Abstract
The present study evaluated the ability and optimal concentration of tetramethylpyrazine (TMP) to induce human umbilical cord-derived mesenchymal stem cells (hUMSCs) to differentiate into neuron-like cells in vitro. Human umbilical cords from full-term caesarean section patients were used to obtain hUMSCs by collagenase digestion after removal of the umbilical artery and vein. The surface antigen expression profile of cultured hUMSCs was monitored by flow cytometry. After amplification, cells of the 5th passage were divided into experimental groups A–C treated with TMP at 4.67, 2.34 and 1.17 mg/ml, respectively, in low glucose-Dulbecco's Modified Eagle's Medium (L-DMEM) (induction medium), while group D (control) was exposed to L-DMEM culture medium only. Differentiation of hUMSCs into neuron-like cells and morphological changes were observed every 0.5 h with an inverted phase contrast microscope for 6 h. After the 6-h induction period, proportions of cells expressing neuronal markers neuron-specific enolase (NSE), neurofilament protein (NF-H) and glial fibrillary acidic protein (GFAP) were detected by immunohistochemistry. The optimal concentration of TMP was selected on the basis of neuron-like cell positive rate. Western blotting and RT-polymerase chain reaction were applied to detect the expression of NSE, NF-H, and GFAP of the group of optimal concentration in each point-in-time. Results showed that most primary cells were adherent 12 h after seeding and first appeared as diamond or polygon shapes. Thereafter, they gradually grew into long spindle-shaped cells and finally in a radiating or swirling pattern. The cells maintained a strong proliferative capacity after continuous passage. Flow cytometry analysis of cultured hUMSCs at the 3rd, 5th and 10th passages expressed CD73, CD90 and CD105, but not CD11b, CD19, CD34, CD45 or human leukocyte antigen-DR. After 6 h of TMP treatment, typical neuron-like cells with many protrusions connected into a net-like pattern were observed in all experimental groups. These neuron-like cells were positive for NSE and NF-H, but negative for GFAP. Among the tested treatment groups, group A with TMP at 4.67 mg/ml had the highest expression of NSE and NF-H. By contrast, no change was found after induction in the control group. The mRNA expression of cells expressing neuronal markers as well as GAPDH was observed, with the relative NSE transcript levels of 0, 1.303±0.031, 1.558±0.025, 1.927±0.019 and 2.415±0.033 after 0, 1, 2, 4 and 6 h of treatment, respectively; the mRNA expression of NH-F was 0, 1.429±0.025, 1.551±0.024, 1.930±0.042 and 1.398±0.014 after 0, 1, 2, 4 and 6 h of treatment, respectively. There was no expression of GFAP before or after induction and all the groups showed high expression of GAPDH at each time point. Protein expression was also observed on cells expressing neuronal markers as well as GAPDH at each time point. The protein expression of NSE was 0, 0.717±0.097, 0.919±0.056, 1.097±0.143 and 1.157±0.055 in proper order; the protein expression of NH-F was 0, 0.780±0.103, 0.973±0.150, 1.053±0.107 and 0.753±0.094 in proper order. There was no expression of GFAP before or after induction, and all the groups showed high expression of GAPDH at each tested time point. Our results demonstrated that TMP can induce hUMSCs to differentiate into neuron-like cells effectively with the optimal concentration of 4.67 mg/ml. After induction, the NSE and NF-H of the neuron-like cells were positive, but the GFAP-2 was negative.
Keywords: tetramethylpyrazine, human umbilical cord-derived mesenchymal stem cells, differentiation, neuron-like cells
Introduction
Mesenchymal stem cells (MSCs) derived from the mesoderm in early development are multipotential stem cells which have the properties of self-renewal and multilineage differentiation. They are widely present in all types of tissues and can differentiate in vitro into cells such as neurons, osteoblasts, chondrocytes, myocytes and adipocytes under certain conditions. Therefore, they are highly valuable in applications of cell replacement therapy and tissue engineering. In 1991, McElreavey et al isolated and cultured fibroblasts with the capability of multilineage differentiation from Wharton's jelly of the human umbilical cord (1). These human umbilical cord-derived MSCs (hUMSCs) have been shown to have greater advantages than MSCs derived from bone marrow, placenta and other tissues. Firstly, ethical concerns regarding the source of the stem cells (umbilical cord) are diminished, and they can be obtained at low cost. Secondly, hUMSCs have been determined not to cause teratomas and can inhibit cancer cells. Additionally, due to their low immunogenicity and genetic stability, as well as functions in immunoregulation, stroma support, paracrine signaling and migration, hUMSCs have good clinical therapeutic potential.
There have been literature reports using antioxidants, such as thioglycerol, 2-mercaptoethanol, dimethylsulphoxide (DMSO) and butylhydroxsanisole, to experimentally induce MSCs to differentiate into neuron-like cells (2). However, these chemicals cannot be used in live animals due to toxicity. Other researchers proposed traditional Chinese medicine and compound preparations with no or low cytotoxicity to induce bone marrow-derived MSCs (BMSCs) to differentiate into neuron-like cells and achieved satisfactory results (3). However, reports on the use of a traditional Chinese medicine to induce hUMSCs to differentiate into neuron-like cells are rare.
