Abstract
Increased incidence of upper cervical metastases and higher life expectancy resulted in higher operative rates in patients. The purpose of this study was to explore the methods and the clinical outcomes of palliative surgery for cervical spinal metastases.
A systematic review of a 15-case series of upper cervical metastases treated with palliative surgery was performed. All cases underwent palliative surgery, including anterior tumor resection and internal fixation in 3 cases, posterior tumor resection and internal fixation in 10 cases, and combined anterior and posterior tumor resection and internal fixation in 2 cases. Patients were followed-up clinically and radiologically after the operation, and visual analog scale (VAS) and activities of daily living scores were calculated. In addition, a literature review was performed and patients with upper cervical spine metastases were analyzed.
The mean follow-up period was 12.5 months (range, 3–26 months) in this consecutive case series. The pain was substantially relieved in 93.3% (14/15) of the patients after the operation. The VAS and Japanese Orthopedic Association scores showed improved clinical outcomes, from 7.86 ± 1.72 and 11.13 ± 2.19 preoperatively to 2.13 ± 1.40 and 14.26 ± 3.03 postoperatively, respectively. The mean survival time was 9.5 months (range, 5–26 months). Dural tear occurred in 1 patient. Wound infections, instrumentation failure, and postoperative death were not observed. Among our cases and other cases reported in the literature, 72% of the patients were treated with simple anterior or posterior operation, and only 12% of the patients (3/25) underwent complex combined anterior and posterior operation.
Metastatic upper cervical spine disease is not a rare occurrence. Balancing the perspective of patients on palliative surgery concerning the clinical benefits of operation versus its operative risks can assist the decision for surgery.
INTRODUCTION
Spine is the most common location for cancer metastasis in the skeletal system, and occurs in approximately 33% of the metastatic cancer patients.1 In the skeletal system, most metastases are located in the thoracic and lumbar segments, whereas cervical metastatic cancer only constitutes 8% to 20% of the metastatic spine disease cases,2,3 with less frequent occurrence of upper cervical spine metastases. However, there are over 1.5 million newly diagnosed cancer cases each year, and metastatic lesions within the cervical spine are not uncommon, with an average survival time of 3 to 18 months.4,5 Lung, prostate, and breast carcinomas are the 3 main tumors that metastasize to the spine.6,7
The most common symptom of upper cervical metastasis is localized nonmechanical pain.8,9 The other symptoms include mechanical pain10 and neurologic dysfunction.11,12 Nonmechanical pain occurs in approximately 89% to 93% of patients,8,9,13 does not have any relationship with daily activities, and worsens in the night. In contrast, mechanical pain is exacerbated by instability of the upper cervical spine and relieved by stabilization. Traditionally, surgical intervention is not generally recommended for patients with cervical metastases, because operative outcomes outweigh its benefits.14 During the past decades, instrumentation for the upper cervical spine has undergone tremendous advancements, which has transformed from wire fixation and external immobilization to screw and rod fixation techniques.15 These advances have helped surgeons handle increasingly complicated upper cervical spine conditions, including cervical metastasis.
In the recent years, we have been able to recommend an aggressive surgical intervention for patients with cervical metastases who satisfied the criteria for surgical eligibility. These criteria were an expected survival time of more than 3 months, better function, and prolonged survival after surgery.16–19 The main goals of palliative surgery for cervical spinal metastases are to relieve pain, decompress the spinal cord, restore spinal stability, and achieve a definitive pathological diagnosis. In the present study, we described the cases of patients who underwent cervical palliative surgery for a known painful upper cervical spine metastasis.
PATIENTS AND METHODS
Case Series
A retrospective review of 15 cases was performed at the Union Hospital for Orthopedics. All patients who underwent palliative surgery for upper cervical spine metastasis, C1, C2, or C3, between 2009 and 2015 were included. The inclusion criteria were intensive local or radiating pain caused by the instability in the cervical spine; failure to relieve symptoms by nonsurgical treatment; detection of metastatic lesions in the cervical spine causing paraplegia; severe nerve compression syndrome or compression neuropathy caused by the direct compression of the bone or fractured bone fragments; single metastasis to the upper cervical spine insensitive to chemotherapy or radiotherapy; and an expected survival time longer than 3 months. The exclusion criteria were patients who cannot tolerate surgery; malignant tumor with severe heart, lung, liver, or kidney disease; high fever, infection, or other serious complications; and an extensively widespread metastasis of malignant tumor with a survival period shorter than 3 months. The majority of the patients underwent surgery by single anterior or combined anterior and posterior approach. Patients with isolated cancer of the upper cervical spine were excluded from the study. This series included 9 men and 6 women with an average age of 55.8 years (range, 43–78 years). The primary cancers were lung cancer (7 cases), breast cancer (2 cases), gastrointestinal tract cancer (2 cases), and 1 case per liver, prostate, kidney, and thyroid cancer.
All involved patients presented with neck pain that could not be relieved with conservative treatment for more than 6 weeks. The average preoperative visual analog scale (VAS) score was 7.86 ± 1.72 (range, 4–10).
