Abstract
Objectives
To investigate whether changes to the apparent diffusion coefficient (ADC) of primary tumour in the early period after starting chemotherapy can predict progression-free survival (PFS) or overall survival (OS) in patients with unresectable pancreatic adenocarcinoma.
Methods
Subjects comprised 43 patients with histologically confirmed unresectable pancreatic cancer treated with first-line chemotherapy. Minimum ADC values in primary tumour were measured using the selected area ADC (sADC), which excluded cystic and necrotic areas and vessels, and the whole tumour ADC (wADC), which included whole tumour components. Relative changes in ADC were calculated from baseline to 4 weeks after initiation of chemotherapy. Relationships between ADC and both PFS and OS were modelled by Cox proportional hazards regression.
Results
Median PFS and OS were 6.1 and 11.0 months, respectively. In multivariate analysis, sADC change was the strongest predictor of PFS (hazard ratio (HR), 4.5; 95 % confidence interval (CI), 1.7–11.9; p = 0.002). Multivariate Cox regression analysis for OS revealed sADC change and CRP as independent predictive markers, with sADC change as the strongest predictive biomarker (HR, 6.7; 95 % CI, 2.7–16.6; p = 0.001).
Conclusion
Relative changes in sADC could provide a useful imaging biomarker to predict PFS and OS with chemotherapy for unresectable pancreatic adenocarcinoma.
Key Points
• Relative change in ADC value can predict survival in unresectable pancreatic cancer.
• ADC change could determine a chemosensitivity of pancreatic cancer.
• ADC values should be measured by excluding cystic, necrotic areas and vessels.
Keywords: Pancreatic cancer, Apparent diffusion coefficient, Biomarker, Prognostic factor, MRI
Introduction
Patients with unresectable pancreatic cancer show a poor prognosis, with overall survival of 5.6–10.1 months. Currently, gemcitabine or oral fluoropyrimidines, including capecitabine and S-1, are recommended as first-line chemotherapies [1–4]. The choice of first-line chemotherapy is thus currently determined by the individual physician. Pancreatic cancer is an aggressive disease and patient condition often deteriorates rapidly after the failure of first-line chemotherapy. Median survival with second-line chemotherapies is only 3–5 months [5, 6]. Choice of an effective first-line chemotherapy is thus vital to improve the patient’s prognosis.
Determining the chemosensitivity of an unresectable pancreatic cancer represents a major challenge. If the therapeutic effect can be predicted in the early period after starting chemotherapy, appropriate treatment can be provided while avoiding unnecessary toxicities.
The apparent diffusion coefficient (ADC) calculated from diffusion-weighted imaging (DWI) has been proposed as a non-invasive imaging biomarker [7–11]. DWI is sensitive to structural properties at the cellular level, and thus has the potential to detect and quantify cellular changes in the tumour microenvironment arising after the initiation of cancer therapy [12]. Several authors have reported promising results demonstrating that changes in ADC from baseline could provide early prediction of therapeutic effects with treatments such as chemoradiotherapy for head and neck cancer and chemotherapy for liver metastases [13–16]. Two investigators reported the usefulness of pretreatment ADC values for prediction of response to chemotherapy or chemoradiotherapy in pancreatic cancer [17, 18]. However, to date, the usefulness of a relative change in ADC during treatment of pancreatic cancer has not yet been evaluated. This study was therefore conducted to investigate whether changes in ADC on DWI could provide a sensitive biomarker to predict progression-free survival (PFS) and overall survival (OS) in the early chemotherapeutic period for patients with unresectable pancreatic cancer.
Materials and methods
Study design
All study protocols for this single-centre prospective trial were approved by the institutional review board of our hospital. Patients who had been diagnosed with unresectable pancreatic adenocarcinoma underwent DWI before and 4 weeks after initiation of chemotherapy and ADC values of the primary pancreatic tumour were determined. Chemotherapy continued until tumour progression based on a clinical practice protocol. The goal of this study was to assess the correlations between relative changes in ADC and PFS or OS using uni- and multivariate Cox regression analyses.
Study subjects
Written informed consent was obtained from all patients. All tumours were histologically confirmed as adenocarcinoma by fine-needle biopsy before treatment. The eligibility criteria were as follows: (i) no prior treatment; (ii) maximum diameter of pancreas tumour >1 cm; (iii) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2; and (iv) adequate bone marrow, kidney and liver function. The exclusion criterion was the presence of a contraindication for magnetic resonance imaging (MRI).
