Abstract
Rheumatic mitral valve stenosis (RMS) is a complication of rheumatic heart disease (RHD) and leads to significant morbidity and mortality. RHD is a chronic inflammatory and autoimmune disease that is associated with cytokine activities. The etiology of RMS is not fully understood yet. Interleukin (IL)-17 and IL-23 have a key role in development of the autoimmunity. The expression of these cytokines in RMS remains unclear. In this study, we investigated the serum levels of IL-17 and IL-23 in RMS patients compared to healthy subjects.
A total of 35 patients admitted to cardiology outpatient clinic between December 2014 and May 2015 who were diagnosed with RMS formed the study group. Age- and gender-matched 35 healthy subjects were included as the control group. Statistical analyses were performed using SPSS 18.0 and P value <0.05 was considered as statistically significant.
The patients with RMS had higher WBC count, hsCRP, systolic pulmonary artery pressure (PAPs), left atrial diameter (LAD), IL-17, and IL-23 levels compared to the control subjects. The levels of IL-17 (P = 0.012) and IL-23 (P = 0.004) were significantly higher in the RMS group. Correlation analysis revealed that IL-17 and IL-23 levels had a significant correlation with each other and with hsCRP and LAD.
We demonstrated that serum levels of IL-17 and IL-23 are significantly higher in patients with RMS compared to those of healthy subjects. IL-17 and IL-23 expression may have a possible role in inflammatory processes that result in RMS development.
INTRODUCTION
Rheumatic heart disease (RHD) is a major cause of cardiovascular death in children and young adults in developing countries.1 RHD is the most serious complication of rheumatic fever which can lead to chronic valvular lesions. Rheumatic mitral valve stenosis (RMS) is the main presentation of RHD that leads to significant morbidity and mortality.2 Mitral stenosis usually develops as a result of persistent or recurrent valvulitis with bicommissural fusion.3 The etiology of RHD is not fully understood yet. Studies have shown that RHD is mediated by humoral and cellular autoimmune responses that occur as a consequence of long-term sequelae of Group A Streptococcus (GAS) infection.4,5 Previous studies suggest that RHD is an autoimmune disease 6,7 that is associated with cytokine activities.8 Inflammatory cytokines are key regulators in immune processes.9
It has been shown that interleukin (IL)-17 and IL-23 are potent proinflammatory molecules 10 that have important effects in mediating chronic inflammation, and in development of autoimmune diseases including multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus, asthma, psoriasis, and many other autoimmune diseases.11–13 Neutralization of these cytokines is expected to be a potent therapeutic strategy in some autoimmune diseases.10 Higher levels of IL-17 and IL-23 expression has been shown in a number of autoimmune disorders.13 However, the expression of these cytokines in RHD remains unclear. In this study, we aimed to explore serum levels of IL-17 and IL-23 in RMS patients and compare with those of healthy subjects.
METHODS
Study Population
A total of 35 patients admitted to cardiology outpatient clinic between December 2014 and May 2015 who were diagnosed with RMS formed the study group. None of the patients had experienced rheumatic fever (RF) attack within last 12 months. Thirty of 35 RMS patients were on regular benzathine penicilin-G (BP-G) treatment for acute RF prophylaxis. One patient had a penicillin allergy history and 4 were not on prophylaxis regimen because of patient-incompliance. Age- and gender-matched 35 healthy subjects were included as the control group. Patients with heart failure, autoimmune disorders (such as systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, asthma, or psoriasis), hematologic or rheumatologic disorders, hypertension, diabetes mellitus, acute infection, chronic inflammatory disorders, coronary artery disease, pregnancy, hypersensitivity, pulmonary, renal or hepatic diseases and malignancies were excluded from the study. All patients gave informed written consent before enrollment to the study.
Serum Collection
Blood samples were collected in citric acid containing tubes and stored at −80 °C after centrifugation. The serum IL-17 and IL-23 levels were measured by using the ELISA kits with respect of the instructions provided by the manufacturer (Sunredbio, Shanghai, PRC).