A thorough history and physical examination is valuable for focusing on the disease at a segmental level and assessing the neurologic deficits. Indications for surgical intervention are spinal instability, intractable pain, and neurologic dysfunction. Written informed consent was obtained from each patient before the operation.
Imaging
Imaging was performed and data were collected. X-ray is the basic imaging technique used for assessment. Computed tomography (CT) and magnetic resonance imaging (MRI) scans could provide important and beneficial supplementary information for diagnosis and evaluation of the bone metastases. CT scans provide more information on the involved bony lesions, the bony anatomy of the vertebra, the course of the vertebral arteries (VAs), and the dimensions of the pedicles. MRI is the gold standard for assessing spinal metastases, and is extremely sensitive and specific for pathological changes in the bone and the surrounding soft tissues.4,20 It can provide detailed information on the contents of the lesion, the surrounding soft tissues, and the spinal cord compression.
Procedures
All patients underwent palliative surgery under general anesthesia, including anterior tumor resection and internal fixation, posterior tumor resection and internal fixation, and combined anterior and posterior tumor resection and internal fixation. For the anterior procedures, a horizontal skin incision was made to expose the lesions, and the destroyed vertebral body and the adjacent intervertebral disc were removed. In cases of complete spinal cord decompression, bone graft fusion with titanium mesh cage and self-locked internal titanium plate fixation were used to achieve anterior column stability. For the posterior procedures, a posterior midline incision was made to expose the posterior structure of the occipitocervical area. After the resection of the lamina, the tumors inside and outside of the spinal canal were removed. The spinal cord, nerve root, and the VA must be carefully preserved during this operation. Either lateral mass or pedicle screws were implanted using the free-hand technique under the guidance of the intraoperative C-arm monitoring system for instrumented fixation and fusion. In patients with apparent instability of the upper cervical spine, skull traction must be performed before anesthesia. In addition, electromyography was recommended for monitoring-evoked potentials and somatosensory-evoked potentials, which could be helpful in the implantation of the instrumentation.21
Assessment Index
Postoperatively, clinical and radiological outcomes were obtained and assessed. Patients were evaluated 24 h after the operation, 2 weeks after discharge, and 1, 3, 6, and 12 months after the operation. The Japanese Orthopedic Association (JOA) score is a widely accepted method in the assessment of neurologic function for various cervical diseases, including clinical signs, subjective symptoms, and restriction of activities of daily living. The VAS score is used for the assessment of pain status before and after the operation. A score of 10 indicates maximal pain and 0 indicates the absence of pain. The clinical follow-up consisted of the JOA and the VAS scores. For the radiological follow-up, X-ray images were obtained every time.
RESULTS
Summary of Cases
All patients underwent palliative surgery for metastasis at the upper cervical spine. Three patients were treated using the single anterior procedure, 2 patients using the combined anterior and posterior procedures, and 10 patients using the posterior procedure. The mean operative time for the 15 cases was 4.2 h (range, 2.5–6 h), and the mean blood loss was 1240 mL (range, 760–2200 mL). Details of the demographic and surgical characteristics of patients are shown in Tables 1 and 2.
TABLE 1.
Demographic and Clinical Characteristics of the Patients (n = 15)
TABLE 2.
Treatment-Related Data
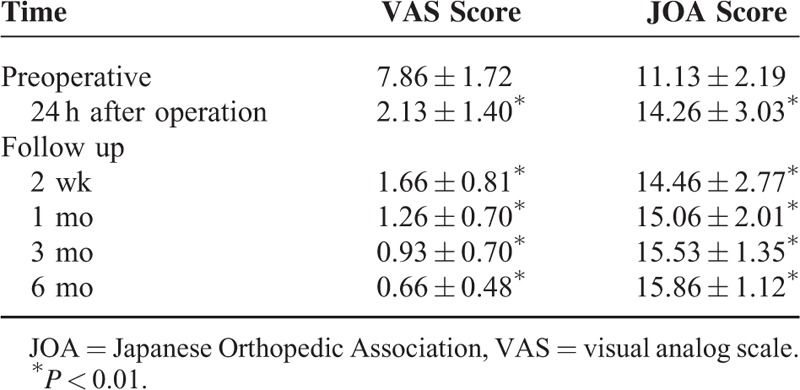
The mean follow-up period was 12.5 months (range, 3–26 months). All the patients presented with neck pain before the operation. This pain was substantially relieved in 93.3% (14/15) of the patients after the operation, mainly attributable to the adopted fixation techniques. Symptoms of myelopathy were presented in 13 (86.7%) patients, improved in 9 (69.2%) of them, and remained unchanged in 4 (30.7%). None of the patients had deteriorating conditions after the surgery. The VAS and JOA scores showed improved clinical outcomes, from 7.86 ± 1.72 and 11.13 ± 2.19 preoperatively to 2.13 ± 1.40 and 14.26 ± 3.03 postoperatively, respectively (Table 3). The VAS scores remained low during the whole follow-up period (Table 3). The mean survival time was 9.5 months (range, 5–26 months).
