Abstract
Background
The skeletal muscle has the ability to regenerate after injury. This process is mediated mainly by the muscle specific stem cells, that is, satellite cells. In case of extensive damage or under pathological conditions, such as muscular dystrophy, the process of muscle reconstruction does not occur properly. The aim of our study was to test whether mobilized stem cells, other than satellite cells, could participate in skeletal muscle reconstruction.
Methods
Experiments were performed on wild‐type mice and mice lacking the functional Pax7 gene, that is, characterized by the very limited satellite cell population. Gastrocnemius mice muscles were injured by cardiotoxin injection, and then the animals were treated by stromal derived factor‐1 (Sdf‐1) with or without granulocyte‐colony stimulating factor (G‐CSF) for 4 days. The muscles were subjected to thorough assessment of the tissue regeneration process using histological and in vitro methods, as well as evaluation of myogenic factors' expression at the transcript and protein levels.
Results
Stromal derived factor‐1 alone and Sdf‐1 in combination with G‐CSF significantly improved the regeneration of Pax7−/− skeletal muscles. The Sdf‐1 and G‐CSF treatment caused an increase in the number of mononucleated cells associated with muscle fibres. Further analysis showed that Sdf‐1 and G‐CSF treatment led to the rise in the number of CD34+ and Cxcr4+ cells and expression of Cxcr7.
Conclusions
Stromal derived factor‐1 and G‐CSF stimulated regeneration of the skeletal muscles deficient in satellite cells. We suggest that mobilized CD34+, Cxcr4+, and Cxcr7+ cells can efficiently participate in the skeletal muscle reconstruction and compensate for the lack of satellite cells.
Keywords: Differentiation, Myoblast, G‐CSF, Regeneration, Sdf‐1 (CXCL12), Skeletal muscle, Stem cells
Introduction
Skeletal muscle fibres contain several hundred nuclei within a continuous cytoplasm. Fibres are accompanied by muscle specific stem cells, that is, satellite cells that are localized between the sarcolemma and the basal lamina. These cells are responsible for postnatal muscle growth and regeneration (reviewed in Relaix and Zammit1). Responding to muscle injury, satellite cells become activated, become myoblasts, and fuse to form myotubes and then new muscle fibres. Simultaneously, with the activation of satellite cells, cytokines and chemokines released locally upon injury attract inflammatory cells, which infiltrate injured muscle and remove damaged fibres. Neutrophils are the first population of leukocytes that appear within the injured muscle and participate in the removal of muscle debris as well as exacerbate satellite cell activation. Then, macrophages accumulate and phagocyte apoptotic and necrotic cells and fibres, and they mediate the remodelling of the extracellular matrix, the formation of new vessels, and activation of myogenic precursors (reviewed in Rigamonti et al. 2).
The ability of satellite cells to reconstruct the damaged muscle is remarkable. For example, it has been demonstrated that satellite cells connected to a single muscle fibre can recreate the number of myoblasts equal to the number of nuclei forming the parent fibre within just 4 days following a muscle injury.3 Moreover, satellite cells are capable of self‐renewing their population in adult muscles, which was first postulated by Moss and Leblond.4 The capacity of satellite cells to self‐renew was experimentally proved by Collins and coworkers, who transplanted a single muscle fibre together with few satellite cells to an injured skeletal muscle that was depleted of endogenous satellite cells by irradiation.5 As a result of such grafting, the functional muscle fibres were reconstructed, and also a few hundred new satellite cells were observed.5 These results were confirmed by Sacco and coworkers, who showed that even a single satellite cell transplantation allowed the reconstruction of the injured muscle and the restoration of the satellite cell pool.6 Importantly, these cells were functional, that is, could orchestrate further rounds of muscle regeneration. Therefore, satellite cells and myoblasts resulting from them have been considered as myogenic cells, which could be used in cell‐based muscle therapy. The ability of transplanted myoblasts to participate in muscle regeneration and restoration of dystrophin expression was shown using dystrophin deficient mdx mice.7 However, the results of clinical trials using myoblasts for human Duchenne muscular dystrophy were disappointing (e.g. Karpati et al. 8). Major limitations in the application of satellite cells or myoblasts in the management of muscular dystrophy is the restricted ability of these cells to migrate through vasculature and effectively engraft the injured muscle, as well as the rapid cell death following transplantation, which could be overcome by immunosuppression (reviewed in Briggs and Morgan9). On the other hand, high‐density injection protocols allow to avoid the issue of limited migration of transplanted cells, but are only applicable to easily accessible small muscle groups.10 Apart from satellite cells, many other populations of muscle‐derived stem cells, bone marrow, or peripheral blood cells manifest myogenic potential in vitro and in vivo. The mesoangioblasts,11 pericytes,12 and CD133+ cells13 make up the most interesting cells from the therapeutic point of view. However, the majority of data on the use of various stem cells in skeletal muscle therapy refers to the transplantation of exogenous cells into the injured muscle. The functions of endogenous cells, other than satellite cells, during skeletal muscle regeneration remain largely obscure.
In the present study, we attempted to mobilize stem cells into the site of the injury using stromal derived factor‐1 (Sdf‐1) and to increase the number of mobilized stem cells using granulocyte‐colony stimulating factor (G‐CSF). Bone marrow is the main source of Sdf‐1, but this cytokine is also expressed in a wide variety of organs, such as the heart, liver, spleen, kidney, and brain (reviewed in Cencioni et al. 14). It is well established that Sdf‐1 induces migration of different stem cell types, that is, satellite cells,15 CD34+ haematopoietic progenitor cells,16 or mesenchymal stem cells.17 Sdf‐1 mobilizes transplanted stem cells to tissues affected by ischemia as it was shown for limbs and hearts (e.g. Elmadbouh et al. and Kuliszewski et al. 18, 19). G‐CSF is the other factor involved in the mobilization of stem cells from the bone marrow to peripheral blood (reviewed in Alvarez et al. 20). G‐CSF was shown to increase the level of cytokines in serum, reduce the numbers of bone marrow macrophages, inhibit the activity of osteoblasts, which are the main source of Sdf‐1 in bone marrow, increase the number of osteoclasts that release cathepsin K, and activate the CD26 protease that degrades Sdf‐1 (reviewed in Hoggatt and Pelus21). Thus, the main mechanism of G‐CSF action relies on down‐regulation of Sdf‐1 level in the bone marrow. G‐CSF and erythropoietin induce neovascularization of infracted rat myocardium, however, it is not clear whether it involves the mobilization of endothelial progenitor cells (EPCs). G‐CSF also inhibits apoptosis of cardiomyocytes and reduces the development of fibrosis in heart muscle.22
To study the impact of Sdf‐1 and G‐CSF on stem cell mobilization to the injured muscle, we used mice lacking functional Pax7 transcription factor (Pax7−/−). These mice are characterized by a very limited satellite cell population23 and cannot efficiently regenerate injured muscles.24 Thus, the induction of skeletal muscle regeneration in Pax7−/− mice must be dependent on cells other than satellite cells. We aimed to verify whether the endogenous (not transplanted) stem cells, mobilized from bone marrow, could participate in muscle regeneration and compensate for the lack of satellite cells.
Materials and methods
Mice
All performed experiments were approved by the Local Ethical Commission No 1 in Warsaw—permission No. 186/2011.
The 7‐day‐old male or female F1 (C57BI6N x 129Sv) Pax7+/+ (wt) and F1 (C57BI6N x 129Sv) Pax7−/− mice were used. C57BI6N Pax7+/− have been generated with the insertion of a sequence encoding the neomycin gene into the first exon of the paired box domain of one Pax7 locus according to the method described previously.25 Such mutation leads to the abolishment of DNA‐binding activity of the Pax7 protein. Next, C57BI6N Pax7+/− mice were crossed with 129Sv mice, and F1 (C57BI6N x 129Sv) were generated.