Transthoracic Echocardiography
Transthoracic echocardiography was performed in all patients with a commercially available ultrasound system (GE Vivid S5, Vingmed System Five, Horton, Norway) according to the recommendations of the American Society of Echocardiography.14
The mitral valve area was measured with both the planimetric and the pressure half time (PHT) methods, and the transmitral gradients were measured with a continuous wave (CW) Doppler in apical 4-chamber view. Following echocardiographic criteria were used to establish the diagnosis of rheumatic mitral stenosis:15 mitral valve area ≤ 2 cm2, the presence of commissural fusion, leaflet thickening, and alteration of the subvalvular apparatus. Mitral valve anatomy was assessed with the Wilkins score.16
Statistical Analysis
Continuous variables are presented as mean ± standard deviation or median and interquartile ranges, whereas categorical variables are given as number and percentages. The Kolmogorov–Smirnov test was used to verify the normality of the distribution of continuous variables. The independent sample t test or the Mann–Whitney U test was used for the continuous variables, and the chi-square test was used for categorical variables. Correlation analysis was assessed with the Spearman rank test. Receiver-operating characteristic (ROC) curve analysis was used to determine the optimum cutoff levels of IL-17 and IL-23 that would predict the presence of RMS, and results are shown as odds ratios with 95% confidence intervals (CIs). Statistical analyses were performed using SPSS 18.0 (SPSS Inc, Chicago, IL). P value <0.05 was considered as statistically significant.
RESULTS
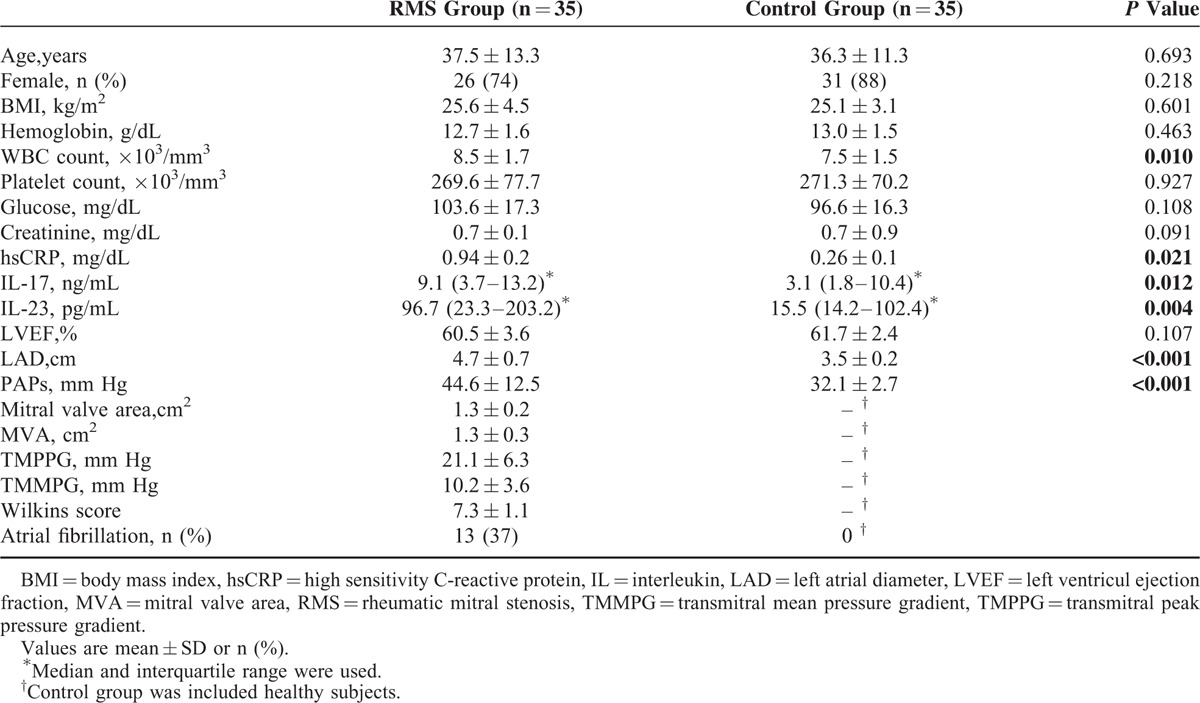
There were no differences between groups in terms of age, gender, body mass index (BMI), left ventricular ejection fraction (LVEF), creatinine, hemoglobin, platelet count, and glucose measures (Table 1). The patients with RMS had higher WBC count, high sensitivity C-reactive protein (hsCRP), systolic pulmonary artery pressure (PAPs), left atrial diameter (LAD), IL-17 and IL-23 levels compared to the healthy controls (Table 1).
