Abstract
Background
A need exists for topical treatments in managing more severe inflammatory acne.
Objectives
The objectives of this study were to evaluate the efficacy and safety of adapalene 0.3 %/benzoyl peroxide 2.5 % (0.3 % A/BPO) topical gel in subjects with moderate and severe inflammatory acne.
Methods
This was a multicenter, randomized, double-blind, parallel-group study. Randomization was stratified by acne severity (50 % moderate and 50 % severe). Subjects received 0.3 % A/BPO, 0.1 % A/BPO (benchmark), or vehicle (comparator) once daily for 12 weeks. Co-primary efficacy endpoints were success rate at week 12 (the percentage of subjects rated ‘clear’ or ‘almost clear’ with at least a 2-grade improvement on Investigator’s Global Assessment [IGA]) and change in inflammatory (IN) and noninflammatory (NIN) lesion counts from baseline to week 12. Secondary efficacy endpoints were percent changes in IN and NIN lesion counts. Safety endpoints were incidence of adverse events (AEs) and local tolerability signs/symptoms.
Results
A total of 503 subjects were randomized: 217, 217, and 69 subjects in the 0.3 % A/BPO, 0.1 % A/BPO, and vehicle groups, respectively. For success rate (subjects rated ‘clear’ or ‘almost clear’ with ≥2-grade improvement in IGA), 0.3 % A/BPO was superior to vehicle, with a treatment difference of 22.7 % (33.7 vs. 11.0 %; 95 % confidence interval [CI] 12.8–32.6, p < 0.001). At week 12, 0.3 % A/BPO was superior to vehicle for mean reduction from baseline in IN (27.0 vs. 14.4) and NIN lesion counts (40.2 vs. 18.5), as well as for percentage reduction from baseline in IN (68.7 vs. 39.2 %) and NIN lesion counts (68.3 vs. 37.4 %) (all p < 0.001). Among subjects with severe inflammatory acne (IGA = 4), 0.1 % A/BPO did not reach statistical significance for success rate compared with vehicle (p = 0.443), whereas 0.3 % A/BPO demonstrated significantly greater efficacy (p = 0.029, requiring ≥3-point IGA improvement). Additionally, 0.3 % A/BPO was safe and well-tolerated.
Conclusions
Results of this clinical trial demonstrate the significantly greater efficacy of adapalene 0.3 % A/BPO topical gel compared with vehicle as well as a good safety profile in the treatment of moderate to severe inflammatory non-nodulocystic acne, which increases patients’ treatment options.
Clinicaltrials.gov identifier
Key Points
| Few studies with validated topical treatment options exist for the management of severe acne. Results of this multicenter, randomized, double-blind, parallel-group clinical trial demonstrate the significantly greater efficacy of adapalene 0.3 %/benzoyl peroxide 2.5 % topical gel compared with vehicle as well as a good safety profile in the treatment of moderate to severe inflammatory non-nodulocystic acne, and particularly high efficacy in subjects with severe inflammatory acne, which increases patients’ treatment options. |
| A higher concentration of A/BPO will increase treatment options for patients with moderate and severe inflammatory acne. |
Introduction
Acne represents a substantial disease burden, and thus effective treatment is crucial. The Global Burden of Disease study covering 187 countries found acne to be among the top ten most prevalent diseases worldwide in 2010, and skin conditions were one of the top causes of years lived with disability [1]. There is growing evidence that acne negatively affects quality of life [2]. Early, effective treatment of acne is essential and is important to avoid later complications, including scarring and psychosocial issues [3].
A need exists for topical treatments in the management of patients with more severe inflammatory acne. A variety of topical therapies already exist for the treatment of mild to moderate acne vulgaris, and a variety of treatments are used in combination [4]. The efficacy and safety of adapalene 0.1 % gel has been demonstrated in multiple randomized controlled trials (RCTs). A higher concentration of the formulation, adapalene 0.3 % gel, expanded treatment options and, in phase III studies, demonstrated superior efficacy and a safety profile similar to that of adapalene 0.1 % gel and its vehicle [5, 6]. Its long-term safety and efficacy have been shown over 12 months [7]. Moreover, noninferior efficacy and superior tolerability were found compared with tazarotene 0.1 % gel [8].
The rationale for combination therapy with a topical retinoid and antimicrobial agent is well-documented, particularly for adapalene 0.1 %/benzoyl peroxide (BPO) 2.5 % fixed-dose combination gel (hereafter, 0.1 % A/BPO, Epiduo®, Galderma Laboratories, LP, Fort Worth, TX, USA), in the interests of limiting antibiotic resistance and simplifying therapy by targeting several etiopathogenic factors of acne. BPO is the gold standard bactericidal agent and is not associated with antibiotic resistance. Adapalene exerts comedolytic, anti-comedogenic effects (the microcomedo being the precursor lesion targeted by retinoids), and demonstrates anti-inflammatory properties in both in vitro and in vivo research as well as clinical studies [8–12]. Moreover, adapalene has been found to down-regulate the expression of toll-like receptor 2 (TLR2) [10, 12], a receptor involved in the pathogenesis of acne lesions through Propionibacterium acnes-mediated activation, which subsequently induces proinflammatory cytokines [13].
Combining a topical retinoid with BPO is supported by the Global Alliance to Improve Outcomes in Acne guidelines [4, 14] and European guidelines for acne treatment [15]. To date, topical therapies alone, including 0.1 % A/BPO, have been recommended for the treatment of mild to moderate papulopustular acne, and in combination with an oral antibiotic for moderate to severe papulopustular acne. According to more recent pediatric treatment guidance from Eichenfield et al. [16], the recommendations diverge for treatment of moderate acne, where topical combination therapy (topical retinoid and BPO with or without a topical antibiotic) is advised as an additional option to the traditional oral antibiotic with topical retinoid and BPO. All of these guidelines suggest the following limited choices in severe, non-nodulocystic acne treatment: either oral isotretinoin or a topical combination (retinoid and BPO) with oral antibiotics used for all but the most severe forms of acne. Thus, a topical therapy in a single formulation that is strong enough to improve severe inflammatory acne as a standalone or adjunct alternative treatment choice for a significant number of acne patients without the need for oral isotretinoin (and its associated side effects) or concomitant antibiotic therapy (to reduce microbial resistance) represents a substantial unmet need.
