Supplemental Digital Content is available in the text
Abstract
Enhanced recovery after surgery (ERAS) pathways are multimodal, evidence-based approaches to optimize patient outcome after surgery. However, the use of ERAS protocols to improve morbidity and recovery time without compromising safety following pancreaticoduodenectomy (PD) remains to be elucidated.
We conducted a systemic review and meta-analysis to assess the safety and efficacy of ERAS protocols compared with conventional perioperative care (CPC) in patients following PD.
PubMed, Medline, Embase, and Science Citation Index Expanded and Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library were searched between January 2000 and June 2015.
The patients who underwent PD with ERAS protocols or CPC were eligible. The studies that compared postoperative length of hospital stay (PLOS), postoperative complications, or in-hospital costs in the 2 groups were included.
A meta-analysis, meta-regression, sensitivity analysis, and subgroup analysis were performed to estimate the postoperative outcomes between the 2 groups and identified the potential confounders. We used the methodological index for nonrandomized studies checklist to assess methodological qualities. Weighted mean differences (WMD) or odds ratios (OR) were calculated with their corresponding 95% confidence intervals (CI). The publication bias tests were also performed through the funnel plots.
In total, 14 nonrandomized comparative studies with 1409 ERAS cases and 1310 controls were analyzed. Implementation of an ERAS protocol significantly reduced PLOS (WMD: −4.17 days; 95%CI: −5.72 to −2.61), delayed gastric emptying (OR: 0.56; 95%CI: 0.44–0.71), overall morbidity (OR: 0.63; 95% CI: 0.54–0.74), and in-hospital costs compared to CPC (all P < 0.001). There were no statistically significant differences in other postoperative outcomes. Age, gender, and ERAS component implementation did not significantly contribute to heterogeneity for PLOS as shown by meta-regression analysis.
Our study suggested that ERAS was as safe as CPC and improved recovery of patients undergoing PD, thus reducing in-hospital costs. General adoption of ERAS protocols during PD should be recommended.
INTRODUCTION
High standards of perioperative management in conjunction with expert surgery are the corner stones of postoperative recovery. A formalized enhanced recovery after surgery (ERAS) program was first implemented in elective colorectal surgery.1 ERAS involves a multidisciplinary team approach and thoughtful review of all aspects of operative and perioperative care, such as optimal pain control (including regional anesthesia), minimally invasive techniques, and aggressive postoperative rehabilitation (including nutritional support and ambulation).2,3 It has been further developed for joint,4 breast,5 and colorectal6 surgeries, consistently demonstrating significantly accelerated postoperative recovery and shortened postoperative length of hospital stay (PLOS), as well as reduced in-hospital costs, while maintaining similar safety profiles compared with conventional perioperative care (CPC) alone.7 As a result, there is a consensus agreement that ERAS should be a standard practice in elective colorectal surgery.8
Guidelines published by the ERAS group also recommend its use in pancreaticoduodenectomy (PD); however, this recommendation was based on a very limited number of studies.9 A subsequent meta-analysis7 was only able to include 4 studies and found that ERAS reduced overall morbidity without affecting readmission rates or mortality.10–13 The effect of ERAS on PLOS and in-hospital costs after PD remains unexplored. This systematic review and meta-analysis incorporates the most recent published literature on this topic and aims to evaluate the effects of implementing an ERAS program following PD.
METHODS
Data Sources and Search Strategy
Major public medical and scientific databases including Medline, Embase, and Science Citation Index Expanded and Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library were searched for studies published in the English language comparing ERAS with CPC after PD, from January 2000 to June 2015. The following search terms were used, in all possible combinations: “Whipple,” “pancreaticoduodenectomy,” “pancreatoduodenectomy,” “pancreatoduodenal resection,” “ERAS program,” “enhanced recovery,” “fast track,” “critical pathway,” and “clinical pathway.” Reference lists of selected articles were further examined for relevant articles during the initial search. Only comparative clinical trials with full-text descriptions were included. Final inclusion of articles was determined by consensus of 3 authors. The reporting of this systematic review is conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.14
Inclusion and Exclusion Criteria
Inclusion criteria were: articles published in English in peer-reviewed journals; human studies; studies reporting at least one outcome of interest as defined below; and where multiple studies by the same institute and/or authors had overlapping enrollment times, only the higher quality study was included in the analysis.
Exclusion criteria were: abstracts, letters, editorials, reviews or guidelines, and case reports; noncomparative studies; and studies including patients undergoing procedures other than PD.
Outcomes of Interest
The primary outcome was PLOS. The secondary outcomes were rates of postoperative pancreatic fistula (POPF), delayed gastric emptying (DGE), overall morbidity, readmission, reoperation, mortality, and in-hospital costs.
PLOS was defined as the postoperative time interval in days. POPF was defined as per the International Study Group of Pancreatic Fistula (ISGPF) definition15 or as defined by the study's authors in studies conducted prior to 2005. DGE was defined according to the International Study Group of Pancreatic Surgery (ISGPS) definition16 or author's own definitions. Overall morbidity was defined as all complications from operation to discharge or within 30 days. Readmissions were defined as any hospital admission for any reason within 30 days of discharge. Reoperation was defined as the need for laparotomy as a consequence of the 1st operation within 30 days. Mortality was defined as death from any cause prior to discharge from hospital or within 30 days.
Data Extraction and Quality Assessment
Data were extracted by 2 independent observers using standardized forms. The recorded data included characteristics of included study, baseline parameters of patients, inclusion criteria for ERAS, elements of ERAS protocol, postoperative outcomes, and in-hospital costs. Means and standard deviations of the outcomes were used for meta-analysis unless otherwise mentioned. Methodologies for estimating means and standard deviations from medians and ranges have been described previously.17,18 The quality of included studies was assessed using the Methodological Index for Non-Randomized Studies (MINORS) checklist.19 This instrument scores 8 methodological items for noncomparative studies and an additional 4 criteria for comparative studies. The items are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The global ideal scores were 16 for noncomparative studies and 24 for comparative studies.