TABLE 1.
Baseline Demographic, Clinical, and Laboratory Characteristics of the Study Groups
IL-17 was 9.1 ng/mL (3.7–13.2 ng/mL) in the RMS group and 3.1 ng/mL (1.8–10.4 ng/mL) in the control group (P = 0.012) (Figure 1). IL-23 was 96.7 pg/mL (23.3–203.2 pg/mL) in the RMS group and 15.5 pg/mL (14.2–102.4 pg/mL) in the control group (P = 0.004) (Figure 2).
FIGURE 1.
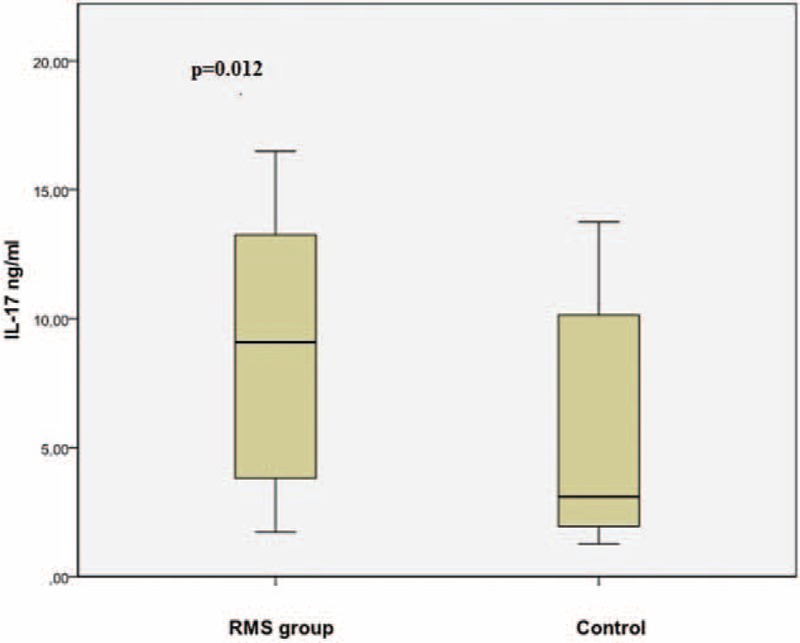
The comparison of serum IL-17 levels between RMS and control group. RMS = rheumatic mitral stenosis.
FIGURE 2.