Data from three RCTs among 3855 subjects in total, of whom 983 received 0.1 % A/BPO, demonstrated that it provided synergistic and significantly greater efficacy than its monotherapies in the treatment of acne vulgaris, with an acceptable safety profile [17]. Synergism was defined as the benefit of the combination greater than the sum of benefits of adapalene and BPO monotherapies [17]. Furthermore, the benefit of A/BPO relative to vehicle has been shown to increase with higher lesion counts at baseline [18]. A synergistic and potent anti-inflammatory effect could thus be inferred with a higher concentration of adapalene combined with BPO, which would be essential in a more severe population.
To date, topical treatments alone are not perceived as suited for treatment of the severe papulopustular, non-nodulocystic acne population. To evaluate the potential for a higher concentration of adapalene in a fixed-dose combination with BPO in meeting this need in a broader segment of patients, the current study aimed to evaluate the efficacy and safety of adapalene 0.3 %/benzoyl peroxide 2.5 % (0.3 % A/BPO) gel as single-agent therapy in subjects with moderate and severe inflammatory acne.
Methods
Study Design
This was a multicenter, randomized, double-blind, parallel-group, 12-week study (Clinicaltrials.gov identifier: NCT01880320) conducted at 31 centers in the USA and Canada from July 2013 to March 2014, with vehicle gel used as a comparator. The objectives of this study were to analyze the efficacy and safety evaluation of 0.3 % A/BPO in the overall (moderate and severe) population as well as in the severe population (Investigator’s Global Assessment [IGA] score of 4 [the IGA being a 5-point scale from 0 = ‘clear’ to 4]). The 0.1 % A/BPO group was used as a benchmark; since this study was neither designed nor powered for superiority testing between the active groups, the results presented herein focus on 0.3 % A/BPO and vehicle. Although comparison between 0.1 % A/BPO and vehicle was not a specific objective of this study, results of this comparison are exploratory and are provided herein for completeness. Following a screening visit, study visits were planned at baseline, and at weeks 1, 2, 4, 8, and 12.
Subjects
Male and female subjects with moderate to severe inflammatory facial acne, meaning a score of 3 (moderate) or 4 (severe) on the IGA, the presence of 20–100 inflammatory (IN) lesions, 30–150 non-inflammatory (NIN) lesions (including the nose), and up to two nodules on the face. Randomization was stratified by acne severity, with 50 % having moderate and 50 % severe acne. Main exclusion criteria included acne conglobata, acne fulminans, nodulo-cystic acne, or acne requiring systemic treatment. A urine pregnancy test was required for female subjects at the screening, baseline, and the week 4, 8, and 12 study visits.
Study Treatment
Subjects were randomized in a 3:3:1 ratio to receive either 0.3 % A/BPO, 0.1 % A/BPO, or vehicle once daily for 12 weeks to the face (and trunk if applicable) in the evening after washing. Each subject received both verbal and written instructions for proper dosing and study treatment application techniques. Subjects were requested to use a gentle cleanser and moisturizer throughout the study. They were instructed to use the cleanser twice daily and apply the moisturizer at least once daily, and liberal application of moisturizer was encouraged (e.g., up to three times daily). If a subject experienced persistent dryness or irritation, the investigator was permitted to consider less frequent applications of the study product as required for the symptomatic relief of skin dryness or irritation. If the once-daily treatment regimen was altered to every other day, the investigator had to attempt to return the subject to once-daily treatment within 2 weeks.
Efficacy Endpoints
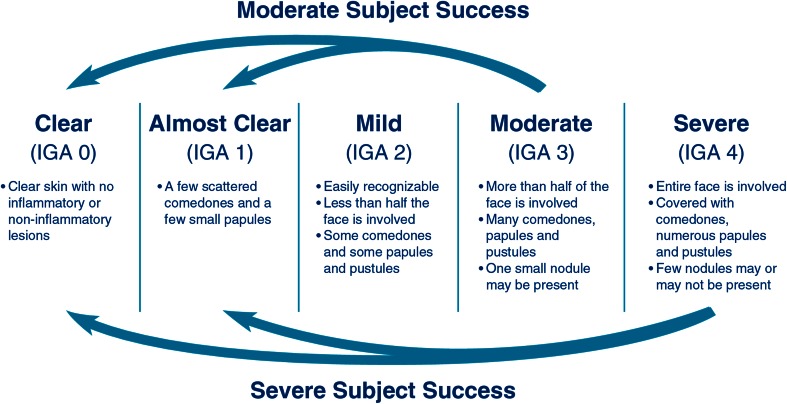
The co-primary efficacy endpoints were success rate at week 12 (defined as the percentage of subjects rated ‘clear’ or ‘almost clear’, with at least a 2-grade improvement on the IGA for subjects rated as moderate, and at least a 3-grade improvement on the IGA for subjects rated as severe at the baseline visit (Fig. 1), and change in IN and NIN lesion counts from baseline to week 12. Secondary efficacy endpoints were percent changes in IN and NIN lesion counts.
Fig. 1.
Investigator’s Global Assessment (IGA) scale
Safety Endpoints
Safety endpoints were the incidence of adverse events (AEs) reported throughout the duration of the study, as well as local tolerability signs and symptoms (erythema, scaling, dryness, and stinging/burning) evaluated by investigators at each visit on a 4-point scale from 0 (none) to 3 (severe).