Genotyping
Genomic DNA was isolated from the tails of 4‐ to 5‐day‐old mice. Briefly, tail tips were cut and heated for 10 min at 95°C in 50 mM NaOH. Then, 1 M Tris (pH 8) was added, and samples were centrifuged, 1 μL of supernatant was used for PCR. Reactions were performed with RedTaq Ready Mix (Sigma‐Aldrich, St. Louis, MO, USA) at the following conditions: 32 cycles of (30 s at 94°C, 30 s at 65°C, and 90 s at 72°C) preceded by 60 s at 94°C, and followed by 10 min at 72°C. Genotypes of Pax7−/− mice were confirmed by PCR reaction using the following primers: [GGGCTTGCTGCCTCCGATATAGC, GTGGGGTCTTCATCAACGGTC, TCGTGCTTTACGGTATCGCCGCTCCCG]. Wild‐type allele was represented by 200 kb product, while the Pax7‐ allele by 700 kb product.26
Muscle injury and treatments
Intact muscles: gastrocnemius muscles of 8‐day‐old F1 (C57BI6N x 129Sv) Pax7+/+ (wt) or Pax7−/− male or female mice were injected with 10 ng/10 μL (wt) or 5 ng/5 μL (Pax7−/−) of Sdf‐1 (Life Technologies, Van Allen Way Carlsbad, CA, USA) dissolved in 0.9% NaCl (saline). Contralateral muscles were injected with the same volume of saline. Next, 6 days after Sdf‐1 treatment, wt and Pax7−/− mice were sacrificed and the muscles were isolated. Injured muscles: both gastrocnemius muscles of 7‐day‐old F1 (C57BI6N x 129Sv) Pax7+/+ (wt) or Pax7−/− male or female mice were injected with 5 μL (wt) or 3 μL (Pax7−/−) of cardiotoxin (ctx, 0.2 mM, Sigma‐Aldrich). Twenty four hours post injury muscles were injected with 10 ng/10 μL (wt) or 5 ng/5 μL (Pax7−/−) of Sdf‐1. Contralateral muscles were injected with the same volume of saline. Next, 4 or 7 days after injury, mice were sacrificed and muscles were isolated. Additionally, 4 days after injury, peripheral blood of the animals was collected. Sdf‐1 and G‐CSF treatment: both gastrocnemius muscles of 7‐day‐old F1 (C57BI6N x 129Sv) Pax7+/+ (wt) or Pax7−/− male mice were injected with 5 μL (wt) or 3 μL (Pax7−/−) of ctx. Starting from the day of injury, animals were subcutaneously injected with G‐CSF (250 ng per 1 g of body weight).27 G‐CSF was administrated twice a day, every 12 h, for 4 days. Twenty four hours post injury muscles were injected with 10 ng/10 μL (wt) or 5 ng/5 μL (Pax7−/−) of Sdf‐1. Contralateral muscles were injected with the same volume of saline. Next, 4 or 7 days after injury, mice were sacrificed and muscles were isolated. Additionally, peripheral blood mice was drawn from the animals at day 4 following injury. Figure 1A presents the experimental scheme.
Figure 1.
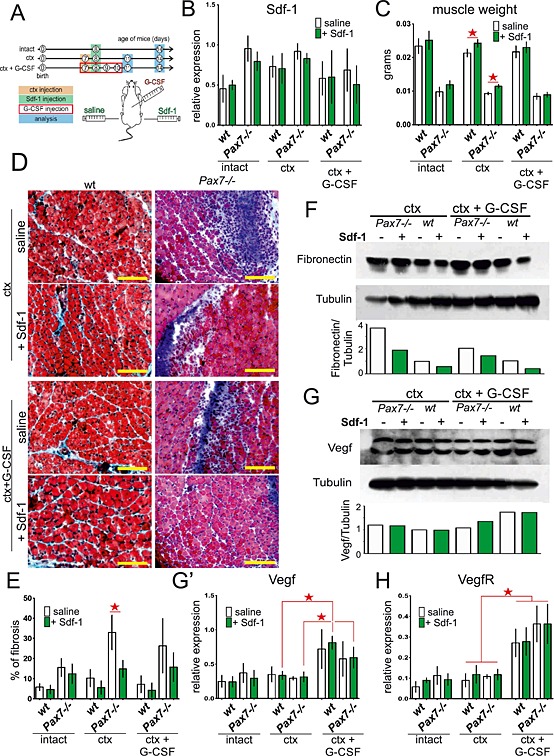
Regeneration of intact and injured wt and Pax7−/− gastrocnemius muscles treated with stromal derived factor‐1 (Sdf‐1) or granulocyte‐colony stimulating factor (G‐CSF) and Sdf‐1. Analyses were performed at day 7 of regeneration. (A) Experimental design. (B) The level of Sdf‐1 mRNA normalized to the level of housekeeping genes. Data are the means ± SD. No statistically important differences were observed. (C) The muscle weight. The statistically important differences are marked with stars. (D) The muscle cross sections stained with Masson's trichrome. Bar 100 µm. (E) The level of fibrosis showed as proportion of tissue area occupied by connective tissue. The statistically important differences are marked with stars. (F) The level of fibronectin and tubulin protein. (G) The level of vascular endothelial growth factor (Vegf) and tubulin protein. (G') The level of Vegf mRNA normalized to the level of housekeeping genes. (H) The level of vascular endothelial growth factor receptor (VegfR) mRNA normalized to the level of housekeeping genes. The statistically important differences are marked with stars.
Analysis of cell numbers associated with individual fibres
Four days after injury, muscle fibres were isolated from wt and Pax7−/− mice gastrocnemius muscles, according to the Rosenblatt method.28 Briefly, muscles were digested with collagenase I for 1.5 h at 37°C. Next, single fibres were transferred to single wells of 96‐well plates coated with 10% Matrigel characterized by reduced growth factors concentration (Becton Dickinson Bioscience, Franklin Lakes, NJ, USA) and cultured in DMEM (Life Technologies) supplemented with 10% horse serum (HS, Life Technologies), 20% fetal bovine serum (FBS, Life Technologies), 0.5% chicken embryo extract (CEE, Sera Laboratories), and 1% antibiotic (AB, Life Technologies). After 48 h of culture, the numbers of cells that migrated off the individual fibres were counted. After 72 h of culture, the morphology of the cells was analyzed. Each culture was repeated five times.
Bone marrow‐derived stem cells migration assay
Whole population of bone marrow cells was obtained from wt and Pax7−/− mice. Bone marrow cells were washed in phosphate buffered saline (PBS) and centrifuged in Histopaque 1077 gradient (Sigma‐Aldrich) to remove erythrocytes. Obtained mononucleated cells were seeded on culture inserts (8 µm pore size) coated with Matrigel containing reduced growth factor concentration (Becton Dickinson Bioscience) and cultured in α‐MEM (Sigma‐Aldrich) supplemented with 2 mM l‐glutamine (Life Technologies), 20% FBS, and 1% antibiotic (penicillin and streptomycin, Life Technologies). After 48 h of culture, non‐adherent cells were removed. Next, culture medium present in the lower chamber was supplemented with Sdf‐1 (50 ng/mL). After an additional 48 h of culture, the cells were fixed with methanol and stained with Giemsa (Merck, Darmstadt, Germany), according to the manufacturer's protocol. The number of cells that migrated through the insert pores and localized either at the insert surface facing the lower dish or at the bottom of the lower chamber was counted. Analysis was performed in triplicates.