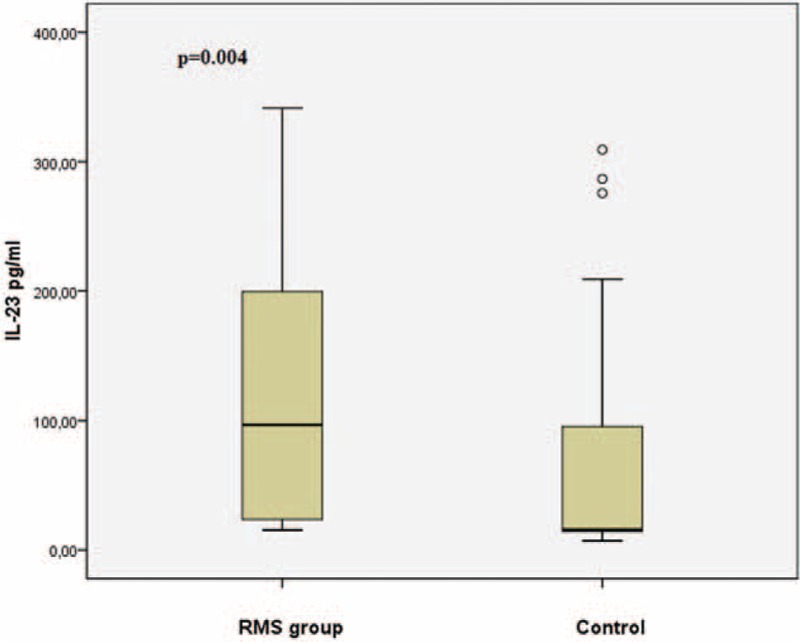
The boxplot graph showing the comparison of serum IL-23 levels between RMS and control group. RMS = rheumatic mitral stenosis.
Of the 35 patients with RMS, mean MVA was 1.3 ± 0.3 cm2, transmitral peak gradient was 21.1 ± 6.3 mm Hg, transmitral mean gradient was 10.24 ± 3.62 mm Hg, left atrial diameter was 4.7 ± 0.7 cm, systolic pulmonary arterial pressure was 44.6 ± 12.5 mm Hg. Mean of the Wilkins score was 7.3 ± 1.1 in the study group. Atrial fibrillation was present in 13 patients.
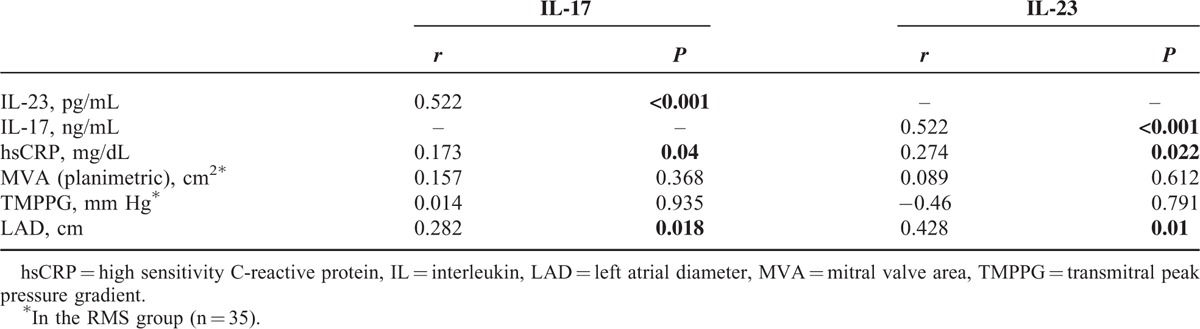
In the ROC curve analysis, a cut-off value of 3.66 ng/mL for the serum IL-17 level predicted the presence of RMS with a sensitivity of 77% and specificity of 64% (ROC area under curve: 0.723, 95% CI: 0.59–0.85; Figure 3). A cut-off value of 20.3 pg/mL, for serum IL-23 level predicted the presence of RMS with a sensitivity of 86% and specificity of 65% (ROC area under curve: 0.762, 95% CI: 0.63–0.88; Figure 3). Correlation analysis revealed that IL-17 and IL-23 had a significant correlation with hsCRP (r = 0.173, P = 0.04 and r = 0.274, P = 0.022, respectively) and LAD (r = 0.282, P = 0.018 and r = 0.428, P = 0.01 respectively). Additionally, IL-17 and IL-23 were correlated with each other (r = 0.522, P < 0.01) (Table 2).
FIGURE 3.