Tetramethylpyrazine (TMP) is an active alkaloid (2,3,5,6-tetramethylpyrazine) separated and purified from a Chinese medicine called Ligusticum wallichii, which belongs to the family Umbelliferae. The saturation concentration of TMP in aqueous solution at 37°C was 4.67 mg/ml. TMP has been demonstrated to promote expansion of small arteries and enhance blood circulation to prevent blood stasis and antiplatelet aggregation. It has also been shown to antagonize calcium ion flow and to exhibit antithrombotic, antioxidant and antifibrotic properties (4). Although the mechanisms of action have not been clearly defined, it was suggested that the protective effects and related mechanism of TMP against central lesions may largely be attributed to inhibition of calcium channels, antioxidation, resistance of neuronal apoptosis and increase in heat shock protein expression (5). In this study, we examined various doses of TMP to induce hUMSCs to differentiate into neuron-like cells and determined the optimal inductive concentration. Possible induction mechanism is also discussed.
Materials and methods
Materials
Full-term pregnancy cesarean neonatal human umbilical cords were provided by Department Obstetrics/Gynecology, The Second Affiliated Hospital of Hebei Medical University (Hebei, China). This study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University. The following reagents were obtained commercially: TMP (20 mg; purity: HPLC ≥98%; batch no. 120919 Chengdu Preferred Biological Technology Co., Ltd, Chengdu, China); low glucose Dulbecco's Modified Eagle's Medium (L-DMEM)/F-12, high glucose-DMEM (H-DMEM)/F-12 and fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA); trypsin (Amresco, LLC, Solon, OH, USA); EDTA and DMSO (Sigma-Aldrich, St. Louis, MO, USA); Triton X-100 (Sinopharm Chemical Reagent Co., Ltd., Beijing, China); penicillin-streptomycin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China); paraformaldehyde (Tianjin Institute of Chemical Preparations, Tianjin, China); epidermal growth factor (EGF) (human EGF, PHG0314; Beijing Jiamei North Biological Technology Co., Ltd., Beijing, China); fluorescein isothiocyanate (FITC)-CD19, FITC-CD34, phycoerythrin (PE)-CD11b, PE-CD73, PE-CD90, PE-CD45, PE-CD105 and glial fibrillary acidic protein (GFAP) (BD Biosciences, Franklin Lakes, NJ, USA); neurofilament protein (NF-H), neuron-specific enolase (NSE) (Cell Signaling Technology, Inc., Danvers, MA, USA); anti-rabbit immunoglobulin G (IgG) (1:2,000; Affinity Biosciences, Cincinnati, OH, USA); PS immunohistochemistry kit (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China); Taq polymerase chain reaction (PCR) star mix (Beijing GenStar Biosolutions Co., Ltd., Beijing, China); EasyScript First-Strand cDNA synthesis supermix (TransGen Biotech Co., Ltd., Beijing, China); cell RIPA lysis buffer and phenylmethylsulfonyl fluoride (Beijing Solarbio Science & Technology Co., Ltd.).
Isolation and culture of hUMSCs
Umbilical cords were placed in H-DMEM/F-12 culture medium under aseptic conditions, stored at 4°C and then timely transported to a cell culture room to carry out the following steps. Each umbilical cord was rinsed thoroughly with D-Hank's medium. After removing the blood sample, umbilical artery and umbilical vein, the umbilical cord mesenchymal tissue was cut into 1 mm3 pieces, digested with 0.2% collagenase II and placed in a culture flask containing 2 ng/ml EGF, 20% FBS, 25 mM L-Glu and 100 U/ml penicillin streptomycin/mixture at 37°C with 5% CO2 and saturation humidity to obtain primary cells. Half of the culture medium was replaced after 24 h, and all the medium was replenished every 3 days. When the cells achieved 80–90% confluency, the medium was removed, and cells were rinsed two times with phosphate-buffered saline (PBS) and then digested with trypsin (0.25%)-EDTA (0.2 g/l) into single cells for passaging at the ratio of 1:3. The culture medium was H-DMEM/F-12 containing 100 U/ml of a penicillin-streptomycin mixture and 10% FBS.
Analysis of cellular phenotype of hUMSCs
In the logarithmic phase of growth, hUMSCs were digested with trypsin and rinsed with PBS, and then the single cell suspension was aliquotted into 10 microcentrifuge tubes at 1×106/tube. Separately, mouse anti-human monoclonal antibodies CD11-PE, CD45-PE, CD73-PE, CD90-PE, CD105-PE, human leukocyte antigen (HLA)-DR-PE, CD19-FITC and CD34-FITC (each 5 μl) were added to eight of the tubes, while anti-mouse IgG1-PE and anti-mouse IgG1-FITC (each 7 μl) were added to the other two tubes as isotype controls. After mixing the contents thoroughly, the tubes were incubated at 4°C for 30 min. Thereafter, the cells were rinsed with PBS and then centrifuged. The supernatant was discarded, and the cells were resuspended with 400 μl of PBS for analysis by flow cytometry.