Other Endpoints
At week 12, subjects completed both a questionnaire about their satisfaction regarding treatment and a questionnaire regarding their assessment of acne improvement on a scale of 0 (complete improvement) to 5 (worse).
Sample Size
The study was powered primarily to detect efficacy in the ‘severe’ population, with 90 % power. This resulted in a 99 % power for the combined analysis of moderate and severe subjects. Based on previous data, we expected that the success rate among subjects with moderate to severe acne would be 35.5 % for 0.3 % A/BPO and 10 % for vehicle. A sample size of 213 subjects in the 0.3 % A/BPO group and 71 subjects in the vehicle group would ensure 99 % power to detect a statistically significant difference in success rate between the 0.3 % A/BPO and vehicle groups for the per protocol (PP) population, assuming that 15 % of subjects would not be evaluable for the PP population.
Randomization and Blinding
Prior to the start of the study, a randomization list was generated by a statistician. At the baseline visit, study personnel used an Interactive Response Technology (IRT) system to allocate unique kit numbers to eligible subjects. Randomization was stratified in order to reach an equal proportion (50 %) of subjects with moderate and severe acne. The study design was considered double-blind based on the following: the topical medication was packaged in identical tubes, medication was dispensed by a third party, and the randomization list was locked, with access restricted to designated personnel.
Statistical Methods
We used the Statistical Analysis System (SAS Institute Inc., Cary, NC, USA) and performed statistical analyses on the following subject populations: intent-to-treat (ITT; all randomized subjects), PP (ITT subjects who met all major protocol criteria), and safety (ITT subjects who applied the study drug at least once). For the primary efficacy endpoints, success rates were analyzed via the Cochran–Mantel–Haenszel (CMH) methodology using center and acne severity as strata. Changes in lesion counts were evaluated through analysis of covariance (ANCOVA) using baseline counts as a covariate and acne severity, center, and treatment as factors. For the secondary efficacy endpoints (percent changes in IN and NIN lesion counts), the CMH test row mean difference statistic using the relative to an identified distribution (RIDIT) score and stratified by center and acne severity was used, and control of multiplicity was achieved using the Hochberg procedure. Multiple imputation (MI) methodology was used as the primary imputation method for missing values. The questionnaires were analyzed by CMH test row mean difference statistic using the RIDIT score.
Results
Subject Disposition and Demographics/Clinical Characteristics
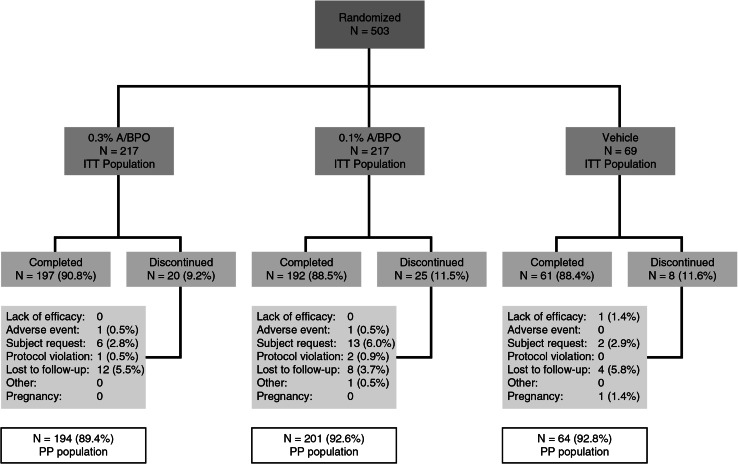
After screening, 503 subjects from 31 sites were randomized, with 217, 217, and 69 subjects randomized into the 0.3 % A/BPO, 0.1 % A/BPO, and vehicle groups, respectively. The rate of study completion was high (N = 450; 89.5 % overall, ranging from 88.4 % [vehicle] and 88.5 % [0.1 % A/BPO] to 90.8 % [0.3 % A/BPO]), with two subjects discontinuing early due to AEs: one (0.5 %) in the 0.3 % A/BPO group (atopic dermatitis flare) and one (0.5 %) in the 0.1 % A/BPO group (worsening of acne). The PP population comprised 459 subjects (91.3 %) (Fig. 2). The most common reason for early study discontinuation was loss to follow-up (3.7–5.8 % across groups). Baseline demographics and clinical characteristics were similar between groups (Table 1). Slightly over half of subjects (52.3 %) were female, and the majority (77.3 %) were Caucasian, with a mean age of 19.6 years. Over one-third (36.5–41 % across groups) of subjects had phototypes IV to VI. Approximately 50 % of subjects had moderate acne (IGA = 3) and the other half severe acne (IGA = 4). Additionally, a large proportion of subjects were Hispanic or Latino (from 20.3 % for vehicle to 27.2 % for 0.3 % A/BPO).
Fig. 2.
Subject disposition. A adapalene, BPO benzoyl peroxide, ITT intent to treat, PP per protocol
Table 1.