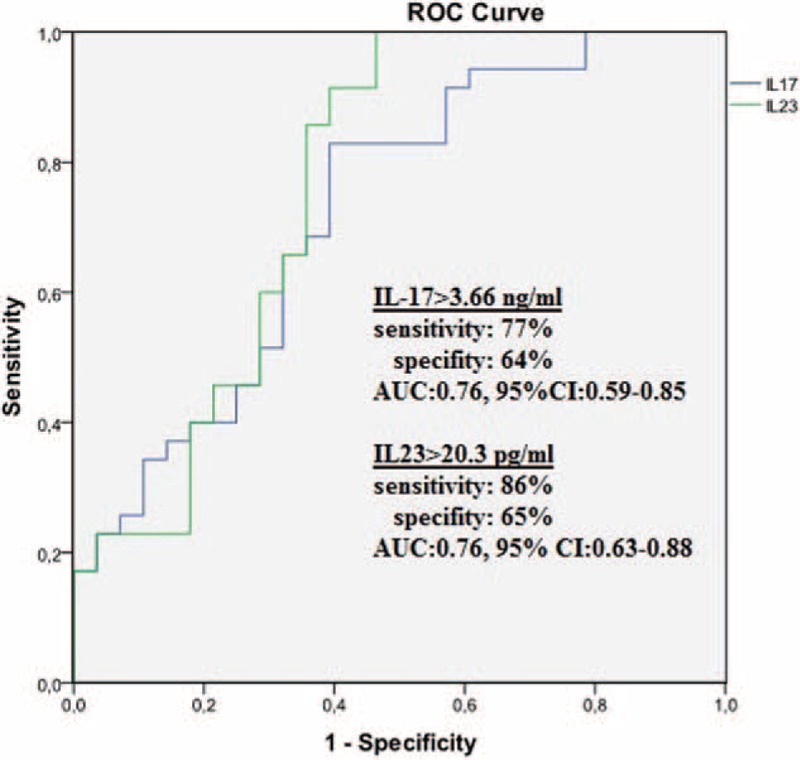
The receiver-operating characteristic (ROC) curve analysis of IL-17 and IL-23 for predicting mitral stenosis. AUC = area under curve, CI = confidence interval.
TABLE 2.
Correlation Analysis for IL-17 and IL-23
DISCUSSION
In this study, we aimed to compare the serum levels of IL-17 and IL-23 between patients with RMS and healthy subjects. According to our results, serum IL-17 and IL-23 levels were significantly higher in patients with RMS compared to healthy controls. Also, the serum hsCRP level was significantly higher in the patient group. Correlation analysis revealed that IL-17 and IL-23 levels had a significant correlation with hsCRP and LAD. Additionally, IL-17 and IL-23 were significantly correlated with each other.
Rheumatic heart disease is the most serious complication of RF and lead to chronic valvular lesions. Rheumatic mitral valve stenosis is a complication of RHD that leads to significant morbidity and mortality.2 RHD still remains an important health burden in many developing countries.17
The role of chronic inflammation in RMS has been shown in previous reports. There is a persistent inflammation that causes the damage of the valvular tissue.8 There are many studies that investigated the markers of chronic inflammation in RHD patients. Golbasi et al have reported higher serum level of hsCRP in patients with chronic rheumatic valvular disease compared to healthy participants.18 In another study that were conducted on patients with RMS, hsCRP levels were significantly higher in patients with RMS, and hsCRP values were correlated with the Wilkins score.19
Additionally, in a study by Polat et al reported increased serum levels of hsCRP and Pentraxin-3 as markers of inflammation levels in RMS patients compared to healthy subjects20 and Pentraxin-3 was significantly correlated with severity of mitral valve stenosis. Our results were compatible with aforementioned studies as the serum hsCRP levels were significantly higher in our RMS patients compared to healthy subjects. Additionally, we found that IL-17 and IL-23 were significantly correlated with hsCRP.
In another study,21 the neutrophyl-to-lymphocyte ratio (NLR) as a recently emerged marker of inflammation was significantly higher in patients with severe RMS when compared to patients with mild to moderate RMS. It was concluded that the NLR may be useful in predicting the presence and severity of RMS.
