Abstract
Angiotensin II receptor blockers (ARB) are widely used drugs that are proven to reduce cardiovascular disease events; however, several recent meta-analyses yielded conflicting conclusions regarding the relationship between ARB and cancer incidence, especially when ARB are combined with angiotensin-converting enzyme inhibitors (ACEI).
We investigated the risk of cancer associated with ARB at different background ACEI levels.
Search of PubMed and EMBASE (1966 to December 17, 2015) without language restriction.
Randomized, controlled trials (RCTs) had at least 12 months of follow-up data and reported cancer incidence was included.
Study characteristics, quality, and risk of bias were assessed by 2 reviewers independently.
Nineteen RCTs including 148,334 patients were included in this study. Random-effects model meta-analyses were used to estimate the risk ratio (RR) of cancer risk. No excessive cancer risk was observed in our analyses of ARB alone versus placebo alone without background ACEI use (risk ratio [RR] 1.08, 95% confidence interval [CI] 1.00–1.18, P = 0.05); ARB alone versus ACEI alone (RR 1.03, 95%CI 0.94–1.14, P = 0.50); ARB plus partial use of ACEI versus placebo plus partial use of ACEI (RR 0.97, 95%CI 0.90–1.04, P = 0.33); and ARB plus ACEI versus ACEI (RR 0.99, 95%CI 0.79–1.24, P = 0.95).
Lack of long-term data, inadequate reporting of safety data, significant heterogeneity in underlying study populations, and treatment regimens.
ARB have a neutral effect on cancer incidence in randomized trials. We observed no significant differences in cancer incidence when we compared ARB alone with placebo alone, ARB alone with ACEI alone, ARB plus partial use of ACEI with placebo plus partial use of ACEI, or ARB plus ACEI combination with ACEI.
INTRODUCTION
In the last decades, renin–angiotensin system blockers have been proven in randomized controlled trials (RCT) to prevent cardiac events. One of the most widely used drug classes among the renin–angiotensin system blockers are the angiotensin II receptor antagonists (angiotensin II receptor blockers [ARB]). ARB are considered to be important therapeutic and preventive tools in multiple clinical settings because of their good tolerability and safety.1 However, there are conflicting conclusions about the relationship between ARB and cancer incidence. A 2010 meta-analysis of 5 trials reported a modestly increased risk of new cancer occurrence associated with ARB (relative risk [RR] 1.08, 95% confidence interval [CI] 1.01–1.15).2 Later, another 2 meta-analyses performed by Bangalore et al3 of 14 RCTs and the ARB Trialists Collaboration4 of 15 RCTs showed no excessive risk of cancer associated with ARB (odds ratio [OR] 0.98, 95%CI 0.93–1.03 and OR 1.00, 95%CI 0.95–1.04, respectively). Moreover, the results regarding cancer risk associated with the combination of angiotensin-converting enzyme inhibitors (ACEI) and ARB therapy reported by 2 meta-analyses were also inconsistent. In Bangalore et al's study,3 this combination was associated with increased cancer risk compared to placebo (OR 1.14, 95%CI 1.02–1.28) in 1 model but not in other models (random-effects models). This increased risk was not observed in the ARB Trialists Collaboration study.4 A combination of ACEI and ARB was commonly used in trials included in these analyses due to background ACEI use either by design or as concomitant therapy.5–10 With the aim of investigating the risk of cancer in patients taking ARB at different background ACEI levels, we conducted a meta-analysis of published RCT.
METHODS
Study Selection
We performed a systematic literature search of PubMed and EMBASE from 1966 to December 17, 2015. Our search strategy used the following medical subject headings and text keywords: “ARB,” “angiotensin receptor blocker,” “angiotensin receptor blockers,” “angiotensin receptor antagonists,” “angiotensin receptor antagonist,” “angiotensin II receptor blocker,” “angiotensin II receptor blockers,” “candesartan,” “eprosartan,” “irbesartan,” “losartan,” “olmesartan,” “tasosartan,” “telmisartan,” and “valsartan.” Searches included a filter to limit studies to those that included humans and RCTs. No language or additional limits were included. Reference lists of reviews and included articles were also examined for additional studies.