Differentiation of hUMSCs into neuron-like cells
Cells were obtained from the 3rd passage in logarithmic growth phase with good growth conditions, hUMSCs were rinsed twice with PBS and digested with trypsin (0.25%)-EDTA (0.2 g/l). The digestion was terminated with FBS, and then the cells were centrifuged, resuspended in culture medium and seeded at 1×105/ml into five 6-well cell culture plates coated with poly-lysine. The plates were divided randomly into groups A–D and then placed in the incubator. When the cells achieved 70–80% confluency, TMP induction medium was added to cells of groups A–C at the concentrations of 4.67, 2.34 and 1.17 mg/ml, respectively. Group D was treated with L-DMEM only and served as control. All the plates were cultured in an incubator at 37°C with 5% CO2 and saturation humidity for 6 h. Morphological changes were observed with an inverted phase contrast microscope each 0.5 h.
Identification of differentiated cells
After induction for 6 h, expression levels of neuronal-specific proteins NSE, NF-H and GFAP were detected by immunocytochemistry. Briefly, after discarding the culture medium from the culture plates, the cells were rinsed once with PBS gently and fixed with 4% paraformaldehyde for 20 min at room temperature. After this step and subsequent steps, the cells were rinsed with PBS three times for 5 min each time, unless otherwise noted. The cell membranes were disrupted with 0.5% Triton X-100 in PBS for 15 min at room temperature away from light and then incubated with 3% H2O2 at room temperature for 5 min. Normal goat serum was added to the cells for blocking at room temperature for 15 min and then removed without washing. Primary antibodies to NSE, GFAP and NF-H were added at a 1:200 dilution. After incubation at 4°C overnight, the cells were incubated with the appropriate biotinylated secondary antibody at room temperature for 15 min, followed by horseradish peroxidase-conjugated streptavidin at room temperature for 15 min. Finally, the cells were stained with freshly prepared DAB for 1 min and counterstained with hematoxylin before finally rinsed repeatedly with water.
RT-PCR
Total RNA was extracted by TriPure Reagent (Aidlab Biotechnologies Co., Ltd., Beijing, China) following the manufacturer's instructions and quantified with a Ultramicro ultraviolet visible light meter (Gene Company, Ltd., Hong Kong, China). cDNA was synthesized from total RNA with an EasyScript First-Strand cDNA synthesis supermix (TransGen Biotech Co., Ltd.) following the manufacturer's instructions. Semi-quantitative PCR was performed using Taq PCR star mix (Beijing GenStar Biosolutions Co., Ltd.) with the following reaction conditions: 1 cycle of 94°C for 3 min; 30 cycles of 94°C for 30 sec, and 72°C for 30 sec; and a final extension at 72°C for 10 min. A 10 μl sample of the PCR product was analyzed by electrophoresis, and the gene expression levels were calculated using GAPDH as the internal standard. The primers were designed using Primer 5.0 software and are shown in Table I.
Table I.
The primer sequences used for RT-polymerase chain reaction analysis in this study.
| Genes | Primer sequences | Sizes (bp) | Temperature (°C) |
|---|---|---|---|
| NSE | F: GGCACTCTACCAGGACTTTG R: GCGATGACTCACCATAACCC |
398 | 61.7 |
| NF-H | F: TGAACACAGACGCTATGCGCTCAG R: CACCTTTATGTGAGTGGACACAGAG |
286 | 61.2 |
| GAPDH | F: AGAAGGCTGGGGCTCATTTG R: AGGGGCCATCCACAGTCTTC |
258 | 64.0 |
NSE, neuron-specific enolase; NF-H, neurofilament protein; F, forward; R, reverse.
Western blot analysis
Cell lysates were prepared with cell RIPA lysis buffer and phenylmethylsulfonyl fluoride (Beijing Solarbio Science & Technology Co., Ltd.), and protein concentrations were determined with an ultramicro-ultraviolet visible light meter (Gene Company, Ltd.). Total protein (16 mg) of each lysate was electrophoresed in a 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride membrane (Beijing Solarbio Science & Technology Co., Ltd.). The membranes were blocked in 10% non-fat milk for 2 h and incubated with the GFAP, NF-H and NSE primary antibodies at 4°C overnight, After rinsing with PBS for 5 min, the membranes were incubated with anti-rabbit IgG (1:2,000; Affinity Biosciences) for 2 h at room temperature and visualized by SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA) using the Image AlphaEaseFC system (Alpha Innotech Co., San Leandro, CA, USA).
Calculation of frequency of neuron-like cells
A total of 10 non-overlapping representative fields under an inverted microscope were selected in each group, in which the total number of cells and neuron-like cells were counted. The proportion of neuron-like cells was calculated and averaged from the 10 fields for each group. Results were presented as mean ± standard deviation.