Treatment regimens
The following three chemotherapy regimens were used [1, 4, 19, 20]: gemcitabine alone (at a dose of 1,000 mg/m2 once weekly for 3 weeks, followed by 1 week of rest); S-1 alone (at a dose of 80 mg/m2 in divided doses twice daily for 28 days, followed by 14 days of rest); or gemcitabine plus S-1 (gemcitabine at a dose of 1,000 mg/m2 once weekly for 2 weeks followed by 1 week of rest; S-1 at a dose of 60 mg/m2 in divided doses twice daily during 14 days followed by 7 days of rest). The chemotherapy regimen was decided by physicians’ preferences, i.e. surgeons or medical oncologists. All treatments were continued until disease progression as judged by Response Evaluation Criteria in Solid Tumour (RECIST) criteria [21] or the appearance of severe toxicity.
Magnetic resonance imaging (MRI) technique
MRI was performed using a 1.5-T system (Magnetom Symphony Sonata; Siemens Medical Systems, Erlangen, Germany) with a four-channel phased array coil before and 4 weeks after the start of chemotherapy. Conventional imaging of the upper abdomen was acquired for all patients using the following sequences: T1-weighted breath-hold gradient-echo (repetition time (TR), 153 ms; echo time (TE), 2.44 ms; flip angle, 90°; field of view (FOV), 350 mm; matrix, 256 × 256; number of slices, 19; thickness, 8 mm; acquisition time, 20 s) and T2-weighted breath-hold turbo spin-echo (TR, 4,400 ms; TE, 95 ms; echo train length, 33; FOV, 350 mm; matrix, 256 × 256; number of slices, 19; thickness, 8 mm; acquisition time, 24 s). DWI was obtained using a free-breathing single-shot echo-planar sequence (TR, 1,400 ms; TE, 67 ms; EPI factor, 128; FOV, 350 mm; matrix, 128 × 128; PAT factor, 2; PAT mode, parallel imaging with GRAPPA; number of slices, 26; thickness, 7 mm; number of excitations, 2; fat saturation, CHESS method; acquisition time, 2 min 41 s). DWI was obtained under free breathing with virtual respiratory monitoring using navigator echoes (prospective acquisition correction technique). Diffusion gradients were applied with three b-values (b = 0, 500 and 1,000 s/mm2) in three directions and combined to produce a trace dataset to minimize the effect of diffusion anisotropy, which was the same technique as that used in a previous study [22]. ADC maps were calculated automatically by the MRI system. ADC was calculated using the following formula:
where b1 and b2 are the motion-probing gradient factors (diffusion factors) of sequences S1 and S2, and SI1 and SI2 are the signal intensities in these sequences.
Analysis of apparent diffusion coefficient (ADC) data
ADC values were measured using the following two techniques; (1) the selected area ADC (sADC): five regions of interest (ROIs) with a mean size of 1.55 cm2 (range; 1.14–2.76) were manually placed on the same slice of the largest tumour diameter, excluding cystic and necrotic areas and vessels, which were detected by contrast enhanced CT and MRI obtained before treatment. Their minimum value was defined as a sADC value. (2) the whole tumour ADC (wADC): ROIs were manually measured by encompassing the whole tumour including cystic and necrotic areas and vessels on the largest tumour diameter. The minimum ADC value inside of the ROIs was defined as a wADC value. These measurements were determined by agreement of experienced diagnostic radiologists (H.N. and N.M.). The relative change in ADC was determined using the following formula: the change in ADC = (ADC at 4 weeks – ADC pre)/(ADC pre × 100 %). An increase in ADC value was defined as a positive ADC change and the same or a decrease in ADC value was defined as a negative ADC change.
Assessment of therapeutic effect and evaluation
The CT protocol included a non-enhanced scan followed by arterial and delayed-phase acquisitions after intravenous injection of iodized contrast medium (100–150 mL; range of iodine concentration, 300–370 mgI/mL) at a flow rate of 3–4 mL/s. Tumour response was evaluated according to RECIST criteria using CT performed every 2 months. PFS was calculated as the time interval from the initiation of treatment to the date of disease progression. If no disease progression was observed, PFS was calculated from the initiation of treatment to the last day of follow-up or the date of death. OS was calculated as the time interval from the initiation of treatment to the date of death or to the last day of the follow-up period.
Selection of other predictive biomarkers
Based on previous reports [21–23], the following additional biochemical markers were evaluated: serum carbohydrate antigen (CA) 19-9 (CA19-9), carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH) and C-reactive protein (CRP). Relative changes in these biochemical markers at 4 weeks after initiation of treatment compared to baseline were calculated. In addition, changes in the longest diameter of pancreatic tumour between baseline and 4 weeks after initiation of chemotherapy were evaluated using contrast-enhanced CT.