Baseline demographics and clinical characteristics
| Demographics | 0.3 % A/BPO (N = 217) | 0.1 % A/BPO (N = 217) | Vehicle (N = 69) |
|---|---|---|---|
| Female sex | 113 (52.1 %) | 114 (52.5 %) | 36 (52.2 %) |
| Age, years (min, max) | 20.1 ± 7.6 (12, 57) | 19.4 ± 6.8 (12, 49) | 18.5 ± 5.7 (12, 36) |
| Race | |||
| Caucasian | 171 (78.8 %) | 166 (76.5 %) | 52 (75.4 %) |
| Black/African American | 35 (16.1 %) | 37 (17.1 %) | 13 (18.8 %) |
| Asian | 5 (2.3 %) | 3 (1.4 %) | 2 (2.9 %) |
| Other | 6 (2.8 %) | 10 (4.6 %) | 1 (1.4 %) |
| Ethnicity | |||
| Hispanic or Latino | 59 (27.2 %) | 56 (25.8 %) | 14 (20.3 %) |
| Skin phototype | |||
| I | 8 (3.7 %) | 5 (2.3 %) | 6 (8.7 %) |
| II | 54 (24.9 %) | 59 (27.2 %) | 20 (29.0 %) |
| III | 66 (30.4 %) | 74 (34.1 %) | 17 (24.6 %) |
| IV | 52 (24.0 %) | 37 (17.1 %) | 11 (15.9 %) |
| V | 17 (7.8 %) | 21 (9.7 %) | 9 (13.0 %) |
| VI | 20 (9.2 %) | 21 (9.7 %) | 6 (8.7 %) |
| Baseline clinical characteristics | |||
| IGA | |||
| 3 = moderate | 111 (51.2 %) | 105 (48.4 %) | 35 (50.7 %) |
| 4 = severe | 106 (48.8 %) | 112 (51.6 %) | 34 (49.3 %) |
| Total lesion count | 98.5 ± 39.0 | 98.0 ± 39.2 | 97.6 ± 36.6 |
| IN lesion counts | 39.2 ± 18.6 | 37.7 ± 16.2 | 36.4 ± 16.5 |
| NIN lesion counts | 58.9 ± 26.9 | 59.9 ± 29.4 | 60.7 ± 28.2 |
| Baseline lesions on trunk (yes) | 107 (49.3 %) | 107 (49.3 %) | 35 (50.7 %) |
Data are presented as mean ± standard deviation or number (percentage)
A adapalene, BPO benzoyl peroxide, IGA Investigator’s Global Assessment, IN inflammatory, NIN noninflammatory
Efficacy
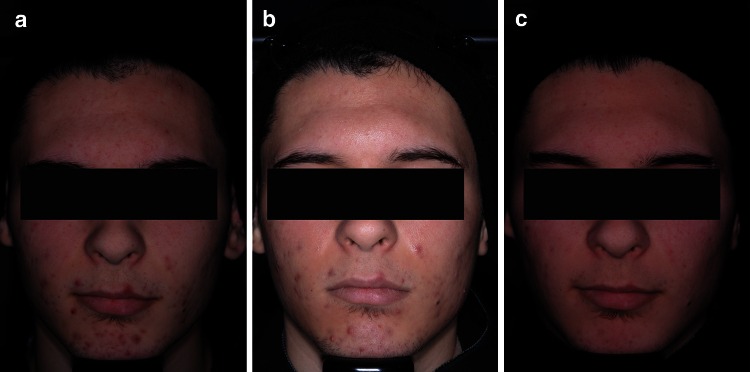
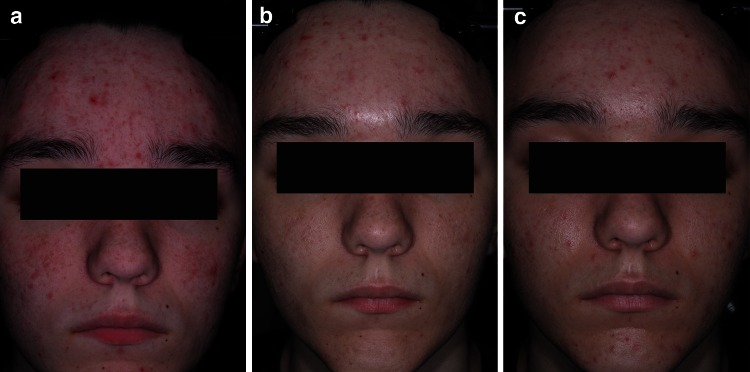
For success rate (subjects rated ‘clear’ or ‘almost clear’, with at least 2-grade improvement in IGA), 0.3 % A/BPO was superior to vehicle (MI, ITT population), with a treatment difference of 22.7 % (33.7 vs. 11.0 %; 95 % confidence interval [CI] 12.8–32.6, p < 0.001) (Table 2). This marked difference was also significant at week 8, where the success rate was about 10 % superior over vehicle (p = 0.017). Furthermore, a treatment difference of 16.3 % over vehicle was observed for 0.1 % A/BPO in terms of success rate. Figures 3 and 4 depict two subjects who experienced treatment success and failure, respectively.
Table 2.
Co-primary efficacy endpoint: success ratea (% IGA clear/almost clear, ITT)
| Success rate | ||
|---|---|---|
| 0.3 % A/BPO | Vehicle | |
| Week 1 | 1 | 0 |
| Week 2 | 3.0 | 0 |
| Week 4 | 4.3 | 1.5 |
| Week 8 | 13* | 3.1 |
| Week 12 | 33.7** | 11 |
A adapalene, BPO benzoyl peroxide, IGA Investigator’s Global Assessment, ITT intent to treat population
* p < 0.05 vs. vehicle
** p < 0.001 vs. vehicle
aMultiple imputation data; post hoc analysis performed prior to week 12
Fig. 3.
Photographs of subject in 0.3 % A/BPO group with severe acne (IGA = 4) at (a) baseline, (b) week 1, and (c) week 12 with treatment success (IGA = 1, ‘almost clear’). A adapalene, BPO benzoyl peroxide, IGA Investigator’s Global Assessment
Fig. 4.