All potentially relevant articles were reviewed independently by 2 investigators (Y-TZ and P-YL). To be eligible for inclusion in this meta-analysis, trials had meet the following criteria: RCT, placebo- or ACEI-treatment controlled, mean or median follow-up of at least 1 year, at least 100 patients enrolled, and data reported regarding the incidence of cancer diagnosis.
As the present meta-analysis was performed based on previous published studies, ethical approval and patient consent were not necessary.
Data Extraction
All data were independently abstracted and verified by 2 investigators (Y-TZ and P-YL). The following information was extracted from each study: year of publication, study population, age, sex, smoking status, sample size, duration of patient follow-up, specific ARB used, and number of cancers. In cases in which there was more than 1 published report on the same population or group of patients, the most recent article was selected for analysis.
Trial eligibility and risk of bias and trial data were assessed independently by Y-TZ and LW. Any disagreements between the assessors were resolved by discussing the item until a consensus was reached. Y-TZ and LW assessed the risk of bias in the trials by considering the following questions regarding potential sources of bias, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions:11 How was the group allocation sequence generated? How was group allocation concealed? How were participants, personnel, and outcome assessors blinded with respect to allocation? Was the data regarding outcome complete? Was there selective reporting of outcome? Did further sources of bias exist? If a trial had a high or unclear risk of bias with respect to the first 3 potential sources of bias, we placed it in the “high risk of bias” category. If a trial did not appear to have a high or unclear risk of bias with respect to the first 3 potential sources of bias, we considered it to be at low risk for bias.