Statistical Analysis
Meta-analysis was conducted by using Review Manager Version 5.3 software (Version 5.3 for Windows, The Cochrane Collaboration, 2014). Continuous and categorical variables were calculated as weighted mean differences or odds ratios (ORs) with their corresponding 95% confidence interval (CI), respectively. Heterogeneity was assessed using a Chi-square test, where P < 0.1 was considered significant. I2 values were used for the evaluation of statistical heterogeneity; an I2 value of 50% or more indicated the presence of heterogeneity.20 The fixed-effects analysis21 was initially used for all outcomes, while the random-effects model22 was calculated when homogeneity of studies was not supported by the test. Data otherwise unsuitable for meta-analysis were described in the text.
Subgroup and sensitivity analyses were carried out by excluding each study out of each outcome measure. Subgroup analyses were performed by separately analyzing only high quality studies (MINORS score ≥13), studies conducted in Western or Eastern countries, and studies in which n > 100. Sensitivity analyses were conducted to evaluate effects of operative technique (PD or PD with pylorus-preserving pancreatoduodectomy [PPPD]), pancreatic texture, or matching preoperative nutritional status. Meta-regression was carried out to assess the impact of age, gender, and implementation of ERAS elements (not used in >2 studies) on heterogeneity using Stata SE Version 13 Software (Stata Corp LP, TX) with a P < 0.05 considered significant. Funnel plots23 were constructed to evaluate potential publication bias based on the PLOS, readmission, and other secondary outcomes.
Ethics, Standards of Reporting, Data Availability
This systematic review was not submitted to any biomedicalethical committee for approval, and no additional consent was sought from individuals analyzed. It was performed and reported according to the PRISMA standard. All primary outcome data are fully available from the published papers. Other data are partially available and are pointed out in the tables.
RESULTS
Description of Trials Included in the Meta-analysis
The search strategy initially generated 436 relevant clinical trials. No randomized clinical trials were identified. Figure 1 shows the process of selecting comparative studies using the PRISMA statement for meta-analyses.
FIGURE 1.
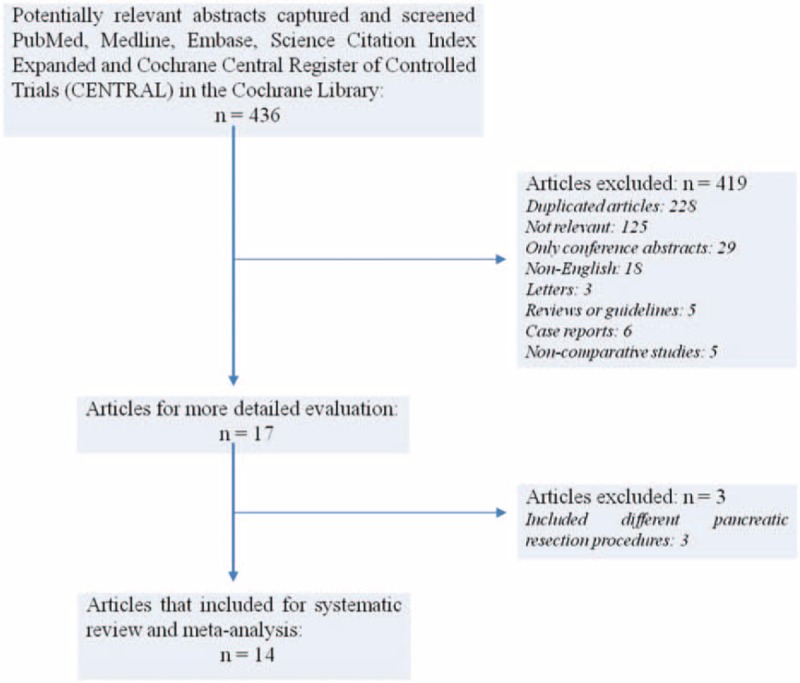
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram depicting the process of identification and inclusion of selected studies.
Application of inclusion/exclusion criteria led to 17 articles10–13,24–36 being subjected to detailed analysis. Of these studies, a further three10,32,33 were excluded as they included pancreatic resections other than PD, leaving 14 studies11–13,24–31,34–36 for data extraction. Patients in Sutcliff et al's study34 were divided into high- and low-risk according to the risk of POPF using drain fluid amylase. As only low-risk patients were selected for all other ERAS programs, we included the patients at low-risk of POPF for analysis.
