chakraborty S, Das T. Theaflavins retard human breast cancer cell migration by inhibiting NF-kappaB via p53-ROS cross-talk. FEBS Lett. 2010;584:7–14. doi: 10.1016/j.febslet.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 16.Schuck AG, Ausubel MB, Zuckerbraun HL, Babich H. Theaflavin-3,3′-digallate, a component of black tea: An inducer of oxidative stress and apoptosis. Toxicol In Vitro. 2008;22:598–609. doi: 10.1016/j.tiv.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhang G, Miura Y, Yagasaki K. Suppression of adhesion and invasion of hepatoma cells in culture by tea compounds through antioxidative activity. Cancer Lett. 2000;159:169–173. doi: 10.1016/S0304-3835(00)00545-0. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan KD, Gallant-Behm CL, Henry RE, Fraikin JL, Espinosa JM. The p53 circuit board. Biochim Biophys Acta. 18252012:229–244. doi: 10.1016/j.bbcan.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine AJ, Hu W, Feng Z. The P53 pathway: What questions remain to be explored? Cell Death Differ. 2006;13:1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 20.Kao CL, Hsu HS, Chen HW, Cheng TH. Rapamycin increases the p53/MDM2 protein ratio and p53-dependent apoptosis by translational inhibition of mdm2 in cancer cells. Cancer Lett. 2009;286:250–259. doi: 10.1016/j.canlet.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Abedini MR, Muller EJ, Bergeron R, Gray DA, Tsang BK. Akt promotes chemoresistance in human ovarian cancer cells by modulating cisplatin-induced, p53-dependent ubiquitination of FLICE-like inhibitory protein. Oncogene. 2010;29:11–25. doi: 10.1038/onc.2009.300. [DOI] [PubMed] [Google Scholar]
- 22.Miura K, Satoh M, Kinouchi M, Yamamoto K, Hasegawa Y, Kakugawa Y, Kawai M, Uchimi K, Aizawa H, Ohnuma S, et al. The use of natural products in colorectal cancer drug discovery. Expert Opin Drug Discov. 2015;10:411–426. doi: 10.1517/17460441.2015.1018174. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Landen CN, Li Y, Alvarez RD, Tollefsbol TO. Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp Cell Res. 2013;319:697–706. doi: 10.1016/j.yexcr.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Jie G, Dong F, Xu Y, Watanabe N, Tu Y. Radical-scavenging abilities and antioxidant properties of theaflavins and their gallate esters in H2O2-mediated oxidative damage system in the HPF-1 cells. Toxicol In Vitro. 2008;22:1250–1256. doi: 10.1016/j.tiv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Gao Y, Rankin GO, Rojanasakul Y, Cutler SJ, Tu Y, Chen YC. Chaetoglobosin K induces apoptosis and G2 cell cycle arrest through p53-dependent pathway in cisplatin-resistant ovarian cancer cells. Cancer Lett. 2015;356(2 Pt B):418–433. doi: 10.1016/j.canlet.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu W, Wang F, Tang J, Liu X, Yuan Z, Nie C, Wei Y. Proapoptotic protein Smac mediates apoptosis in cisplatin-resistant ovarian cancer cells when treated with the anti-tumor agent AT101. J Biol Chem. 2012;287:68–80. doi: 10.1074/jbc.M111.271205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson TR, Johnston PG, Longley DB. Anti-apoptotic mechanisms of drug resistance in cancer. Curr Cancer Drug Targets. 2009;9:307–319. doi: 10.2174/156800909788166547. [DOI] [PubMed] [Google Scholar]
- 28.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SY, Lee DH, Song X, Bartlett DL, Kwon YT, Lee YJ. Role of Bcl-xL/Beclin-1 in synergistic apoptotic effects of secretory TRAIL-armed adenovirus in combination with mitomycin C and hyperthermia on colon cancer cells. Apoptosis. 2014;19:1603–1615. doi: 10.1007/s10495-014-1028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Hannafon B, Gill L, Kelly W, Benbrook D. Flex-Hets differentially induce apoptosis in cancer over normal cells by directly targeting mitochondria. Mol Cancer Ther. 2007;6:1814–1822. doi: 10.1158/1535-7163.MCT-06-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozören N, El-Deiry WS. Cell surface death receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003;13:135–147. doi: 10.1016/S1044-579X(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 32.Lahiry L, Saha B, Chakraborty J, Adhikary A, Mohanty S, Hossain DM, Banerjee S, Das K, Sa G, Das T. Theaflavins target Fas/caspase-8 and Akt/pBad pathways to induce apoptosis in p53-mutated human breast cancer cells. Carcinogenesis. 2010;31:259–268. doi: 10.1093/carcin/bgp240. [DOI] [PubMed] [Google Scholar]