Statistical analysis
All statistical analyses were conducted using SPSS version 17.0 statistical software (SPSS; Chicago, IL, USA). PFS and OS were estimated using the Kaplan-Meier method, and 95 % confidence intervals (CIs) were provided for proportions. Uni- and multivariate Cox regression analyses were performed to assess predictive markers for PFS and OS, including relative changes to ADC, CA19-9, CEA, LDH, CRP and tumour size. Cut-off values for these biomarkers were defined as 0 % in ADCs and tumour size, and 20 % in CA19-9, CEA, LDH and CRP based on previous reports [23–25]. Survival curves for PFS and OS were compared between groups using the log-rank test. Multivariate analysis was undertaken for variables demonstrating values of P < 0.10 after univariate analysis, and P < 0.05 was considered statistically significant.
Results
Patient and tumour characteristics
Patient and tumour characteristics are summarized in Table 1. A total of 45 patients were initially enrolled, but two patients dropped out because they could not undergo follow-up DWI. The remaining 43 patients (19 women, 24 men; median age, 67 years; range, 40–82 years) were analysed. Tumour stage according to the TNM classification system published by the International Union Against Cancer, 6th edition was stage III disease in ten patients (23.3 %) and stage IV disease in 33 patients (76.7 %). Liver metastases were observed in 22 patients (51.2 %), lymph node metastases in nine patients (20.9 %), lung metastases in seven patients (16.3 %) and peritoneal dissemination in six patients (14 %) at the time of primary diagnosis before initiation of therapy. Median pretreatment pancreatic tumour size was 4.0 cm in diameter (range, 2.1–7.3 cm). Median baseline levels of CA19-9 and CEA were 761 U/mL (range, 1–28,487 U/mL) and 7.8 ng/mL (range, 1.6–311 ng/mL), respectively.
Table 1.
Patient characteristics (N = 43)
| Characteristics | Patients (%) |
|---|---|
| Age (years) | |
| Median (range) | 67 (40–82) |
| Sex (%) | |
| Male | 24 (55.8) |
| Female | 19 (44.2) |
| ECOG PS (%) | |
| 0 | 16 (37.2) |
| 1 | 22 (51.2) |
| 2 | 5 (11.6) |
| Primary site (%) | |
| Head | 22 (51.2) |
| Body and tail | 21 (48.8) |
| Stage (%) | |
| III | 10 (23.3) |
| IV | 33 (76.7) |
| Chemotherapy regimen | |
| Gemcitabine | 24 (55.8) |
| TS-1 | 7 (16.2) |
| Gemcitabine plus TS-1 | 12 (28.0) |
| Previous CA19-9 (U/mL) | |
| Median (range) | 761 (1–28,487) |
| Previous CEA (ng/ml) | |
| Median (range) | 7.8 (1.6–311) |
ECOGPS = Eastern Cooperative Oncology Group performance status, CA19-9 = carbohydrate antigen 19-9, CEA = carcinoembryonic antigen
Therapeutic effect and survival
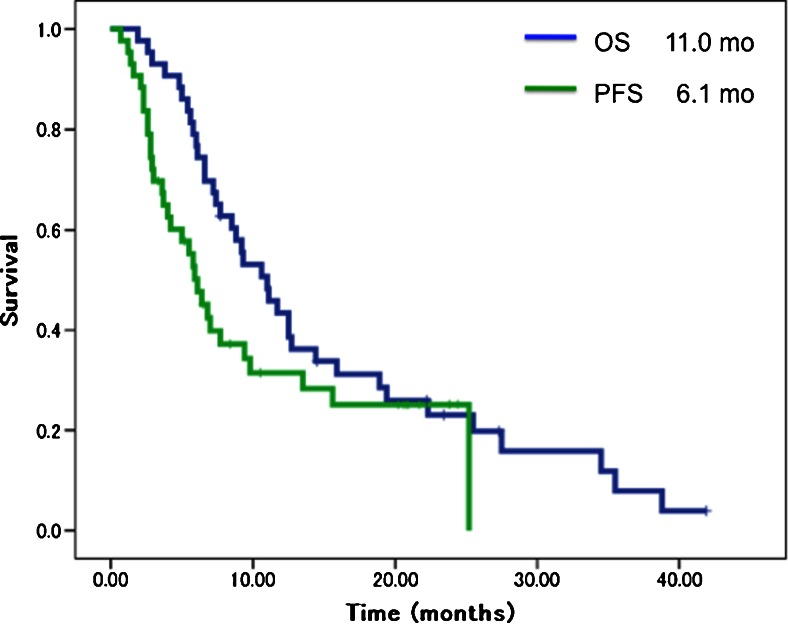
Median duration of follow-up was 11.0 months (range, 2.6–42.1 months). Five patients (11.6 %) achieved partial response, whereas the remaining 38 patients were judged as showing stable disease or progressive disease. Median PFS was 6.1 months (95 % CI, 4.5–7.7 months) and median OS was 11.0 months (95 % CI, 7.9–14.1 months) (Fig. 1). After failure of first-line chemotherapy, 28 patients (65.1 %) received second-line therapy, as follows: gemcitabine plus S-1 in ten patients (35.7 %); S-1 in 11 patients (39.3 %); S-1 plus oxaliplatin in three patients (10.7 %), gemcitabine plus hepatic arterial chemoinfusion in two patients (7.1 %), and gemcitabine plus radiotherapy in two patients (7.1 %). Mean duration of treatment with second-line chemotherapy was 2.8 months. Two patients (4.7 %) who had obtained marked tumour shrinkage with first-line chemotherapy underwent curative surgical resection. The remaining 13 patients (30.2 %) received best supportive care.
