Abstract
The aim of the study was to determine the predictive role of breast cancer subtypes in the efficacy and prognosis of neoadjuvant chemotherapy (NCT) regimens combining taxanes and anthracyclines.
Data from 240 patients with breast cancer who received surgery after 4 to 6 weeks of NCT were retrospectively analyzed. The patients were classified into luminal A, luminal B, HER2 overexpression, and triple negative breast cancer (TNBC) as well as low Ki67 (≤ 14%) and high Ki67 (> 14%) expression groups using immunohistochemistry. NCT outcome parameters were pathological complete response (pCR), clinical complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) 4 weeks after surgery. Long-term outcome parameters were disease-free survival (DFS) with a follow-up time of 3 to 56 months.
pCR rates were 1.6%, 13.4%, 22.6%, and 23.8% in patients with luminal A, luminal B, HER2, and TNBC cancers, respectively. High pCR rates correlated with high Ki67 expression (> 40%) (P < 0.001, HR = 0.17, 95% CI: 0.074–0.37) and negative estrogen receptor (ER) status (P < 0.001, HR = 3.74, 95% CI: 1.71–8.12) in a multivariate analysis. However, the DFS rate of luminal A breast cancer was the highest compared to all other groups, but only significantly higher compared to luminal B (P = 0.035, HR = 1.480, 95% CI: 1.060–1.967) patients and correlated with Ki67 expression > 40% (P = 0.005).
Luminal A type patients derived the least benefit from neoadjuvant chemotherapy but had better long-term prognoses. ER status and Ki67 expression served as efficacy predictors for NCT, whereas only Ki67 expression > 40% correlated with long-term treatment outcomes.
INTRODUCTION
Breast cancer accounted for ∼21% of all cancer cases worldwide from 1995 to 2009,1,2 and subsequent studies have noted a rapid increase in the incidence of breast cancer in China,1,3,4 especially among women 20 to 45 years of age. Thus, breast cancer has become one of the most common types of malignancy in Chinese women.3,5 The earliest classification into estrogen and progesterone receptor positive and negative breast cancers has been extended to the human epidermal growth factor receptor 2 (HER2) expressing types. The 2011 and 2013 St. Gallen Consensus Conference added Ki-67 for the determination of proliferation rates to the markers for breast cancer subtype categorization into luminal A, luminal B, as well as triple negative basal-like (ER neg, PgR neg, and HER2 neg) and HER2 overexpressing types.6,7 These breast cancer molecular subtypes have been proposed to serve as risk factor and prognosis indicators, but their role in evaluating risk and prognosis of an individual patient is limited.8,9 In addition, threshold values for defining high and low Ki-67 expression are not clearly defined and vary between laboratories.7,10,11 However, particularly for endocrine therapies, a classification of breast tumors is crucial, so avoiding the unnecessary burden of ineffective therapies for patients with resistant tumors.12 Neoadjuvant chemotherapy (NCT), initially adopted to downgrade inoperable cancers into operable cancers, became a well-accepted treatment option for breast cancer to minimize tumor size13 and to evaluate the efficiency of a regime with respect to micrometastases14 as well as for adjuvant chemotherapy medication adjustments.15 It has been shown that neoadjuvant therapy of breast cancer was equivalent to adjuvant therapy regarding survival and the overall disease progression,16 but it has been suggested that patients reaching pCR after neoadjuvant chemotherapy have favorable outcomes.17 However, whether pCR after neoadjuvant breast cancer therapy can serve as a surrogate endpoint marker for long-term outcomes is still under debate.18 In the present study, we analyzed molecular breast cancer subtypes related pathologically complete response (pCR) and disease-free survival (DFS) outcomes after neoadjuvant chemotherapies in a Chinese cohort of patients in order to identify specific efficacy predictors and thus improve individualized treatment of breast cancer.
PATIENTS AND METHODS
Patients
In a retrospective study, we included 240 female breast cancer patients without metastasis who were admitted to our hospital between January 2009 and January 2014. The median age was 48 years (23–73 years), the clinical stage was II or III, the Eastern Cooperative Oncology Group (ECOG) scores ranged from 0 to 1, and 98% of patients were diagnosed with invasive ductal carcinoma according to pathological examinations after core needle biopsies. The research protocol was approved by the medical ethical committee of the Cancer Hospital of the Chinese Academy of Medical Sciences and informed consent was obtained from all participants.