Statistical analysis
Statistical analysis was performed by using SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). Comparison on the experimental data with a completely randomized design was tested by analysis of variance among groups and the Student-Newman-Keuls method (q-test). Difference in the proportion of neuron-like cells among groups was analyzed by the Chi-square test. P<0.05 was considered to indicate a statistically significant difference.
Results
Growth and morphological changes of hUMSCs
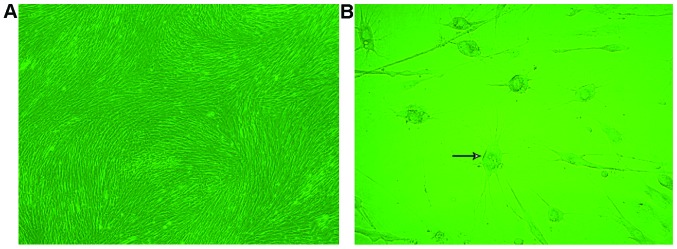
Primary cultured cells were passaged at the ratio of 1:1, and most cells were adherent after 12 h. However, the adherent cells were not outstretched, but appeared triangular or diamond in shape. The cell culture medium was replaced to remove non-adherent cells after 24 h. The adherent cells proliferated rapidly and became significantly larger and more uniform, long fusiform cells after 48 h of culture. They achieved 80–90% confluency on the 7th day as a monolayer arranged in a radial or spiral shaped pattern. After digestion, the hUMSCs cells floated as spherical single cells in the culture medium and were passaged at the ratio of 1:3 after centrifugation. Most cells were adherent 24 h after passaging and achieved 80–90% confluency on the 7th day with a radial or spiral shaped arrangement (Fig. 1A). The hUMSCs of the 10th passaged maintained a strong proliferative capacity.
Figure 1.
Morphology of human umbilical cord-derived mesenchymal stem cells (hUMSCs). (A) Arrangement of spiral shaped hUCMSCs ((magnification, x40). (B) The arrow points to a representative neuron-like cell viewed at a higher magnification (magnification, ×100).
Cellular phenotype of hUMSCs
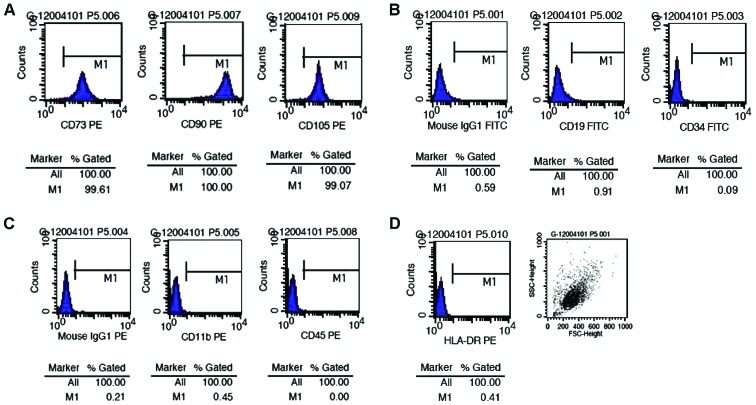
We detected the cellular phenotype of the 2nd, 5th and 10th passages of hUMSCs by flow cytometry and found that all generations of the cells tested co-expressed CD105, CD90 and CD73, but not CD11b, CD34, CD19, CD45 and histocompatibility antigen HLA-DR (major histocompatibility complex class II) (Fig. 2).
Figure 2.
Surface marker expression in human umbilical cord-derived mesenchymal stem cells (hUMSCs) detected by flow cytometry. The percentages of (A) hUMSCs expressing CD73, CD90 and CD105 were 99.61, 100 and 99.07%, respectively. However, the percentages of (B and C) hUMSCs expressing CD19, CD34, CD11b and CD45, and (B and D) the expression of isotype controls and human leukocyte antigen (HLA)-DR were very low. These results confirmed that the cells we cultured were mesenchymal stem cells, and not hematopoietic stem cells. PE, phycoerythrin; IgG, immunoglobulin G; FITC, fluorescein isothiocyanate.
