Abstract
Purpose
To assess long-term outcomes of men with favorable-risk prostate cancer in a prospective, active-surveillance program.
Methods
Curative intervention was recommended for disease reclassification to higher cancer grade or volume on prostate biopsy. Primary outcomes were overall, cancer-specific, and metastasis-free survival. Secondary outcomes were the cumulative incidence of reclassification and curative intervention. Factors associated with grade reclassification and curative intervention were evaluated in a Cox proportional hazards model.
Results
A total of 1,298 men (median age, 66 years) with a median follow-up of 5 years (range, 0.01 to 18.00 years) contributed 6,766 person-years of follow-up since 1995. Overall, cancer-specific, and metastasis-free survival rates were 93%, 99.9%, and 99.4%, respectively, at 10 years and 69%, 99.9%, and 99.4%, respectively, at 15 years. The cumulative incidence of grade reclassification was 26% at 10 years and was 31% at 15 years; cumulative incidence of curative intervention was 50% at 10 years and was 57% at 15 years. The median treatment-free survival was 8.5 years (range, 0.01 to 18 years). Factors associated with grade reclassification were older age (hazard ratio [HR], 1.03 for each additional year; 95% CI, 1.01 to 1.06), prostate-specific antigen density (HR, 1.21 per 0.1 unit increase; 95% CI, 1.12 to 1.46), and greater number of positive biopsy cores (HR, 1.47 for each additional positive core; 95% CI, 1.26 to 1.69). Factors associated with intervention were prostate-specific antigen density (HR, 1.38 per 0.1 unit increase; 95% CI, 1.22 to 1.56) and a greater number of positive biopsy cores (HR, 1.35 for one additional positive core; 95% CI, 1.19 to 1.53).
Conclusion
Men with favorable-risk prostate cancer should be informed of the low likelihood of harm from their diagnosis and should be encouraged to consider surveillance rather than curative intervention.
INTRODUCTION
Widespread prostate-specific antigen (PSA)– based screening for prostate cancer in the United States led to concerns about the overdiagnosis and overtreatment of a disease with a long natural history.1 The US Preventive Services Task Force concluded that harms likely outweigh benefits and issued a grade D recommendation to discourage the use of PSA-based screening for prostate cancer2; others have recommended shared decision making for men most likely to benefit.3,4 If treatment follows diagnosis for most men, as is the case in the United States, overtreatment rates will be high.5
Although underused in the United States, active surveillance (AS) is one approach to address the overtreatment of prostate cancer.6 Recent evidence suggests growing acceptance of AS as a means of reducing overtreatment of risk-based screening–detected prostate cancers.7 Furthermore, disease-specific survival rates of AS have been reported to be consistent with those associated with immediate curative treatment,8 which may lead to improved acceptance of this approach. The National Institutes of Health identified evaluation of short- and long-term outcomes of men receiving AS as a top research priority.9 Therefore, we report on the follow-up of a cohort of men receiving AS that used a clearly defined protocol for enrollment, monitoring, and intervention since 1995.
METHODS
Prospectively Defined Study Design
Beginning in January 1995, AS was offered as a management strategy to men with very low-risk (VLR) prostate cancer, as described by Epstein et al10 and endorsed by the National Comprehensive Cancer Network.11 VLR criteria include clinical stage T1c disease, PSA density (PSAD) less than 0.15 ng/mL, biopsy Gleason score ≤ 6, two or fewer positive biopsy cores, and a maximum of 50% involvement of any biopsy core with cancer. We have not used a PSA cut point of 10 ng/mL to exclude men from the VLR category if the PSAD criterion was met, because no clinically meaningful PSA threshold could be identified to predict biopsy reclassification.12 Because of patient preference, older men with low-risk (LR) disease (ie, clinical stage ≤ T2a, PSA < 10 ng/mL, and Gleason score ≤ 6) were also enrolled in this program. Our surveillance protocol included semiannual PSA measurement for PSA decrease/increase and digital rectal examination as well as an annual 12- to 14-core biopsy for most men. Curative intervention was recommended for disease reclassification, defined as biopsy findings no longer meeting the inclusion criteria. This study was approved by the institutional review board at the Johns Hopkins medical institutions.