Characteristics and Quality Assessment of Included Studies
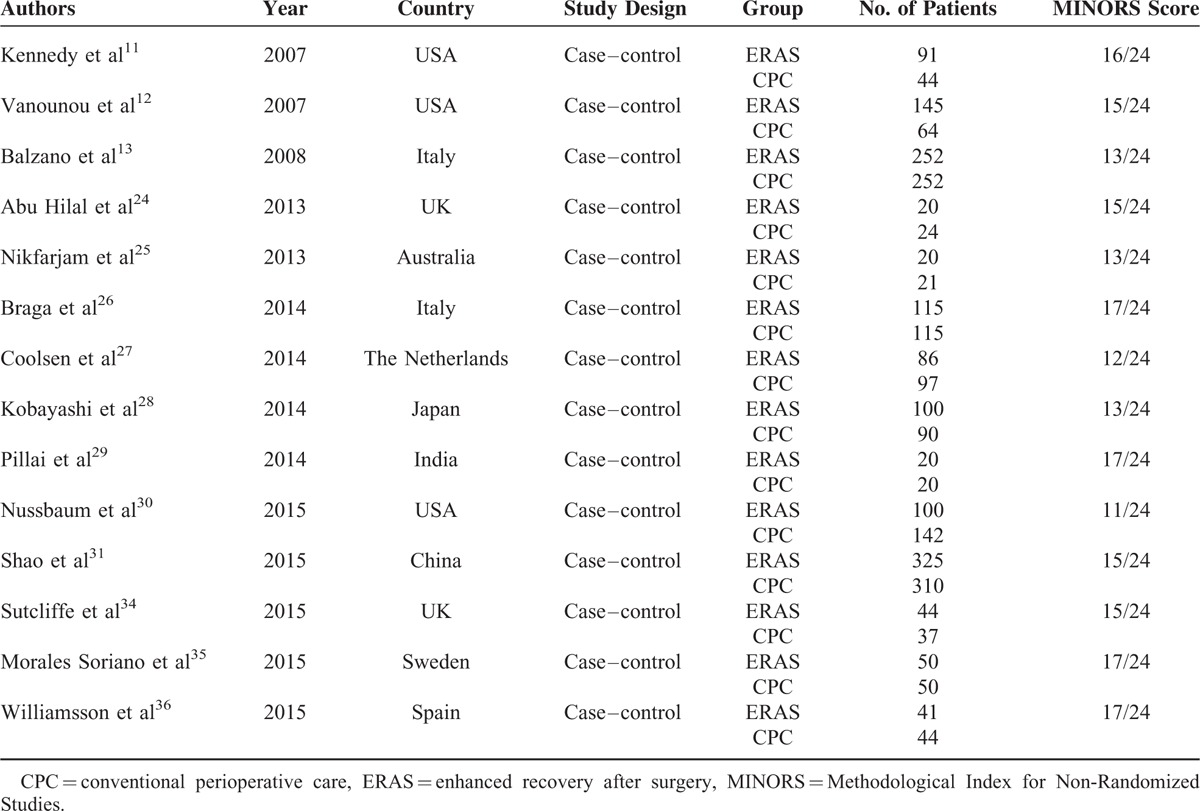
Detailed study characteristics and quality assessments are shown in Table 1. All the included studies were retrospective case–control series. Eleven studies11–13,24–27,30,34–36 were conducted in Western populations, and 8 studies11–13,26–28,30,31 had sample sizes >100. A total of 2719 patients were included with 1409 and 1310 patients in the ERAS group and CPC group, respectively. There were 12 studies11–13,24–26,28,29,31,34–36 with MINORS scores ≥13.
TABLE 1.
Study Characteristics and Quality Assessment
Baseline Parameters of Patients and Inclusion Criteria for Surgery
The baseline parameters and inclusion criteria for ERAS are shown in Table 2. The mean or median age ranged from 44.2 to 70. The gender, body mass index, American Society of Anesthesiologist score, pancreatic texture, and duct status distribution were equal for ERAS and CPC groups in most of the studies. Malignant pancreatic diseases accounted for 50% to 95% of the indications for surgery. Patients with benign pancreatic tumor, chronic pancreatitis, and other pancreatic disorders were also included for surgery.
TABLE 2.
Baseline Parameters of Patients and Inclusion Criteria for Surgery
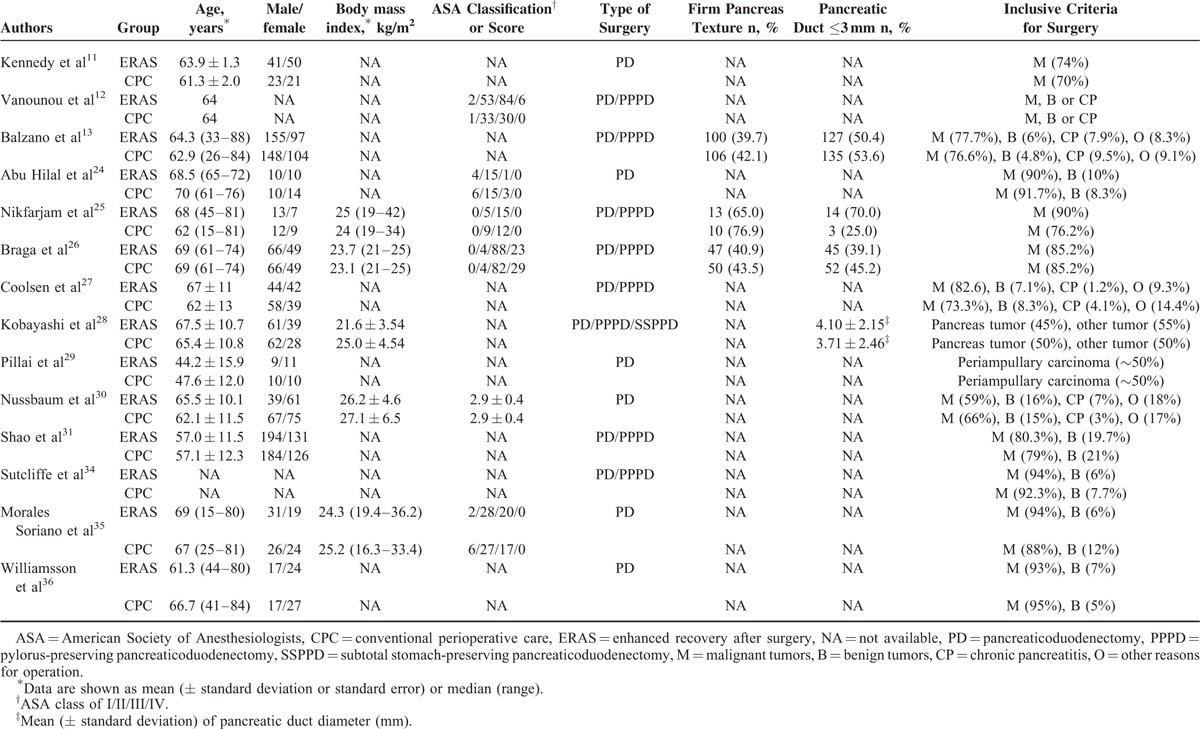
In 6 studies11,24,29,30,35,36 only PD was performed, while PPPD was used as well as PD in 8 studies.12,13,25–28,31,34 One Japanese study28 included both PPPD and subtotal stomach-preserving PD alongside PD. Eleven studies13,24,26–31,34–36 used the ISGPF definition for POPF. Kennedy et al11 defined POPF as drainage of >30 mL with serum amylase level >3-fold for more than 10 days after surgery, while the diagnostic criteria were not described in the remaining 2 studies.12,25 Eight studies7,24,28–31,35,36 defined DGE as per ISGPS guidance, 3 studies11,13,26 defined it as a need for nasogastric decompression, persistent vomiting or vomiting occurring after the 10th postoperative day, and the remaining 3 studies12,25,34 did not state their criteria.