Medications and Surgery
Neoadjuvant Medications
Chemotherapy doses were epirubicin 75 mg/m2 IV day 1, paclitaxel 175 mg/m2 IV day 2, or docetaxel 75 mg/m2 IV day 2 in 21 days for 1 cycle. A total of 160 patients received epirubicin + paclitaxel and 80 patients received epirubicin + docetaxel.
Adjuvant Medication
Postoperative adjuvant chemotherapies (6–8 cycles) were administered to 166 patients, out of which the regimens changed for 67 patients. Also, 21 HER2-positive patients received postoperative trastuzumab for 1 year and 10 patients were treated with paclitaxel + platinum-based drugs + trastuzumab after operation. However, 170 patients who were positive for the estrogen receptor were treated with adjuvant endocrine therapy.
All patients were operated within 1 month after the end of NCT (2–6 cycles, median 6 cycles, depending on their responses, but in the case of PD after 2 cycles, NCT was discontinued) and the interventions were modified radical mastectomy for 223 and breast-conserving operations for 23 women. Also, 180 patients received postoperative radiotherapy.
Efficacy Evaluation
NCT Outcomes 4 Weeks After Surgery
RECIST version 1.119 was used to assess the treatment response. NCT outcome parameters were a pathologically complete response (pCR), clinical complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), 4 weeks after surgery. pCR was defined as no histological evidence of malignancies or only in situ residuals in breast tissue after surgery, and complete disappearance of lymph node metastasis. CR was defined as disappearance of all known lesions for >4 weeks. PR was defined as at least a 30% decrease in the sum of the largest diameters of target lesions for >4 weeks. PD was defined as at least a 20% increase in the sum of the largest diameters of target lesions or new lesions detected. SD was defined as a reduction in the largest sum diameters of tumors by no more than 30% or an increase of no more than 20% for 4 weeks.
Long-Term Outcomes With a Follow-Up Time of 3 to 56 Months (Median 29 Months)
Disease-free survival (DFS) refers to the time from start of NCTs to the appearance of local recurrence, regional metastasis, second primary cancer, distant metastasis, or death.
Breast Cancer Classification
Breast cancer staging was performed according to the American Joint Committee on Cancer (AJCC) TNM system.20 The tumors were categorized into luminal A and luminal B, as well as HER2 and triple negative types according to Goldhirsch et al6 (Table 1).
TABLE 1.
Molecular Breast Cancer Subtypes Based on Immunohistochemistry
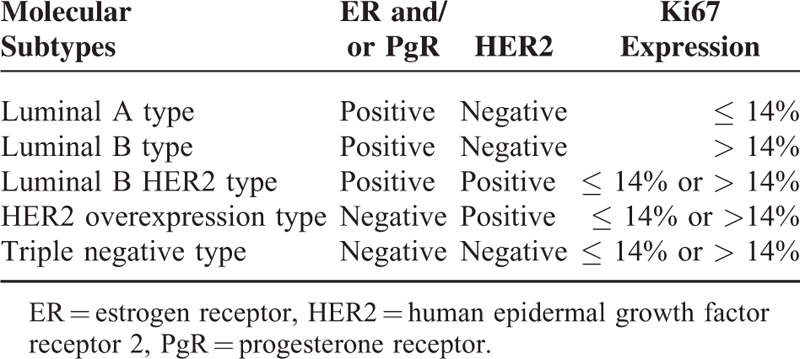
Estrogen receptor (ER) and progesterone receptor (PgR) positivity was conservatively defined to those cells with tumor nuclear staining of > 10%.21,22 A positive HER2 result was defined as a +++ staining of > 30% of invasive tumor cells and a fluorescent in situ hybridization (FISH) result of > 6 HER2 gene copies per nucleus; a negative result was defined as an immunohistochemistry (IHC) staining of 0 or + and a FISH result of < 4 HER2 gene copies per nucleus.23 To identify Ki67-positive tumor cells we used the method described by Bukholm et al.24 Briefly, 10 fields of cell nuclei Ki67-stained cells (pale yellow or brownish yellow) were randomly chosen and 500 cells were counted under each field. Then, the percentages of Ki67-positive cells were calculated. Ki67 ≤ 14% was defined as low expression and Ki67 > 14% as high expression6,25 for the classifications, whereas for pCR and DFS correlation estimates different expression percentages were used for the calculations in order to evaluate the best suitable cut-off value.