RMS is a complex disease. Chronic inflammation and the autoimmune reactions constitute the main mechanisms of the pathogenesis. Molecular mimicry, between antigens of the host and GAS, has been thought to be the triggering factor leading to the disease. Both T-cells and crossreactive antibodies have important roles in the cross-recognition between streptococcal antigens and human proteins leading to inflammation and autoimmunity.22 In a rat study, Lymbury et al had immunized the rats with pooled synthetic peptides from the conserved C-region of the GAS M5 protein. They demonstrated that there were inflammatory lesions in myocardium and valve tissue by histological examination of cardiac tissue obtained from immunized rats. They suggested that the results were indicated a role for GAS M protein-specific autoreactive T cells in the development of cardiac lesions.23
An inflammatory cascade mediates the development of heart lesions with overexpression of a number of inflammatory cytokines including IL-17 and IL-23. Autoreactive CD4 + T cells infiltrate the heart tissue and trigger autoimmune reactions through molecular mimicry.24
Although the etiology of RHD is not completely understood, studies have shown that RHD is mediated by humoral and cellular autoimmune responses4 and associated with cytokine activities.8 T helper 17 (Th17) cells have been recently identified preferential producers of IL-17, IL-17A, IL-17F, IL-21, and IL-22. IL-23 is responsible for the differentiation of Th17 cells from naive CD4 + T cells. IL-17 is a proinflammatory cytokine that is produced by activated T-cells. IL-17 production is increased in response to IL-23 stimulation.25 In a study by Bas et al reported that the percentage of peripheral blood Th17 cells and the Th17/regulatuar T cell (Treg) ratio were increased significantly in RHD patients. Th17 and Treg cells play opposite roles in the immune tolerance and autoimmune diseases.26 Although Th17 cells and related cytokines have an important effects in defending against various infections, especially extracellular bacterial infections, they have a key role in mediating chronic inflammation and in the development of autoimmune diseases.11,27
Previous studies have indicated that Th17 cells and associated cytokines participate in the pathogenesis of various autoimmune disorders such as multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus, and asthma. The overexpression of Th17-associated cytokines has been shown in some autoimmune diseases.13 However, the expression of these cytokines in RMS has not been studied yet. Therefore, in this study, we evaluated serum IL-17 and IL-23 levels in patients with RMS.
The relation between interleukins and chronic inflammation is well known, as well as RHD development. Davutoglu et al have reported that the patients with RMS had increased plasma levels of IL-6, IL-8, IL-2, tumor necrosis factor-alpha (TNFα), and hsCRP as indicators of ongoing inflammation compared with the healthy subjects.28
Additionally in a rat study Wen et al have reported that the expression of IL-17, IL-21, IL-6, and IL-23 were significantly increased in mitral valve tissues in rats with RHD compared with normal group and the serum IL-17 and IL-6 concentrations were significantly higher in RHD rats.13 They thought that the expression of Th17 cell-associated cytokines is not induced by acute infection of GAS. However, cytokines are overexpressed in the chronic stable stage of RHD, so the autoimmune injury induced by Th17 cells was thought to be a key factor.