Photographs of subject in 0.3 % A/BPO group with severe acne (IGA = 4) at (a) baseline, (b) week 1, and (c) week 12 with treatment failure (IGA = 3, ‘moderate’). A adapalene, BPO benzoyl peroxide, IGA Investigator’s Global Assessment
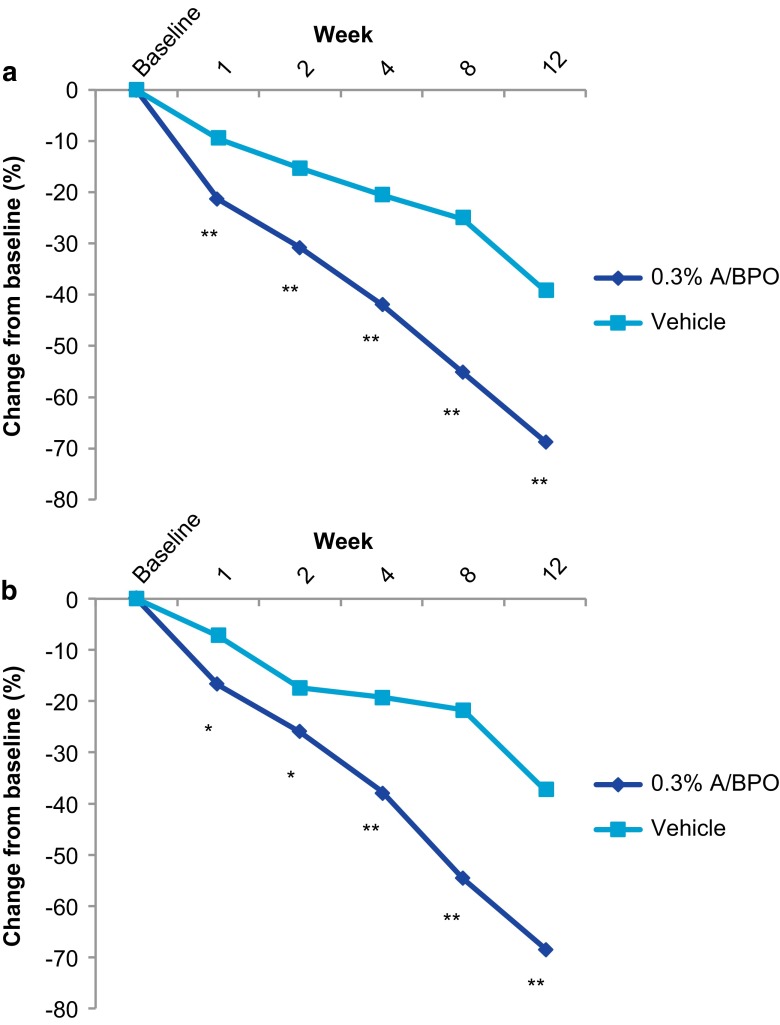
At week 12, 0.3 % A/BPO was superior to vehicle for least squares (LS) mean reduction from baseline in IN (27.0 vs. 14.4) and NIN (40.2 vs. 18.5) lesion counts, as well as for 0.1 % A/BPO (all p < 0.001 vs. vehicle). As shown in Fig. 5 for 0.3 % A/BPO, this was also demonstrated for mean percentage reduction from baseline in IN (68.7 % for 0.3 % A/BPO vs. 39.2 % for vehicle, or about 30 % greater) and NIN lesion counts (68.3 vs. 37.4 %, a 31 % difference) (all p < 0.001). These efficacy results were confirmed by PP analysis. At week 12, 0.1 % A/BPO also reduced IN and NIN lesion counts by 69.3 and 68 %, respectively. As of week 1, 0.3 % A/BPO showed an early onset of action in reducing lesion counts. From weeks 1 to 8, the mean difference from vehicle ranged from approximately 12–30 % higher for IN (p < 0.001 at all timepoints), and 10–33 % higher for NIN (p < 0.05 for weeks 1 and 2, and p < 0.001 at remaining timepoints).
Fig. 5.
Mean percent change (MI data; post hoc analysis performed prior to week 12) in (a) inflammatory lesions and (b) non-inflammatory lesions (ITT; MI). A adapalene, BPO benzoyl peroxide, ITT intent to treat, MI multiple imputation. *p < 0.05, **p < 0.001
In the severe population (IGA = 4), 0.3 % A/BPO continued to be efficacious, meeting the requirement for the success rate of at least a 3-grade change to ‘almost clear’ and ‘clear.’ Its statistical superiority over vehicle was demonstrated for not only success rate, with a treatment difference of 20.1 % (31.9 vs. 11.8 %, p = 0.029), but also for changes in IN (−35.17 vs. −15.46, p < 0.001) and NIN (−45.61 vs. −17.25, p < 0.001). However, for 0.1 % A/BPO, the treatment effect difference compared with vehicle for success rate was only 8.7 %, and this exploratory comparison did not demonstrate statistical significance versus vehicle (p = 0.443).
Safety
There were few treatment-related AEs (15 in 12 subjects [5.5 %] vs. two in one subject [0.5 %] for 0.3 and 0.1 % A/BPO, respectively), and they were generally dermatologic and mild to moderate in severity. In the 0.3 % A/BPO group, the most common treatment-related AEs were skin irritation (six [2.8 %]) subjects) and skin burning sensation (two [0.9 %] subjects). One subject (0.5 %) in the 0.3 % A/BPO group discontinued treatment due to a treatment-related AE: an atopic dermatitis flare of moderate severity that resolved after 10 days. One subject in the 0.1 % A/BPO group discontinued due to worsening of acne. No serious treatment-related AEs were reported.
Overall, 0.3 % A/BPO was well tolerated, with a local tolerability profile similar to that of 0.1 % A/BPO. Mean scores of tolerability signs/symptoms (erythema, scaling, dryness, and stinging/burning) peaked at week 1, decreasing over time with continued treatment as shown in Fig. 6. Their severity was mainly mild or moderate, with few subjects (≤6.1 %) experiencing severe signs/symptoms (see Table 3 for comparison between groups). Only 27 (6.2 %) subjects in the active-product groups needed to use the every-other-day regimen: 21 (9.7 %) subjects in the 0.3 % A/BPO group, with the majority having moderate severity at baseline and six (2.8 %) in the 0.1 % A/BPO group, with half having moderate severity at baseline. Moreover, local tolerability profiles were similar in the severe acne population (baseline IGA = 4).