Statistical Analysis
SPSS19.0 software (SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.) was used for all calculations. A χ2 test was used to analyze the relationship between molecular subtypes and clinicopathological features and for univariate analysis of clinicopathological indicators and pCR. The Kaplan–Meier method was used for survival analysis and a bivariate logistic regression model for multivariate analysis. A Cox multivariate regression model was used to determine and analyze risk factors effecting prognosis (DFS). P < 0.05 was considered to be statistically significant.
RESULTS
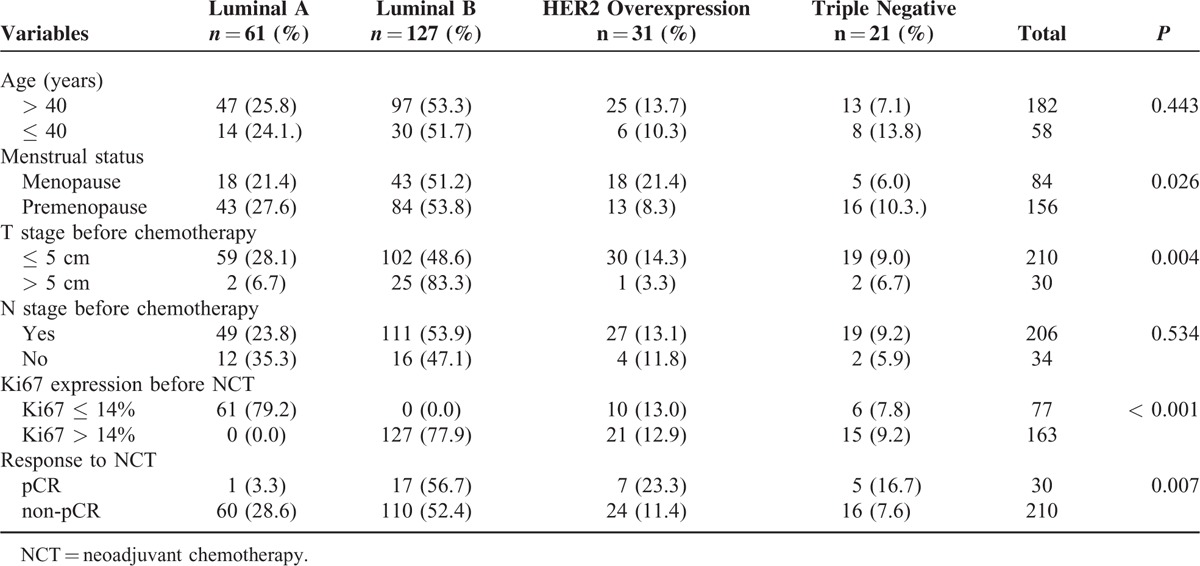
Among the 240 breast cancer patients, there were 61 (25.4%) with luminal A, 127 (52.9%) with luminal B type, 31 (12.6%) with HER2 overexpression, and 21 (8.8%) were triple negative (TN) types. As shown in Table 2, age and N stage before chemotherapy did not correlate with molecular subtypes, whereas menstrual status (P = 0.026), T stage before chemotherapy (P = 0.004), and Ki67 expressions with a cut-off threshold of 14% (P < 0.001) were significantly differently distributed between the molecular subtypes, being the highest in luminal B type cancers. The response rates to neoadjuvant chemotherapies (pCR vs non-pCR) also significantly differed (P = 0.007) between the subgroups (Table 2).
TABLE 2.