Fig. 1.
Overall survival (OS) and progression-free survival (PFS) curves. Median overall PFS and OS were 6.1 months (95 % CI, 4.5–7.7) and 11.0 months (95 % CI, 7.9–14.1), respectively
Predictive biomarkers
The sADC values before treatment and at 4 weeks after initiation of chemotherapy were 1.10 ± 0.14 mm2/sec and 1.15 ± 0.20 × 10-3 mm2/sec, respectively. The wADC values before and 4 weeks after treatment were 1.03 ± 0.14 mm2/sec and 1.09 ± 0.19 × 10-3 mm2/sec, respectively. In sADC, 28 positive ADC changes and 15 negative ADC changes were seen, while in wADC, 27 positive ADC changes and 16 negative ADC changes were seen.
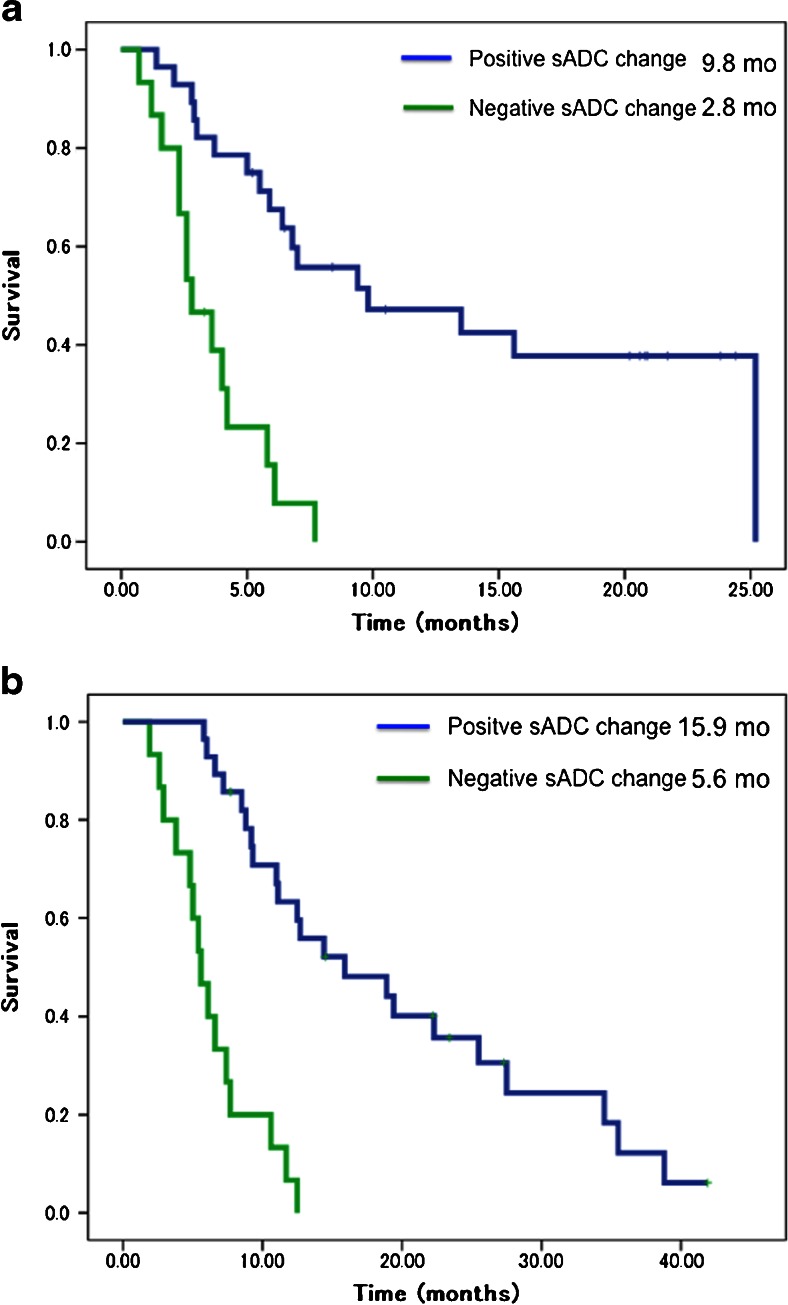
Uni- and multivariate analyses of early predictive markers for PFS are shown in Table 2. Multivariate analysis revealed sADC change, CRP and tumour size as independent predictive markers for PFS, and sADC change was the strongest of these (HR, 4.5; 95 % CI, 1.7–11.9; p = 0.002). Figure 2a shows Kaplan-Meier curves for PFS in the positive sADC change group (n = 28) and the negative sADC change group (n = 15). Median PFS was 9.8 months (95 % CI, 0.4–19.2) in the positive sADC change group, compared to 2.8 months (95 % CI, 1.6–4.0) in the negative sADC change group (p = 0.001).