TABLE 3.
Pre and Postoperative Clinical Outcomes
The incidence rate of complications was consistent with existing literature. An increased risk of the injury of superior laryngeal nerve is associated with upper cervical tumors, which occurred in 2 cases and recovered within 2 to 4 weeks after the operation. Dural tear occurred in 1 patient and was repaired during the operation. Pulmonary infection occurred in 1 patient and was resolved with intravenous antibiotic therapy. Wound infections, instrumentation failure, or postoperative death did not occur. None of the patients required revision surgery during the follow-up.
Illustrative Cases
Case 1
The patient was a 51-year-old woman with neck pain dating back to 3 months before the discovery of the cervical tumor. Four years preceding the discovery of the cervical tumor, the patient was operated for breast cancer. After admission, X-ray, CT, and MRI images were obtained. CT scans indicated extensive osteolytic destruction of the C2 vertebra and the laminae. MRI showed vertebral lesions and a soft tissue mass. The patient underwent posterior tumor resection and internal fixation. The removed tumor was sent for pathological analysis, which confirmed breast cancer (Figure 1).
FIGURE 1.
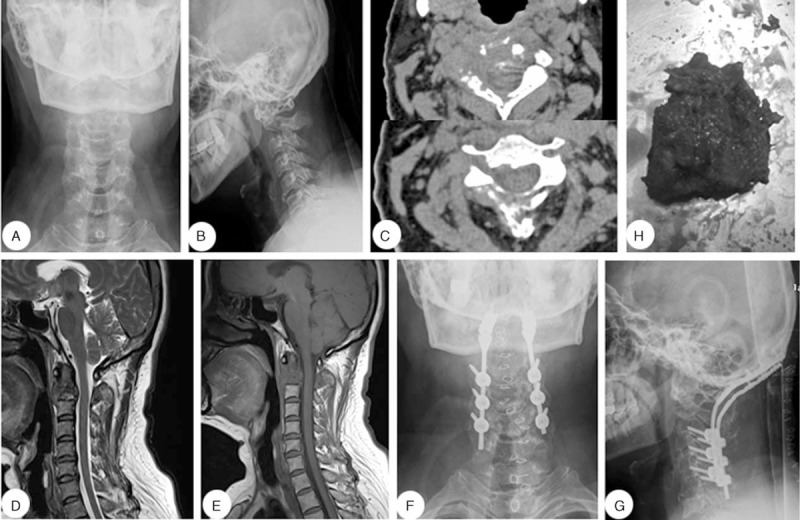
A 51-y-old woman diagnosed with a C2 vertebral metastasis. (A, B) Preoperative lateral and anteroposterior cervical X-rays. (C) Preoperative computed tomography scan. (D, E) Preoperative sagittal T1- and T2-weighted magnetic resonance imaging scans. (F, G) Postoperative lateral and anteroposterior cervical X-ray images.
Case 2
The patient was a 47-year-old man with neck pain dating back to 6 months before the discovery of the cervical tumor. X-ray, CT, and MRI images were obtained for diagnostics. Preoperative CT and MRI showed bone destruction and a soft tissue mass surrounding the C3/4 vertebra. After detailed examination and complete preparation, the patient underwent combined anterior and posterior tumor resection and internal fixation. Pathological analysis of the tumor sample revealed a gastrointestinal tract tumor (Figure 2).
FIGURE 2.
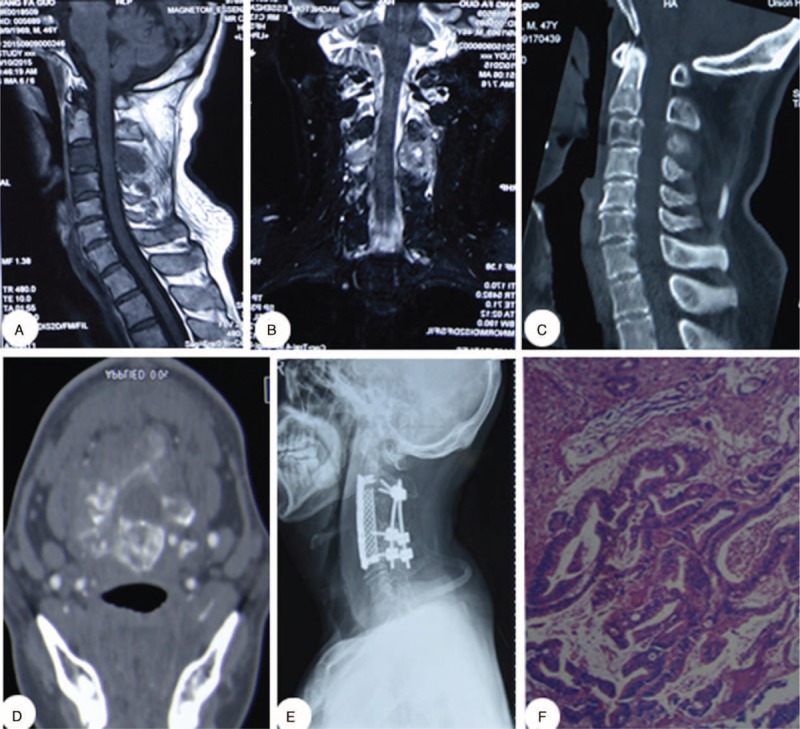
A 47-y-old man diagnosed with a C3/4 vertebral metastasis. (A, B) Preoperative sagittal and coronal magnetic resonance imaging scans. (C, D) Preoperative sagittal and axial computed tomography scans. (E) Postoperative lateral cervical radiographic images. (F) Postoperative pathological examination (hematoxylin and eosin, ×100).
