Abstract
Many observational studies showed hogh-density lipoprotein cholesterol (HDL-C) is a strong inverse predictor of cardiovascular (CV) outcome. However, recent large clinical trials evaluating therapies to raise HDL-C level in those already on statin therapy have been discouraging. This complexity is not well-known.
A total of 28,357 acute myocardial infarction (AMI) patients were enrolled in the Korea Acute Myocardial Infarction Registry (KAMIR), which was a prospective, multicenter, nationwide, web-based database of AMI in Korea. From this registry, we evaluated 3574 patients with AMI who have follow-up HDL-C level to investigate its association with clinical outcomes. The primary endpoint was the relationship between follow-up change in HDL-C and a 12-month composite of major adverse cardiac events (MACEs).
Patients with initial HDL-C ≥ 40 mg/dL showed significantly lower rates of 12-month MACEs, especially cardiac and all-cause mortalities (P < 0.001). When patients were stratified into 4 groups according to the change of HDL-C, patients with decreasing HDL-C showed significantly higher rates of 12-month MACEs as comparable with patients with increasing HLD-C. A multivariate analysis indicated that HDL-C level was a significant predictor of CV events (hazard ratio, 1.38; 95% confidence interval, 1.12–1.71) after correcting for confounding variables.
The follow-up change in HDL-C level was significantly related with CV outcomes in patients with AMI.
INTRODUCTION
Lowering low-density lipoprotein cholesterol (LDL-C) level with statins has been shown to reduce the cardiovascular (CV) events.1 However, significant residual CV risk remains even after achieving optimal LDL-C levels with statin therapy.2,3 These ongoing events have driven researchers to identify factors that contribute to the CV risk that persists in patients taking a statin. Over the last few years, epidemiological studies have provided evidence that low concentrations of high-density lipoprotein cholesterol (HDL-C) are associated with an increased risk of coronary artery disease (CAD) and CV events.4–6 Indeed, a meta-analysis of 4 large prospective studies concluded that every 1-mg/dL decrease in HDL-C is associated with a 2% to 3% increase in CV events.1 However, clinical trials on agents that increase HDL-C levels have failed to improve clinical outcomes despite substantial increases in HDL-C levels.2,7,8 Further studies are needed to re-evaluate the role of HDL-C in patients with acute myocardial infarction (AMI). Additionally, very few studies have evaluated the clinical impacts of follow-up changes of HDL-C in patients taking a statin. The purposes of this study were to re-investigate the relationship between HDL-C level and CV events in patients with AMI and to analyze the impacts of increasing or decreasing HDL-C level during statin therapy on CV events.
METHODS
Study Population
The Korea Acute Myocardial Infarction Registry (KAMIR) database is a Korean prospective, open, observational, multicenter, on-line registry to investigate risk factors for mortality in patients with AMI and to establish universal management for preventing AMI with support from the Korean Circulation Society. It began in November 2005, and 53 centers that were capable of performing a large number of primary percutaneous coronary interventions (PCIs) participated. The study protocol was approved by the institutional review board at each participating center.
As shown in Figure 1, a total of 28,357 patients with AMI were identified from the KAMIR. Of these patients, 3365 patients with insufficient initial lipid profile data and 6740 who had not been followed up clinically for 12 months were excluded and the remaining 18252 patients were analyzed for the effects of initial HDL-C level (HDL-C ≥40 mg/dL or HDL-C <40 mg/dL) on CV events (phase 1 analysis). Among them, 3574 patients had sufficient 12-month lipid profile and clinical outcome data. Using these data, we analyzed the effects of a follow-up change in HDL-C level after statin therapy on CV events (phase 2 analysis). The patients were classified into 4 groups according to the follow-up change in HDL-C level (group 1: initial HDL-C ≥40 mg/dL and follow-up HDL-C ≥40 mg/dL, n = 1446; group 2: initial HDL-C ≥40 mg/dL and follow-up HDL-C <40 mg/dL, n = 592; group 3: initial HDL-C <40 mg/dL and follow-up HDL-C ≥40 mg/dL, n = 503; and group 4: initial HDL-C <40 mg/dL and follow-up HDL-C <40 mg/dL, n = 1033).
FIGURE 1.