Study Cohort
Since inception in 1995 through analysis in June 2014, 1,298 men with favorable-risk (ie, VLR and LR) prostate cancer were enrolled onto AS. Most patients were white (88.4%), 7.4% were African American, and 4.2% were of other ethnic backgrounds. Enrollment occurred at a median age of 66 years (range, 41 to 92 years). In total, 926 men (71%) met all criteria for VLR cancer, whereas 372 (29%) met criteria for LR cancer. Of men with LR disease, 188 (50.5%, 14.5% of the total) did not meet VLR criteria because of a PSAD greater than 0.15 ng/mL, whereas 184 (49.5%, 14.2% of the total) did not meet at least one VLR biopsy criterion. No patient had a biopsy Gleason score of 7 or greater.
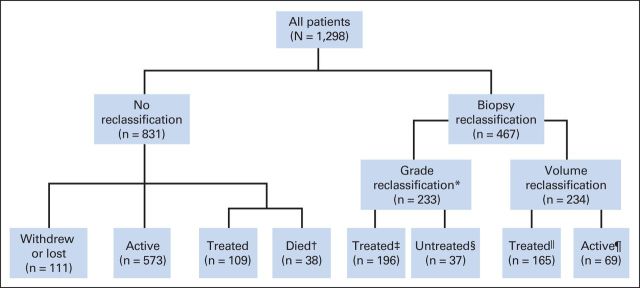
Median follow-up time was 4.0 years (range, 0.01 to 18.0 years) for patients who remained at risk and 5.0 years (range, 0.01 to 18.0 years) for all patients from enrollment to the most recent follow-up time. A total of 650 men underwent at least 5 years of follow-up, and 184 men received at least 10 years of follow-up. The median interval between surveillance biopsies was 1.0 year (range, 0 to 11 years; Table 1). As of June 2014, 642 men (50%) were active in the program, 470 (36%) underwent curative intervention, and 186 (14%) were undecided about treatment modality, withdrew, were lost to follow-up, or died. Forty-seven died as a result of causes other than prostate cancer, and two died as a result of prostate cancer (Fig 1).
Table 1.
Demographic Characteristics of the Study Group at Diagnosis
| Characteristic | Overall Cohort (N = 1,298) |
Very Low Risk (n = 926) |
Low Risk (n = 372) |
|||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Age at diagnosis, years | 66 | 62-69 | 66 | 62-69 | 66 | 62-70 |
| Year of diagnosis | 2008 | 2004-2010 | 2007 | 2003-2010 | 2009 | 2005-2011 |
| PSA level, ng/mL | 4.8 | 3.6-6.2 | 4.5 | 3.4-5.8 | 5.6 | 4.2-7.4 |
| PSA density | 0.1 | 0.07-0.14 | 0.09 | 0.07-0.12 | 0.18 | 0.14-0.22 |
| No. of cores positive for cancer | 1 | 1-2 | 1.0 | 1-1 | 1.0 | 1-2 |
| Maximum involvement of any core with cancer, % | 5 | 1-10 | 5.0 | 1-10 | 5.0 | 1-20 |
| No. of repeat biopsies after diagnosis | 2 | 1-4 | 2 | 1-4 | 2 | 1-3 |
| Interval between surveillance biopsies, years | 1 | 1-1 | 1 | 1-1 | 1 | 1-1 |
| Follow-up in men at risk, years | 4 | 2-7 | 5 | 2-7 | 3 | 1-5 |
Abbreviations: IQR, interquartile range; PSA, prostate-specific antigen.
Fig 1.
CONSORT diagram for the Johns Hopkins active surveillance program. (*) Treatment is recommended in all cases of grade reclassification (ie, Gleason score upgrading). (†) Death from nonprostate cancer (PCA) causes. (‡) Includes five men who died of non-PCA causes after treatment. (§) Includes 27 men who were undecided on treatment at the time of analysis, eight men with no treatment data available, and two men who were found to have metastatic disease shortly after grade reclassification and subsequently died of PCA without treatment of their local disease. (‖) Includes four men who died of non-PCA causes after treatment. (¶) Includes 54 men who elected continued observation and 15 men with no treatment data available.