Quantitation of mRNA levels
Total RNA was extracted from intact and injured muscles of wt and Pax7−/− mice using the RNasy Midi Kit (QIAGEN, Venlo, Netherlands), according to the manufacturer instruction. 500 ng of total RNA was reverse transcribed using the Transcriptor First Stand cDNA Synthesis Kit (Roche Applied Science, Penzberg, Upper Bavaria, Germany), according to the manufacturer's protocol. mRNA levels were examined using custom PCR array based on Universal ProbeLibrary (Roche Applied Science) for the following genes: Cxcr4, Cxcr7, Sdf‐1, CD34, CD45, CD133, Myf5, m‐cadherin, muscle creatine kinase (m‐CK), vascular endothelial growth factor (VEGF), VEGF receptor (VEGFR), and embryonic myosin heavy chains (eMyHC). Hypoxanthine phosphoribosyltransferase 1 (Hprt 1), glyceraldehydes‐3‐phosphate dehydrogenase (GAPDH), and beta‐2‐microglobulin (B2m) were used as the candidate reference genes. Quantitative real‐time PCR analysis was performed using the LightCycler 480 Probes Master 9.0 (Roche Applied Sciences) and LightCycler 480 (Roche Applied Sciences), according to the PCR array manufacturer protocol. Threshold‐cycle (Ct) values of the analyzed amplicons were determined using the LightCycler 480 Software (Roche Applied Science). Expression levels were calculated with the 2‐(ΔCT) formula using the relative quantification tool in the LightCycler 480 Software. All the candidate reference genes displayed high expression stability and were therefore used for the normalization of the expression data. RNAs isolated from three muscles of each group were analyzed.
Western blot analysis
The protein lysates from muscles of 14‐day‐old wt and Pax7−/− mice were obtained using Complete Lysis‐M EDTA‐free reagent (Roche Applied Science) and denatured by boiling in Laemmli buffer. Next, 25 µg of protein samples were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Roche Applied Science), according to previously described protocol.15 Membranes were washed in Tris‐buffered saline (TBS), incubated in 5% non‐fatty dry milk in TBS at room temperature for 1 h, followed by the overnight incubation in primary antibody diluted in 5% non‐fatty dried milk in TBST (1:2 000) at 4°C. Next, the membranes were exposed for 2 h to HRP‐conjugated secondary antibody diluted in 5% non‐fatty dry milk in TBST (1:10 000 or 1:20 000) at room temperature. Protein bands were visualized using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and exposed to chemiluminescence positive film (Amersham Hyperfilm ECL, GE Healthcare, Little Chalfont, UK). The experiment was performed in triplicates, that is, three muscles of each group were independently analyzed. The protein level was quantified using the Gel Doc XR+ (BioRad, Hercules, CA, USA) and Image Software System version 5.1 (BioRad). The optical density of analyzed protein bands was compared with tubulin bands. The following primary antibodies were used: rabbit polyclonal anti‐Cxcr4 (Abcam, Cambridge, UK), rat monoclonal (MEC 14.7) anti‐CD34 (Abcam), rabbit polyclonal anti‐Cxcr7 (Abcam), rabbit polyclonal anti‐m‐CK (Sigma‐Aldrich), mouse monoclonal (EP5) anti‐fibronectin (Santa Cruz Biotechnology, Dallas, TX, USA), and mouse monoclonal (B‐5‐1‐2) anti‐tubulin (Sigma‐Aldrich), rabbit polyclonal anti‐VEGF (Millipore, Billerica, MA, USA), and goat polyclonal anti‐VEGFR (Santa Cruz Biotechnology). The following secondary antibodies were used: goat anti‐mouse Igg (Sigma‐Aldrich, dilution 1:20 000), goat anti‐rabbit (Sigma‐Aldrich, dilution 1:10 000), and goat anti‐rat (Calbiochem, dilution 1:10 000).
Histochemical staining
Six days after Sdf‐1 injection to intact and injured gastrocnemius muscles of wt and Pax7−/− mice, the muscles were dissected. Next, muscles were frozen in isopentane cooled with liquid nitrogen, transferred into −80°C, and cut into 7 µm‐thick sections using cryomicrotome (Microm HM505N). Sections were hydrated in deionized water and fixed in Bouin's reagent (Sigma‐Aldrich) overnight. Masson's Trichrome (Sigma‐Aldrich) staining was performed according to the manufacturer protocol. Finally, sections were dehydrated and mounted with Entellan (Merck). Sections were photographed using Nikon Eclipse, TE200 microscope equipped with Hoffman contrast. Fields of view (10 per each variant) were analyzed using GIMP ImageJ to evaluate the percentage of the tissue area occupied by connective tissue. Five muscles collected during independent experiments were analyzed.
Immunolocalization
Gastrocnemius muscles of wt and Pax7−/− mice were frozen in isopentane cooled with liquid nitrogen, transferred into −80°C, cut into 7 µm‐thick sections using cryomicrotome (Microm HM505N). Muscle sections were hydrated in PBS and fixed in 3% PFA for 10 min. Next, samples were washed with PBS, permeabilized with 0.05% TritonX100 (Sigma‐Aldrich) in PBS, incubated in 0.25% glycine (Sigma‐Aldrich) in PBS, followed by 1 h incubation in 3% BSA in PBS (Sigma‐Aldrich) containing 2% donkey serum (Sigma‐Aldrich) at room temperature. Next, all the samples were incubated at 4°C in primary antibodies diluted in 3% bovine serum albumin (BSA) in PBS (1:100), overnight. Subsequently, the specimen were incubated for 2 h with secondary antibodies conjugated with Alexa Fluor 488, 568, or 594 (Life Technologies) diluted in 1.5% BSA/PBS (1:200), at room temperature. In order to visualize the nuclei, samples were incubated for 5 min at room temperature with DraQ5 (Biostatus Limited, Leicestershire, GB) diluted in PBS (1:1000). Finally, the samples were mounted with Fluorescent Mounting Medium (DakoCytomation, Glostrup, Denmark).
Single muscle fibres, isolated according to the Rosenblatt method, were fixed for 10 min with 3% PFA in PBS. Then fibres were permeabilized in 0.5% TritonX100 (Sigma‐Aldrich) for 10 min, incubated in 15 mM NH4Cl for 20 min, followed by 30 min incubation with 10% HS (Life Technologies) at room temperature. Next, fibres were incubated overnight at 4°C with primary antibodies diluted in 10% HS in PBS (1:50), which was followed by 1 h of incubation with secondary antibodies conjugated with Alexa Fluor 488 or 594 (Life Technologies) diluted in 10% HS/PBS (1:100), at room temperature. Nuclei were visualized with DraQ5 (Biostatus Limited) diluted in PBS (1:1000), incubated at room temperature, for 5 min. Fibres were mounted with Fluorescent Mounting Medium (DakoCytomation).
The following primary antibodies were used: mouse monoclonal anti‐CD45 (Santa Cruz Biotechnology), rabbit polyclonal anti‐Cxcr4 (Abcam), rat monoclonal (MEC 14.7) anti‐CD34 (Abcam), rabbit polyclonal anti‐Cxcr7 (Abcam), rabbit monoclonal (SP6) anti‐Ki67 (Abcam), mouse monoclonal (12G4) anti‐m‐cadherin (Abcam), mouse monoclonal (F1.652) anti‐eMyHC (Hybridoma Bank), and rabbit polyclonal anti‐laminin (Sigma‐Aldrich). The following secondary antibodies were used: donkey anti‐mouse Igg Alexa 594 conjugated (Life Technologies), donkey anti‐rabbit Igg Alexa 488 conjugated (Life Technologies), donkey anti‐rabbit Igg Alexa 594 conjugated (Life Technologies), and goat anti‐rat Igg Alexa 568 conjugated (Life Technologies). Appropriate controls of secondary antibodies were performed. Each analysis was repeated three times.