Statistical Analysis
We performed statistical analyses with 2 × 2 tables on the basis of an intent-to-treat analysis. To estimate heterogeneity, we used I2, which measures the percentage of total variation across trials. I2 was calculated as follows: 100.0% × (Q − df)/Q, where Q is the Cochran heterogeneity statistic. I2 percentages of >25% and >50% were interpreted as indicators of moderate and substantial heterogeneity, respectively.
Pooled RRs were estimated by a random-effects model with the Mantel–Haensel method, which considers between-study heterogeneity. We assessed publication bias using the Begg funnel plot and Egger test. If publication bias exists, the Begg funnel plot is asymmetric or the Egger test is P < 0.05. We assessed publication bias with a funnel plot and the Begg rank correlation method (P < 0.05 indicates significant bias).12
All reported P values are 2-sided, with significance set at P < 0.05. Stata version 11.0 (Stata Corp, College Station, TX) and RevMan software (Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) were used for all calculations.
RESULTS
Search Results
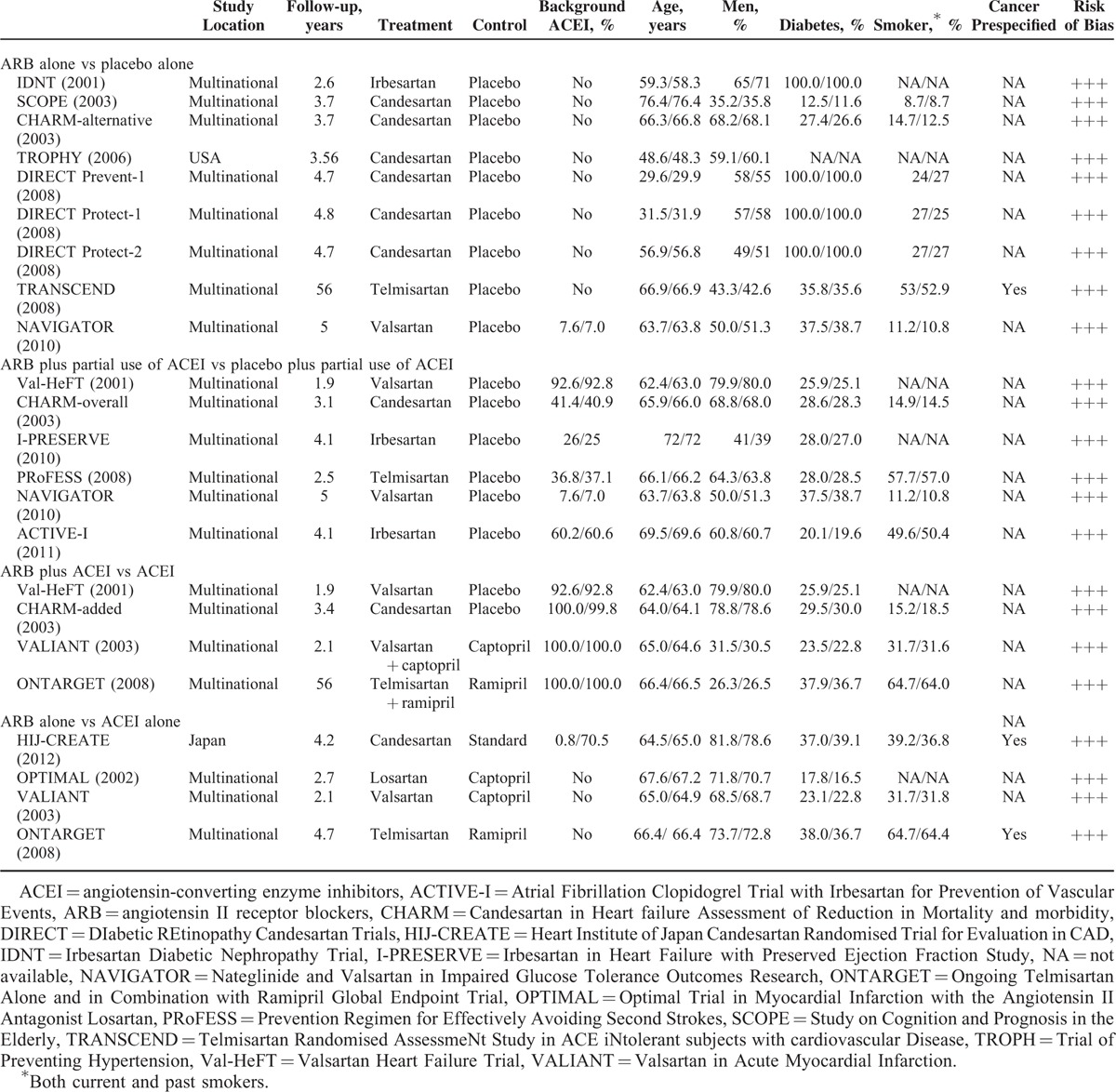
Figure 1 shows the stages of the systematic review process, which was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.13 Of the 2754 citations initially identified after duplicate citations were removed, full-text versions of 36 potentially relevant studies were retrieved for detailed evaluation. Ultimately, 19 RCTs met the inclusion criteria and were included in our systematic review5–10,14–24 (Figure 1). All trials included reports of the incidence of cancer diagnosis. Patient enrollment ranged from 772 to 20,332. The mean patient age range was 31.7 to 69.6 years, and the participants were mostly men. All trials randomized patients to active ARB, placebo, ACEI, or a combination of ARB and ACEI. Characteristics of the trials are summarized in Table 1.
FIGURE 1.