Correlation Between Molecular Subtypes and Clinicopathological Features (Case Numbers [%])
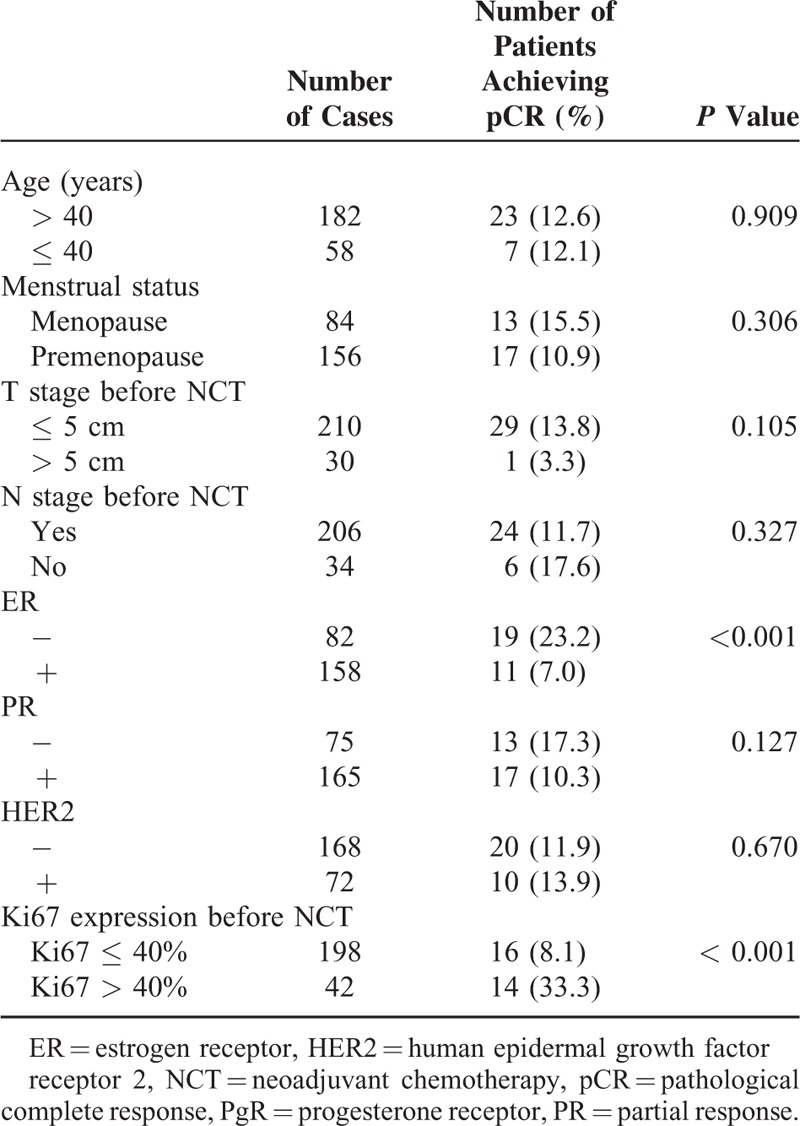
According to the pathological response after using taxanes in combination with anthracyclines, 96.3% (231/240) of patients reached PR + CR + SD, 3.7% (9/240) reached PD, and 30 patients (12.5%) achieved pCR, whereas 53 (22.1%) of patients achieved pCR in breast lesions. According to a univariate analysis of clinicopathological indicators and pCR, ER (P < 0.001) and Ki67 (P < 0.001) statuses correlated significantly with pCR, with the most significant percentage cut-off value for Ki67 expression being 40% (Table 3). pCR rates were higher in ER-negative than in ER-positive patients (23.2% vs 7.0%, P < 0.001) and in patients with Ki67 > 40% compared to those with Ki67 ≤ 40% (33.3% vs 8.1%, P < 0.001). No significant correlation was found between pCR and age, size of breast tumors, PR, menstrual status, and condition of axillary lymph nodes before chemotherapy or HER2 overexpression (Table 3).
TABLE 3.
Univariate Analysis of Clinical Indicators and pCR
Also the results of a bivariate logistic regression analysis of the correlations between pCR and clinical stages, molecular subtypes, ER status and Ki67 index before chemotherapy, showed significant correlations for ER (P = 0.014, HR = 3.341, 95% CI: 1.280–8.724) as well as Ki67 index (Ki67 of 40% as the threshold level, P < 0.001, HR = 0.189, 95% CI: 0.079–0.448) and pCR rates.
Luminal A type patients had the lowest pCR and response rates to chemotherapy, followed by luminal B type patients. The pCR rate was the highest in patients with HER2 expression followed by TNBC patients (Table 4). The 240 patients were followed for 3 to 56 months, with a median follow-up time of 29 months. Until May 2014 as the last follow-up, 26 patients had recurrent and metastatic lesions and 14 patients died. DFS rates were significantly superior (P = 0.035) in patients with luminal A type than in those with luminal B type breast cancer, with a median DFS of 35 and 26 months, respectively (Figure 1).
TABLE 4.