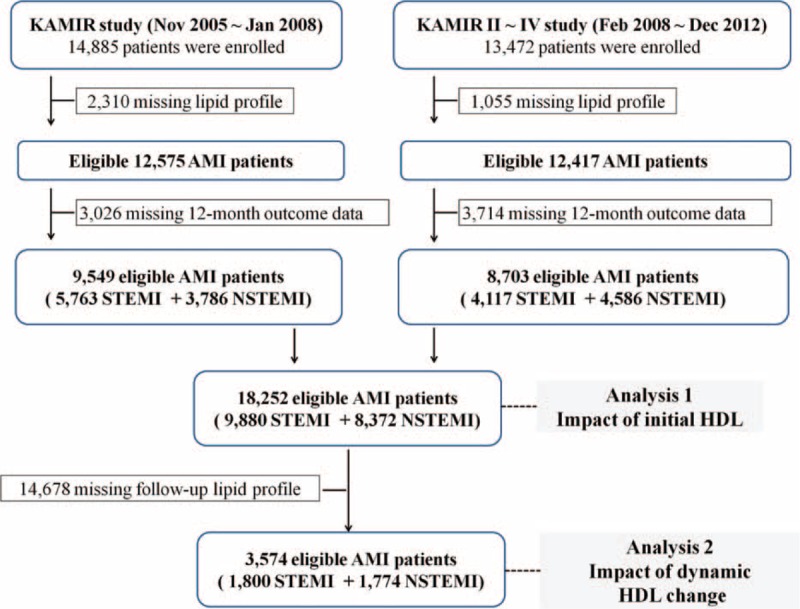
Flow chart of patient enrollment. AMI = acute myocardial infarction, HDL = high-density lipoprotein, KAMIR = Korea Acute Myocardial Infarction database, NSTEMI = non ST-elevation myocardial infarction, STEMI = ST-elevation myocardial infarction.
Study Endpoints
All definitions of the clinical outcomes in this study were based on the recommendations of the Academic Research Consortium.9 The primary endpoint was the relationship between follow-up change in HDL-C and a 12-month composite of major adverse cardiac events (MACEs), which was composed of all-cause mortality, recurrent MI, and repeat revascularization. The secondary endpoints were individual components of the primary endpoint, cardiac death, and coronary artery bypass grafting from any cause.
Statistical Analysis
Statistical analyses were carried out using the SPSS Statistics for Windows ver. 17.0 software (SPSS Inc, Chicago, IL). A 2-sided P < 0.05 was considered significant. Continuous variables are presented as means ± standard deviations and were evaluated for normality of the distribution and compared using Student t test or analysis of variance accordingly. The continuous parameters with a skewed distribution were logarithmically transformed. Categorical variables are presented as frequencies and percentages and were compared using the χ2 or Fisher exact test, when appropriate. The Kaplan–Meier method and log-rank test were used to assess 12-month event-free survival for the MACEs. Multivariate Cox proportional hazards regression analyses were performed to determine independent variables associated with 12-month MACEs. All variables (age, sex, body mass index [BMI], Killip class, hypertension, DM, dyslipidemia, smoking, previous CAD, lipid profile, high-sensitivity C-reaction protein [hsCRP], and NT-proBNP) were entered en bloc, and the results are expressed with a hazard ratio (HR) and 95% confidence interval (CI).