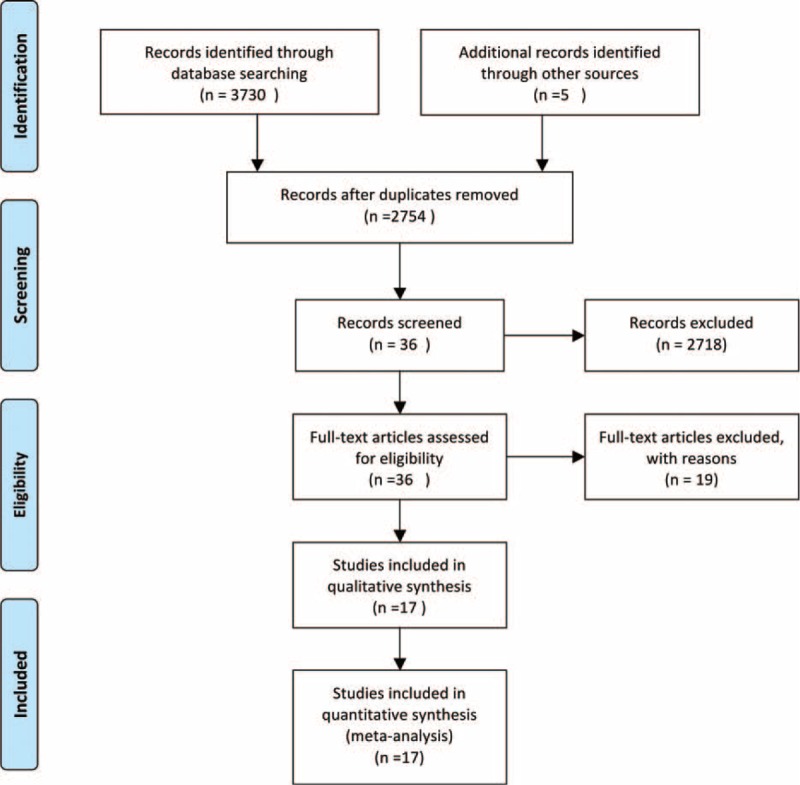
Flow diagram of included studies. ACEI = angiotensin-converting enzyme inhibitors, ARB = angiotensin II receptor blockers.
TABLE 1.
Characteristics of Randomized Controlled Trials Included in the Meta-Analysis
In consideration of the background ACEI therapy bias and previous reported uncertain risk in the ARB and ACEI combination therapy group, we conducted comparisons of the ARB and control groups by dividing the combination therapy group into 3 subgroups: ARB alone versus placebo alone, ARB alone versus ACEI alone, ARB versus placebo with partial use of ACEI in both groups, and combination therapy versus ACEI.
ARB Alone Versus Placebo Alone (Without Background ACEI)
Seven trials (Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity [CHARM]-alternative,14 DIabetic REtinopathy Candesartan Trials overall,15,16 Irbesartan Diabetic Nephropathy Trial,17 Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR),8 Study on Cognition and Prognosis in the Elderly,18 Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease,19 and Trial of Preventing Hypertension)20 were included in the ARB alone versus placebo alone analysis; 6 of them had no ACEI used as background therapy after randomization. The NAVIGATOR8 trial had a background ACEI therapy ratio of <10% at baseline (ARB group and placebo group 7.6% and 7.0%, respectively); thus, it was also included in this comparison group. The pooled effect on total cancer incidence was borderline significant, with an RR of 1.08 (95%CI 1.00–1.18, P = 0.05). A total of 2028 cancer incidences were detected among the 29,214 participants. No heterogeneity across studies was detected in the analysis (I2 = 0%). Sensitivity analyses limited to 6 trials without background ACEI therapy did not change the results (5.6% with ARB alone vs 5.0% with placebo alone, I2 = 4%, RR 1.13, 95%CI 1.00–1.27, P = 0.05) (Figure 2).
FIGURE 2.