Table 2.
Uni- and multivariate analyses of predictive markers for progression-free survival
| Factors | Pts | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| P | HR | 95%CI | P | HR | 95%CI | ||
| sADC (0% =>) | 15 | 0.001 | 5.3 | 2.3- 11.9 | 0.002 | 4.5 | 1.7- 11.9 |
| wtADC (0%=>) | 16 | 0.525 | 1.3 | 0.6- 2.6 | |||
| CA 19–9 (20%<) | 10 | 0.097 | 1.9 | 0.9- 4.3 | 0.345 | 1.6 | 0.6- 3.9 |
| CEA (20%<) | 10 | 0.216 | 1.7 | 0.7- 3.8 | |||
| CRP (20%<) | 12 | 0.001 | 3.7 | 1.7- 7.9 | 0.011 | 2.9 | 1.3- 6.4 |
| LDH (20%<) | 13 | 0.765 | 1.1 | 0.5- 2.5 | |||
| Tumour size (0%<) | 5 | 0.001 | 20.6 | 5.3- 79.9 | 0.029 | 5.7 | 1.2- 27.1 |
CA19-9 = carbohydrate antigen 19-9, sADC = selected area apparent diffusion coefficient, wADC = whole tumour apparent diffusion coefficient, Pts = patients, HR = hazard ratio, CI = confidence interval, CEA = carcinoembryonic antigen, LDH = lactate dehydrogenase, CRP = C-reactive protein
Fig. 2.
(a) Progression-free survival (PFS) per relative change in selected area apparent diffusion coefficient (sADC). Patients with positive sADC change (n = 28) showed a significantly better median PFS than patients with a negative sADC change (n = 15) (9.8 months, 95 % CI, 0.4–19.2, vs. 2.8 months, 95 % CI, 1.6–4.0 months; p = 0.001). (b) Overall survival (OS) per relative change in sADC. Patients with a positive sADC change (n = 28) had a significantly better median survival than patients with a negative sADC change (n = 15) (15.9 mo, 95 % CI, 5.9–25.8, vs. 5.6 mo, 95 % CI, 4.2– 7.0; p = 0.001)
Regarding the correlation with OS, multivariate analysis identified sADC change and CRP as independent predictive markers, with sADC change as the strongest (HR, 6.7; 95 % CI, 2.7–16.6; p = 0.001) (Table 3). Median OSs in the positive sADC change and negative sADC change groups were 15.9 months (HR, 5.1; 95 % CI, 5.9–25.8, n = 28) and 5.6 months (HR, 0.70; 95 % CI, 4.2–7.0, n = 15; p = 0.001), respectively (Fig. 2b).
Table 3.