Case 3
The patient was a 43-year-old man with neck pain that lasted for 1 year and aggravated in the last 2 months before the discovery of the cervical tumor. Physical examination revealed weakness and numbness of the left limbs. X-ray, CT, and MRI images were obtained. CT scans showed extensive osteolytic destruction of the C2/3 vertebra. The MRI scan showed vertebral lesions and a soft tissue mass extending from C2 to C3. After complete preparation, the patient underwent posterior tumor resection and posterior internal fixation (Figure 3).
FIGURE 3.
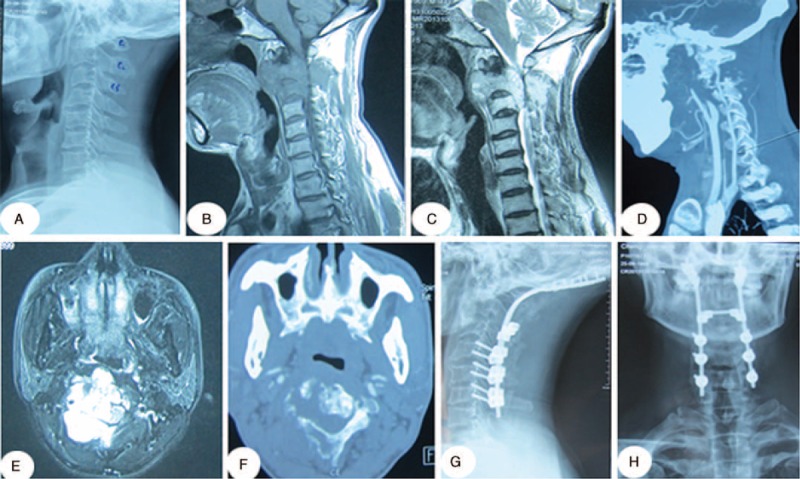
A 43-y-old man diagnosed with a C2/3 vertebral metastasis. (A) Preoperative lateral cervical X-rays. (B, C) Preoperative sagittal T1- and T2-weighted MRI images. (D) Cervical computed tomographic angiography (CTA). (E) Axial MRI scans. (F) Axial computed tomography scans. (G, H) Postoperative lateral and anteroposterior cervical X-ray images. Pathological examination of the tumor sample revealed lung cancer. MRI = magnetic resonance imaging.
Case 4
The patient was a 77-year-old man with a neck pain that lasted for half a year and aggravated in the last 2 weeks before the discovery of the cervical tumor. No neurological deficits were detected upon physical examination. X-ray and CT images were obtained. The CT scans showed extensive osteolytic destruction and collapse of the C3 vertebra. After complete preparation, the patient underwent anterior tumor resection and internal titanium plate fixation (Figure 4).
FIGURE 4.
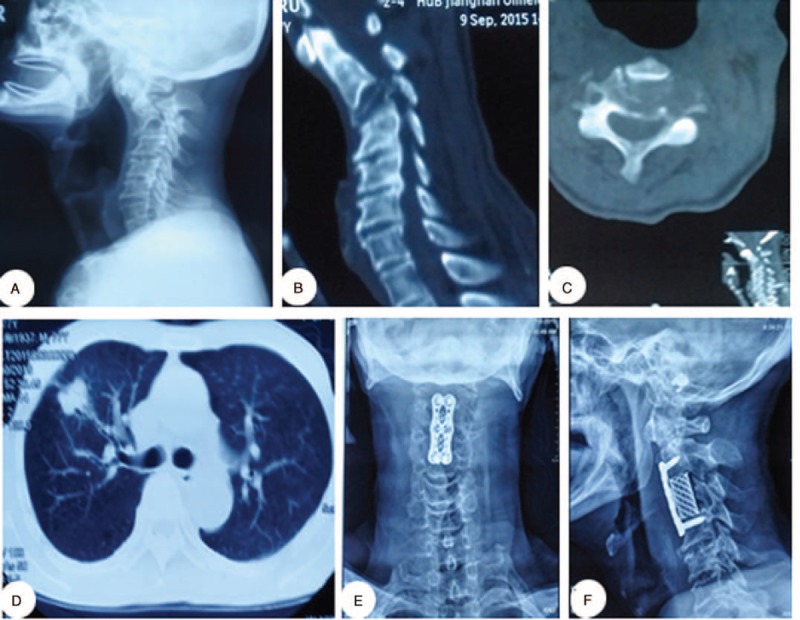
A 77-y-old man diagnosed with a C3 vertebral metastasis. (A) Preoperative lateral cervical radiographic images. (B, C) Preoperative sagittal and axial CT scans. (D) CT scans revealed a lung lesion. (E, F) Postoperative lateral and anteroposterior cervical X-ray images. Pathological examination of the tumor sample revealed lung cancer. CT = computed tomography.