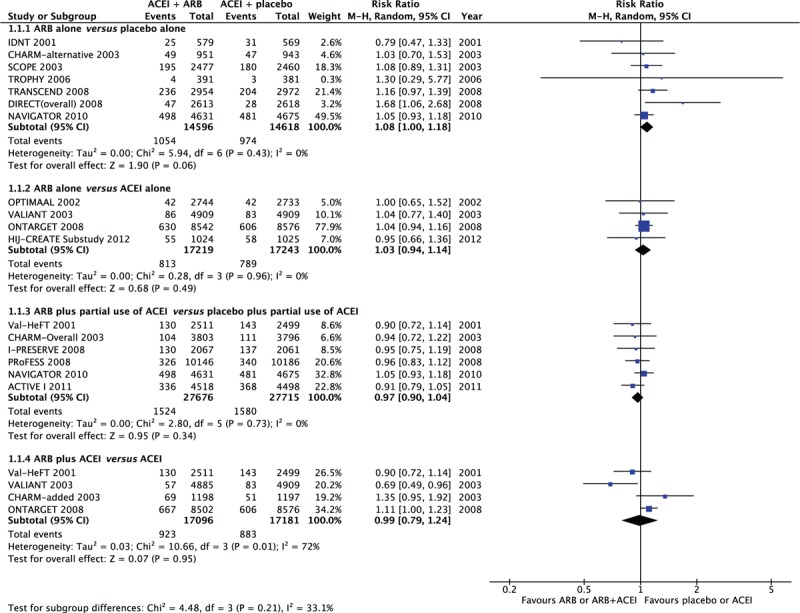
Cancer risk and ARBs, stratified by different background ACEI therapy. ACEI = angiotensin-converting enzyme inhibitors, ARB = angiotensin II receptor blockers.
ARB Alone Versus ACEI Alone
A comparison was made between patients randomized to ARB alone and those treated with ACEI alone in 4 trials: Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET),22 Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan,23 Valsartan in Acute Myocardial Infarction [VALIANT],24 and the Heart Institute of Japan Candesartan Randomised Trial for Evaluation in CAD (HIJ-CREATE) Substudy.21 In the HIJ-CREATE Substudy,21 patients were randomized to standard therapy (with 70.5% background ACEI treatment) or candesartan-based therapy (with 0.8% background ACEI treatment); therefore, it was also included in this subgroup. In the other 3 trials, patients were randomized to ARB alone or ACEI alone without concomitant therapy. No excess risk of cancer was observed in this comparison: 4.7% for ARB alone versus 4.6% for ACEI alone (RR 1.03, 95%CI 0.94–1.14, P = 0.50). When the comparison was restricted to the 3 trials ONTARGET,22 Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan,23 and VALIANT,24 the calculated effects estimate did not change (4.7% with ARB alone vs 4.5% with ACEI alone, I2 = 0%, RR 1.04, 95%CI 0.94–1.15, P = 0.43) (Figure 2).
ARB Plus Partial Use of ACEI Versus Placebo Plus Partial Use of ACEI
There was partial use of background ACEI in 6 trials (Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events [ACTIVE-I],5 CHARM-overall,6 Valsartan Heart Failure Trial [Val-HeFT],10 Irbesartan in Heart Failure with Preserved Ejection Fraction Study [I-PRESERVE],7 NAVIGATOR,8 and Prevention Regimen for Effectively Avoiding Second Strokes [PRoFESS]),9 ranging from 7.3% to 92.7%). Cancer incidence was 5.23% in patients randomized to ARB plus partial use of ACEI and 5.26% in those receiving placebo plus partial use of ACEI (RR 0.97, 95%CI 0.90–1.04), with no significant difference between the 2 groups. There was no heterogeneity in this analysis (I2 = 0%) (Figure 2).
ARB Plus ACEI Versus ACEI
Data from 4 trials (Val-HeFT,10 CHARM-added,25 VALIANT,24 and ONTARGET)22 were used for comparisons of combination therapy versus ACEI. All of these trials had a background ACEI therapy percentage of almost 100%, except for Val-HeFT,10 where it was 92.6% and 92.8% in the ARB and placebo groups, respectively. There was no significant difference with respect to the development of new cancer in patients randomized to combination therapy: 5.4% with ARB plus ACEI versus 5.1% with ACEI (RR 0.99, 95%CI 0.79–1.24, P = 0.95) by random-effects model. A high level of heterogeneity (I2 = 72%, P = 0.01) was detected in these trials. Sensitivity analyses that excluded Val-HeFT10 showed similar results: 5.4% with ARB plus ACEI versus 5.0% with ACEI (I2 = 77%, RR 1.02, 95%CI 0.74–1.40, P = 0.90) (Figure 2).