The actions of IL-17 and IL-23 in autoimmune diseases make them important therapeutic targets in autoimmune disorders.10 Previous studies have shown that blocking TNFα, IL-6, IL-23, IL-17, or their corresponding receptors by using the neutralizing antibodies is highly effective in the treatment of some autoimmune diseases such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease.9,27 Additionally, new antibody drugs targeting IL-17A, IL-17RA, IL-17F, IL-17A/TNF, IL-23 are being tested in psoriasis, psoriatic arthritis, ankylosing spondylitis, rheumatoid arthritis, autoimmune uveitis, asthma, and multiple sclerosis. The antibody drugs that neutralize IL-23 or IL-17A have shown a significant efficacy in the treatment of psoriasis. These agents also show hopeful results in ankylosing spondylitis and multiple sclerosis.27
There is yet no specific treatment to prevent the progression of RHD. Primary prevention of acute RF consists of early diagnosis and treatment of GAS tonsillopharyngitis with penicillin.29 In patients with a prior episode of RF, secondary prevention is critically important. Recurrent pharyngeal GAS infection can trigger a severely exaggerated immune response in these patients and the recurrent RF is associated with a higher incidence of carditis.30 Secondary prevention with intramuscular injection of BP-G every 3 to 4 weeks is stil recommended,31 but the efficacy of secondary prevention is limited in prevention of RHD progression; however, the compliance of patients with BP-G treatment is relatively low.32 For this reason, new strategies and therapies are needed to prevent the relapse of acute RF and the progression of RHD.
Neutralizing inflammatory cytokines or antagonizing their receptor function has been considered as a useful therapeutic strategy to treat autoimmune diseases. In this respect, new therapies targeting IL-17 and IL-23 and their reseptors as studied in some autoimmune diseases may promise a new approach for patients with RHD.
To our best knowledge, this is the first study to evaluate serum levels of IL-17 and IL-23 in patients with RMS.
Study Limitations
Relatively small sample size was the main limitation of our study. Another limitation was the absence of other associated cytokines. The findings cannot be generalized to overall population. These results need to be confirmed by multicentre studies with larger sample sizes.
CONCLUSION
We demonstrated that serum levels of IL-17 and IL-23 are significantly higher in patients with RMS compared to those of healthy subjects. IL-17 and IL-23 expression may have a possible role in inflammatory processes that result in RMS development. Further large-scale studies are required to confirm our results.
Footnotes
Abbreviations: BMI = body mass index, BP-G = benzathine penicilin-G, GAS = group A streptococcus, hsCRP = high sensitivity C-reactive protein, IL = interleukin, LAD = left atrial diameter, LVEF = left ventricul ejection fraction, MVA = mitral valve area, RF = rheumatic fever, RHD = rheumatic heart disease, RMS = rheumatic mitral stenosis, TMMPG = transmitral mean pressure gradient, TMPPG = transmitral peak pressure gradient.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005; 5:685–694. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012; 42:S1–44. [DOI] [PubMed] [Google Scholar]
- 3.Marcus RH, Sareli P, Pocock WA, et al. The spectrum of severe rheumatic mitral valve disease in a developing country. Correlations among clinical presentation, surgical pathologic findings, and hemodynamic sequelae. Ann Intern Med 1994; 120:177–183. [DOI] [PubMed] [Google Scholar]
- 4.Guilherme L, Kalil J. Rheumatic fever: from sore throat to autoimmune heart lesions. Int Arch Allergy Immunol 2004; 134:56–64. [DOI] [PubMed] [Google Scholar]
- 5.Rheumatic fever and rheumatic heart disease. World Health Organ Tech Rep Ser 2004; 923:1–122. [PubMed] [Google Scholar]
- 6.Perricone C, Rinkevich S, Blank M, et al. The autoimmune side of rheumatic fever. Isr Med Assoc J 2014; 16:654–655. [PubMed] [Google Scholar]