Morphological and quantitative changes of neuron-like cells in each group
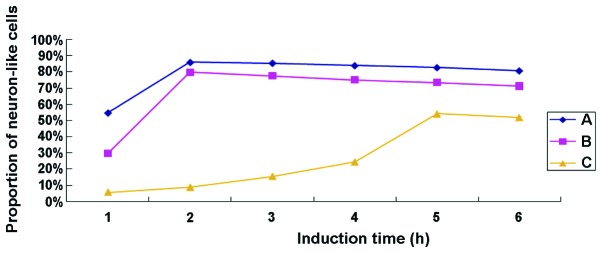
After treatment with TMP for 0.5 h, 40% of cells in group A contracted into an oval shape and extended protuberances from their cell bodies (Fig. 1B). After treatment for 1 h, the ratio of neuron-like cells reached 55%, with the cell bodies contracted and the protuberances lengthened further. At this time, the cells appeared connected in a net-like pattern. After treatment for 1.5 h, ~85% of the cells appeared as neuron-like cells. After treatment for 4 h, some of the neuron-like cells began to detach, and the ratio was reduced to ~80% in the 6th h. In group B, ~45% of the cells were neuron-like cells after treated with TMP for 1.5 h, and the ratio increased to 80% until the 2nd h. Thereafter, some of the neuron-like cells started to become non-adherent, and the ratio was reduced to 70% in the 6th h. In group C, ~30% of the cells were neuron-like cells after treatment with TMP for 4.5 h; however, some became detached, and the ratio was ~55% at the 6th h. The control group D had no significant changes before or after treated with medium alone. As shown in Fig. 3, the proportion of TMP-induced neuron-like cells was, from high to low, group A >group B >group C at each time point, and it declined gradually over the induction time indicating that the neuron-like cells were continually becoming detached. The neuron-like cells in group C required more time to reach the highest ratio, and the detaching cells could also be seen during the process of differentiation. Treatment of TMP at the concentration of 4.67 mg/ml in group A was employed in the subsequent experiment to detect the neuronal-specific markers by western blotting.
Figure 3.
The proportion of tetramethylpyrazine-induced neuron-like cells was, from high to low, group A >group B >group C at each time point, and it declined gradually over the induction time indicating that the neuron-like cells were continually becoming detached. The neuron-like cells in group C required more time to reach the highest ratio, and the detaching cells could also be seen during the process of differentiation.
Immunocytochemical detection of neuronal-specific markers
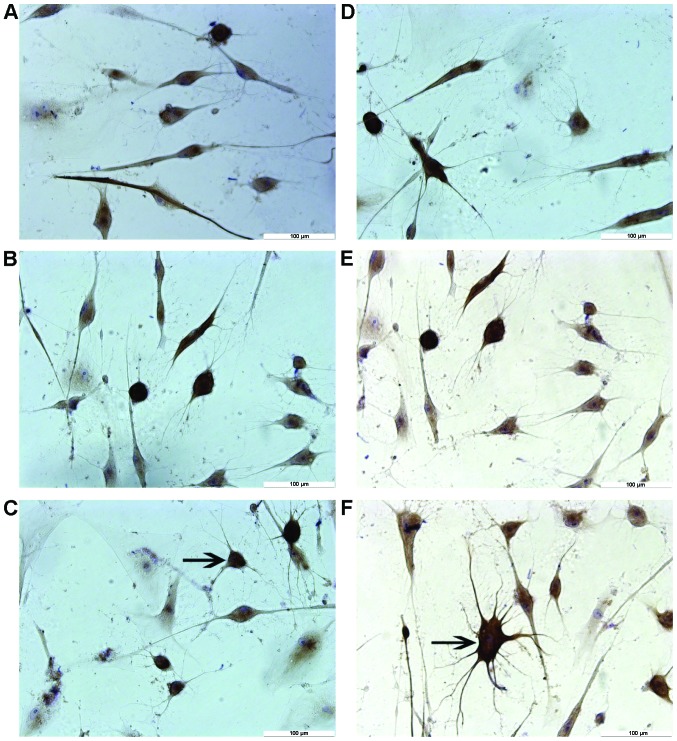
Results from immunocytochemical staining showed that most neuron-like cells treated by different concentrations of TMP for 6 h were positive for NF-H and NSE, but negative for GFAP (Fig. 4). By contrast, no cells were found positively expressing these markers in the control group. Negative staining was evidenced when PBS was used to replace the primary antibody (data not shown). As shown in Table II, differences in the expression levels of NF-H and NSE among these groups were significant (P<0.01).
Figure 4.
Different concentrations of tetramethylpyrazine (TMP) can induce differentiation of human umbilical cord-derived mesenchymal stem cells into neuron-like cells in vitro. Immunohistochemical staining of neuron-like cells for (A–C) neurofilament protein and (D–F) MAP-2 in groups treated with TMP at the concentrations of 4.67 mg/ml (group A, A and D), 2.34 mg/ml (group B, B and E), and 1.17 mg/ml (group C, C and F). The arrows in C and F indicate the neuron-like cells.
Table II.
Comparison of antigen expression in neuron-like cells induced from hUMSCs after 6 h of TMP treatment (%, n=3).
| Group | NF-H | NSE |
|---|---|---|
| A-TMP 4.67 mg/ml | 80.79±4.36 | 79.76±2.46 |
| B-TMP 2.34 mg/ml | 71.30±1.94a,b | 69.83±4.42a,c |
| C-TMP 1.17 mg/ml | 52.01±3.66a,c | 50.18±4.07a,c |
| D-Control | 0 | 0 |
Data are presented as the mean ± standard deviation.
P<0.01.
Compared to group A for NF-H and NSE, respectively.
hUMSCs, human umbilical cord-derived mesenchymal stem cells; TMP, tetra-methylpyrazine; NSE, neuron-specific enolase; NF-H, neurofilament protein.