DISCUSSION
In contrast to previous meta-analysis,2–4 we used stricter criteria in the present study: first, we excluded studies that included the use of active medication other than ACEI as a control. For example, the LIFE26 study, which randomized patients to losartan and atenolol, was not included in our analysis; however, the data from this study were used in three previous meta-analyses.2–4 Second, due to discrepancies in the cancer incidence in association with combination therapy of ARB and ACEI in earlier meta-analyses, we subdivided trials into 3 groups based on their background ACEI level (no ACEI use, partial ACEI use, and 100% ACEI use). Third, the Valsartan in a Japanese population with hypertension and other cardiovascular disease (JIKEI)27 and KYOTO Heart28 studies included in the meta-analysis by Bangolore et al3 were retracted from publications due to unreliable data and were thus excluded from our analysis. Therefore, our meta-analysis provided further evidence that ARB use is not associated with a decreased or increased risk of cancer compared to control groups with or without background ACEI use. Similarly, treatment with ARB compared to ACEI was not associated with detectable differences in cancer incidence.
We did not find a significant increase in cancer risk among patients who took ARB alone compared to those who took placebo alone, either by primary analysis or additional sensitivity analysis. This finding differed from the findings reported by Sipahi et al2 and was in line with the results from 2 more recent meta-analyses.3,4 Our study differed from all of these earlier meta-analyses in that we restricted our analysis to trials that compared ARB to placebo only with background ACEI contamination of <10%. In sensitivity analyses, we limited the included studies to placebo-controlled trials without any background ACEI used in patient arms; this did not change the results.
In Sipahi et al's study,2 only the ONTARGET study was used to compare combination therapy with ACEI alone. In the ART meta-analysis,4 data from seven trials (ONTARGET,22 PRoFESS,9 ACTIVE I,5 I-PRESERVE,7 Val-HeFT,10 VALIANT,24 and CHARM-Added)25 were pooled together. However, in our study, trials with low levels of ACEI use were excluded (PRoFESS9 36.8% and 37.0%, ACTIVE I5 60.2% and 60.4%, and I-PRESERVE7 25% and 25% for ARB and control, respectively). Thus, only 4 trials (ONTARGET22 100% and 100%, Val-HeFT10 92.6% and 96.8%, VALIANT24 100% and 100%, and CHARM-Added25 100% and 99.8% for ARB and control, respectively) were included in this comparison. Moreover, our sensitivity analyses were limited to trials (ONTARGET,22 VALIANT,24 and CHARM-Added)25 with almost 100% ACEI use in both arms.
Our comparison of ACEI alone with ARB alone yielded results that were consistent with those of a previous study by the ARB Trialists Collaboration.4 Despite the similar results, the trials selected for this comparison in our study were different from those included in the ARB Trialists Collaboration.4 Background ACEI use contamination was not allowed in our analysis, except for the HIJ-CREATE Substudy,21 which was excluded from our sensitivity analysis.
Angiotensin II binds to different subtypes of the receptors AT1 and AT2.29 Experimental data30–32 have demonstrated that angiotensin II may have a role in cell growth and proliferation and in angiogenesis, mainly through angiotensin II type I receptor (AT1R) signaling. Long-term antagonism of the AT1 receptor by ARB may result in persistent activation of AT2 receptor signaling, the role of which has not yet been established in cancer.29 Some studies33–35 suggest that AT2 receptor stimulation results in an antitumor effect, while others indicate that AT2 has protumor effects.36,37 Additionally, Dabul et al38 elucidated that candesartan and valsartan were the most potent at blocking angiotensin II-induced β-arrestin-1 activation at AT1 receptor. Meanwhile, there are increasing evidences that nuclear β-arrestin-1 contributes to tumor growth, invasion, and metastasis in multiple malignancies such as breast cancer, colorectal cancer, lung cancer, and prostate cancer. A possible explanation for these contradictory observations is that AT2 receptors may achieve an AT1 receptor-like phenotype under pathological conditions.39 The complicated biological effects underlying the blocking of AT1 and the activation of AT2 by means of ARB could explain its neutral effects on cancer risk.