Fig. 6.
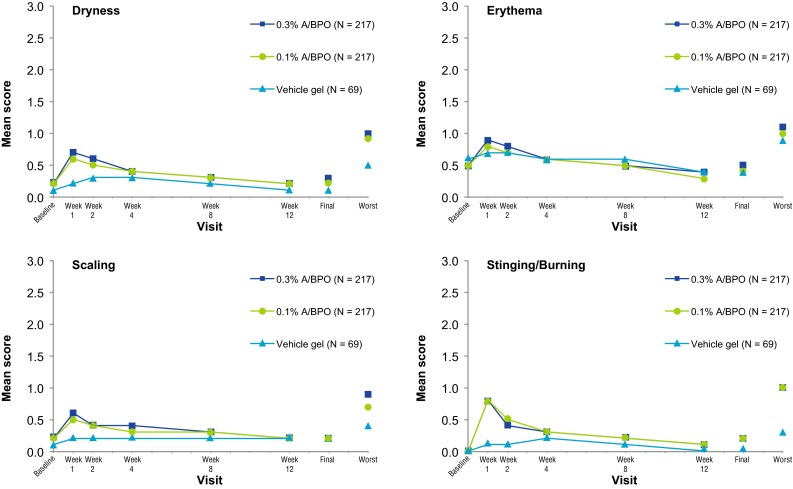
Local tolerance parameters (safety population). A adapalene, BPO benzoyl peroxide, final last data observed during the post-baseline period for a subject, worst highest severity score observed during the post-baseline period for a subject
Table 3.
Local tolerability evaluations for all subjects during and at end of treatment
| Maximum severity during treatment | End-of-treatment severity (final score) | |||
|---|---|---|---|---|
| Moderate | Severe | Moderate | Severe | |
| A/BPO 0.3 % gel (N = 213) | ||||
| Erythema | 20 | 1 | 4 | <1 |
| Scaling | 17 | 1 | 1 | <1 |
| Dryness | 15 | 2 | 3 | <1 |
| Stinging/burning | 19 | 6 | 1 | 1 |
| A/BPO 0.1 % (N = 212) | ||||
| Erythema | 15 | 1 | 2 | <1 |
| Scaling | 12 | <1 | 2 | 0 |
| Dryness | 13 | 1 | 2 | 0 |
| Stinging/burning | 14 | 9 | 3 | 0 |
| Vehicle gel (N = 68) | ||||
| Erythema | 6 | 1 | 1 | 0 |
| Scaling | 6 | 0 | 1 | 0 |
| Dryness | 4 | 1 | 1 | 0 |
| Stinging/burning | 3 | 1 | 0 | 0 |
Data are presented as % of patients
A adapalene, BPO benzoyl peroxide
Subject Satisfaction Questionnaire
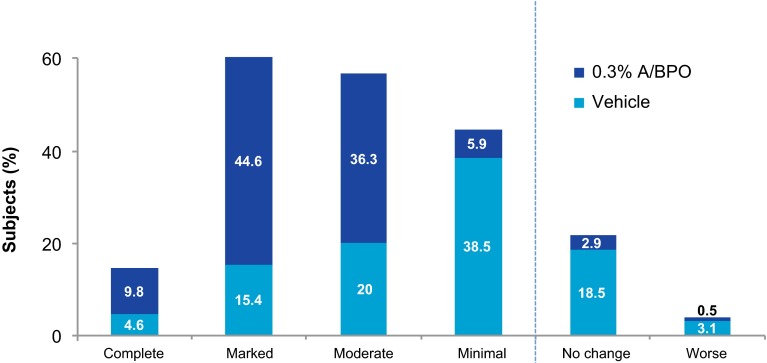
For the subject’s assessment of acne improvement, results for 0.3 % A/BPO (n = 204) were significantly superior to vehicle (n = 65). As illustrated in Fig. 7 for 0.3 % A/BPO, the majority of subjects (90.7 %) reported moderate to complete improvement in their acne at week 12 vs. only 40 % for vehicle (p < 0.001). Regarding the subject’s satisfaction questionnaire, more than twice as many subjects in the 0.3 % A/BPO group were satisfied with the product than in the vehicle group (71.6 vs. 32.3 %, respectively). Over half of subjects (about 53 %) considered that 0.3 % A/BPO did not lead to skin irritation compared with 76.9 % for vehicle.
Fig. 7.
Subject’s assessment of acne improvement (week 12, intent-to-treat population). A adapalene, BPO benzoyl peroxide
Discussion
These are the primary results for the first topical drug developed for severe acne. In this study, 0.3 % A/BPO as single-agent therapy was superior to vehicle in the co-primary endpoints of IGA success rate (clear/almost clear, at least a 2-grade change) and change in IN and NIN lesion counts (consistently p < 0.001) at week 12, which was confirmed in the PP population. In addition, an early onset of action was shown as of week 1, with significantly larger reductions in lesions relative to vehicle. Furthermore, in the severe inflammatory acne population (IGA = 4), where a rigorous 3-grade IGA improvement was required, 0.3 % A/BPO continued to demonstrate statistical superiority over vehicle (p = 0.029). The product was also safe and well-tolerated, and side effects subsided quickly, as can be expected with any retinoid-containing product. The safety profile of 0.3 % A/BPO was similar to that of 0.1 % A/BPO. Furthermore, no increase in incidence of AEs or local tolerability effects for subjects with baseline severe IGA 4 acne was observed compared with the moderate population.