Flow cytometry analysis
Four days after the injury of gastrocnemius muscles of wt and Pax7−/− mice, the peripheral blood (250 μL) was collected into the heparin containing tubes. The erythrocytes were lysed using lysis buffer (0,17 M Tris HCl, 0,16 M NH4Cl, pH 7.2). Simultaneously, gastrocnemius muscles were dissected and incubated for 2 h at 37°C in 0.2% collagenase I (Sigma‐Aldrich), followed by 40 min incubation in 0.05% Dispase (Becton Dickinson Bioscience) at 37°C. Next, muscles were fragmented by pipetting using 20G needle. Obtained suspensions of cells and fibre fragments were filtered through a 40 µm cell strainer (Becton Dickinson Bioscience). Collected cells were incubated for 30 min with 0.5% BSA supplemented with 1% FBS in PBS, at room temperature. Next, cells were transferred to anti‐CD34‐PE (Abcam) or anti‐Cxcr4 (Abcam) primary antibodies diluted 1:100 in PBS with 0.5% BSA and incubated for 30 min at 4°C, washed in PBS, and then incubated for 30 min with secondary Alexa 488 conjugated antibody diluted 1:200 (Life Technologies) at room temperature. Finally, both blood and muscle‐derived cells were fixed for 10 min in 2% PFA in PBS, at room temperature. Samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson Bioscience). Control samples were incubated with isotype fluorochrome matched immunoglobulin antibodies. Each analysis was repeated five times.
Statistical analysis
Data was presented as means ± standard deviation. Student's t‐test was used for statistical comparisons. P < 0.05 was considered significant and marked with an asterisk.
Results
Stromal derived factor‐1 improves Pax7−/− muscles regeneration
Our previous studies documented that Sdf‐1 is produced during skeletal muscles regeneration29, and exogenous Sdf‐1 can improve this process.15 Because this cytokine can increase the mobilization of stem cells into injured muscle, we decided to check whether it can also improve growth and regeneration of skeletal muscles of Pax7−/− mice, that is, mice lacking satellite cells. Thus, by using Pax7−/− mice, we analyzed whether the endogenous stem cells would be mobilized by Sdf‐1 and would be able to substitute missing satellite cells. We also mobilized stem cells from bone marrow to peripheral blood using G‐CSF. It allowed us to increase the number of stem cells possibly able to engraft the injured muscles. To this point, intact or ctx damaged gastrocnemius muscles of wt and Pax7−/− mice were injected either with saline (control) or Sdf‐1. Ctx injured muscles were also treated with G‐CSF (Figure 1A). To sumarize, the muscles of wt and Pax7−/− mice were analyzed in the following experimental variants: (1) intact control and Sdf‐1 injected (intact); (2) ctx injured control and Sdf‐1 injected (ctx); and (3) ctx injured and G‐CSF treated control and Sdf‐1 injected (ctx + G‐CSF) (Figure 1A). Three and 6 days after Sdf‐1 administration, that is, in either 11 or 14‐day‐old mice, we compared the progress and the ‘quality’ of growth as well as muscle regeneration.
The levels of Sdf‐1 transcripts (endogenous Sdf‐1) did not differ between the muscles of 14‐day‐old mice, that is, at day 7 of regeneration after ctx injection (Figure 1B). Importantly, the Pax7−/− muscle mass and muscle fibres diameter were smaller when compared with the wt muscles. All wt and Pax7−/− muscles, that is, intact and regenerating that were treated with Sdf‐1, had a tendency to increase their mass when compared with the control, that is, saline injected ones (Figure 1C). Statistically significant differences (P < 0.05) were observed only in the case of wt and Pax7−/− mice that were injured and treated with only Sdf‐1 (Figure 1C). G‐CSF treatment did not have an effect on muscle mass. However, treatment with Sdf‐1 and G‐CSF, alone or in combination, significantly increased the ability of Pax7−/− muscle to reconstruct its structure, as shown in muscle cross sections stained with Masson's trichrome (Figure 1D). The regeneration of untreated injured Pax7−/− muscles (ctx and saline; Figure 1D) was defective, clearly indicating regeneration deficit at this stage (14‐day‐old mice, day 7 of regeneration after ctx injection). At the same time, regeneration of injured Pax7−/− muscles treated with Sdf‐1 proceeded more efficiently (Figure 1D). Visualization of connective tissue in wt and Pax7−/− muscles with Masson's trichrome showed limited fibrosis in Sdf‐1 treated muscles (Figure 1D). Importantly, the connective tissue area had a tendency to decrease in all injured muscles injected with Sdf‐1, regardless of mice genotype (Figure 1E). Statistically significant differences (P < 0.05) in the fibrosis level were observed between injured Pax7−/− muscles and treated with Sdf‐1 muscles. In addition, the level of fibronectin, which is characteristic for connective tissue, was lower in Sdf‐1 injected muscles (Figure 1F). In order to verify whether Sdf‐1 and G‐CSF treatment enhances angiogenesis, we assessed the angiogenic factor levels, that is, the transcript levels of Vegf and its receptor VegfR. The levels of Vegf and VegfR did not differ significantly between saline and Sdf‐1 treated muscles of 14‐day‐old mice, that is, at day 7 of regeneration (Figure 1G, 1G′, and 1H and Figure S1A in the Supporting Information). Thus, it suggests that Sdf‐1 did not influence the blood vessel formation in regenerating muscles. Importantly, G‐CSF stimulation significantly increased the level of Vegf and VegfR, implying that G‐CSF stimulation improves angiogenesis (Figure 1G, 1G′, 1H and Figure S1A).
Many lines of evidence showed that eMyHC is synthesized in newly formed muscle fibres.30, 31 For this reason, the augmentation of eMyHC expression indicates an increase of newly formed fibres number within the muscle. In intact wt and Pax7−/− muscles, the level of mRNA encoding eMyHC was very low (Figure 2A). At the seventh day of regeneration, ctx injury caused the increase in eMyHC transcript level in wt and Pax7−/− muscles (Figure 2A). Sdf‐1 treatment insignificantly decreased eMyHC transcript levels in Pax7−/− muscles. Interestingly, G‐CSF stimulation resulted in dramatic increase of eMyHC mRNA level in Sdf‐1 treated Pax7−/− (P < 0.05) but not wt muscles (Figure 2A). Immunolocalization of eMyHC protein in regenerating wt and Pax7−/− muscles confirmed results of qPCR analysis at the protein level. The number of muscle fibres expressing eMyHC is presented in Figure 2B and C as their percentage in a total number of muscle fibres in the field of view (six muscles, 10 fields of view per muscle). Analysis of ctx injured wt muscles revealed low number of eMyHC positive fibres that decreased as a consequence of Sdf‐1 treatment. Pax7−/− muscles were characterized by significantly higher number of eMyHC positive fibres, which also decreased following Sdf‐1 treatment (Figure 2B and C). Surprisingly, G‐CSF and Sdf‐1 injections led to the dramatic increase in the formation of new, that is, immature, muscle fibres in Pax7−/− but not in wt muscles (Figure 2B and 2C). Moreover, the level of m‐CK, which expression is associated with muscle fibres maturation, was higher in ctx injured and Sdf‐1 treated wt muscles compared with untreated muscles (Figure 2D and 2E). Taken together, this data indicates that Sdf‐1 accelerates skeletal muscle regeneration in wt and Pax7−/− mice. G‐CSF stimulation followed by Sdf‐1 treatment dramatically changed the process of Pax7−/− muscle regeneration and led to massive formation of new immature muscle fibres (Figure 2B and 2C). Importantly, maturation of these newly formed muscle fibres was delayed in Pax7−/− mice.
Figure 2.