Western blot detection of neuronal-specific markers
On the basis of neuron-like cell positive rate, we selected the TMP treatment at a concentration of 4.67 mg/ml to detect the expression of NSE, NF-H, GFAP using western blotting. Protein expression of cells expressing neuronal markers as well as GAPDG was observed after TMP treatment. The calculated relative transcript level for protein expression of NSE was 0, 0.717±0.097, 0.919±0.056, 1.097±0.143 and 1.157±0.055 before treatment, for 1, 2, 4 and 6 h, respectively; whereas, the relative level for NH-F expression was 0, 0.780±0.10, 0.973±0.150, 1.053±0.107 and 0.753±0.094, respectively. No expression of GFAP was detected before or after induction, whereas all tested samples showed high expression of GAPDH after TMP treatment (Fig. 5).
Figure 5.
Detection and quantitative analysis of protein expression in tetramethylpyrazine (TMP)-inducing neuron-like cells. Protein expression of neuron-specific enolase (NSE) and neurofilament protein (NF-H) in neuron-like cells was confirmed after TMP treatment by western blotting (top panel). The lower panels show the normalized protein expression levels for NSE (lower-left panel) and NF-H (lower-right panel).
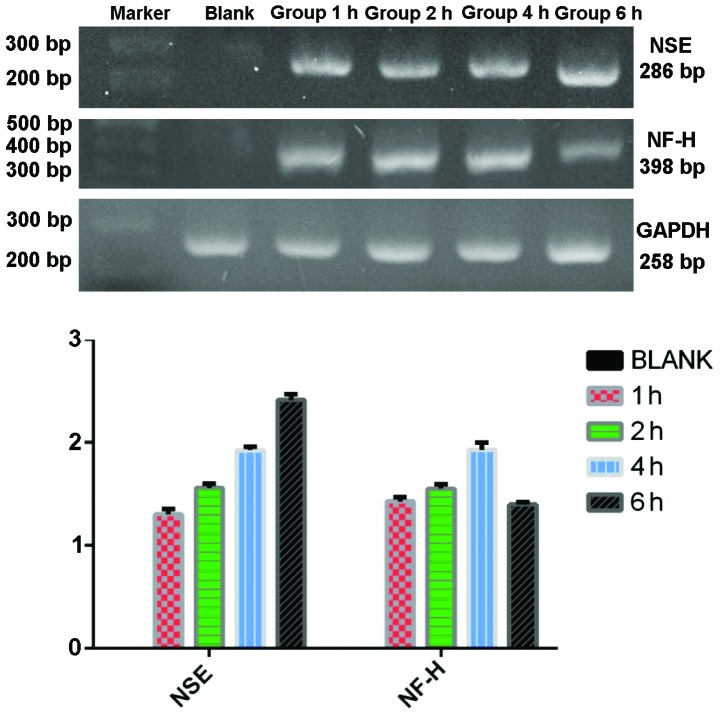
RT-PCR detection of neuronal-specific markers
RT-PCR was used to detect the expression of NSE, NF-H, and GFAP of the group C in each time point after TMP treatment. The mRNA expression of NSE was 0, 1.303±0.031, 1.558±0.025, 1.927±0.019 and 2.415±0.033 in proper order. The mRNA expression of NF-H was 0, 1.429±0.025, 1.551±0.024, 1.930±0.042 and 1.398±0.014 in proper order. There was no expression of GFAP before or after induction and all the groups showed high expression of GAPDH at each time point (Fig. 6).
Figure 6.
mRNA expression of neuron-specific enolase (NSE) and neurofilament protein (NF-H) in tetramethylpyrazine-inducing neuron-like cells before differentiation (blank), and up to 6 h of differentiation. Top panel shows electrophoresis of the polymerase chain reaction-amplified products expressing NSE and NF-H genes. Quantitative mRNA expression levels of genes normalized by GAPDH are presented in the lower panel.
Discussion
The plasticity of stem cells refers to the ability of adult stem cells to lose their phenotype of a specific tissue or germ layer and differentiate into other types of cells (6–8). Typically, MSCs exhibit cell plasticity by being able to differentiate into bone, cartilage, smooth muscle, skeletal muscle and cardiac muscle, as well as cells from other germ layers such as skin and liver (9–19). Woodbury et al (2) first reported in 2000 that BMSCs can differentiate into neuron-like cells under certain conditions, a finding that has attracted significant attention. Soon afterwards, numerous domestic and foreign laboratories carried out in vitro and in vivo studies on the neural differentiation of MSCs from different species and sources. These studies have shown that MSCs of rats, mice, humans, rabbits and other mammals can be induced to differentiate into neuron-like cells under certain conditions. In this context, newborn umbilical cords, as a reliable source of MSCs that can be obtained non-invasively and without ethical constraints, have been widely used in stem cell transplantation therapy and experiments of neural differentiation.