- 7.Guilherme L, Kalil J. Rheumatic heart disease: molecules involved in valve tissue inflammation leading to the autoimmune process and anti-S. pyogenes vaccine. Front Immunol 2013; 4:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilherme L, Cury P, Demarchi LM, et al. Rheumatic heart disease: proinflammatory cytokines play a role in the progression and maintenance of valvular lesions. Am J Pathol 2004; 165:1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai Y, Dong C. Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases. Int Immunol 2015; pii:dxv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherlock JP, Taylor PC, Buckley CD. The biology of IL-23 and IL-17 and their therapeutic targeting in rheumatic diseases. Curr Opin Rheumatol 2015; 27:71–75. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008; 28:454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen Y, Zeng Z, Gui C, et al. Changes in the expression of Th17 cell-associated cytokines in the development of rheumatic heart disease. Cardiovasc Pathol 2015; 24:382–387. [DOI] [PubMed] [Google Scholar]
- 14.Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002; 15:167–184. [DOI] [PubMed] [Google Scholar]
- 15.Horstkotte D, Niehues R, Strauer BE. Pathomorphological aspects, aetiology and natural history of acquired mitral valve stenosis. Eur Heart J 1991; 12 (Suppl B):55–60. [DOI] [PubMed] [Google Scholar]
- 16.Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988; 60:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralph AP, Carapetis JR. Group a streptococcal diseases and their global burden. Curr Top Microbiol Immunol 2013; 368:1–27. [DOI] [PubMed] [Google Scholar]
- 18.Golbasi Z, Ucar O, Keles T, et al. Increased levels of high sensitive C-reactive protein in patients with chronic rheumatic valve disease: evidence of ongoing inflammation. Eur J Heart Fail 2002; 4:593–595. [DOI] [PubMed] [Google Scholar]
- 19.Alyan O, Metin F, Kacmaz F, et al. High levels of high sensitivity C-reactive protein predict the progression of chronic rheumatic mitral stenosis. J Thromb Thrombolysis 2009; 28:63–69. [DOI] [PubMed] [Google Scholar]
- 20.Polat N, Yildiz A, Alan S, et al. Association of pentraxin-3 with the severity of rheumatic mitral valve stenosis. Acta Cardiol 2015; 70:409–413. [DOI] [PubMed] [Google Scholar]
- 21.Polat N, Yildiz A, Yuksel M, et al. Association of neutrophil-lymphocyte ratio with the presence and severity of rheumatic mitral valve stenosis. Clin Appl Thromb Hemost 2014; 20:793–798. [DOI] [PubMed] [Google Scholar]
- 22.Guilherme L, Fae K, Oshiro SE, et al. Molecular pathogenesis of rheumatic fever and rheumatic heart disease. Expert Rev Mol Med 2005; 7:1–15. [DOI] [PubMed] [Google Scholar]
- 23.Lymbury RS, Olive C, Powell KA, et al. Induction of autoimmune valvulitis in Lewis rats following immunization with peptides from the conserved region of group A streptococcal M protein. J Autoimmun 2003; 20:211–217. [DOI] [PubMed] [Google Scholar]
- 24.Guilherme L, Kohler KF, Postol E, et al. Genes, autoimmunity and pathogenesis of rheumatic heart disease. Ann Pediatr Cardiol 2011; 4:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal S, Ghilardi N, Xie MH, et al. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 2003; 278:1910–1914. [DOI] [PubMed] [Google Scholar]
- 26.Bas HD, Baser K, Yavuz E, et al. A shift in the balance of regulatory T and T helper 17 cells in rheumatic heart disease. J Investig Med 2014; 62:78–83. [DOI] [PubMed] [Google Scholar]
- 27.Gaffen SL, Jain R, Garg AV, et al. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014; 14:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davutoglu V, Celik A, Aksoy M. Contribution of selected serum inflammatory mediators to the progression of chronic rheumatic valve disease, subsequent valve calcification and NYHA functional class. J Heart Valve Dis 2005; 14:251–256. [PubMed] [Google Scholar]
- 29.Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation 2009; 119:1541–1551. [DOI] [PubMed] [Google Scholar]
- 30.Rayamajhi A, Sharma D, Shakya U. First-episode versus recurrent acute rheumatic fever: is it different? Pediatr Int 2009; 51:269–275. [DOI] [PubMed] [Google Scholar]
- 31.Remenyi B, Carapetis J, Wyber R, et al. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol 2013; 10:284–292. [DOI] [PubMed] [Google Scholar]
- 32.Marijon E, Mirabel M, Celermajer DS, et al. Rheumatic heart disease. Lancet 2012; 379:953–964. [DOI] [PubMed] [Google Scholar]