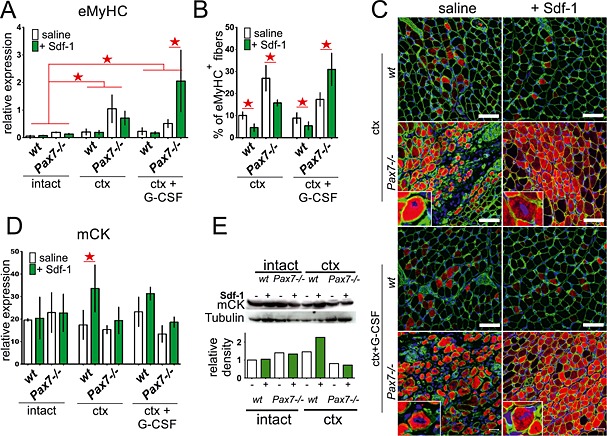
Expression and localization of muscle specific factors in intact and injured wt and Pax7−/− gastrocnemius muscles treated with stromal derived factor‐1 (Sdf‐1) or granulocyte‐colony stimulating factor (G‐CSF) and Sdf‐1. Analyses were performed at day 7 of regeneration. (A) The level of embryonic myosin heavy chains (eMyHC) mRNA normalized to the level of housekeeping genes. Data are the means ± SD. The statistically important differences marked with stars. (B) The proportion of muscle fibres number expressing eMyHC protein (eMyHC+ fibres) of all muscle fibres at cross section. The statistically important differences marked with stars. (C) Immunolocalization of eMyHC in newly formed muscle fibres. Green—laminin, red—eMyHC, and blue—nuclei. Bar 50 µm. (D) The level of muscle creatine kinase (mCK) mRNA normalized to the level of housekeeping genes. Data are the means ± SD. The statistically important differences marked with stars. (E) The level and analysis of mCK and tubulin protein.
Granulocyte‐colony stimulating factor and stromal derived factor‐1 treatment increases the mobilization of the stem cells into injured muscles
Next, we attempted to identify endogenous stem cells that would replace lacking satellite cells and improve skeletal muscle regeneration in Pax7−/− mice. Histological analysis revealed that Sdf‐1 injection resulted in the increase of mononucleated cells localized between muscle fibres (Figure 1D), as compared with control ones (Figure 3A). G‐CSF treatment further increased the number of mononucleated cells that engrafted ctx injured muscles, as compared with those treated only with Sdf‐1 (Figure 3A). Next, the expression of mRNAs encoding stem cells markers, that is, CD34, Cxcr4, and Cxcr7, was significantly increased in G‐CSF and Sdf‐1 injected regenerating muscles, as compared with saline treated ones (Figure 3B). The level of mRNA encoding myogenic cells marker, that is, Myf5, was very low in Pax7−/− as compared with wt muscles. In intact and ctx injured muscles, neither Sdf‐1 alone nor in combination with G‐CSF increased Myf5 expression, regardless of mice genotype (Figure 3B). Analysis of mRNA encoding m‐cadherin, another satellite cells marker, revealed that its level was increased only in Sdf‐1‐treated regenerating wt muscles (Figure 3B). In Pax7−/− muscles, m‐cadherin expression did not change, proving that their regeneration depends on cells other than satellite cells or myoblasts derived from them (Figure 3B). Histological analysis revealed that the number of CD34 and Cxcr4 expressing cells and levels of these proteins in tissue lysates were higher in both wt and Pax7−/− muscles treated with Sdf‐1, as well as co‐stimulated with G‐CSF and Sdf‐1 as compared with untreated muscles (Figure 4A and 4B). The effect of G‐CSF and Sdf‐1 treatment on Cxcr7 expression in muscles was less pronounced (Figure 4B).
Figure 3.
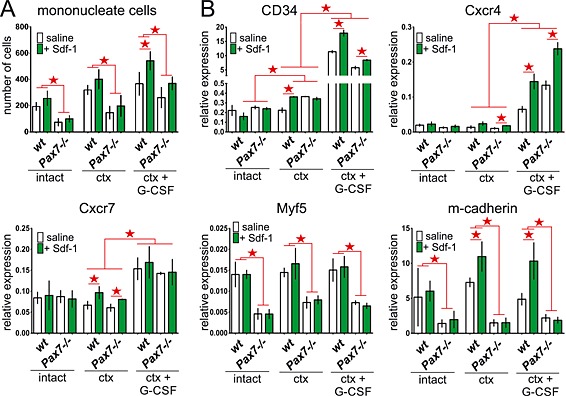
Analysis of mononucleated cells present in intact and injured wt and Pax7−/− gastrocnemius muscles treated with stromal derived factor‐1 (Sdf‐1) or granulocyte‐colony stimulating factor (G‐CSF) and Sdf‐1. Analyses were performed at day 7 of regeneration. (A) The number of mononucleated cell counted on muscle cross sections stained with Masson's trichrome. The statistically important differences marked with stars. (B) The level of CD34, Cxcr4, Cxcr7, Myf5 and m‐cadherin mRNA normalized to the level of housekeeping genes. The statistically important differences marked with stars.
Figure 4.
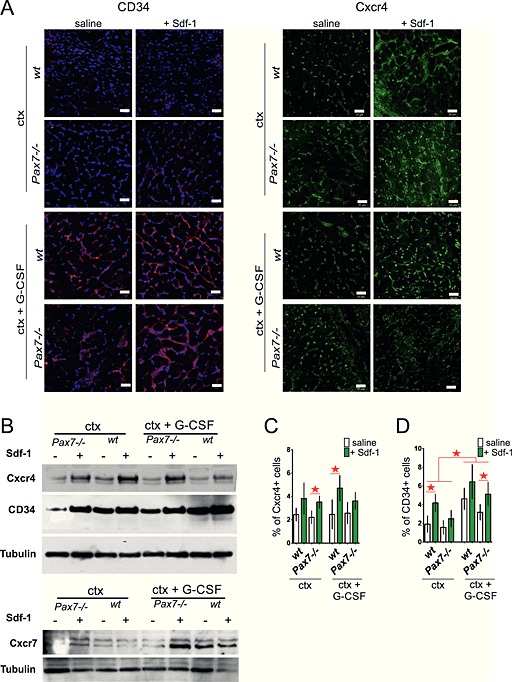
Analysis of localization and level of CD34 and Cxcr4 in cells present in intact and injured wt and Pax7−/− gastrocnemius muscles treated with stromal derived factor‐1 (Sdf‐1) or granulocyte‐colony stimulating factor (G‐CSF) and Sdf‐1. A. The presence of CD34 and Cxcr4 positive cells in muscles at day 7 of regeneration. Green—Cxcr4, red—CD34, and blue—nuclei. Bar 20 µm. (B) The level of Cxcr4, CD34, Cxcr7, and tubulin protein at day 7 of regeneration. (C) The proportion of Cxcr4 positive cells of all cells isolated from regenerating muscle measured by fluorescence‐activated cell sorting at day 4 of regeneration. (D) The proportion of CD34 positive cells of all cells isolated from regenerating muscle measured by FACS at day 4 of regeneration.