Some inducers, including chemical inducer, neurotrophic factor, and Chinese medicine active ingredients and their preparations, have been shown to be able to differentiate MSCs into neuron-like cells expressing surface antigen markers of neural cells. Our study confirmed that the TMP monomer (2,3,5,6-tetramethylpyrazine), an active ingredient in Chinese medicine, could effectively induce hUMSCs to differentiate into neuron-like cells in vitro and express NSE and NF-H, but not GFAP. Moreover, the optimal concentration of TMP for obtaining these inductive effects was determined to be 4.67 mg/ml, which is the saturation concentration of TMP in aqueous solution at 37°C (20).
Different inducers have different mechanisms of facilitating neuronal cell differentiation. The common feature of chemical inducers is their ability to increase the intracellular concentration of cAMP, suggesting that the second messenger is involved in the induction of MSCs to differentiate into neural precursor cells (21). Butylated hydroxyanisole, β-mercaptoethanol and other antioxidants promote an increase of intracellular cAMP in different ways and then activate the PKA pathway and phosphorylation of downstream target proteins. Moreover, PKC has an important role in the induction process to maintain cell survival. The MEK-ERK signaling pathway also plays an important role in the process of neural cell induction from MSCs. Neurotrophic factor inducers include basic fibroblast growth factor (bFGF), EGF, retinoic acid (RA), nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). In the neuronal differentiation of mouse MSCs, the medium used by Kohyama et al (22) included a demethylation agent (5-azaC), NGF, NT-3 and BDNF, while Jin et al (23) successfully used EGF, bFGF, RA and NGF. The mechanism by which neurotrophic factors promote neural differentiation of MSCs may involve their high concentrations which can potentially simulate the microenvironment of embryonic developmental stages of neurogenesis, thereby promoting the differentiation of MSCs into neural cells. Previous studies have shown that neurotrophic factors increase the expression of MSC membrane proteins TrkA, TrkB and TkrC, which are neurotrophin receptors. The binding of neurotrophin and its receptor initiates changes in some gene expression (24). Traditional Chinese medicines may have antioxidant and anti-ischemic properties and other effects, as well as improve microcirculation. Previously, we also found that they have protective effects against nerve cell injury (25). TMP may play a role as antioxidant in promoting the increase in the intracellular second messenger cAMP, which subsequently activates the PKA pathway and the MEK-ERK signaling pathway, and thus plays a role in the neural induction process. Liu et al (26) and others have pointed out that TMP as a Ca2+ chelator, via the inhibition of the intracellular Ca2+ signal, can upregulate the expression of NSE and Nurrl, thereby accelerating the differentiation of hUMSCs into nerve cells. Zhao et al (27) indicated that sub-totipotent stem cells still express sub-totipotent genes after the embryo has developed into adulthood, but they gradually lose part of the original stem cell phenotype. If the tissue-specific gene expression programs of such cells were activated in an appropriate microenvironment, they can differentiate into various histocytes. hUMSCs are sub-totipotent stem cells, but whether the microenvironment provided by TMP can activate the specific gene expression program of nerve cells to further differentiate into neural cells will require further study.
The ultimate goal of inducing MSCs to differentiate into nerve cells is to use them in vivo as a replacement therapy. However, electrophysiological evidence of whether the induced cells can function as nerve cells is still lacking. Some researchers have claimed that the morphological change of cells was initiated by the toxicity of the inducers, and the long protuberances were caused by the reduction of cytoplasm due to destruction of the actin cytoskeleton. According to this argument, the expression of nerve cell antigen markers is more likely to be result of an abnormal combination of protein interactions rather than a series of genetic events (28). Moreover, most induced nerve cells live for only a short period of time in vitro, which creates a barrier to their effective use in transplantation therapies.
In conclusion, our study demonstrated that TMP can induce hUMSCs to effectively differentiate into neuron-like cells with the optimal concentration of 4.67 mg/ml. After induction, the NSE and NF-H of the neuron-like cells were positive but the GFAP-2 was negative. Future studies will verify whether the differentiated cells are bona fide nerve cells, and the extra-cellular environment required to maintain neuron-like cells after induction in vitro also need further investigation, which may provide additional evidence and rationale for using in vitro induced and differentiated hUMSCs in potential nerve cell transplantation.
Acknowledgements
This study was supported by the Topic Outstanding Youth Science Foundation of Natural Science Fund of Hebei (no. C2009001547), the Natural Science Fund of Hebei (no. H2013206399), and the Medical Science Research Key Project of Hebei (no. 20130240).