SYSTEMATIC LITERATURE REVIEW
Review Strategy and Study Selection
A review of the literature was performed using MEDLINE, Embase, and OVID to search articles published between January 2005 and October 2015. The search terms included cervical spine, cervical cancer, palliative, metastatic/metastasis, and operation/surgery. The inclusion criteria for the search were papers written in English concerning the patients with upper cervical spine metastases. The exclusion criteria were articles written in a language other than English, which included studies of cases with cervical primary cancer or cases with no extractable specific data.
Summary of the Literature Review
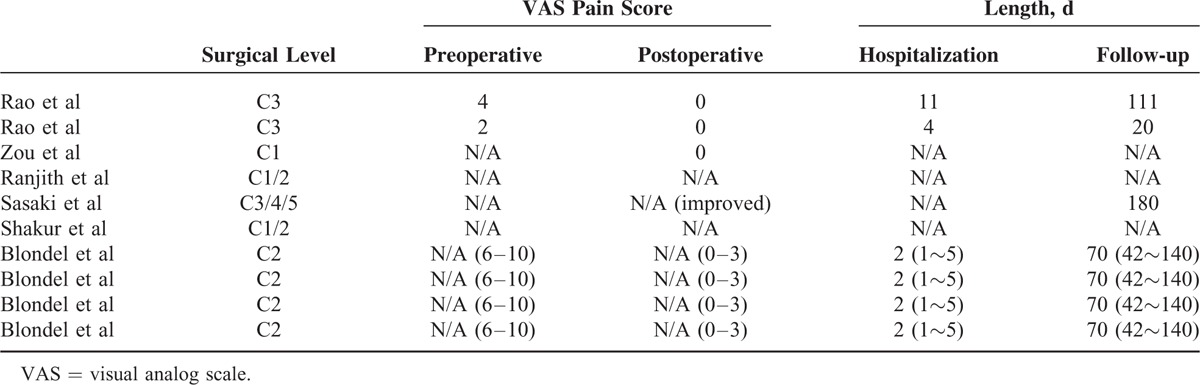
The following data were extracted from the literature according to the inclusion criteria mentioned above. Finally, 6 articles describing 10 cases of metastasis to the upper cervical spine (Tables 4 and 5) were included. One of the patients was treated with combined anterior and posterior tumor resection and internal fixation, 1 patient was treated with only anterior operation, 4 patients were operated using posterior tumor resection and fixation, and the remaining 4 cases were treated with minimally invasive kyphoplasty. Among our cases and the literature reported cases, 56% of the cases (14/25) were treated with simple posterior cervical removal decompression and internal fixation, and 16% of the cases (4/25) were treated with anterior tumor resection and internal titanium plate fixation, both of which constituted 71% of the patients (18/25). In contrast, only 12% of the patients (3/25) were treated with complex combined anterior and posterior surgery. In addition, the other 4 cases were treated invasively with kyphoplasty. Moreover, of the 25 cases, almost all patients displayed significant improvement in pain status after the operation.
TABLE 4.
Comparison the Data From Literature
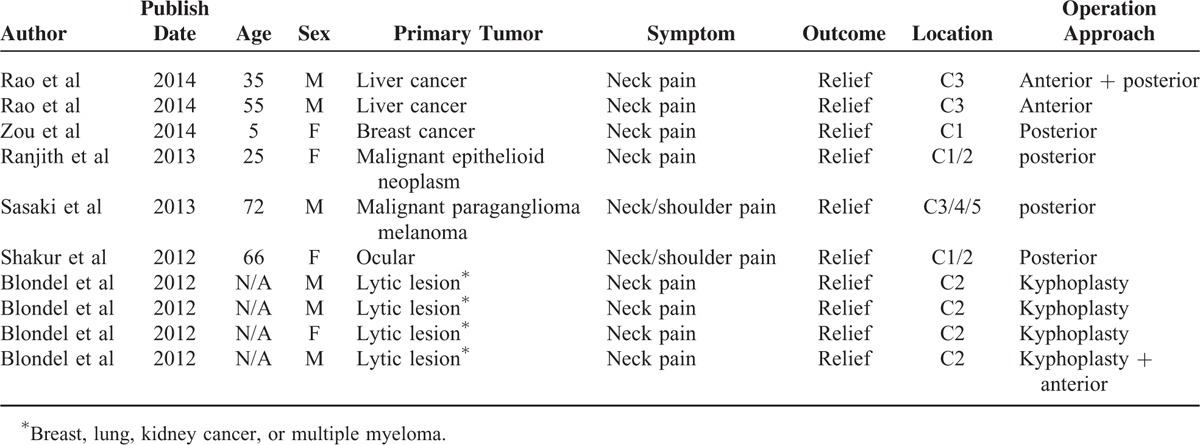
TABLE 5.