RESULTS
Baseline Characteristics
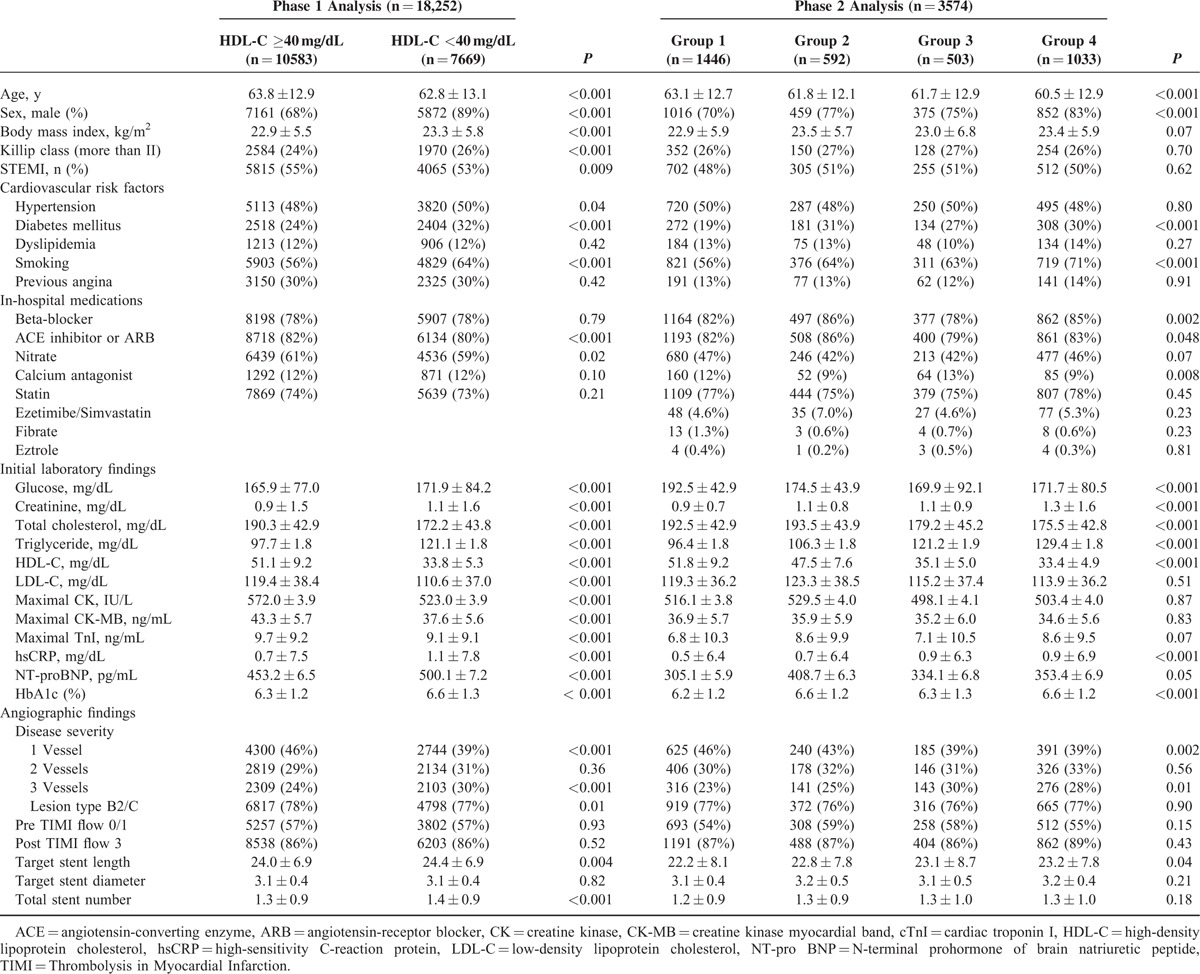
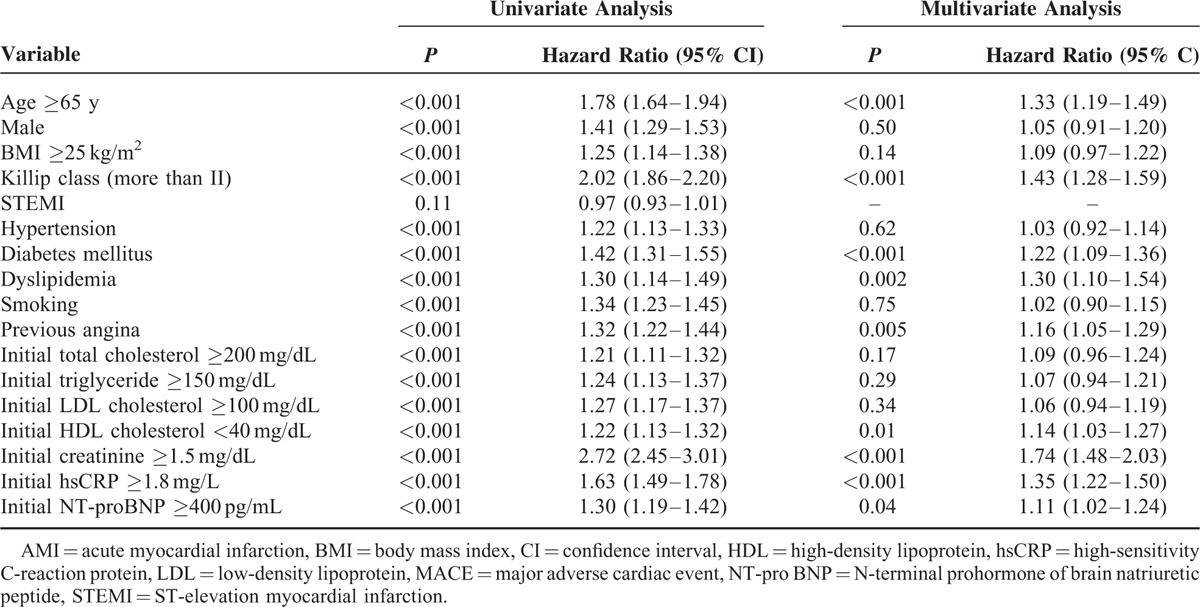
The baseline characteristics, CV risk factors, in-hospital medications, initial laboratory findings, and angiographic findings according to initial HDL-C level are summarized in Table 1. Patients with a low initial HDL-C level (<40 mg/dL) were younger, more male, had a higher BMI, a poorer Killip class, less ST-segment elevation MI, and higher rates of hypertension, diabetes mellitus (DM), and smoking than those in the higher HDL-C group. This group also showed higher levels of creatinine, hsCRP, and NT-proBNP, but lower maximal levels of creatine kinase-MB and troponin I. Patients with a low initial HDL-C level showed more 3-vessel disease and stent implantation on angiography than those with a higher initial HDL-C level.
TABLE 1.
Baseline Patient Characteristics
Clinical Impact of Initial HDL Level on Clinical Outcomes