To exclude the possibility that Sdf‐1 increased the expression of Cxcr4 and CD34 in cells already present within the muscle, we decided to isolate mononucleated cells from wt and Pax7−/− muscles at day 4 after injury and determine the proportion of Cxcr4 or CD34 expressing cells by fluorescence‐activated cell sorting (FACS) analysis (Figure 4C and 4D). In all samples obtained from either Sdf‐1 or G‐CSF and Sdf‐1 treated muscles, we observed the tendency that the number of Cxcr4 and CD34 positive cells was higher, as compared with non‐treated muscles. To exclude the possibility that Sdf‐1 and G‐CSF stimulate cell proliferation but not their migration, we assessed the number of proliferating cells, that is, expressing Ki67, in regenerating muscles. The number of Ki67 expressing cells was found to be comparable in all the analyzed muscles at day 7 of regeneration (Figure 5A). Thus, neither G‐CSF nor Sdf‐1 affected the proliferation rate of resident cells but increased the number of cells by promoting the homing of circulating cells into the regenerating muscles. This result was confirmed by the analysis of muscle fibres that were isolated from wt and Pax7−/− gastrocnemius muscles at day 7 of regeneration. Co‐localization of Ki67 and m‐cadherin in the cells that were attached to muscle fibres showed that the proportion of double immunostained ones, that is, expressing both Ki67 and m‐cadherin, was comparable regardless of mice genotype and type of muscle treatment (Figure 5B). Thus, we confirmed that G‐CSF and Sdf‐1 did not affect the proliferation of resident cells, that is, satellite cells. In addition, we checked the presence of so‐called inflammatory cells within the analyzed muscles. The level of mRNA encoding CD45, which is characteristic for inflammatory cells, did not change in response to G‐CSF and Sdf‐1 (Figure 5C), indicating that the cells present within the muscle were not inflammatory ones. Additionally, immunostaing for CD45 (Figure S1B) showed that CD45+ cells were rarely observed within the analyzed muscle. Thus, we suggest that at day 7 of regeneration, infiltration of muscle tissue by immune system cells is low. Moreover, the level of CD133 marker characteristic for circulating stem cells (AC133+) did not differ between control and treated muscles (Figure 5D).
Figure 5.
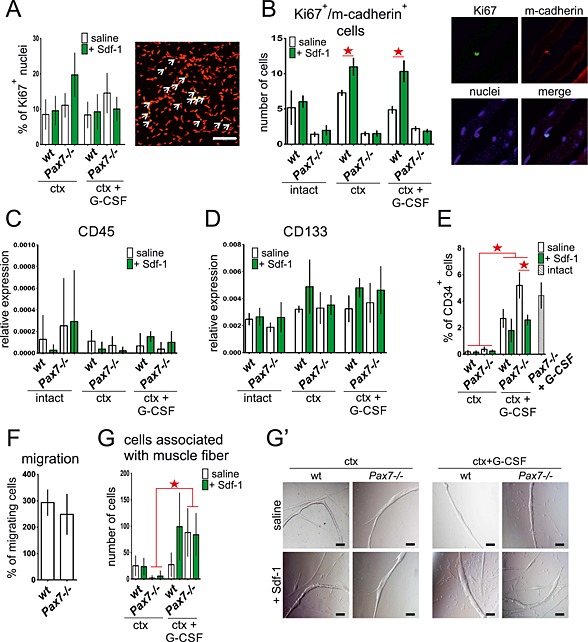
Analysis of Ki67 positive cells (Ki67+) in intact and injured wt and Pax7−/− gastrocnemius muscles treated with stromal derived factor‐1 (Sdf‐1) or granulocyte‐colony stimulating factor (G‐CSF) and Sdf‐1 (A and B). (E) Mobilization and migration ability of cells isolated at day 4 and 7 of regeneration (C–G′). (A) The proportion of Ki67 positive nuclei (graph) of all nuclei at cross section and representative immunolocalization of Ki67 in wt muscle at day 7 of regeneration. Red—nuclei, green—Ki76, and yellow—colocalization. Bar 50 µm. The statistically important differences marked with stars. (B) The number of Ki67 and m‐cadherin positive cells connected with freshly isolated muscle fibres (graph) and immunolocalization of Ki67 and m‐cadherin at day 7 of regeneration. Bar 10 µm. The statistically important differences marked with stars. (C) The level of CD45 mRNA at day 7 of regeneration, normalized to the level of housekeeping genes. The statistically important differences marked with stars. (D) The level of CD133 mRNA at day 7 of regeneration, normalized to the level of housekeeping genes. The statistically important differences marked with stars. (E) The proportion of CD34 positive cells (CD34+) in peripheral blood at day 4 measured by fluorescence‐activated cell sorting. The statistically important differences marked with stars. (F) The migration of wt and Pax7−/− BMSCs in Sdf‐1 gradient (transmembrane migration assay). The statistically important differences marked with stars. (G) The number of cells associated with isolated at day 4 of regeneration muscle fibres after 48 h of in vitro culture. The statistically important differences marked with stars. (G′) The presence of cells associated with muscle fibre after 48 h of in vitro culture. Transmitted light images. Bar 100 µm.
The source of the stem cells mobilized by stromal derived factor‐1 and granulocyte‐colony stimulating factor into injured muscles
To verify whether in our hands G‐CSF promotes the release of bone marrow stem cells (BMSCs) into circulation, we analyzed the peripheral blood for the presence of CD34 expressing cells. We did not detect any increase in CD34 positive cells in the blood of wt and Pax7−/− mice following muscle injury with ctx, regardless of Sdf‐1 treatment. However, G‐CSF was found to cause an increase in the number of CD34 expressing cells in the peripheral blood of wt and Pax7−/− mice, analyzed at day 4 of regeneration (Figure 5E). The number of these cells was lower in those mice in which ctx‐treated muscles were Sdf‐1 injected, suggesting their increased homing to the site of injury. We also observed that G‐CSF caused an increase in the number of CD34 positive cells in blood of Pax7−/− mice which muscles were not injured (Figure 5E, grey bar). Next, we compared ability of BMSCs of wt and Pax7−/− mice to migrate in Sdf‐1 gradient. Transmembrane migration assay showed that wt and Pax7−/− BMSCs cells did not differ in their migration potential (Figure 5F). Further analysis covered the isolation of muscle fibres and cells from wt and Pax7 −/− muscles at day 4 of regeneration. These fibres were cultured individually for 48 h, and cells that migrated out of them were counted (Figure 5G and 5G′). The number of cells present in the vicinity of the analyzed fibres was higher in fibres of mice injected with G‐CSF and Sdf‐1, as compared with fibres isolated from Sdf‐1 or saline injected mice, regardless of their genotype.
Discussion
Our previous studies provided the evidence that Sdf‐1 stimulation improves skeletal muscle regeneration. This effect can be linked to the presence of Cxcr4 and CD34 expressing cells and also to the increase of myoblast migration capacity in metalloproteinase‐2 (MMP‐2) and MMP‐9‐dependent manner.15 Available data, however, do not provide straightforward proof showing that regeneration process can depend on endogenous cells other than satellite cells. To verify such notion, we studied mice carrying a targeted mutation in the Pax7 gene (Pax7−/−). Pax7 has been shown to inhibit satellite cells differentiation by inhibiting Myod1‐dependent activation of myogenin that is a crucial myogenic transcription factor.32 Another myogenic transcription factor, that is, Myf5, is also a direct target gene of Pax7.33 Previous study showed that Pax7−/− mice are significantly smaller than wt and are characterized by a limited lifetime.34 We also showed here that Pax7−/− muscles, such as gastrocniemus, had significantly lower mass comparing with wt ones. This phenotype is caused by the reduced number of satellite cells present in the skeletal muscles of Pax7−/− mice. Satellite cells from Pax7−/− mice are characterized by cell cycle arrest, inability to proliferate, and precocious differentiation.23, 24, 35, 36 Loss of satellite cells in Pax7−/− mice is a progressive process starting soon after the animal birth. In 10‐day‐old Pax7−/− mice, satellite cell number drops to 15–25% of the number present in newborn mice.35, 36 As a result, the satellite cells are hardly detectable. In adult Pax7−/− mice, that is, 8 weeks old.24 Pax7−/− mice are also characterized by reduced muscle fibre size. This suggests defective phase of postnatal skeletal muscle growth in these animals, while it is known that this phase in wt mice is mediated by satellite cells.23, 35, 36
As shown by Lepper and coworkers, the role of Pax7 could be dependent on mouse age. Ablation of Pax7 expressing cells during postnatal growth (between 7 and 18 days of age) leads to the complete inhibition of regeneration. However, in adult mice (older than 21 days), Pax7 positive cells appear not to be essential for muscle regeneration, and their ablation does not inhibit regeneration.37 On the other hand, Rudnicki's group showed that inactivation of Pax7 in satellite cells of adult mice, that is, 40 days old, markedly impaired muscle regeneration.38 Other authors report that in Pax7−/− mice older than 21 days, the regeneration process is reduced24, 36 proving the substantial role of satellite cells in skeletal muscle regeneration. This function of satellite cells was postulated from the very moment of their discovery.39 The essential role of satellite cells in adult muscle regeneration was also demonstrated in other studies.40, 41
Our work proves that defects in Pax7−/− muscle regeneration can be ameliorated. By analyzing regenerating muscles of 14‐day‐old Pax7−/− mice, we showed that their regeneration is impaired and associated with the development of fibrosis. However, by using G‐CSF and then Sdf‐1, we were able to induce the mobilization of stem cells to the injured muscles, ameliorated fibrosis and, in a consequence, made the skeletal muscle regeneration effective, even in the absence of satellite cells. The improvement of regeneration was manifested in better muscle architecture, higher creatine kinase levels, and lower fibrosis. Moreover, we observed that G‐CSF and Sdf‐1 treatment led to the increase of VegfR level that could suggest amelioration of angiogenesis. Indeed, it was previously shown that combination of G‐CSF and Sdf‐1 treatments enhanced neovascularization in ischemic muscles.42 This angiogenic effect can be another factor, apart from mobilization of stem cells, improving reconstruction of regenerating muscles.