Molecular Subtypes and pCR
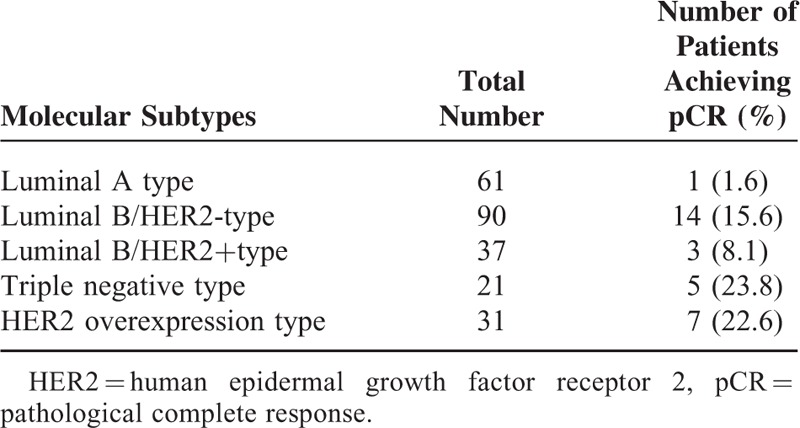
FIGURE 1.
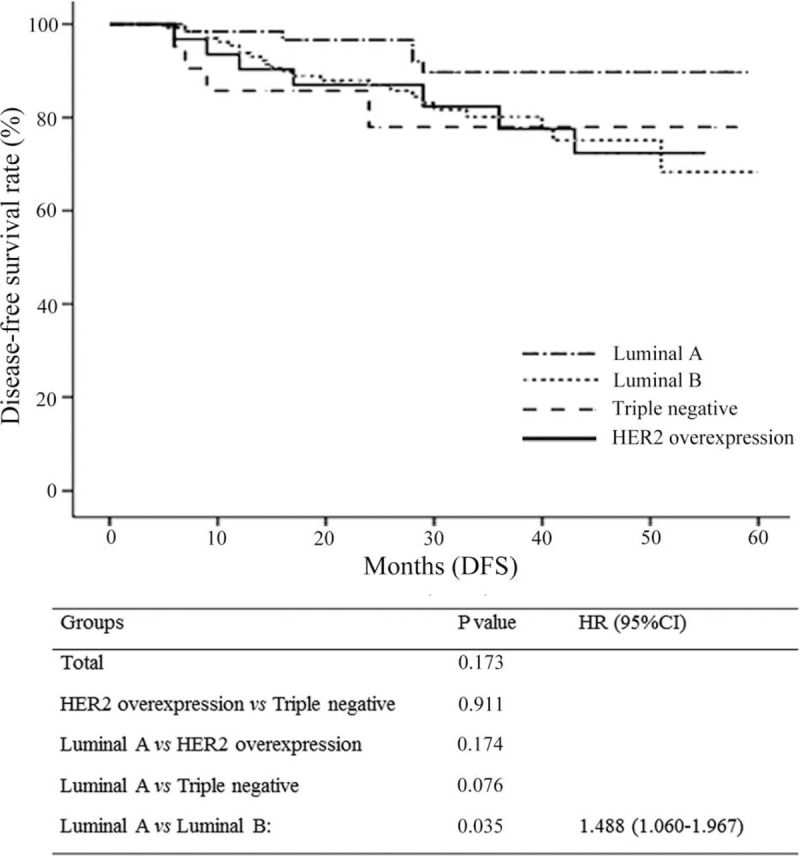
Comparison of disease-free survival rates among the indicated breast cancer groups.
A Cox regression model was used to analyze factors affecting DFS, including age, menstrual status, Ki67 expression, ER, PR, HER2, lymph node status before chemotherapy, and molecular subtypes. Only the Ki67 expression level before chemotherapy was an independent prognostic factor, with a significantly higher DFS rate in patients with a Ki67 expression ≤ 40% compared to those with > 40% before NCT (P = 0.005).