As shown in Figure 2A and Table 2, subjects with a higher HDL-C level at admission showed significantly lower rates of 12-month MACEs, particularly cardiac and all-cause mortality. These beneficial effects were observed in the rates of in-hospital mortality and 1-month MACEs during the early period after AMI. The Cox-regression analysis revealed that low HDL-C level at admission was an important predictor for 12-month MACEs before and after adjusting for other important covariates (age, sex, body mass index [BMI], Killip class, hypertension, DM, dyslipidemia, smoking, previous CAD, lipid profile, hsCRP, and NT-proBNP), as shown in Figure 3A. Additionally, older age (≥65 years), poorer Killip class, DM, dyslipidemia, previous CAD, chronic kidney disease, and high levels of hsCRP and NT-proBNP were significant independent predictors for 12-month MACEs (Table 3).
FIGURE 2.
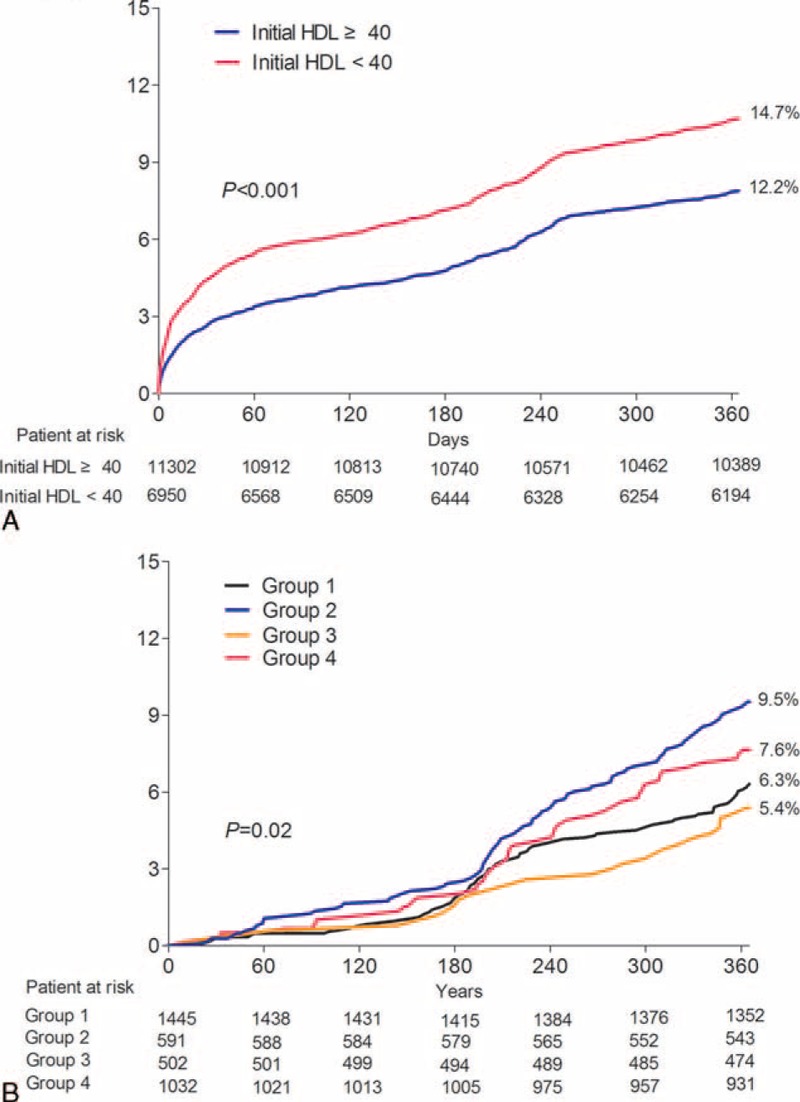
Kaplan–Meier curve of the prevalence of 1-year MACEs. (A) Initial HDL-C. (B) quartiles of HDL-C change at 6 months. HDL-C = high-density lipoprotein cholesterol, MACE = major adverse cardiac event.