Our meta-analysis has several limitations. First, most of the RCT had a limited follow-up period of 1.9 to 5 years. The time frame required for cancer development may exceed the follow-up time in many of the RCT. Second, despite the differences among studies with respect to the drugs and dosages administered, all of the drugs, both ARB and ACEI, have historically been regarded as being very similar. Pharmacologically, this is incorrect and may, therefore, have had a variety of effects on risk. Third, there was a large amount of heterogeneity between the ARB plus ACEI versus the ACEI group. We could not determine the origin of this heterogeneity. A possible explanation is the limited number of RCT included in this analysis group.
In conclusion, the results of our meta-analysis suggest that treatment with ARB had a neutral effect on cancer incidence in RCT. Moreover, no significant increases were observed in cancer incidence when we compared ARB with placebo or with, without, or with partial background use of ACEI.
Footnotes
Abbreviations: ACEI = angiotensin-converting enzyme inhibitors, ACTIVE-I = Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events, ARB = angiotensin II receptor blockers, CHARM = Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity, CI = confidence interval, HIJ-CREATE = Heart Institute of Japan Candesartan Randomised Trial for Evaluation in CAD, I-PRESERVE = Irbesartan in Heart Failure with Preserved Ejection Fraction Study, NAVIGATOR = Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research, ONTARGET = Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial, OR = odds ratio, PRoFESS = Prevention Regimen for Effectively Avoiding Second Strokes, RCT = randomized controlled trials, RR = relative risk, Val-HeFT = Valsartan Heart Failure Trial, VALIANT = Valsartan in Acute Myocardial Infarction.
Brief Summary: Angiotensin II receptor blockers (ARB) are proven to reduce cardiovascular disease (CVD) events in several clinical settings; however, the relationship between ARB and cancer incidence, especially when combined with angiotensin-converting enzyme inhibitors (ACEI) were inconsistent in previous meta-analyses. We conducted a meta-analysis of the risk of cancer in patients taking ARB at different background ACEI levels. We discovered no excessive cancer of ARB versus placebo with, without, or with partial background use of ACEI.
Y-TZ and P-YL contributed equally to the writing of this article and share primary authorship.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Volpe M, Tocci G, Pagannone E. Fewer mega-trials and more clinically oriented studies in hypertension research? The case of blocking the renin-angiotensin-aldosterone system. J Am Soc Nephrol 2006; 17:S36–S43. [DOI] [PubMed] [Google Scholar]
- 2.Sipahi I, Debanne SM, Rowland DY, et al. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol 2010; 11:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangalore S, Kumar S, Kjeldsen SE, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol 2011; 12:65–82. [DOI] [PubMed] [Google Scholar]
- 4.Collaboration ARBT. Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens 2011; 29:623–635. [DOI] [PubMed] [Google Scholar]
- 5.Investigators AI, Yusuf S, Healey JS, et al. Irbesartan in patients with atrial fibrillation. N Engl J Med 2011; 364:928–938. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003; 362:759–766. [DOI] [PubMed] [Google Scholar]
- 7.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 8.Group NS, McMurray JJ, Holman RR, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010; 362:1477–1490. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn JN, Tognoni G. Valsartan Heart Failure Trial I. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S. Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Chichester, England; Hoboken, NJ: Wiley-Blackwell; 2008. [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003; 362:772–776. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi N, Porta M, Klein R, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet 2008; 372:1394–1402. [DOI] [PubMed] [Google Scholar]