DISCUSSION
In our study, we analyzed the efficacy difference of neoadjuvant chemotherapy regimens in 240 breast cancer patients and found that patients with luminal A (1.6%) and luminal B (13.4%) types had the lowest pCR rates followed by HER2 overexpressing (22.6%) and triple negative (23.8%) types (Table 4). This is in agreement with previous reports, which noted that triple negative and HER2+ subtypes were more sensitive to anthracycline-based neoadjuvant chemotherapies than luminal breast cancers.26,27 Also, others reported that the response to neoadjuvant chemotherapy was significantly higher in patients with endocrine nonresponsive tumors,28 with pCR rates of 24% in hormone receptor (HR)-negative and 8% in HR-positive tumors.29 Patients with luminal B/HER2-subtypes had a higher pCR rate than those with the luminal B/ HER2+ subtypes (15.6% and 8.1%, respectively), which is in accordance with a recent report in which 25% of patients with luminal B/HER2- and only 8% with luminal B/ HER2+ subtypes achieved pCR after neoadjuvant chemotherapies.30 Interestingly, with neoadjuvant medication achieved pCR in luminal B/HER2-, HER2 overexpressing and triple negative subtypes, which showed the highest pCR rates in our study, but not for luminal A and luminal B/HER2+ subtypes, which showed the lowest pCR rates, has been proposed as a surrogate end point for favorable prognosis in a previous meta-analysis.31 On the other hand, a meta-regression analysis of 29 randomized prospective studies revealed that pCR is not a surrogate end point for outcomes in patients with breast cancer.32 In our DFS analysis, luminal A subtype patients had the lowest pCR rates and the best prognosis (Figure 1). Luminal A type breast cancer is the most common and least aggressive type with the lowest mortality rate.33 In addition, luminal A mortality rates were reported to be constant over time with mortality rates of luminal B HER2-positive and nonluminal subtypes tending to peak within 5 years after diagnosis which then declined over time,34 findings also reflected in our data of significantly better DFS rates of luminal A versus luminal B type patients (P = 0.035). The Ki67 index, which indicates the cell proliferation rate, has been the focus of various studies and is recognized as a prognostic predictor for breast cancer.35 Patients with a high expression of Ki67 are more sensitive to chemotherapy, have higher pCR rates, and benefit more from chemotherapy compared to those with a low Ki67 expression.36 In a study conducted by Ohno et al, 477 patients with locally advanced breast cancer were given 4 weeks of neoadjuvant chemotherapy with fluorouracil + epirubicin + cyclophosphamide (FEC) and then randomized into docetaxel + capecitabine (TX) or docetaxel monotherapy (T) groups. They found that the pCR rate was higher in patients with a high Ki67 expression (Ki67 > 10%) than in patients with a low expression (Ki67 ≤ 10%) (12.3% vs 6.5%, P = 0.0004).37 Also in our study, patients with Ki67 > 40% were more sensitive to chemotherapy and had significantly higher pCR rates compared with lower Ki67 ≤ 40% expressing patients (33.3% vs 8.1%, P < 0.001). In addition, Ki67 expression < 40% was a marker for favorable DFS rates, which is in agreement with a previous study in which patients with Ki-67 values > 45 % had reduced DFS.38 A limitation of our study was the relatively short follow-up period of 3 to 56 months (median 29 months), which did not cover the different recurrence patterns of the breast cancer molecular subtypes, and that our study was retrospective.
In conclusion, patients with different types of breast cancer had different responses to NCT regimens. High Ki67 expression and ER status were factors determining neoadjuvant chemotherapy pCR outcomes, whereas only Ki67 expression significantly correlated with DFS rates. However, though luminal A cancer patients had the lowest pCR rate after NCT, they had the highest DFS rate, which was significantly superior to that of luminal B patients. The evaluation of the role of molecular subtype for prognosis of breast cancer NCT regimens needs further long-term studies with more patient-orientated NCT regimens.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CR = clinical complete response, DFS = disease-free survival, ECOG = Eastern Cooperative Oncology Group, ER = estrogen receptor, FEC = fluorouracil + epirubicin + cyclophosphamide, FISH = fluorescent in situ hybridization, HER2 = human epidermal growth factor receptor 2, IHC = immunohistochemistry, NCT = neoadjuvant chemotherapy, pCR = pathological complete response, PD = progressive disease, PgR = progesterone receptor, PR = partial response, RECIST = Response Evaluation Criteria in Solid Tumors, SD = stable disease, T = docetaxel monotherapy, TN = triple negative, TNBC = triple negative breast cancer, TX = docetaxel + capecitabine.
JW and DS equally contributed to this study.