TABLE 2.
Clinical Outcomes According to HDL Level
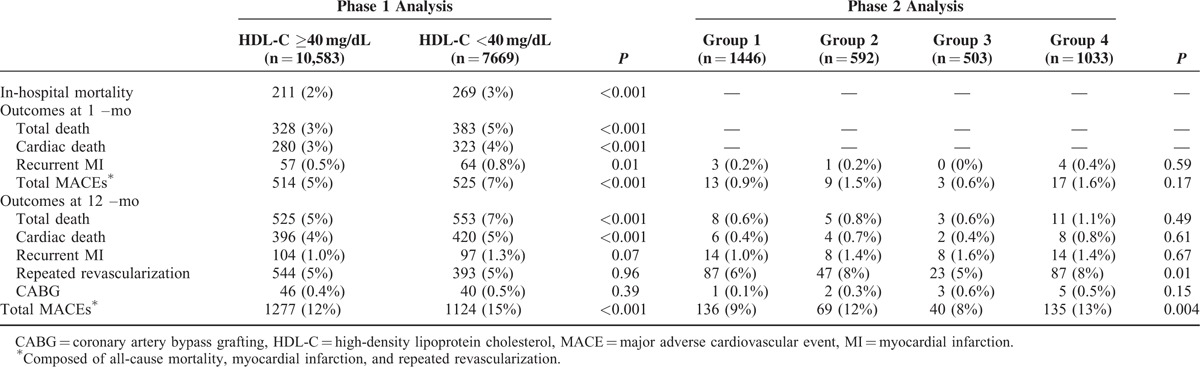
FIGURE 3.
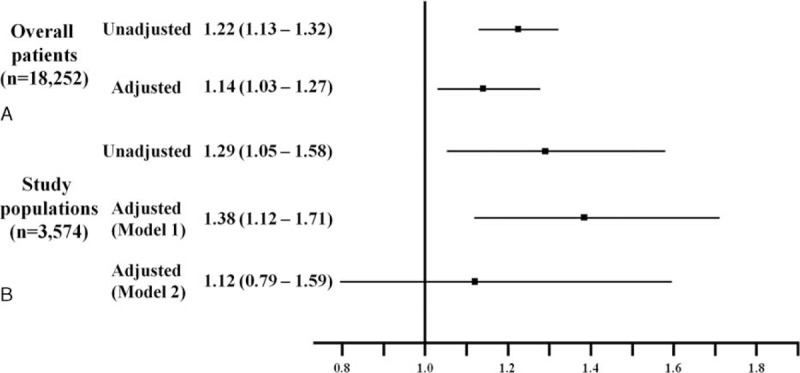
Estimates of adjusted and unadjusted hazard ratios. (A) Overall patients, (B) study populations; (model 1), adjusted for age, sex, BMI, Killip class; presence or absence of hypertension, DM, dyslipidemia, follow-up lipid profile, previous CAD, smoking, (model 2), added follow-up hsCRP and NT-proBNP levels. BMI = body mass index, CAD = coronary artery disease, DM = diabetes mellitus, hsCRP = high sensitivity C-reactive protein, NT-proBNP = N-terminal prohormone of brain natriuretic peptide.