Patient Outcomes
DISCUSSION
Our findings showed a significant improvement in our ability to surgically treat patients with painful upper cervical spine metastases. Once an upper cervical spinal metastasis is diagnosed, it is impossible to cure it indefinitely while improving the quality of life. Nevertheless, it should still be treated with palliative surgery to relieve pain, decompress the spinal canal, and stabilize the cervical spine, thereby increasing the life expectancy of the patient while improving the quality of life.
As reported in the literature, only 10% of the patients with cancer will exhibit symptomatic spinal metastasis,22,23 with a 17% prevalence for the cervical spine, compared with the 64% for the lumbar spine.24 However, upper cervical spine metastasis is almost limited to case reports, representing <1% of all the spinal metastases.25,26 The main symptom of the upper cervical metastases is neck pain (90%), followed by neurologic deficits (50%).27–29 The patients presented with nonmechanical neck pain and/or radical pain of the shoulders, especially worsening at night. Along with the performance-related improvements in medical care and prolonged life span, the diagnosis rate for spinal metastases has increased.30
General treatments for cervical spinal metastasis include nonsurgical therapy and surgical treatments. Nonsurgical methods for the treatment of cervical spine metastasis include radiotherapy, chemotherapy, and biotherapy, which were proven to be successful treatments; however, they could not stabilize the unstable cervical spine.31 Therefore, reconstructive surgical intervention is necessary for the treatment of a patient with spinal instability that causes extensive osteolytic lesions. The surgical management of cervical spine tumors is determined by the type of the tumor, the compartment involved, the extent of the lesion, the presence of signs and symptoms, and the patients’ health status. Generally, surgical options include palliative and curative methods. If the disease is a primary tumor, a curative surgical procedure is suggested (i.e., en bloc resection).32 In contrast, metastatic tumors are usually treated with palliative surgeries.33,34 En bloc resection of the vertebra involved and the surrounding structures with negative margins can lead to the achievement of satisfactory clinical outcomes and decreased metastatic recurrence.35–37 At present, en bloc resection is the standard procedure for thoracic and lumbar primary tumors; however, it is difficult to remove cervical tumors using this method, because of the special structures of the VAs and the intricate bony architecture. Radical operation for upper cervical spinal metastasis is usually unsuitable, because the operation risk increased and the patients’ survival rate could not be improved. In addition, because of these factors, as well as longer operative times, high rates of perioperative morbidity, and high risk of postoperative recurrence and metastasis, this procedure is not suitable for the management of upper cervical metastases.
The existing literature on the role of palliative surgery for the management of cervical spine metastasis is limited. Palliative surgical management of upper cervical spinal metastases includes tumor resection and internal fixation, single neurologic decompression, and vertebral body reinforcement. For cervical metastatic cancer, surgical indications are intractable neck pain, progressive neurologic deficits, and spinal instability. The vertebral column is the most frequent location of metastatic involvement and bears nearly 80% of the axial load of the cervical spine; therefore, anterior decompression and stabilization of the cervical spine is a good surgical approach.10 The goals of surgery are to relieve pain and restore neurologic function. Kyphoplasty is a recently developed minimally invasive spine surgery technique, which has been extended to vertebral compression fractures, hemangioma, and osteolytic metastasis of the spine with highly satisfactory results.38–41 According to the literature, highly satisfactory results have been achieved for the diseased vertebra below T5.40–42 In recent years, kyphoplasty has been introduced as a method of treatment for the cervical spine with the advantages of quick pain relief and timely spinal stabilization. In the upper cervical spine, this procedure is challenging for the surgeon to control using the anterolateral, posterolateral, translateral, and direct transoral approaches.43–48 Indications for kyphoplasty include painful vertebral metastases without spinal instability, and spinal cord and spinal nerve root compression. Therefore, the purpose of the operation is to stabilize the cervical spine and relieve intractable pain.
The surgeon must be aware of the risks and benefits of the surgical methods used in the treatment of the upper cervical spine malignancies, and pick the correct surgical technique to avoid complications and optimize clinical outcomes. As our results indicated, only 12% of the patients were treated using the combined anterior and posterior approaches to remove the tumor and reconstruct the cervical spine stability, and 88% of the patients were treated with more invasive procedures. Because of bad treatment effects and limited life expectancy, major operative procedures should be avoided. In conclusion, palliative surgery is valuable for a patient with upper cervical spinal metastasis, to relieve pain, stabilize the cervical spine, ameliorate and restore neurologic function, and control local lesions. Although, the management of upper cervical spine metastases is still a clinical challenge, and there is no doubt that palliative surgery improves life expectancy, in spite of the risks involved.