Funding: this study was supported by grants from the National Natural Science Foundation of China (30972934) and the National Natural Science Foundation of China (81000991).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015; 385:977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol 2009; 33:315–318. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Parkin DM, Ferlay J, et al. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev 2005; 14:243–250. [PubMed] [Google Scholar]
- 4.Yang L, Parkin DM, Li LD, et al. Estimation and projection of the national profile of cancer mortality in China: 1991–2005. Br J Cancer 2004; 90:2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linos E, Spanos D, Rosner BA, et al. Effects of reproductive and demographic changes on breast cancer incidence in China: a modeling analysis. J Natl Cancer Inst 2008; 100:1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22:1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013; 24:2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Tamimi RM, Collins LC, et al. The association between vascular endothelial growth factor expression in invasive breast cancer and survival varies with intrinsic subtypes and use of adjuvant systemic therapy: results from the Nurses’ Health Study. Breast Cancer Res Treat 2011; 129:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou W, Jirstrom K, Amini RM, et al. Molecular subtypes in ductal carcinoma in situ of the breast and their relation to prognosis: a population-based cohort study. BMC Cancer 2013; 13:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polley MY, Leung SC, McShane LM, et al. An international Ki67 reproducibility study. J Natl Cancer Inst 2013; 105:1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polley MY, Leung SC, Gao D, et al. An international study to increase concordance in Ki67 scoring. Mod Pathol 2015; 28:778–786. [DOI] [PubMed] [Google Scholar]
- 12.Larionov AA, Miller WR. Challenges in defining predictive markers for response to endocrine therapy in breast cancer. Future Oncol 2009; 5:1415–1428. [DOI] [PubMed] [Google Scholar]
- 13.Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol 2012; 23 Suppl 10:x231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998; 16:2672–2685. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GF, Hortobagyi GN. Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26–28, 2003, Philadelphia, Pennsylvania. Cancer 2004; 100:2512–2532. [DOI] [PubMed] [Google Scholar]
- 16.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 2005; 97:188–194. [DOI] [PubMed] [Google Scholar]
- 17.Kong X, Moran MS, Zhang N, et al. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 2011; 47:2084–2090. [DOI] [PubMed] [Google Scholar]
- 18.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384:164–172. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–247. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 21.Regan MM, Viale G, Mastropasqua MG, et al. Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst 2006; 98:1571–1581. [DOI] [PubMed] [Google Scholar]
- 22.Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol 2008; 26:1059–1065. [DOI] [PubMed] [Google Scholar]
- 23.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007; 25:118–145. [DOI] [PubMed] [Google Scholar]
- 24.Bukholm IR, Bukholm G, Holm R, et al. Association between histology grade, expression of HsMCM2, and cyclin A in human invasive breast carcinomas. J Clin Pathol 2003; 56:368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009; 101:736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007; 13:2329–2334. [DOI] [PubMed] [Google Scholar]
- 27.Salim DK, Mutlu H, Eryilmaz MK, et al. Molecular types and neoadjuvant chemotherapy in patients with breast cancer- while molecular shifting is more common in luminal a tumors, the pathologic complete response is most frequently observed in her-2 like tumors. Asian Pac J Cancer Prev 2014; 15:9379–9383. [DOI] [PubMed] [Google Scholar]
- 28.Colleoni M, Viale G, Zahrieh D, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res 2004; 10:6622–6628. [DOI] [PubMed] [Google Scholar]
- 29.Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol 2006; 24:1037–1044. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Dong X, Li R, et al. Evaluation of the pathological response and prognosis following neoadjuvant chemotherapy in molecular subtypes of breast cancer. Onco Targets Ther 2015; 8:1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30:1796–1804. [DOI] [PubMed] [Google Scholar]
- 32.Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 2014; 32:3883–3891. [DOI] [PubMed] [Google Scholar]
- 33.Haque R, Ahmed SA, Inzhakova G, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev 2012; 21:1848–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 2010; 7:e1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Azambuja E, Cardoso F, de Castro G, Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007; 96:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshioka T, Hosoda M, Yamamoto M, et al. Prognostic significance of pathologic complete response and Ki67 expression after neoadjuvant chemotherapy in breast cancer. Breast Cancer 2015; 22:185–191. [DOI] [PubMed] [Google Scholar]
- 37.Ohno S, Chow LW, Sato N, et al. Randomized trial of preoperative docetaxel with or without capecitabine after 4 cycles of 5-fluorouracil- epirubicin-cyclophosphamide (FEC) in early-stage breast cancer: exploratory analyses identify Ki67 as a predictive biomarker for response to neoadjuvant chemotherapy. Breast Cancer Res Treat 2013; 142:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inwald EC, Klinkhammer-Schalke M, Hofstadter F, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 2013; 139:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]