In growing Pax7−/− skeletal muscles, the number of satellite cells progressively decreases. Thus, in case of muscle injury, improved muscular regeneration has to result from stem cells mobilization. This hypothesis was proved by detailed analyses of the cells present within regenerating muscles of mice stimulated with G‐CSF and Sdf‐1. A very limited number of previous studies documented that endogenous (not transplanted) stem cells could, albeit with low frequency, participate in the skeletal muscle reconstruction. First, it was shown that muscle resident pericytes were able to contribute to regeneration and also colonize the satellite cell niche.43 Next, it was demonstrated that endogenous bone marrow cells with very low frequency can took part in the muscle regeneration.44 Our results suggest that Pax7−/− BMSCs are able to react to Sdf‐1 treatment and improve muscle regeneration showing that they can participate in this process regardless of functional Pax7. Furthermore, another study suggested that BMSCs are able to undergo myogenic differentiation in Pax7 independent manner.45
The main population of cells mobilized to injured wt and Pax7−/− skeletal muscles were CD34 and Cxcr4 positive ones (but CD133 and CD45 negative). Importantly, as it was shown by Ieronimakis and coworkers, the proportion of CD34 expressing cells remains constant after mouse muscle injury.46 Apart from the endothelial progenitors and stromal cells, CD34 expression characterizes haematopoietic stem and progenitor cells (reviewed in Lin et al. 47). A low number of CD34 positive cells is present in the peripheral blood and could be significantly increased by a mobilization protocol based on G‐CSF administration (reviewed in Moggatt and Pelus48). The reports concerning the influence of G‐CSF on myoblasts proliferation are conflicting. Some data document that G‐CSF does not influence myoblasts proliferation or differentiation in vitro.49 Other lines of evidence imply that G‐CSF receptor is expressed in C2C12 myoblasts, and that G‐CSF strongly induces myoblasts proliferation in vitro.50 Moreover, the expression of G‐CSF receptor was shown to be elevated in regenerating muscle in mice, starting from day 3 to 8 after ctx injury, that is, at the stages when myoblasts proliferate.50 Importantly, mice lacking functional G‐CSF receptor gene were characterized by diminished regeneration, and G‐CSF administration led to an increase of the number of myoblasts and improved skeletal muscle regeneration.50 Results of our experiments indicate, however, that G‐CSF used together with Sdf‐1 does not affect the proliferation of cells mobilized into the regenerating muscles of Pax7−/− mice at analyzed stage of regeneration. In wt mice, such treatment increased the number of cells expressing Ki67 together with m‐cadherin, suggesting that it stimulates only satellite cells‐derived myoblasts. We did not dissociate, however, the action of G‐CSF from Sdf‐1.
As we have demonstrated, the administration of G‐SCF and Sdf‐1 immediately after muscle injury can be very effective, even in the case of skeletal muscles lacking satellite cells. Fibrosis was diminished, and endogenous cells were mobilized to the regenerating tissue. This observation can be remove used during the development of the treatment of many muscle diseases and impairments. However, as it was shown previously, the frequency of the formation of new fibres with the participation of transplanted bone marrow stem cells is very low [e.g. Corbel et al. 51], suggesting that the major population of mobilized cells is not highly effective in supporting skeletal muscle regeneration. This can be a limiting factor for the therapies based on such cells. In the current study, we showed that single administration of Sdf‐1 could be an effective stimulant increasing the participation of BMSCs in the skeletal muscle regeneration. Additional increase in the number of stem cells present in the blood stream, achieved by G‐CSF injections, allows for the improvement of the regeneration process. To conclude, the muscle regeneration can be improved by treatment with Sdf‐1 alone or combined with G‐CSF, even when the population of skeletal muscle specific stem cells, that is, satellite cells is nearly exhausted. Our results add to the currently existing knowledge on the use of mouse model lacking satellite cells for studying novel therapies of pathological conditions of muscles with reduced satellite cell numbers.
Conflict of interest
K.K., A.R., A.K., S.W., P.A., G.M., C.M.A., and B.E. declare that they have no conflict of interest.
Supporting information
Figure S1. A. Regeneration of intact and injured wt and Pax7−/− gastrocnemius muscles treated with Sdf‐1 or G‐CSF and Sdf‐1. Analyses were performed at day 7 of regeneration and showed the level of VegfR and tubulin protein. B. Localization of CD45 positive cells in wt gastrocnemius muscles at day 7 of regeneration. Blue – cells nuclei, green – laminin, red – CD45.
Supporting info item
Acknowledgements
This research was supported by the Ministry of Science and Higher Education (Iuventus Plus Program, grant number: 0048/IP1/2011/71).
The authors certify that they complied with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.
Kowalski, K. , Archacki, R. , Archacka, K. , Stremińska, W. , Paciorek, A. , Gołąbek, M. , Ciemerych, M. A. , and Brzoska, E. (2016) Stromal derived factor‐1 and granulocyte‐colony stimulating factor treatment improves regeneration of Pax7−/− mice skeletal muscles. Journal of Cachexia, Sarcopenia and Muscle, 7: 483–496. doi: 10.1002/jcsm.12092.