Uni- and multivariate analyses of predictive markers for overall survival
| Factors | Pts | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| P | HR | 95%CI | P | HR | 95%CI | ||
| sADC (0% =>) | 15 | 0.001 | 6.8 | 3.0- 15.2 | 0.001 | 6.7 | 2.7- 16.6 |
| wtADC (0%=>) | 16 | 0.657 | 0.9 | 0.4- 1.7 | |||
| CA19-9 (20%<) | 10 | 0.602 | 1.2 | 0.6- 2.7 | |||
| CEA (20%<) | 10 | 0.396 | 1.4 | 0.6- 3.0 | |||
| CRP (20%<) | 12 | 0.002 | 3.3 | 1.6- 7.0 | 0.016 | 2.6 | 1.2- 5.6 |
| LDH (20%<) | 13 | 0.978 | 1.0 | 0.5- 2.0 | |||
| Tumour size (0%<) | 5 | 0.015 | 3.4 | 1.3- 9.0 | 0.545 | 0.7 | 0.2- 2.1 |
CA19-9 = carbohydrate antigen 19-9, sADC = selected area apparent diffusion coefficient, wADC = whole tumour apparent diffusion coefficient, Pts = patients, HR = hazard ratio, CI = confidence interval, CEA = carcinoembryonic antigen, LDH = lactate dehydrogenase, CRP = C-reactive protein
Discussion
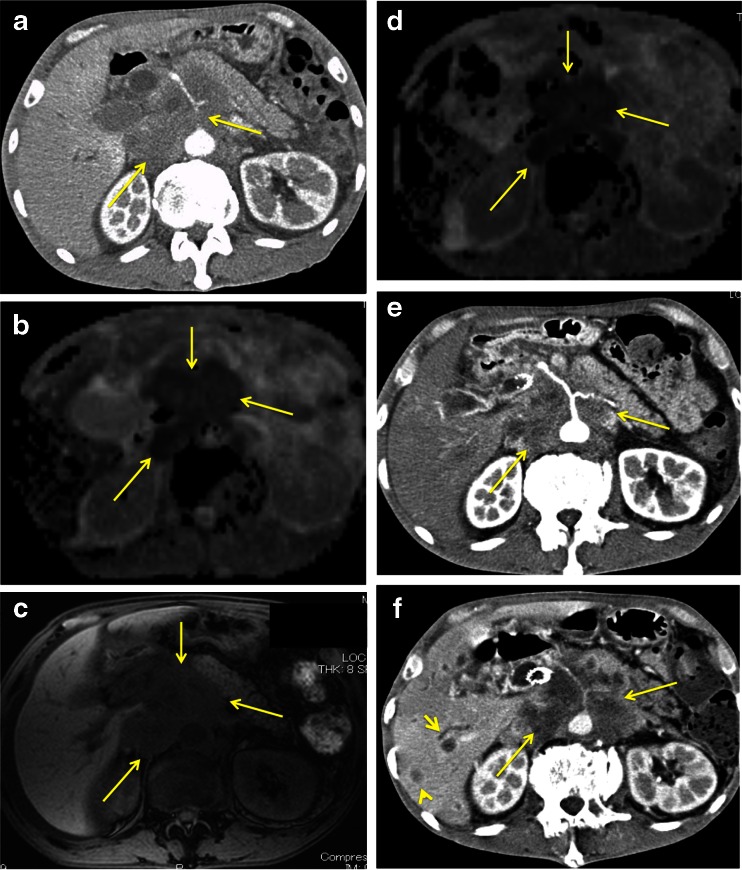
The current results revealed that sADC change was significantly associated with PFS. This means that sADC change can predict the therapeutic efficacy of chemotherapy for unresectable pancreatic cancer at 4 weeks after the initiation of treatment. Our results also demonstrated sADC change as a strong predictive marker of OS. This was because the survival period after the failure of first-line chemotherapy was extremely short. Only 65 % of patients received second-line chemotherapy and, in addition, the mean treatment duration with second-line chemotherapy was only 2.8 months (Fig. 3).
Fig. 3.
A 59-year-old man with pancreatic head cancer. (a) Contrast-enhanced CT before initiation of treatment shows encasement of the coeliac, common hepatic and splenic arteries (arrow), and retroperitoneal invasion (arrow) by a tumour with a diameter of 6.4 cm arising in the pancreatic head. (b) Apparent diffusion coefficient (ADC) map obtained by diffusion-weighted imaging (DWI) before initiation of treatment shows a low selected area apparent diffusion coefficient (sADC) value (0.95 × 10-3 mm2/s) (arrow). (c) T1-weighted image on magnetic resonance imaging after 4 weeks from starting chemotherapy showed no change in primary tumour size (6.4 cm in diameter) (arrow). (d) ADC map obtained by DWI after 4 weeks from starting chemotherapy demonstrated a decrease in sADC value (0.75 × 10-3 mm2/s) (arrow). (e) Contrast-enhanced CT after 8 weeks from starting chemotherapy showed no change in primary tumour size (diameter, 6.4 cm) (arrow). (f) Contrast-enhanced CT after 3 months from starting chemotherapy demonstrated the multiple liver metastases (arrowhead) and the increased primary tumour (arrow). This patient survived for only 5.6 months from the initiation of first-line chemotherapy using gemcitabine
In this study, we evaluated minimum ADC values using two techniques, sADC and wADC. The change in sADC was a significantly predictive factor for survival, whereas the change in wADC was not. These results could indicate the importance of excluding cystic and necrotic areas and vessels from the measurement areas of ADC. The wADC values might be also influenced by susceptibility artefacts due to gastrointestinal air.