TABLE 3.
Univariate and Multivariate Cox Regression Analyses of 1-year MACEs in Patients With AMI (Phase 1)
Clinical Impact of Follow-Up Changes in HDL-C
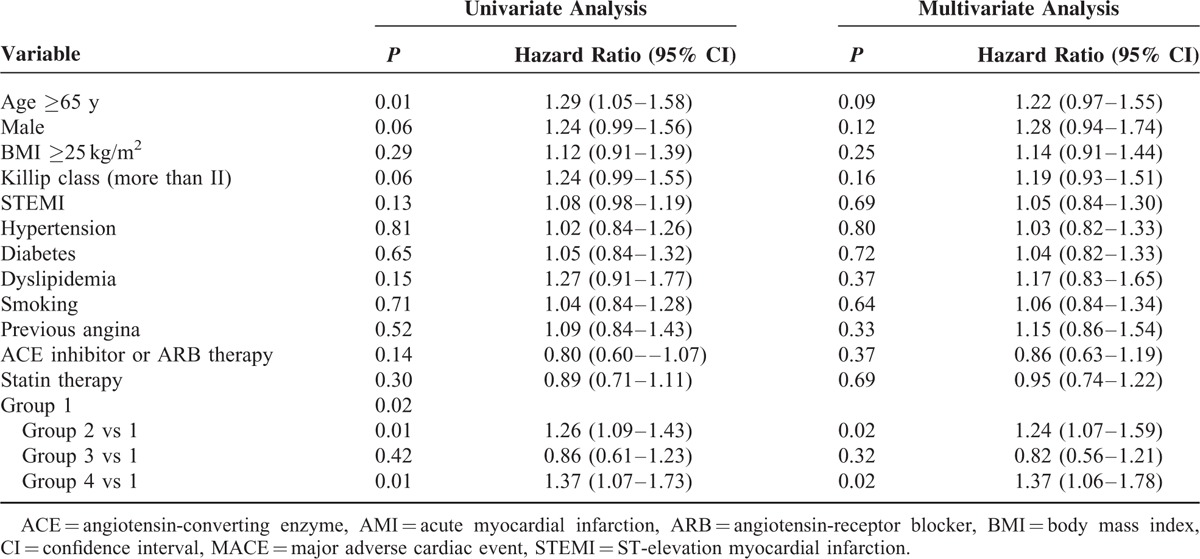
A total of 3,574 patients with lipid profile data at admission and 12 months were stratified into 4 groups according to the follow-up change between the initial and follow-up HDL-C levels using the reference value of 40 mg/dL HDL-C. Group 2 had more females, fewer smokers, and a lower creatinine level than those in group 4 (Table 1). No significant differences in other variables were found, including in-hospital medications or angiographic findings. Group 2 showed significantly higher rates of 12-month MACEs compared with those of group 1 and 3. Poor clinical outcomes of group 2 were similar to those of group 4 (Figure 2B and Table 2). These poorer outcomes were mainly attributed to an increased rate of repeated revascularization. The univariate and multivariate Cox regression analyses showed that the characteristics of groups 2 and 4 were independent predictors for 12-month MACE (Table 4). We also investigated the clinical impact of low follow-up HDL-C level. Figure 3B shows that follow-up HDL-C level was a significant independent predictor after adjusting for age, sex, BMI, Killip class, hypertension, DM, dyslipidemia, smoking, previous CAD, and follow-up lipid profile (model 1). Follow up NT-proBNP level (HR 1.52, 95% CI 1.05–2.19, P = 0.03) was the only significant predictor when model 1 was added to the follow-up hsCRP and NT-pro BNP levels in model 2.
TABLE 4.
Univariate and Multivariate Cox Regression Analyses of 1-year MACEs in Patients With AMI
DISCUSSION
We showed 2 major findings in unselected consecutive patients with AMI from a large number of different hospitals. First, low initial HDL-C level was a powerful predictor of CV risk, similar to previous clinical research. Second, the decreasing follow-up HDL-C level was associated with the risk of MACEs defined as all-cause death, recurrent MI, or any repeat revascularization after 1 year of follow-up in patients with AMI.