Essential ERAS Elements Used in Included Studies
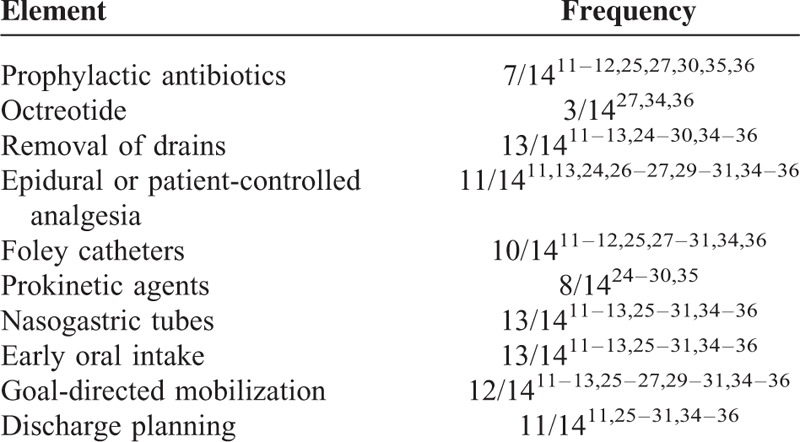
Individual elements of different ERAS protocols used in each study are listed in Table 3. Although there were significant differences in the use of prophylactic antibiotics and octreotide, most studies used epidural and/or patient-controlled analgesia, prokinetic agents, and goal-directed mobilization and had predefined criteria for removal of drains, nasogastric tubes, and catheters as well as a preagreed discharge plan. Early oral intake was encouraged in most of studies.
TABLE 3.
Frequency of Elements Included in Enhanced Recovery After Surgery (ERAS) Protocols for Pancreaticoduodenectomy
Meta-Analysis Outcomes
The postoperative outcomes are detailed in Supplementary Table 1 and meta-analysis results are shown in Figures 2 and 3.
FIGURE 2.
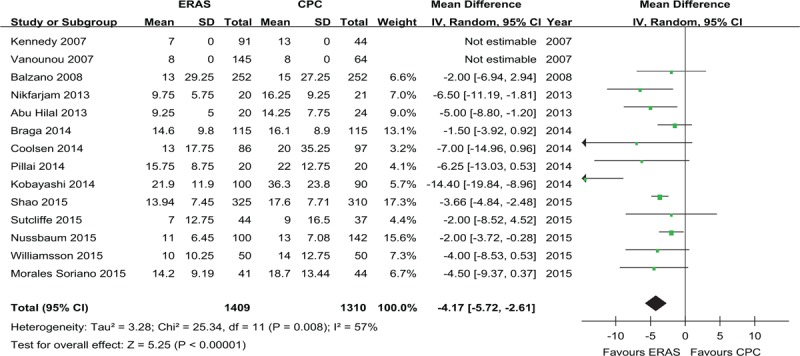
Forest plots demonstrating the primary outcome postoperative length of hospital stay in terms of ERAS versus CPC after pancreaticoduodenectomy. Pooled WMDs with 95% CIs were calculated using the random-effects model. CI = confidence interval, CPC = conventional perioperative care, ERAS = enhanced recovery after surgery, WMD = weighted mean difference.
FIGURE 3.
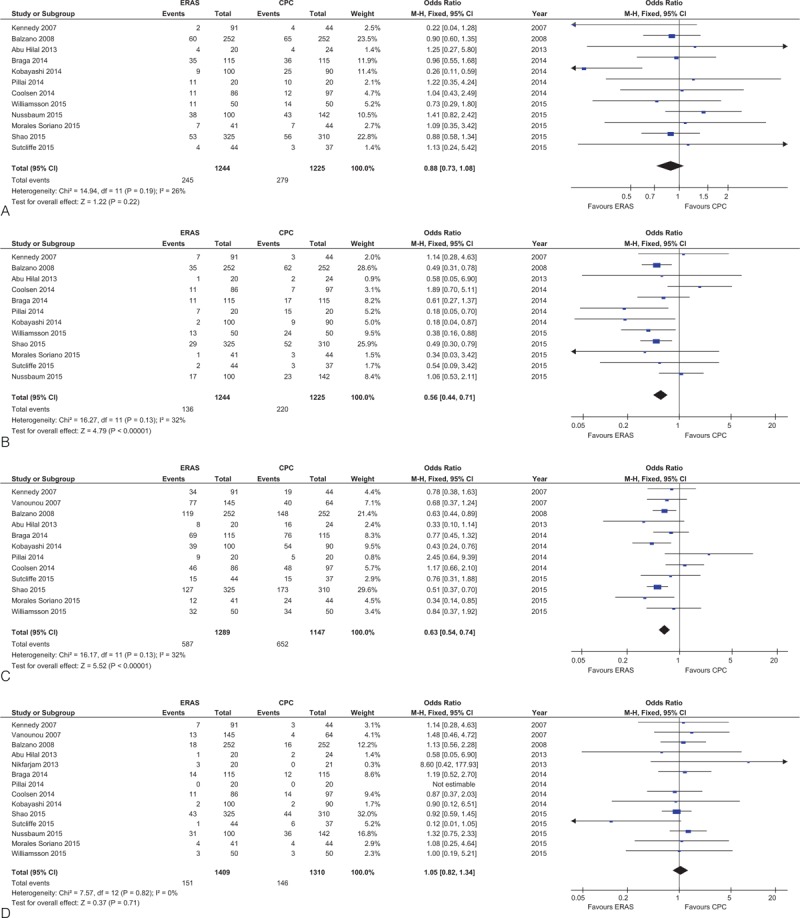
Forest plots demonstrating secondary outcomes in terms of ERAS versus CPC after pancreaticoduodenectomy. (A) Postoperative pancreatic fistula; (B) delayed gastric emptying; (C) overall morbidity; (D) readmission rate; (E) reoperation rate; and (F) mortality. CPC = conventional perioperative care, ERAS = enhanced recovery after surgery.