References
- 1. Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 2012;139:2845–2856. [DOI] [PubMed] [Google Scholar]
- 2. Rigamonti E, Zordan P, Sciorati C, Rovere‐Querini P, Brunelli S. Macrophage plasticity in skeletal muscle repair. Bio Med research international 2014;2014:560629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zammit PS, Heslop L, Hudon V, Rosenblatt JD, Tajbakhsh S, Buckingham ME, et al. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp Cell Res 2002;281:39–49. [DOI] [PubMed] [Google Scholar]
- 4. Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 1971;170:421–435. [DOI] [PubMed] [Google Scholar]
- 5. Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self‐renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005;122:289–301. [DOI] [PubMed] [Google Scholar]
- 6. Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self‐renewal and expansion of single transplanted muscle stem cells. Nature 2008;456:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin‐negative to ‐positive by injection of normal myoblasts. Nature 1989;337:176–179. [DOI] [PubMed] [Google Scholar]
- 8. Karpati G, Ajdukovic D, Arnold D, Gledhill RB, Guttmann R, Holland P, et al. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol 1993;34:8–17. [DOI] [PubMed] [Google Scholar]
- 9. Briggs D, Morgan JE. Recent progress in satellite cell/myoblast engraftment—relevance vfor therapy. FEBS J 2013;280:4281–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skuk D, Goulet M, Roy B, Piette V, Cote CH, Chapdelaine P, et al. First test of a “high‐density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow‐up. Neuromuscul Disord 2007;17:38–46. [DOI] [PubMed] [Google Scholar]
- 11. Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, et al. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol 2006;174: 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2011;2:499. [DOI] [PubMed] [Google Scholar]
- 13. Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D'Antona G, et al. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest 2004;114:182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cencioni C, Capogrossi MC, Napolitano M. The SDF‐1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res 2012;94:400–407. [DOI] [PubMed] [Google Scholar]
- 15. Brzoska E, Kowalewska M, Markowska‐Zagrajek A, Kowalski K, Archacka K, Zimowska M, et al. Sdf‐1 (CXCL12) improves skeletal muscle regeneration via the mobilisation of Cxcr4 and CD34 expressing cells. Biol Cell 2012;104:722–737. [DOI] [PubMed] [Google Scholar]
- 16. Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez‐Ramos JC. The chemokine SDF‐1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 1997;185:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Son BR, Marquez‐Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal‐derived factor‐1‐CXCR4 and hepatocyte growth factor‐c‐met axes and involves matrix metalloproteinases. Stem Cells 2006;24:1254–1264. [DOI] [PubMed] [Google Scholar]
- 18. Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell‐derived factor‐1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol 2007;42:792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong‐Poi H. Vascular gene transfer of SDF‐1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol Ther 2011;19:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvarez P, Carrillo E, Velez C, Hita‐Contreras F, Martinez‐Amat A, Rodriguez‐Serrano F, et al. Regulatory systems in bone marrow for hematopoietic stem/progenitor cells mobilization and homing. BioMed research international 2013;2013:312656, doi:10.1155/2013/312656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoggatt J, Pelus LM. Mobilization of hematopoietic stem cells from the bone marrow niche to the blood compartment. Stem cell research & therapy 2011;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu ZH, Dong W, Gai LY, Wang F, Ding R, Chen YD. [Effect of erythropoietin combined with granulocyte‐colony stimulating factor in the treatment of acute myocardial infarction in rats]. Nan fang yi ke da xue xue bao = J Southern Med Univ 2011;31:17–22. [PubMed] [Google Scholar]
- 23. Seale P, Sabourin LA, Girgis‐Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell 2000;102: 777–786. [DOI] [PubMed] [Google Scholar]
- 24. Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. Embo J 2004;23:3430–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mansouri A, Stoykova A, Torres M, Gruss P. Dysgenesis of cephalic neural crest derivatives in Pax7−/− mutant mice. Development 1996;122:831–838. [DOI] [PubMed] [Google Scholar]
- 26. Lu A, Cummins JH, Pollett JB, Cao B, Sun B, Rudnicki MA, et al. Isolation of myogenic progenitor populations from Pax7‐deficient skeletal muscle based on adhesion characteristics. Gene Ther 2008;15:1116–1125. [DOI] [PubMed] [Google Scholar]
- 27. Sicinska E, Lee YM, Gits J, Shigematsu H, Yu Q, Rebel VI, et al. Essential role for cyclin D3 in granulocyte colony‐stimulating factor‐driven expansion of neutrophil granulocytes. Mol Cell Biol 2006;26:8052–8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim 1995;31:773–779. [DOI] [PubMed] [Google Scholar]
- 29. Brzoska E, Grabowska I, Hoser G, Streminska W, Wasilewska D, Machaj EK, et al. Participation of stem cells from human cord blood in skeletal muscle regeneration of SCID mice. Exp Hematol 2006;34:1262–1270. [DOI] [PubMed] [Google Scholar]
- 30. Karsch‐Mizrachi I, Travis M, Blau H, Leinwand LA. Expression and DNA sequence analysis of a human embryonic skeletal muscle myosin heavy chain gene. Nucleic Acids Res 1989;17:6167–6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Silberstein L, Webster SG, Travis M, Blau HM. Developmental progression of myosin gene expression in cultured muscle cells. Cell 1986;46:1075–1081. [DOI] [PubMed] [Google Scholar]
- 32. Olguin HC, Yang Z, Tapscott SJ, Olwin BB. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol 2007;177:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soleimani VD, Punch VG, Kawabe Y, Jones AE, Palidwor GA, Porter CJ, et al. Transcriptional dominance of Pax7 in adult myogenesis is due to high‐affinity recognition of homeodomain motifs. Dev Cell 2012;22:1208–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mansouri A, Hallonet M, Gruss P. Pax genes and their roles in cell differentiation and development. Curr Opin Cell Biol 1996;8:851–857. [DOI] [PubMed] [Google Scholar]
- 35. Relaix F, Montarras D, Zaffran S, Gayraud‐Morel B, Rocancourt D, Tajbakhsh S, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 2006;172:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 2006;172:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 2009;460:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A 2013;110:16474–16479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 1961;9:493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud‐Morel B, et al. Pax7‐expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 2011;138:3647–3656. [DOI] [PubMed] [Google Scholar]
- 41. Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7‐positive satellite cells in acute injury‐induced skeletal muscle regeneration. Development 2011;138:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan Y, Shao H, Eton D, Yang Z, Alonso‐Diaz L, Zhang H, et al. Stromal cell‐derived factor‐1 enhances pro‐angiogenic effect of granulocyte‐colony stimulating factor. Cardiovasc Res 2007;73:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 2007;9:255–267. [DOI] [PubMed] [Google Scholar]
- 44. Palermo AT, Labarge MA, Doyonnas R, Pomerantz J, Blau HM. Bone marrow contribution to skeletal muscle: a physiological response to stress. Dev Biol 2005;279:336–344. [DOI] [PubMed] [Google Scholar]
- 45. Xynos A, Corbella P, Belmonte N, Zini R, Manfredini R, Ferrari G. Bone marrow‐derived hematopoietic cells undergo myogenic differentiation following a Pax‐7 independent pathway. Stem Cells 2010;28:965–973. [DOI] [PubMed] [Google Scholar]
- 46. Ieronimakis N, Balasundaram G, Rainey S, Srirangam K, Yablonka‐Reuveni Z, Reyes M. Absence of CD34 on murine skeletal muscle satellite cells marks a reversible state of activation during acute injury. PLoS One 2010;5:e10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin CS, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy 2012;14:1159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hoggatt J, Pelus LM. Many mechanisms mediating mobilization: an alliterative review. Curr Opin Hematol 2011;18:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wright CR, Brown EL, Della‐Gatta PA, Ward AC, Lynch GS, Russell AP. G‐CSF does not influence C2C12 myogenesis despite receptor expression in healthy and dystrophic skeletal muscle. Frontiers in physiology 2014;5:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hara M, Yuasa S, Shimoji K, Onizuka T, Hayashiji N, Ohno Y, et al. G‐CSF influences mouse skeletal muscle development and regeneration by stimulating myoblast proliferation. J Exp Med 2011;208:715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Corbel SY, Lee A, Yi L, Duenas J, Brazelton TR, Blau HM, et al. Contribution of hematopoietic stem cells to skeletal muscle. Nat Med 2003;9:1528–1532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A. Regeneration of intact and injured wt and Pax7−/− gastrocnemius muscles treated with Sdf‐1 or G‐CSF and Sdf‐1. Analyses were performed at day 7 of regeneration and showed the level of VegfR and tubulin protein. B. Localization of CD45 positive cells in wt gastrocnemius muscles at day 7 of regeneration. Blue – cells nuclei, green – laminin, red – CD45.
Supporting info item