Footnotes
Abbreviations: ADLa = ctivities of daily living, CT = computed tomography, JOA = Japanese Orthopedic Association, MRI = magnetic resonance imaging, VAs = vertebral arteries, VAS = visual analog pain scale.
This study was supported by grants from the financial support of the National Science Foundation of China (81201393 and 2013YGYL015).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Marchesi DG, Boos N, Aebi M. Surgical treatment of tumors of the cervical spine and first two thoracic vertebrae. J Spinal Disord 1993; 6:489–496. [DOI] [PubMed] [Google Scholar]
- 2.Fehlings MG, David KS, Vialle L, et al. Decision making in the surgical treatment of cervical spine metastases. Spine (Phila Pa 1976) 2009; 34:S108–S117. [DOI] [PubMed] [Google Scholar]
- 3.Brihaye J, Ectors P, Lemort M, et al. The management of spinal epidural metastases. Adv Tech Stand Neurosurg 1988; 16:121–176. [DOI] [PubMed] [Google Scholar]
- 4.Perrin RG, Laxton AW. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am 2004; 15:365–373. [DOI] [PubMed] [Google Scholar]
- 5.Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005; 30:2186–2191. [DOI] [PubMed] [Google Scholar]
- 6.Sundaresan N, Boriani S, Rothman A, et al. Tumors of the osseous spine. J Neurooncol 2004; 69:273–290. [DOI] [PubMed] [Google Scholar]
- 7.Mesfin A, Buchowski JM, Gokaslan ZL, et al. Management of metastatic cervical spine tumors. J Am Acad Orthop Surg. 2015;23:38–46. [DOI] [PubMed] [Google Scholar]
- 8.Mazel C, Balabaud L, Bennis S, et al. Cervical and thoracic spine tumor management: surgical indications, techniques, and outcomes. Orthop Clin North Am 2009; 40:75–92.vi–vii. [DOI] [PubMed] [Google Scholar]
- 9.Jenis LG, Dunn EJ, An HS. Metastatic disease of the cervical spine. A review. Clin Orthop Relat Res 1999; 359:89–103. [DOI] [PubMed] [Google Scholar]
- 10.Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine. 2010;13:94–108. [DOI] [PubMed] [Google Scholar]
- 11.Sciubba DM, Gokaslan ZL. Diagnosis and management of metastatic spine disease. Surg Oncol 2006; 15:141–151. [DOI] [PubMed] [Google Scholar]
- 12.Phillips E, Levine AM. Metastatic lesions of the upper cervical spine. Spine (Phila Pa 1976) 1989; 14:1071–1077. [DOI] [PubMed] [Google Scholar]
- 13.Lesoin F, Rousseaux M, Pellerin P, et al. The anterior retropharyngeal intermaxillohyoid approach and acrylic prosthesis in metastatic lesions of the upper cervical spine (C1–C2). J Chir (Paris) 1986; 123:343–346. [PubMed] [Google Scholar]
- 14.Findlay GF. Adverse effects of the management of malignant spinal cord compression. J Neurol Neurosurg Psychiatry 1984; 47:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bransford RJ, Lee MJ, Reis A. Posterior fixation of the upper cervical spine: contemporary techniques. J Am Acad Orthop Surg. 2011;19:63–71. [DOI] [PubMed] [Google Scholar]
- 16.Meyer SA, Singh H, Jenkins AL. Surgical treatment of metastatic spinal tumors. Mt Sinai J Med. 2010;77:124–129. [DOI] [PubMed] [Google Scholar]
- 17.Eleraky M, Papanastassiou I, Vrionis FD. Management of metastatic spine disease. Curr Opin Support Palliat Care. 2010;4:182–188. [DOI] [PubMed] [Google Scholar]
- 18.Laufer I, Sciubba DM, Mcadera M, et al. Surgical management of metastatic spinal tumors. Cancer Control. 2012;19:122–128. [DOI] [PubMed] [Google Scholar]
- 19.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005; 366:643–648. [DOI] [PubMed] [Google Scholar]
- 20.Khaw FM, Worthy SA, Gibson MJ, et al. The appearance on MRI of vertebrae in acute compression of the spinal cord due to metastases. J Bone Joint Surg Br 1999; 81:830–834. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Hilibrand AS, Lim MR, et al. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine (Phila Pa 1976) 2006; 31:1916–1922. [DOI] [PubMed] [Google Scholar]
- 22.Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer 1997; 80:1614–1627. [DOI] [PubMed] [Google Scholar]
- 23.Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery 1991; 29:645–650. [DOI] [PubMed] [Google Scholar]
- 24.Gokaslan ZL, Aladag MA, Ellerhorst JA. Melanoma metastatic to the spine: a review of 133 cases. Melanoma Res 2000; 10:78–80. [PubMed] [Google Scholar]