FIGURE 3 (Continued).
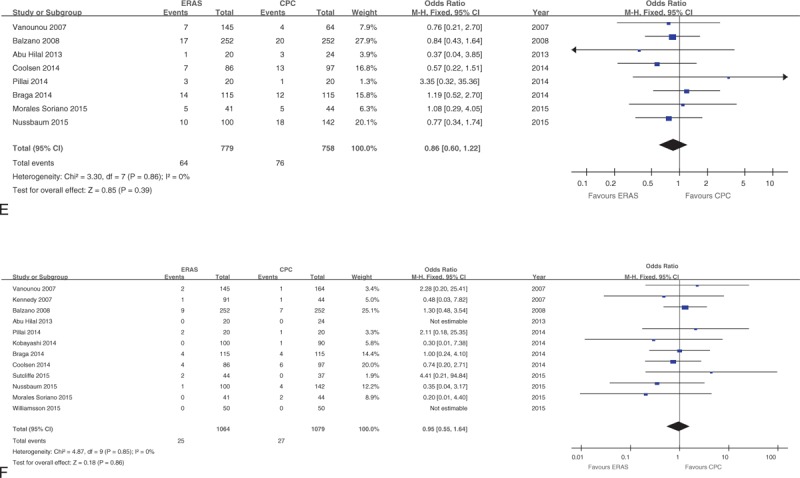
Forest plots demonstrating secondary outcomes in terms of ERAS versus CPC after pancreaticoduodenectomy. (A) Postoperative pancreatic fistula; (B) delayed gastric emptying; (C) overall morbidity; (D) readmission rate; (E) reoperation rate; and (F) mortality. CPC = conventional perioperative care, ERAS = enhanced recovery after surgery.
Primary Outcome
All studies reported the primary outcome: PLOS. Meta-analysis including 2719 patients showed that patients in the ERAS group had a shorter PLOS compared with those in the CPC group (weighted mean differences: −4.17 days; 95%CI: −5.72 to −2.61, P < 0.00001; Figure 2), although a moderate degree of heterogeneity was observed (I2 = 57%, P = 0.008).
Secondary Outcomes
The rates of POPF in all studies (OR: 0.88, 95%CI: 0.73–1.08; P = 0.22; Figure 3 A) or only those using the ISGPF definition (OR: 0.90, 95%CI: 0.74–1.10; P = 0.30) were similar between ERAS and CPC groups. Furthermore, there was no significant difference in POPF B/C (OR: 0.87, 95%CI: 0.66–1.14; P = 0.32) between ERAS and CPC groups.
Compared to CPC, the incidence of DGE (OR: 0.56; 95% CI: 0.44–0.71, P < 0.0001; Figure 3 B) was lower in the ERAS group. This difference remained statistically significant when only including studies adhering to the ISGPS definition (OR: 0.57, 95%CI: 0.42–0.77; P = 0.003).
The incidence of overall morbidity (OR: 0.63; 95%CI: 0.54–0.74, P < 0.00001; Figure 3 C) was lower in the ERAS group.
There were no statistically significant differences in rates of readmission (OR: 1.05, 95%CI: 0.82–1.34; P = 0.71; Figure 3 D), reoperation (OR: 0.86, 95%CI: 0.60–1.22; P = 0.39; Figure 3 E), or mortality (OR: 0.95, 95%CI: 0.55–1.64; P = 0.86; Figure 3 F).
In-hospital costs were reported by 4 studies11,12,31,36 and showed that ERAS protocols significantly reduced costs. Kennedy et al11 reported that the respective average costs were US $126,566 ± 4883 in the ERAS group and $240,242 ± 32,490 in the CPC group (P < 0.0001). Vanounou et al12 reported decreased costs when using an ERAS protocol (a reduction from $28,886 to $23,344 following ERAS implementation). Shao et al31 also reported a reduction of costs from RMB 68,663.18 ± 26,639.74 to RMB 58,505.19 ± 34,044.92 (P < 0.001). These findings again were corroborated in the study of Williamsson et al,36 with costs of €10400 (6519–39558) and €14576 (8245–42750) in the ERAS group and CPC group (P < 0.001), respectively.
Subgroup, Sensitivity, and Meta-Regression Analyses
The results of the subgroup analysis are summarized in Table 4. The results of sensitivity analysis and meta-regression analysis are also summarized Supplementary Tables 2 and 3, respectively.
TABLE 4.