HDL-C level is an important predictor of coronary events in patients with known CAD across a broad range of LDL-cholesterol levels, as demonstrated in the following post-hoc analyses of some randomized trials. An analysis of 13,173 patients in the LIPID and CARE trials found that low serum HDL-C level is a significantly stronger predictor of CV events in patients with a LDL-C <125 mg/dL compared with those with LDL-C ≥125 mg/dL.10 The event rate for a 10-mg/dL increase in HDL-C decreased 29% in those with LDL-C <125 mg/dL compared with 10% in those with LDL-C ≥125 mg/dL. Nearly 10,000 patients with established CAD were treated with either high- or low-dose statin therapy in the Treating to New Targets trial.11 A multivariate analysis revealed that HDL-C level was inversely predictive of the time to first major CV event across a spectrum of LDL-C levels, including patients with treated LDL-C levels <70 mg/dL. The rate of all-cause death or MI was 33% lower in the highest compared with the lowest HDL-C quartile in a post-hoc analysis of 2193 subjects with stable disease who participated in the COURAGE trial for optimal medical therapy.12 This relationship was even stronger among subjects with LDL-C levels <70 mg/dL. These results indicate that HDL-C could be a surrogate marker for residual CV risk in patients taking a statin.
The 2013 American College of Cardiology/American Heart Association and 2015 European Society guidelines on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults do not recommend adding therapy to raise low HDL-C in patients who are on maximal statin therapy.13,14 These guidelines only focused on LDL-C and do not recommend follow-up HDL-C level checks because some clinical trials have indicated no improvement in clinical outcome despite treatment to increase HDL-C levels.7,8,15 However, it is important to regularly check HDL-C level, take more intensive statin therapy, modify lifestyle, and quit smoking because follow-up HDL level was a surrogate marker for residual CV risk in our data.
The discrepancy between previous studies and ours may be explained by several reasons. First, most of the previous studies were not performed in the setting of AMI unlike ours. Although dal-OUTCOMES trial was researched in Acute coronary syncrome,8 15% of patients were not an initial clinical presentation of AMI. In addition, previous studies only compared the treatment group with the control group by only whether use of HDL raising agent or not. This point would be most important difference between previous studies and this work. Our study population was collected from a nationwide registry with a large sample size, an unselected population and properly adjusted for major potential confounders. Additionally, their cholesterol treatment was not done by HDL-raising agent, but only by statins. In the statin era, we showed the clinical effect of the absolute value of follow-up HDL-C level on CV risk. Group 4 showed a similar rate of 12-month MACEs compared with that of group 2, which is one of the most important findings.
In contrast, recent clinical studies have suggested that the results of previous studies were owing to abnormal HDL-C particle composition and function.16,17,19 The HDL particle has multiple antiatherogenic properties thought to be mediated by its participation in removing cholesterol from macrophages during “macrophage cholesterol efflux.”17 Furthermore, cholesterol efflux capacity has a strong inverse relationship with CV risk and increasing capacity was expected another therapeutic target.18 In addition, the vascular effects exerted by HDL in patients with a range of CV conditions differ substantially from the properties of HDL in healthy subjects, which have been called “HDL dysfunction.” 19 In the absence of inflammation, HDL has a complement of antioxidant enzymes that work to maintain an anti-inflammatory state. In the presence of systemic inflammation, such as in acute coronary syndrome, these antioxidant enzymes are inactivated and HDL accumulates oxidized lipids and proteins, making it proinflammatory. Other study with mendelian randomization has suggested that some genetic mechanisms that raise HDL-C plasma level do not reduce risk of CV events.20 Some another studies have suggested that measuring the quality and novel functions of HDL could provide an improved means of identifying subjects at increased risk for atherosclerotic events, compared with the current practice of only measuring HDL-C level.21 The quality and function of HDL are attractive targets for emerging therapies. And the other recent studies presented that HDL subclass, especially HDL3-C, had inverse association in coronary heart disease (CHD).22 Until recently, there is no consensus as the HDL subclass function in CHD, but research will become active about HDL subclass.