- 16.Sjolie AK, Klein R, Porta M, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet 2008; 372:1385–1393. [DOI] [PubMed] [Google Scholar]
- 17.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860. [DOI] [PubMed] [Google Scholar]
- 18.Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886. [DOI] [PubMed] [Google Scholar]
- 19.Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008; 372:1174–1183. [DOI] [PubMed] [Google Scholar]
- 20.Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006; 354:1685–1697. [DOI] [PubMed] [Google Scholar]
- 21.Sugiura R, Ogawa H, Oka T, et al. Candesartan-based therapy and risk of cancer in patients with systemic hypertension (Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Artery Disease [HIJ-CREATE] substudy). Am J Cardiol 2012; 109:576–580. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 23.Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002; 360:752–760. [DOI] [PubMed] [Google Scholar]
- 24.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349:1893–1906. [DOI] [PubMed] [Google Scholar]
- 25.McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771. [DOI] [PubMed] [Google Scholar]
- 26.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003. [DOI] [PubMed] [Google Scholar]
- 27.Mochizuki S, Dahlof B, Shimizu M, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet 2007; 369:1431–1439. [DOI] [PubMed] [Google Scholar]
- 28.Sawada T, Yamada H, Dahlof B, et al. Effects of valsartan on morbidity and mortality in uncontrolled hypertensive patients with high cardiovascular risks: KYOTO HEART Study. Eur Heart J 2009; 30:2461–2469. [DOI] [PubMed] [Google Scholar]
- 29.George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 2010; 10:745–759. [DOI] [PubMed] [Google Scholar]
- 30.Suganuma T, Ino K, Shibata K, et al. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res 2005; 11:2686–2694. [DOI] [PubMed] [Google Scholar]
- 31.Kikkawa F, Mizuno M, Shibata K, et al. Activation of invasiveness of cervical carcinoma cells by angiotensin II. Am J Obstet Gynecol 2004; 190:1258–1263. [DOI] [PubMed] [Google Scholar]
- 32.George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 2010; 10:745–759. [DOI] [PubMed] [Google Scholar]
- 33.Doi C, Egashira N, Kawabata A, et al. Angiotensin II type 2 receptor signaling significantly attenuates growth of murine pancreatic carcinoma grafts in syngeneic mice. BMC Cancer 2010; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickel L, Matsuzuka T, Doi C, et al. Over-expression of angiotensin II type 2 receptor gene induces cell death in lung adenocarcinoma cells. Cancer Biol Ther 2010; 9:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benndorf R, Boger RH, Ergun S, et al. Angiotensin II type 2 receptor inhibits vascular endothelial growth factor-induced migration and in vitro tube formation of human endothelial cells. Circ Res 2003; 93:438–447. [DOI] [PubMed] [Google Scholar]
- 36.Kanehira T, Tani T, Takagi T, et al. Angiotensin II type 2 receptor gene deficiency attenuates susceptibility to tobacco-specific nitrosamine-induced lung tumorigenesis: involvement of transforming growth factor-beta-dependent cell growth attenuation. Cancer Res 2005; 65:7660–7665. [DOI] [PubMed] [Google Scholar]
- 37.Clere N, Corre I, Faure S, et al. Deficiency or blockade of angiotensin II type 2 receptor delays tumorigenesis by inhibiting malignant cell proliferation and angiogenesis. Int J Cancer 2010; 127:2279–2291. [DOI] [PubMed] [Google Scholar]
- 38.Dabul S, Bathgate-Siryk A, Valero TR, et al. Suppression of adrenal betaarrestin1-dependent aldosterone production by ARBs: head-to-head comparison. Sci Rep 2015; 5:8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moltzer E, Verkuil AV, van Veghel R, et al. Effects of angiotensin metabolites in the coronary vascular bed of the spontaneously hypertensive rat: loss of angiotensin II type 2 receptor-mediated vasodilation. Hypertension 2010; 55:516–522. [DOI] [PubMed] [Google Scholar]