The study design is also original in that other topical therapies do not typically target such a high proportion of patients with severe inflammatory acne (50 %) as single-agent therapy. The subjects enrolled in this study had a relatively higher number of baseline IN and NIN lesions (about 39 and 59 for the 0.3 % A/BPO group, with a total of 98.5 lesions) compared with other topical acne drug studies (e.g., ranging from 65.5 total lesions for clindamycin 1.2 %/BPO 3.75 % to 79 total lesions for dapsone 0.5 % gel at baseline) [19, 20]. In separate phase III studies regarding clindamycin 1.2 %/BPO 2.5 % gel, clindamycin 1.2 %/BPO 3.75 %, clindamycin 1.2 %/tretinoin 0.025 % gel, and tazarotene foam 0.1 %, a range of about 17–20 % of subjects had severe acne at baseline [19, 21–23]. Clindamycin 1 %/BPO 5 % gel was studied in a population with moderate to moderately severe acne (IGA between 3 and 4), and improvement was only described in terms of at least one grade of improvement [24].
Indeed, 0.3 % A/BPO may fill a gap as a single-agent treatment for moderate to severe inflammatory acne. Pediatric guidelines recommend that an oral antibiotic be started concomitantly with topical combination therapy (i.e., topical retinoid and BPO) promptly to prevent scarring in severe acne, when there is evidence of scarring even in moderate acne, or as a step-up regimen if initial therapeutic response with topical therapy is not adequate [16]. European guidance suggests that for moderate acne, an oral antibiotic should be initiated upon the presentation of more widespread disease [15]. Therefore, 0.3 % A/BPO may be able to substitute antibiotics in the interest of minimizing antibiotic resistance in cases where the addition of systemic antibiotic therapy is considered, particularly as its mechanism of action includes prevention of microcomedone formation. International guidelines for acne management stipulate that oral isotretinoin remains the gold standard for severe acne treatment [4, 14]. Recent guidance for the pediatric population suggests that oral isotretinoin is no longer indicated as first-line treatment, but instead only after inadequate response with initial treatment using combination therapy (topical treatment associated with oral antibiotic) [16]. Therefore, 0.3 % A/BPO may be an appropriate treatment, alone or in combination with other therapies, prior to the potential need for step-up treatment to oral isotretinoin, or while enrolling the patient in the required programs to obtain access to oral isotretinoin. Furthermore, 0.3 % A/BPO may be seen as a replacement for existing topical combination therapies used with oral antibiotics in not only moderate acne but also in severe inflammatory non-nodulocystic acne (although, perhaps not exclusively).
As adapalene has been found to down-regulate the expression of TLR2, β-defensin 4 and interleukin (IL)-8, as well as to increase CD1d expression [10, 12, 25], with a dose-dependent effect on inflammatory markers [12], it seems logical that a higher potency of A/BPO can demonstrate an even greater anti-inflammatory effect in patients with more severe acne, an effect that has not previously been available. Thus, 0.3 % A/BPO may open the door to more treatment options for patients with severe inflammatory acne and can be considered as a step-up option from 0.1 % A/BPO before considering more aggressive systemic therapies. A once-daily topical treatment regimen may be perceived as easier to integrate into the patient’s existing skin care habits. Patient adherence may be improved with a standalone topical treatment versus an often long-term, oral regimen as in this study. In this trial, 0.3 % A/BPO was effective, and patient adherence was maintained throughout the trial (90.8 % for the 0.3 % A/BPO group).
It is noteworthy that this study was conducted in a diverse patient population with about one-fourth Hispanic or Latino subjects, and over one-third with darker skin phototypes (IV–VI). This is important because Hispanics are the fastest-growing minority group in the USA, and acne is reported as the most common dermatologic diagnosis seen in over 20 % of the Hispanic population [26]. Furthermore, in darker skin phototypes, there is a potential for increased cutaneous irritation and postinflammatory hyperpigmentation (PIH) [27]. The use of 0.3 % A/BPO in this population may be particularly warranted to provide quick control of the disease with good tolerability. A limitation of this study was that in the absence of adapalene and BPO arms, the contribution of each individual component could not be determined. Nevertheless, this may be extrapolated from previous data regarding synergy of 0.1 % A/BPO and the advantage of the combination over its components [17].
Conclusion
Treatment with 0.3 % A/BPO topical gel showed superior efficacy to vehicle, along with a good safety profile in moderate to severe inflammatory non-nodulocystic acne. The availability of a higher concentration of A/BPO will increase patients’ treatment options and allow clinicians to further customize acne management according to the disease presentation.
Acknowledgments
The authors wish to thank Galadriel Bonnel, PhD, independent owner of Galadriel Bonnel Consulting, for editorial assistance.
Compliance with Ethical Standards
Funding
This study was funded by Galderma R&D.
Conflict of interest
The investigators received grants for conducting the studies. Dr. Stein Gold is an investigator, consultant, and a member of the speaker’s bureau for Galderma, and is also a consultant and/or investigator for Allergan, Valeant and Ranbaxy. Dr. Weiss is an investigator and has served on advisory boards for Galderma, and also has received grants and/or honoraria from AbbVie, Allergan, Amgen, Celgene, Galderma, Leo, Neothetics, Promius, Sebacia, Valeant, and Xenoport. Dr. Tanghetti has served on advisory boards for Galderma. Dr. Rueda and Ms. Liu are employees of Galderma.