Results of Subgroup Analysis
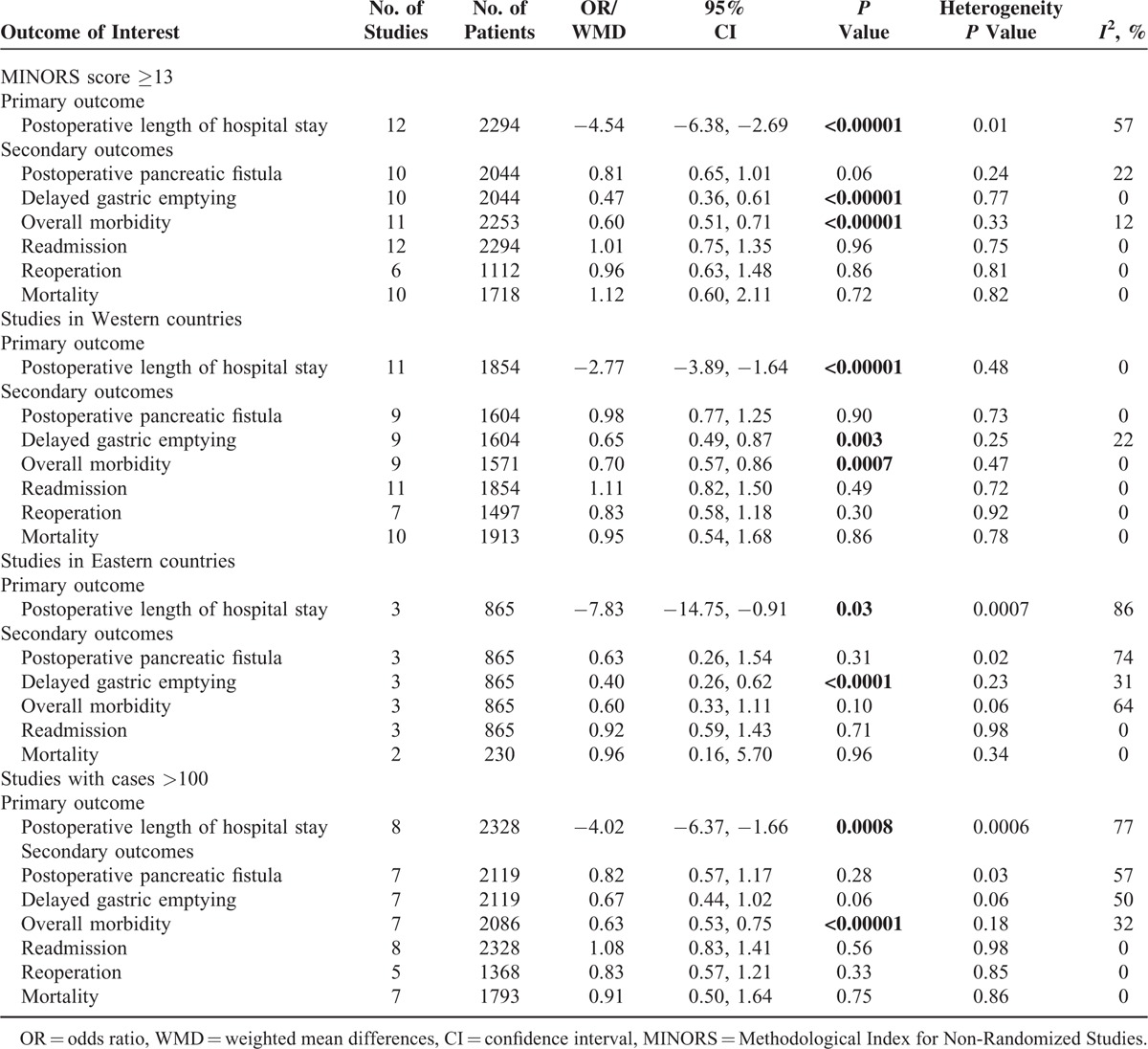
The subgroup analysis including only high quality studies yielded similar results to the primary analysis. When analyzing only studies conducted in Western countries, the results were also the same, but with abolished heterogeneity for PLOS (I2 = 0%); expectedly, there was increased heterogeneity in studies conducted in Eastern countries (I2 = 86%), and furthermore, the reduction of overall morbidity by ERAS was also no longer statistically significant (P = 0.1). The heterogeneity for PLOS in larger studies (n > 100) was increased (77%) with consistent clinical outcomes when compared to the primary analysis. In the sensitivity analysis, studies reporting matching preoperative nutritional status, pancreatic texture, or type of procedure (PD, PD/PPPD) showed reduced heterogeneity for PLOS and a consistent reduction of PLOS by ERAS. Age, gender, and ERAS component implementation did not significantly contribute to heterogeneity for PLOS as shown by meta-regression analysis.
Publication Bias
The funnel plots based on PLOS and readmission are shown in Supplementary Figure 1. There was no evidence of publication bias of PLOS, readmission, and the other secondary outcomes (data not shown).
DISCUSSION
The 1-stage PD, or Whipple procedure, remains one of the most technically challenging general surgical operations with extreme impact on patient physiology.37 Nowadays, despite reductions in mortality of this procedure to around 5% in high-volume specialized centers,38 it is still associated with postoperative morbidity up to 60%.38–40 A previous systematic review41 and meta-analysis7 suggest that using an ERAS protocol in pancreatic resections may help to shorten PLOS and reduced overall morbidity without affecting readmission rates or mortality. When only focusing on PD, a reduction of overall morbidity was noticed without significant differences in rates of readmission and mortality by the implementation of ERAS.7 The PLOS and in-hospital costs, however, were not specifically analyzed.7,41 We included 14 studies in our meta-analysis, demonstrating that implementation of ERAS program following PD can reduce PLOS, DGE, overall morbidity and in-hospital costs without affecting POPF, reoperation, readmission, and mortality rates. The primary outcome and most of the secondary outcomes remained unchanged in the subgroup and sensitivity analyses.