Our study had a number of limitations. First, it was a retrospective study with inherent methodological restrictions. We could not control noncardiac-related medications and other patients’ condition such as nutritional status or lifestyle because of inherent limitations of registry data. Second, our study population was restricted to only 3574 patients because follow-up lipid profiles of only these patients could be obtained. Undetected selection bias and small sample size may remain despite our cautious use of multivariate analysis. Third, we cannot evaluate a subparticle or subclass of HDL-C because this study was nation-wide and web-based large cohort in acute myocardial infarction setting. Finally, we were unable to acquire exact medication data, such as the incidence rates of serious adverse effects or patient compliance. Despite these limitations, we demonstrated that a follow-up change in HDL-C level has important prognostic value in patients with AMI. We also reconfirmed that initial HDL-C level is an important factor in clinical outcome. Our data could play a hypothesis-generating role for future research and better clinical outcomes of patients with AMI who have changes in follow-up HDL-C levels.
In conclusion, lowering follow-up HDL-C level was an important prognostic factor for 1-year MACEs in patients with AMI.
Footnotes
Abbreviations: AMI = acute myocardial infarction, BMI = body mass index, BNP = brain natriuretic peptide, CAD = coronary artery disease, CHD = coronary heart disease, CI = confidence interval, CV = cardiovascular, DM = diabetes mellitus, HDL-C = high-density lipoprotein cholesterol, HDL-C = high-density lipoprotein cholesterol, HR = hazard ratio, hsCRP = high-sensitivity C-reaction protein, KAMIR = Korea Acute Myocardial Infarction Registry, LDL-C = low-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, MACE = major adverse cardiac event, MI = myocardial infarction, NT-pro BNP = N-terminal prohormone of brain natriuretic peptide.
CHL, JSW contributed equally to this study.
Financial support: None declared.
The authors report no conflicts of interest.
This research was supported by Bio & Medical Technology Development Program of the National Research Foundation of the Ministry of Education, Science and Technology (No.: 2012M3A9C6050507).
The authors report no conflicts of interest.
REFERENCES
- 1.Pedersen T, Kjekshus J, Faergerman O, et al. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389. [PubMed] [Google Scholar]
- 2.Jafri H, Alsheikh-Ali AA, Karas RH. Meta-analysis: statin therapy does not alter the association between low levels of high-density lipoprotein cholesterol and increased cardiovascular risk. Ann Intern Med 2010; 153:800–808. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364:226–235. [DOI] [PubMed] [Google Scholar]
- 4.Di Angelantonio E, Gao P, Pennells L, et al. Emerging Risk Factors Collaboration. Lipid-related markers and cardiovascular disease prediction. JAMA 2012; 307:2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007; 357:1301–1310. [DOI] [PubMed] [Google Scholar]
- 6.Castelli WP, Garrison RJ, Wilson PW, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 1986; 256:2835–2838. [PubMed] [Google Scholar]
- 7.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low hdl cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Abt M, et al. for dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012; 367:2089–2099. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007; 115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 10.Sacks FM, Tonkin AM, Craven T, et al. Coronary heart disease in patients with low LDL-cholesterol: benefit of pravastatin in diabetics and enhanced role for HDL-cholesterol and triglycerides as risk factors. Circulation 2002; 105:1424–2428. [DOI] [PubMed] [Google Scholar]
- 11.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007; 357:1301–1310. [DOI] [PubMed] [Google Scholar]
- 12.Acharjee S, Boden WE, Hartigan PM, et al. Low levels of high-density lipoprotein cholesterol and increased risk of cardiovascular events in stable ischemic heart disease patients: A post-hoc analysis from the COURAGE Trial. J Am Coll Cardiol 2013; 62:1826–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129 (25 Suppl 2):S76–S99. [DOI] [PubMed] [Google Scholar]
- 14.Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016; 37:267–315. [DOI] [PubMed] [Google Scholar]
- 15.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999; 341:410–418. [DOI] [PubMed] [Google Scholar]
- 16.Lüscher TF, Landmesser U, von Eckardstein A, et al. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res 2014; 114:171–182. [DOI] [PubMed] [Google Scholar]
- 17.Rosenson RS, Brewer HB, Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navab M, Anantharamaiah GM, Reddy ST, et al. Mechanisms of disease: proatherogenic HDL–an evolving field. Nat Clin Pract Endocrinol Metab 2006; 2:504–511. [DOI] [PubMed] [Google Scholar]
- 19.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014; 371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012; 380:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navab M, Anantharamaiah GM, Reddy ST, et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 2004; 109:3215–3220. [DOI] [PubMed] [Google Scholar]
- 22.Joshi PH, Toth PP, Lirette ST, et al. Association of high-density lipoprotein subclasses and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. Eur J Prev Cardiol 2016; 23:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