RECIST criteria are generally used for the evaluation of chemotherapy for pancreatic cancer. However, the timing of assessment is often delayed because performance status has already become poor by the time the judgement of disease progression is delivered. The patient could thus lose the opportunity to receive effective second-line treatment. Histological changes in tumour tissues occur prior to changes in tumour size. DWI can provide information on tissue cellularity and the integrity of cellular membranes [26]. Several studies have reported that the ADC obtained from DWI is useful to provide an early prediction of tumour response [8–11, 13–18].
Previously, Niwa et al. [17] and Cuneo et al. [18] reported that pretreatment ADC values were useful to evaluate the tumour response in pancreatic cancer. In contrast, our study demonstrated the effectiveness of relative change in ADC value during treatment to predict OS and PFS. The measurement techniques were also different: Niwa et al. [17] and Cuneo et al. [18] used mean ADC value, while we used minimum ADC value.
This study evaluated minimum ADC due to the mechanism of tumour growth for pancreatic cancer. Pancreatic stellate cells, which promote tumour growth, increase deposition of connective tissue in tumour [27]. Muraoka et al. [28] clinically reported that fibrosis of connective tissue in pancreatic tumour, which showed a low ADC value, was a significant representation of malignancy. We therefore considered that changes in the minimum ADC during treatment could represent the tumour response.
Several studies have examined biomarkers for chemotherapy for pancreatic cancer. Although the serum CA19-9 level is the most commonly used in clinical practice, a large-scale prospective trial showed that a decrease in CA19-9 levels at 42 days after starting gemcitabine and/or capecitabine was not significantly associated with OS [29]. Haas et al. [23] reported that changes in CEA and CRP during chemotherapy were independently associated with the prognosis of second-line chemotherapy. Cutsem et al. [30] also identified changes in serum CRP and LDH during first-line chemotherapy as prognostic factors [30]. Our results demonstrated a change in minimum ADC to be the strongest predictive marker when compared with CA19-9, CEA, LDH, CRP and tumour size.
Gemcitabine and oral fluoropyrimidine are currently in common use for pancreatic cancer [1, 2, 4–6]. In cases where gemcitabine is applied as the first-line chemotherapy and fails, oral fluoropyrimidine is used. Likewise, in cases where oral fluoropyrimidine is applied first and fails, gemcitabine is used. To prolong survival, the regimen to which the tumour is more sensitive should be used first because, as mentioned above, the opportunities for second-line chemotherapy appear limited due to the rapid tumour growth with the worsening of the patient’s performance status. To date, no useful chemo-sensitivity tests have been reported for pancreatic cancer. According to our results, DWI could predict chemo-sensitivity in the early period after starting chemotherapy.
Several limitations must be considered when interpreting the results of the present study. First, the patient population was relatively small. Second, the measurement reproducibility of sADC was not assessed in this study. However, we instead measured at least five ROIs in each tumour. Third, we performed evaluations only at 4 weeks after the start of chemotherapy for ethical reasons. Namely, additional MRI examinations could not be approved by our IRB, since results of this study did not contribute to decisions on therapeutic strategy. Because we obtained positive data after 4 weeks, further study is warranted to elucidate whether it is also possible to detect the response by performing DWI after 1 or 2 weeks.
In conclusion, the relative change in sADC at 4 weeks from the initiation of treatment could serve as a sensitive imaging biomarker for the therapeutic effect and prognosis of unresectable pancreatic cancer. Further study is needed to clarify the role of ADC in determining treatment strategies for unresectable pancreatic cancer.
Acknowledgments
The scientific guarantor of this publication is Prof. Kichikawa at Nara Medical University. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work was partially supported by Nara Medical University Grant-in-Aid for Collaborative Research Projects and Pancreas Research Foundation of Japan. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all patients in this study. Some study subjects had been previously presented at the ECR in 2011. Methodology: This was a prospectively prognostic study performed at a single institution.