A large number of factors contribute to the timing of discharge of a patient following major surgery relating both to patient recovery and the healthcare environment. Patient morbidity may significantly contribute to PLOS; however, patient baseline characteristics and inclusion criteria for surgery were over all well balanced in the ERAS and CPC groups, indicating ERAS implementation as the main factor affecting PLOS. From a patient perspective, the reduction in PLOS is associated with reduced DGE rates and earlier return to normal nutrition and enteric function, as well as lower levels of pain and quicker return to preoperative levels of mobility resulting in an overall improvement in the postoperative experience. Although pain and mobility levels were not specifically reported by all individual studies, they were included in the measure of overall morbidity, a reduction of which was seen in the ERAS group in this analysis. Factors relating to the healthcare environment can also delay discharge and, amongst others, these include availability of community support and transportation.42 Healthcare systems also function in entirely different cultural and economical environments. In the UK, it is common practice to discharge patients from hospital early and continue care in the community if required through the use of specialist community nursing and therapy staff. In countries including China and India, not all patients have medical insurance or are able to fund convalescent periods in hospital, contributing to a higher heterogeneity observed in our analysis which was reduced when analyzing only studies from Western centers.
There is substantial and ever increasing evidence for optimal practice relating to individual components of operative and perioperative management, both specific to pancreatic resections and surgery in general. The ERAS programs utilize these data to plan idealized care, limiting variability of outcome while maintaining patient-specific and individualized management through the use of treatment protocols, targets/objectives for staff and patients as well as embedding early warning systems to identify problems. Indeed, members of local multidisciplinary teams often receive specific training relating to ERAS components following its introduction, and this may in part explain the identified patient benefits. It is self-evident, therefore, that each specialist unit included in this meta-analysis has developed and implemented their own individual ERAS protocol, which differs slightly in details (e.g., how early the drain or Foley was removed) to that of all others. This is also a likely source for the observed heterogeneity seen in this meta-analysis. Finally, there was heterogeneity in the definitions of the outcome measures. For addressing this issue, we performed a meta-regression analysis to assess the impact of ERAS elements on heterogeneity for PLOS and found that none of the parameters contributed significantly to heterogeneity.
ERAS pathways have previously been shown to reduce healthcare costs in a number of surgical procedures, including pancreatic surgery.5,11,43 PLOS correlates with costs,44 so it was not surprizing that in the 4 studies reported this outcome there was a reduction of in-hospital costs in each center, resulting in overall reduction in in-hospital costs in the EARS group in our meta-analysis. There was a 10-fold difference between the costliest and the cheapest center, with the greatest reductions reported by the centers with the highest costs. Naturally, clinical pathways require resources to develop, implement and maintain,45 but this cost will be offset somewhat by savings generated by the protocols. Nevertheless, the emphasis when developing a service clearly has to remain with improving patient care, and the reductions in morbidity reported in this meta-analysis supports implementation of ERAS protocols following PD on those grounds.
We base our analysis on best, currently available evidence, consisting exclusively of retrospective studies. Clearly, blinded implementation of such an ERAS protocol is impossible, and simultaneous prospective cohorts of patients impractical, which is why only retrospective cohorts have been published to date. Therefore, to a certain extent, selection bias may affect the results of the study. Prospective study designs such as cross-over trials or use of national and international prospective databases are of course possible, and also allow detailed evaluation of individual elements within protocols.46 However, one could argue that it is not necessary to trial a protocol that simply implements a summation of best available evidence into daily practice. As such, we conclude that implementation of a locally agreed, standardized perioperative protocol designed and implemented in the spirit of ERAS can enhance recovery in patients undergoing PD.
In summary, the present study shows that ERAS protocols reduced PLOS, DGE and overall morbidity without adversely affecting rates of POPF, readmission, reoperation, or mortality during PD, thereby reducing healthcare costs. Following ERAS implementation, data should be collected and published, preferably through the use of national or international databases, allowing future analysis of the relative contributions of individual protocol components.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CPC = conventional perioperative care, DGE = delayed gastric emptying, ERAS = enhanced recovery after surgery, ISGPF = International Study Group of Pancreatic Fistula, ISGPS = International Study Group of Pancreatic Surgery, OR = odds ratios, PD = pancreaticoduodenectomy, PLOS = postoperative length of hospital stay, POPF = postoperative pancreatic fistula, PPPD = pylorus-preserving pancreaticoduodenectomy.
Junjie Xiong, Peter Szatmary and Wei Huang contributed equally as co-first authors.
Xubao Liu, Robert Sutton, Michael G. Raraty and Qing Xia designed the research, corrected, and approved the final manuscript; Junjie Xiong and Wei Huang developed the literature search and carried out the statistical analysis of studies; Junjie Xiong, Wei Huang, Peter Szatmary, Daniel de la Iglesia-Garciaand, Weiming Hu and Quentin M. Nunes carried out the extraction of data; Junjie Xiong, Peter Szatmary and Wei Huang wrote the manuscript.