- 25.Moulding HD, Bilsky MH. Metastases to the craniovertebral junction. Neurosurgery. 2010;66:113–118. [DOI] [PubMed] [Google Scholar]
- 26.Sherk HH. Lesions of the atlas and axis. Clin Orthop Relat Res 1975; 109:33–41. [DOI] [PubMed] [Google Scholar]
- 27.Greenlee RT, Murray T, Bolden S, et al. Cancer statistics, 2000. CA Cancer J Clin 2000; 50:7–33. [DOI] [PubMed] [Google Scholar]
- 28.Wegener B, Muller PE, Jansson V, et al. Cervical spine metastasis of multiple myeloma: a case report with 16 years of follow-up. Spine (Phila Pa 1976) 2004; 29:E368–E372. [DOI] [PubMed] [Google Scholar]
- 29.Liu JK, Apfelbaum RI, Schmidt MH. Surgical management of cervical spinal metastasis: anterior reconstruction and stabilization techniques. Neurosurg Clin N Am 2004; 15:413–424. [DOI] [PubMed] [Google Scholar]
- 30.Bailar JC, III, Gornik HL. Cancer undefeated. N Engl J Med 1997; 336:1569–1574. [DOI] [PubMed] [Google Scholar]
- 31.Ryken TC, Eichholz KM, Gerszten PC, et al. Evidence-based review of the surgical management of vertebral column metastatic disease. Neurosurg Focus 2003; 15:E11. [DOI] [PubMed] [Google Scholar]
- 32.Cloyd JM, Chou D, Deviren V, et al. En bloc resection of primary tumors of the cervical spine: report of two cases and systematic review of the literature. Spine J 2009; 9:928–935. [DOI] [PubMed] [Google Scholar]
- 33.Chaichana KL, Pendleton C, Wolinsky JP, et al. Vertebral compression fractures in patients presenting with metastatic epidural spinal cord compression. Neurosurgery 2009; 65:267–274.discussion 274–265. [DOI] [PubMed] [Google Scholar]
- 34.Chaichana KL, Pendleton C, Sciubba DM, et al. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. Clinical article. J Neurosurg Spine 2009; 11:56–63. [DOI] [PubMed] [Google Scholar]
- 35.Boriani S, De Iure F, Bandiera S, et al. Chondrosarcoma of the mobile spine: report on 22 cases. Spine (Phila Pa 1976) 2000; 25:804–812. [DOI] [PubMed] [Google Scholar]
- 36.Tomita K, Kawahara N, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci 2006; 11:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talac R, Yaszemski MJ, Currier BL, et al. Relationship between surgical margins and local recurrence in sarcomas of the spine. Clin Orthop Relat Res 2002; 397:127–132. [DOI] [PubMed] [Google Scholar]
- 38.Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987; 33:166–168. [PubMed] [Google Scholar]
- 39.Murphy KJ, Deramond H. Percutaneous vertebroplasty in benign and malignant disease. Neuroimaging Clin N Am 2000; 10:535–545. [PubMed] [Google Scholar]
- 40.Mendel E, Bourekas E, Gerszten P, et al. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine (Phila Pa 1976) 2009; 34:S93–S100. [DOI] [PubMed] [Google Scholar]
- 41.Fourney DR, York JE, Cohen ZR, et al. Management of atlantoaxial metastases with posterior occipitocervical stabilization. J Neurosurg 2003; 98:165–170. [DOI] [PubMed] [Google Scholar]
- 42.Ryu KS, Park CK, Kim MC, et al. Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg 2002; 96:56–61. [DOI] [PubMed] [Google Scholar]
- 43.Blondel B, Adetchessi T, Demakakos J, et al. Anterolateral kyphoplasty in the management of cervical spinal metastasis. Orthop Traumatol Surg Res. 2012;98:341–345. [DOI] [PubMed] [Google Scholar]
- 44.Barragan-Campos HM, Vallee JN, Lo D, et al. Percutaneous vertebroplasty for spinal metastases: complications. Radiology 2006; 238:354–362. [DOI] [PubMed] [Google Scholar]
- 45.Wetzel SG, Martin JB, Somon T, et al. Painful osteolytic metastasis of the atlas: treatment with percutaneous vertebroplasty. Spine (Phila Pa 1976) 2002; 27:E493–E495. [DOI] [PubMed] [Google Scholar]
- 46.Yoon JY, Kim TK, Kim KH. Anterolateral percutaneous vertebroplasty at C2 for lung cancer metastasis and upper cervical facet joint block. Clin J Pain 2008; 24:641–646. [DOI] [PubMed] [Google Scholar]
- 47.Sachs DC, Inamasu J, Mendel EE, et al. Transoral vertebroplasty for renal cell metastasis involving the axis: case report. Spine (Phila Pa 1976) 2006; 31:E925–E928. [DOI] [PubMed] [Google Scholar]
- 48.Anselmetti GC, Manca A, Chiara G, et al. Painful osteolytic metastasis involving the anterior and posterior arches of C1: percutaneous vertebroplasty with local anesthesia. J Vasc Interv Radiol 2009; 20:1645–1647. [DOI] [PubMed] [Google Scholar]