References
- 1.McElreavey KD, Irvine AI, Ennis KT, McLean WH. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton's jelly portion of humanumbilical cord. Biochem Soc Trans. 1991;19:29S. doi: 10.1042/bst019029s. [DOI] [PubMed] [Google Scholar]
- 2.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Li SH, Guo PD. Research progress about traditional Chinese medicine (TCM) inducing bone marrow mesenchymal stem cells to differentiate into neural cell. Gansu J Tradit Chin Med. 2009;22:68–69. [Google Scholar]
- 4.Liu YX, Gao JZ. Clinical observation of tetramethylpyrazine early treatment of acute craniocerebral injury. Chin J Clin Neurosurg. 2007;12:754–755. (In Chinese) [Google Scholar]
- 5.Li YQ, Lan TM. Research progress about tetramethylpyrazine in the pharmacokinetics of the nervous system and clinical application. Seek Med Ask The Med. 2011;9:334. (In Chinese) [Google Scholar]
- 6.Xin ZC, Wang L, Lin GT, et al. The markers and tracer marker of mesenchymal stem cells. J Peking Univ. 2013;45:514–517. (In Chinese) [Google Scholar]
- 7.Verfaillie C. Stem cell plasticity. Hematology. 2005;10(Suppl 1):293–296. doi: 10.1080/10245330512331390113. [DOI] [PubMed] [Google Scholar]
- 8.Filip S, Mokrý J, English D, Vojácek J. Stem cell plasticity and issues of stem cell therapy. Folia Biol (Praha) 2005;51:180–187. doi: 10.14712/fb2005051060180. [DOI] [PubMed] [Google Scholar]
- 9.Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98:538–554. doi: 10.1002/jcb.20719. [DOI] [PubMed] [Google Scholar]
- 10.Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109:74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 11.Moscoso I, Centeno A, López E, Rodriguez-Barbosa JI, Santamarina I, Filgueira P, Sánchez MJ, Domínguez-Perles R, Peñuelas-Rivas G, Domenech N. Differentiation ‘in vitro’ of primary and immortalized porcine mesenchymal stem cells into cardiomyocytes for cell transplantation. Transplant Proc. 2005;37:481–482. doi: 10.1016/j.transproceed.2004.12.247. [DOI] [PubMed] [Google Scholar]
- 12.Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016–3020. doi: 10.3748/wjg.v10.i20.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22:617–624. doi: 10.1634/stemcells.22-4-617. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 15.de la Fuente R, Abad JL, García-Castro J, Fernández-Miguel G, Petriz J, Rubio D, Vicario-Abejón C, Guillén P, González MA, Bernad A. Dedifferentiated adult articular chondrocytes: a population of human multipotent primitive cells. Exp Cell Res. 2004;297:313–328. doi: 10.1016/j.yexcr.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Bosnakovski D, Mizuno M, Kim G, Ishiguro T, Okumura M, Iwanaga T, Kadosawa T, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol. 2004;32:502–509. doi: 10.1016/j.exphem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Bhagavati S, Xu W. Isolation and enrichment of skeletal muscle progenitor cells from mouse bone marrow. Biochem Biophys Res Commun. 2004;318:119–124. doi: 10.1016/j.bbrc.2004.03.192. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger M, Vanguri P, Simonetti D, Young R. Adult mesenchymal stem cells: Potential for muscle and tendon regeneration and use in gene therapy. J Musculoskelet Neuronal Interact. 2002;2:309–320. [PubMed] [Google Scholar]
- 19.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 20.Gao LP, Cai JH, He HH, Huang LS, Fu ZH. Determination of the equilibrium solubility and oil/water apparent partition coefficient (P) of tetramethylpyrazine by HPLC. Strait Pharm J. 2013;25:24–26. [Google Scholar]
- 21.Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- 22.Kohyama J, Abe H, Shimazaki T, Koizumi A, Nakashima K, Gojo S, Taga T, Okano H, Hata J, Umezawa A. Brain from bone: Efficient ‘meta-differentiation’ of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation. 2001;68:235–244. doi: 10.1046/j.1432-0436.2001.680411.x. [DOI] [PubMed] [Google Scholar]
- 23.Jin K, Mao XO, Batteur S, Sun Y, Greenberg DA. Induction of neuronal markers in bone marrow cells: Differential effects of growth factors and patterns of intracellular expression. Exp Neurol. 2003;184:78–89. doi: 10.1016/S0014-4886(03)00133-X. [DOI] [PubMed] [Google Scholar]
- 24.Yuan Y, Yang SY, Han ZC, et al. Amplification and differentiation towards neuron-like cells of human umbilical cord derived mesenchymal stem cells. Chin J Neuromed. 2006;5:230–236. [Google Scholar]
- 25.Sun WX, An HM. The mechanism research concerning protective effects of traditional chinese medicine on nerve system injury caused by oxidative stress. Chin J Chin Med. 2015;4:561–564. (In Chinese) [Google Scholar]
- 26.Liu YY, Zhao XX, Zhao HB, Ge BF, Liu XY, Chen KM. Tetramethylpyrazine induces the differentiation of mouse bone marrow-derived mesenchymal stem cells into nerve cells mediated by Ca2+ signaling. J Gansu Agric Univ. 2010;45:1–5. [Google Scholar]
- 27.Zhao CH, Fang BJ, Han Q, et al. Study about biological property of pluripotent stem cells and transplantation application. J Chin Microcirc. 2004;8:345. [Google Scholar]
- 28.Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: Differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004;77:174–191. doi: 10.1002/jnr.20148. [DOI] [PubMed] [Google Scholar]