References
- 1.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 4.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 5.Xiong HQ, Varadhachary GR, Blais JC, Hess KR, Abbruzzese JL, Wolff RA. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008;113:2046–2052. doi: 10.1002/cncr.23810. [DOI] [PubMed] [Google Scholar]
- 6.Altwegg R, Ychou M, Guillaumon V, et al. Second-line therapy for gemcitabine-pretreated advanced or metastatic pancreatic cancer. World J Gastroenterol. 2012;18:1357–1364. doi: 10.3748/wjg.v18.i12.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth Y, Tichler T, Kostenich G, et al. High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology. 2004;232:685–692. doi: 10.1148/radiol.2322030778. [DOI] [PubMed] [Google Scholar]
- 8.Higano S, Yun X, Kumabe T, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241:839–846. doi: 10.1148/radiol.2413051276. [DOI] [PubMed] [Google Scholar]
- 9.Marugami N, Tanaka T, Kitano S, et al. Early detection of therapeutic response to hepatic arterial infusion chemotherapy of liver metastases from colorectal cancer using diffusion-weighted MR imaging. Cardiovasc Intervent Radiol. 2009;32:638–646. doi: 10.1007/s00270-009-9532-8. [DOI] [PubMed] [Google Scholar]
- 10.De Cobelli F, Giganti F, Orsenigo E, et al. Apparent diffusion coefficient modifications in assessing gastro-oesophageal cancer response to neoadjuvant treatment: comparison with tumour regression grade at histology. Eur Radiol. 2013;23:2165–2174. doi: 10.1007/s00330-013-2807-0. [DOI] [PubMed] [Google Scholar]
- 11.Wagner M, Ronot M, Doblas S, et al. Assessment of the residual tumour of colorectal liver metastases after chemotherapy: diffusion-weighted MR magnetic resonance imaging in the peripheral and entire tumour. Eur Radiol. 2015 doi: 10.1007/s00330-015-3800-6. [DOI] [PubMed] [Google Scholar]
- 12.Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248:894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Loevner L, Quon H, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–994. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun YS, Zhang XP, Tang L, et al. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology. 2010;254:170–178. doi: 10.1148/radiol.2541082230. [DOI] [PubMed] [Google Scholar]
- 16.King AD, Chow KK, Yu KH, et al. Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology. 2013;266:531–538. doi: 10.1148/radiol.12120167. [DOI] [PubMed] [Google Scholar]
- 17.Niwa T, Ueno M, Ohkawa S, et al. Advanced pancreatic cancer: the use of the apparent diffusion coefficient to predict response to chemotherapy. Br J Radiol. 2009;82:28–34. doi: 10.1259/bjr/43911400. [DOI] [PubMed] [Google Scholar]
- 18.Cuneo KC, Chenevert TL, Ben-Josef E, et al. A pilot study of diffusion-weighted MRI in patients undergoing neoadjuvant chemoradiation for pancreatic cancer. Transl Oncol. 2014;7:644–649. doi: 10.1016/j.tranon.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueno H, Okusaka T, Ikeda M, et al. An early phase II study of S-1 in patients with metastatic pancreatic cancer. Oncology. 2005;68:171–178. doi: 10.1159/000086771. [DOI] [PubMed] [Google Scholar]
- 20.Ueno H, Okusaka T, Furuse J, et al. Multicenter phase II study of gemcitabine and S-1 combination therapy (GS Therapy) in patients with metastatic pancreatic cancer. Jpn J Clin Oncol. 2011;41:953–958. doi: 10.1093/jjco/hyr090. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Lee SS, Byun JH, Park BJ, et al. Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging. 2008;28:928–936. doi: 10.1002/jmri.21508. [DOI] [PubMed] [Google Scholar]
- 23.Haas M, Laubender RP, Stieber P, et al. Prognostic relevance of CA 19-9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumour Biol. 2010;31:351–357. doi: 10.1007/s13277-010-0044-6. [DOI] [PubMed] [Google Scholar]
- 24.Sawaki A, Kanemitsu Y, Mizuno N, et al. Practical prognostic index for patients with metastatic pancreatic cancer treated with gemcitabine. J Gastroenterol Hepatol. 2008;23:1292–1297. doi: 10.1111/j.1440-1746.2006.04734.x. [DOI] [PubMed] [Google Scholar]
- 25.Maréchal R, Demols A, Gay F, et al. Prognostic factors and prognostic index for chemonaïve and gemcitabine-refractory patients with advanced pancreatic cancer. Oncology. 2007;73:41–51. doi: 10.1159/000120627. [DOI] [PubMed] [Google Scholar]
- 26.Koh DM, Padhani AR. Diffusion-weighted MRI: a new functional clinical technique for tumour imaging. Br J Radiol. 2006;79:633–635. doi: 10.1259/bjr/29739265. [DOI] [PubMed] [Google Scholar]
- 27.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Muraoka N, Uematsu H, Kimura H, et al. Apparent diffusion coefficient in pancreatic cancer: characterization and histopathological correlations. J Magn Reson Imaging. 2008;27:1302–1308. doi: 10.1002/jmri.21340. [DOI] [PubMed] [Google Scholar]
- 29.Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour–marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132–138. doi: 10.1016/S1470-2045(08)70001-9. [DOI] [PubMed] [Google Scholar]
- 30.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]