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and in compliance with good clinical practices and local regulatory requirements. This study was approved by an institutional review board, and all subjects provided written informed consent before entering the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
References
- 1.Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527–1534. doi: 10.1038/jid.2013.446. [DOI] [PubMed] [Google Scholar]
- 2.Bhate K, Williams HC. What’s new in acne? An analysis of systematic reviews published in 2011-2012. Clin Exp Dermatol. 2014;39(3):273–277. doi: 10.1111/ced.12270. [DOI] [PubMed] [Google Scholar]
- 3.Kligman AM. An overview of acne. J Invest Dermatol. 1974;62:268–287. doi: 10.1111/1523-1747.ep12676801. [DOI] [PubMed] [Google Scholar]
- 4.Thiboutot D, Gollnick H, Bettoli V, et al. Global Alliance to Improve Outcomes in Acne. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(Suppl 5):S1–S50. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: a randomized, multicenter, investigator-blinded, controlled comparison study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76(2):145–151. [PubMed] [Google Scholar]
- 6.Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54(2):242–250. doi: 10.1016/j.jaad.2004.10.879. [DOI] [PubMed] [Google Scholar]
- 7.Weiss JS, Thiboutot DM, Hwa J, Liu Y, Graeber M. Long-term safety and efficacy study of adapalene 0.3% gel. J Drugs Dermatol. 2008;7(6 Suppl):s24–s28. [PubMed] [Google Scholar]
- 8.Thiboutot D, Arsonnaud S, Soto P. Efficacy and tolerability of adapalene 0.3% gel compared to tazarotene 0.1% gel in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7(6 Suppl):s3–s10. [PubMed] [Google Scholar]
- 9.Michel S, Jomard A, Demarchez M. Pharmacology of adapalene. Br J Dermatol. 1998;139(Suppl 52):3–7. doi: 10.1046/j.1365-2133.1998.1390s2003.x. [DOI] [PubMed] [Google Scholar]
- 10.Vega B, Jomard A, Michel S. Regulation of human monocyte toll-like receptor 2 (TLR2) expression by adapalene. J Eur Acad Dermatol Venereol. 2002;16(Suppl 1):123–124. [Google Scholar]
- 11.Cunliffe WJ, Caputo R, Dreno B, et al. Clinical efficacy and safety comparison of adapalene gel and tretinoin gel in the treatment of acne vulgaris: Europe and US multicenter trials. J Am Acad Dermatol. 1997;36:S126–S134. doi: 10.1016/S0190-9622(97)70056-2. [DOI] [PubMed] [Google Scholar]
- 12.Tenaud I, Khammari A, Dreno B. In vitro modulation of TLR-2, CD1d and IL-10 by adapalene on normal human skin and acne inflammatory lesions. Exp Dermatol. 2007;16(6):500–506. doi: 10.1111/j.1600-0625.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 13.Bakry OA, Samaka RM, Sebika H, Seleit I. Toll-like receptor 2 and P. acnes: do they trigger initial acne vulgaris lesions? Anal Quant Cytopathol Histpathol. 2014;36(2):100–110. [PubMed] [Google Scholar]
- 14.Gollnick H, Cunliffe W, Berson D, et al. Global alliance to improve outcomes in acne. management of acne: a report from a global alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(Suppl 1):S1–S37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 15.Nast A, Rosumeck S, Sammain A, Sporbeck B, Rzany B. Methods report on the development of the European S3 guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(Suppl 1):e1–e41. doi: 10.1111/j.1468-3083.2011.04375.x. [DOI] [PubMed] [Google Scholar]
- 16.Eichenfield LF, Krakowski AC, Piggott C, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131(Suppl 3):S163–S186. doi: 10.1542/peds.2013-0490B. [DOI] [PubMed] [Google Scholar]
- 17.Tan J, Gollnick HP, Loesche C, Ma YM, Gold LS. Synergistic efficacy of adapalene 0.1%-benzoyl peroxide 2.5% in the treatment of 3855 acne vulgaris patients. J Dermatolog Treat. 2011;22(4):197–205. doi: 10.3109/09546631003681094. [DOI] [PubMed] [Google Scholar]
- 18.Feldman SR, Tan J, Poulin Y, Dirschka T, Kerrouche N, Manna V. The efficacy of adapalene-benzoyl peroxide combination increases with number of acne lesions. J Am Acad Dermatol. 2011;64(6):1085–1091. doi: 10.1016/j.jaad.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Pariser DM, Rich P, Cook-Bolden FE, Korotzer A. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13(9):1083–1089. [PubMed] [Google Scholar]
- 20.Draelos ZD, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56(3):439.e1–439.e10. doi: 10.1016/j.jaad.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Gold MH. A new, once-daily, optimized, fixed combination of clindamycin phosphate 1.2% and low-concentration benzoyl peroxide 2.5% gel for the treatment of moderate-to-severe acne. J Clin Aesthet Dermatol. 2009;2(5):44–48. [PMC free article] [PubMed] [Google Scholar]
- 22.Dréno B, Bettoli V, Ochsendorf F, et al. Efficacy and safety of clindamycin phosphate 1.2%/tretinoin 0.025% formulation for the treatment of acne vulgaris: pooled analysis of data from three randomised, double-blind, parallel-group, phase III studies. Eur J Dermatol. 2014;24(2):201–209. doi: 10.1684/ejd.2014.2293. [DOI] [PubMed] [Google Scholar]
- 23.Epstein EL, Stein Gold L. Safety and efficacy of tazarotene foam for the treatment of acne vulgaris. Clin Cosmet Investig Dermatol. 2013;6:123–125. doi: 10.2147/CCID.S34054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson JM, Fu JJ, Almekinder JL. A randomized, investigator-blinded trial to assess the antimicrobial efficacy of a benzoyl peroxide 5%/clindamycin phosphate 1% gel compared with a clindamycin phosphate 1.2%/tretinoin 0.025% gel in the topical treatment of acne vulgaris. J Drugs Dermatol. 2010;9(2):131–136. [PubMed] [Google Scholar]
- 25.Zuliani T, Khammari A, Chaussy H, Knol AC, Dréno B. Ex vivo demonstration of a synergistic effect of Adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20(10):850–853. doi: 10.1111/j.1600-0625.2011.01339.x. [DOI] [PubMed] [Google Scholar]
- 26.Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(6 Suppl):S143–S148. doi: 10.1067/mjd.2003.274. [DOI] [PubMed] [Google Scholar]
- 27.Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(2 Suppl):S41–S62. doi: 10.1067/mjd.2002.120790. [DOI] [PubMed] [Google Scholar]