This study was funded by The Science and Technology Support International Program of Sichuan in China (No. 2012HH0029), The Research Special Fund for Public Welfare Industry of Health (No.201202007), and National Institute for Health Research BRU Award, UK/China Postgraduate Scholarships for Excellence and The Royal Colleague of Surgeons of England Fellowship.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002; 183:630–641. [DOI] [PubMed] [Google Scholar]
- 2.Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001; 322:473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005; 24:466–477. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med 2009; 7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber WP, Barry M, Junqueira MJ, et al. Initial experiences with a multidisciplinary approach to decreasing the length of hospital stay for patients undergoing unilateral mastectomy. EJSO 2011; 37:944–949. [DOI] [PubMed] [Google Scholar]
- 6.Khoo CK, Vickery CJ, Forsyth N, et al. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg 2007; 245:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coolsen MM, van Dam RM, van der Wilt AA, et al. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg 2013; 37:1909–1918. [DOI] [PubMed] [Google Scholar]
- 8.Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009; 144:961–969. [DOI] [PubMed] [Google Scholar]
- 9.Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS (R)) Society recommendations. Clin Nutr 2012; 31:817–830. [DOI] [PubMed] [Google Scholar]
- 10.Porter GA, Pisters PW, Mansyur C, et al. Cost and utilization impact of a clinical pathway for patients undergoing pancreaticoduodenectomy. Ann Surg Oncol 2000; 7:484–489. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy EP, Rosato EL, Sauter PK, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution--the first step in multidisciplinary team building. J Am Coll Surg 2007; 204:917–923.discussion 923–914. [DOI] [PubMed] [Google Scholar]
- 12.Vanounou T, Pratt W, Fischer JE, et al. Deviation-based cost modeling: a novel model to evaluate the clinical and economic impact of clinical pathways. J Am Coll Surg 2007; 204:570–579. [DOI] [PubMed] [Google Scholar]
- 13.Balzano G, Zerbi A, Braga M, et al. Fast-track recovery programme after pancreatico- duodenectomy reduces delayed gastric emptying. Br J Surg 2008; 95:1387–1393. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005; 138:8–13. [DOI] [PubMed] [Google Scholar]
- 16.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007; 142:761–768. [DOI] [PubMed] [Google Scholar]
- 17.Xiong JJ, Altaf K, Mukherjee R, et al. Systematic review and meta-analysis of outcomes after intraoperative pancreatic duct stent placement during pancreaticoduodenectomy. Br J Surg 2012; 99:1050–1061. [DOI] [PubMed] [Google Scholar]
- 18.Xiong JJ, Tan CL, Szatmary P, et al. Meta-analysis of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Br J Surg 2014; 101:1196–1208. [DOI] [PubMed] [Google Scholar]
- 19.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73:712–716. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987; 6:341–350. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 23.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001; 323:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu Hilal M, Di Fabio F, Badran A, et al. Implementation of enhanced recovery programme after pancreatoduodenectomy: a single-centre UK pilot study. Pancreatology 2013; 13:58–62. [DOI] [PubMed] [Google Scholar]
- 25.Nikfarjam M, Weinberg L, Low N, et al. A fast track recovery program significantly reduces hospital length of stay following uncomplicated pancreaticoduodenectomy. JOP 2013; 14:63–70. [DOI] [PubMed] [Google Scholar]
- 26.Braga M, Pecorelli N, Ariotti R, et al. Enhanced recovery after surgery pathway in patients undergoing pancreaticoduodenectomy. World J Surg 2014; 38:2960–2966. [DOI] [PubMed] [Google Scholar]
- 27.Coolsen MM, van Dam RM, Chigharoe A, et al. Improving outcome after pancreaticoduodenectomy: experiences with implementing an enhanced recovery after surgery (ERAS) program. Dig Surg 2014; 31:177–184. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Ooshima R, Koizumi S, et al. Perioperative care with fast-track management in patients undergoing pancreaticoduodenectomy. World J Surg 2014; 38:2430–2437. [DOI] [PubMed] [Google Scholar]
- 29.Pillai SA, Palaniappan R, Pichaimuthu A, et al. Feasibility of implementing fast-track surgery in pancreaticoduodenectomy with pancreaticogastrostomy for reconstruction – a prospective cohort study with historical control. Int J Surg 2014; 12:1005–1009. [DOI] [PubMed] [Google Scholar]
- 30.Nussbaum DP, Penne K, Stinnett SS, et al. A standardized care plan is associated with shorter hospital length of stay in patients undergoing pancreaticoduodenectomy. J Surg Res 2015; 193:237–245. [DOI] [PubMed] [Google Scholar]
- 31.Shao Z, Jin G, Ji W, et al. The role of fast-track surgery in pancreaticoduodenectomy: a retrospective cohort study of 635 consecutive resections. Int J Surg 2015; 15:129–133. [DOI] [PubMed] [Google Scholar]
- 32.Yamaki S, Satoi S, Toyokawa H, et al. The clinical role of critical pathway implementation for pancreaticoduodenectomy in 179 patients. J Hepatobiliary Pancreat Sci 2013; 20:271–278. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy EP, Grenda TR, Sauter PK, et al. Implementation of a critical pathway for distal pancreatectomy at an academic institution. J Gastrointest Surg 2009; 13:938–944. [DOI] [PubMed] [Google Scholar]
- 34.Sutcliffe RP, Hamoui M, Isaac J, et al. Implementation of an enhanced recovery pathway after pancreaticoduodenectomy in patients with low drain fluid amylase. World J Surg 2015; 39:2023–2030. [DOI] [PubMed] [Google Scholar]
- 35.Morales Soriano R, Esteve Perez N, Tejada Gavela S, et al. Enhanced recovery after surgery: can we improve the results after pancreatoduodenectomy? Cir Esp 2015; 93:509–515. [DOI] [PubMed] [Google Scholar]
- 36.Williamsson C, Karlsson N, Sturesson C, et al. Impact of a fast-track surgery programme for pancreaticoduodenectomy. Br J Surg 2015; 102:1133–1141. [DOI] [PubMed] [Google Scholar]
- 37.Whipple AO. The rationale of radical surgery for cancer of the pancreas and ampullary region. Ann Surg 1941; 114:612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Wilde RF, Besselink MG, van der Tweel I, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg 2012; 99:404–410. [DOI] [PubMed] [Google Scholar]
- 39.DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 2006; 244:931–937.discussion 937–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 2000; 232:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kagedan DJ, Ahmed M, Devitt KS, et al. Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB 2015; 17:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon TA, Burleyson GP, Tielsch JM, et al. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg 1995; 221:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011; CD007635. [DOI] [PubMed] [Google Scholar]
- 44.Goodney PP, Stukel TA, Lucas FL, et al. Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg 2003; 238:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dy SM, Garg P, Nyberg D, et al. Critical pathway effectiveness: assessing the impact of patient, hospital care, and pathway characteristics using qualitative comparative analysis. Health Serv Res 2005; 40:499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Group EC. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 2015; 261:1153–1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