- 33.Saha P, Banerjee S, Ganguly C, Manna S, Panda CK, Das S. Black tea extract can modulate protein expression of H-ras, c-Myc, p53, and Bcl-2 genes during pulmonary hyperplasia, dysplasia, and carcinoma in situ. J Environ Pathol Toxicol Oncol. 2005;24:211–224. doi: 10.1615/JEnvPathToxOncol.v24.i3.70. [DOI] [PubMed] [Google Scholar]
- 34.Kawabe T. G2 checkpoint abrogators as anticancer drugs. Mol Cancer Ther. 2004;3:513–519. [PubMed] [Google Scholar]
- 35.Duiker EW, Meijer A, van der Bilt AR, Meersma GJ, Kooi N, van der Zee AG, de Vries EG, de Jong S. Drug-induced caspase 8 upregulation sensitises cisplatin-resistant ovarian carcinoma cells to rhTRAIL-induced apoptosis. Br J Cancer. 2011;104:1278–1287. doi: 10.1038/bjc.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu W, Park SK, Jia L, Tiwary R, Scott WW, Li J, Wang P, Simmons-Menchaca M, Sanders BG, Kline K. RRR-gamma-tocopherol induces human breast cancer cells to undergo apoptosis via death receptor 5 (DR5)-mediated apoptotic signaling. Cancer Lett. 2008;259:165–176. doi: 10.1016/j.canlet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasad S, Kaur J, Roy P, Kalra N, Shukla Y. Theaflavins induce G2/M arrest by modulating expression of p21waf1/cip1, cdc25C and cyclin B in human prostate carcinoma PC-3 cells. Life Sci. 2007;81:1323–1331. doi: 10.1016/j.lfs.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 38.Kuo YC, Kuo PL, Hsu YL, Cho CY, Lin CC. Ellipticine induces apoptosis through p53-dependent pathway in human hepatocellular carcinoma HepG2 cells. Life Sci. 2006;78:2550–2557. doi: 10.1016/j.lfs.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Pucci B, Kasten M, Giordano A. Cell cycle and apoptosis. Neoplasia. 2000;2:291–299. doi: 10.1038/sj.neo.7900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green JA, Berns EM, Coens C, van Luijk I, Thompson-Hehir J, van Diest P, Verheijen RH, van de Vijver M, van Dam P, Kenter GG, et al. EORTC Gynaecological Cancer Group. Alterations in the p53 pathway and prognosis in advanced ovarian cancer: A multi-factorial analysis of the EORTC Gynaecological Cancer group (study 55865) Eur J Cancer. 2006;42:2539–2548. doi: 10.1016/j.ejca.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Brown R, Clugston C, Burns P, Edlin A, Vasey P, Vojtĕsek B, Kaye SB. Increased accumulation of p53 protein in cisplatin-resistant ovarian cell lines. Int J Cancer. 1993;55:678–684. doi: 10.1002/ijc.2910550428. [DOI] [PubMed] [Google Scholar]
- 42.Lahiry L, Saha B, Chakraborty J, Bhattacharyya S, Chattopadhyay S, Banerjee S, Choudhuri T, Mandal D, Bhattacharyya A, Sa G, et al. Contribution of p53-mediated Bax transactivation in theaflavin-induced mammary epithelial carcinoma cell apoptosis. Apoptosis. 2008;13:771–781. doi: 10.1007/s10495-008-0213-x. [DOI] [PubMed] [Google Scholar]
- 43.Kalra N, Seth K, Prasad S, Singh M, Pant AB, Shukla Y. Theaflavins induced apoptosis of LNCaP cells is mediated through induction of p53, down-regulation of NF-kappa B and mitogen-activated protein kinases pathways. Life Sci. 2007;80:2137–2146. doi: 10.1016/j.lfs.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Innocente SA, Abrahamson JL, Cogswell JP, Lee JM. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA. 1999;96:2147–2152. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/ AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137:173–179. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Kim CW, Lu JN, Go SI, Jung JH, Yi SM, Jeong JH, Hah YS, Han MS, Park JW, Lee WS, et al. p53 restoration can overcome cisplatin resistance through inhibition of Akt as well as induction of Bax. Int J Oncol. 2013;43:1495–1502. doi: 10.3892/ijo.2013.2070. [DOI] [PubMed] [Google Scholar]