Statistical Analysis
Our primary outcomes of interest were overall, cancer-specific, and metastasis-free survivals. Secondary outcomes were the rates of biopsy reclassification and curative intervention; biochemical recurrence (BCR) was assessed in those who underwent treatment. Incidence rates were calculated and expressed per 100 person-years. We evaluated both primary and secondary outcomes by using the Kaplan-Meier (KM) time-to-event approach and a cumulative-incidence, competing-risks approach. Both methods yielded similar results for the primary outcomes of interest, but the KM approach caused overestimation of risk (underestimation of survival time) for the secondary outcomes, as previously reported.13 Therefore, we used the cumulative-incidence, competing-risks approach to evaluate secondary outcomes and included as competing risks elective treatment in the absence of reclassification, volume reclassification, and death as a result of causes other than prostate cancer. The cumulative incidence of biopsy reclassification, grade reclassification to Gleason score 3 + 4 and 4 + 3 or greater, curative intervention, and BCR were calculated from the date of diagnosis. A competing-risk model was used to evaluate predictors of grade reclassification and curative intervention. The clinical and pathologic variables that were statistically significantly associated with these outcomes on univariable analysis (P < .05) were entered into a multivariable model. To prevent overfitting, variables were assessed for correlation and multicollinearity. Active patients and those lost to follow-up were censored at the time of their last visits. Date and cause of mortality were obtained from the National Death Index.14
BCR, or biochemical failure, was defined as a PSA level greater than 0.2 ng/mL in men who underwent surgery for treatment of prostate cancer, and a PSA level greater than or equal to 2.0 ng/mL higher than the nadir in men who underwent radiation therapy. Freedom from BCR was compared in men who underwent surgery versus radiation by using the log-rank test in patients who had at least 1 year of follow-up after treatment. Statistical significance was set at P < .05, and analyses were performed by using SAS version 9.4 (SAS, Cary, NC) and STATA version 13.1 (StataCorp, College Station, TX).
RESULTS
Primary Outcomes: Mortality and Metastasis
Of the 1,298 men who received follow-up, 49 (4%) died (Fig 1, Table 2). Thirty-eight men (3%) died as a result of causes other than prostate cancer before reclassification or treatment, and nine men (0.7%) died as a result of causes other than prostate cancer after being treated for prostate cancer. Death occurred at a median age of 75.5 years (range, 59.2 to 90.6 years), and the most common overall cause of death was cardiovascular disease. The 47 men who died as a result of causes other than prostate cancer were receiving AS for a median of 7.0 years (range, 1.0 to 18.0 years) before their deaths.
Table 2.
Event Rates During Surveillance Per 100 Person-Years
| Event | Overall (N = 1,298) |
Very Low Risk (n = 926) |
Low Risk (n = 372) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Incidence | 95% CI | Events | Incidence | 95% CI | Events | Incidence | 95% CI | |
| Lymph node or distant metastasis* | 5 | 0.08 | 0.03 to 0.2 | 2 | 0.04 | 0.01 to 0.2 | 3 | 0.2 | 0.06 to 0.6 |
| Cause of death | |||||||||
| Prostate cancer | 2 | 0.03 | 0.01 to 0.1 | 2 | 0.04 | 0.01 to 0.2 | 0 | 0.0 | |
| All causes | 49 | 0.70 | 0.60 to 1.0 | 37 | 0.7 | 0.5 to 1.0 | 12 | 0.7 | 0.4 to 1.3 |
| Biopsy | |||||||||
| Grade reclassification | 233 | 3.8 | 3.3 to 4.3 | 161 | 3.4 | 3.0 to 4.0 | 72 | 4.8 | 3.8 to 6.0 |
| Any reclassification | 467 | 8.7 | 8.0 to 9.5 | 343 | 8.5 | 7.7 to 9.4 | 124 | 9.5 | 8.0 to 11.2 |
| Curative intervention | 471 | 9.0 | 8.3 to 9.9 | 333 | 8.4 | 7.6 to 9.4 | 138 | 10.9 | 9.3 to 12.7 |
Includes two men who died as a result of prostate cancer.
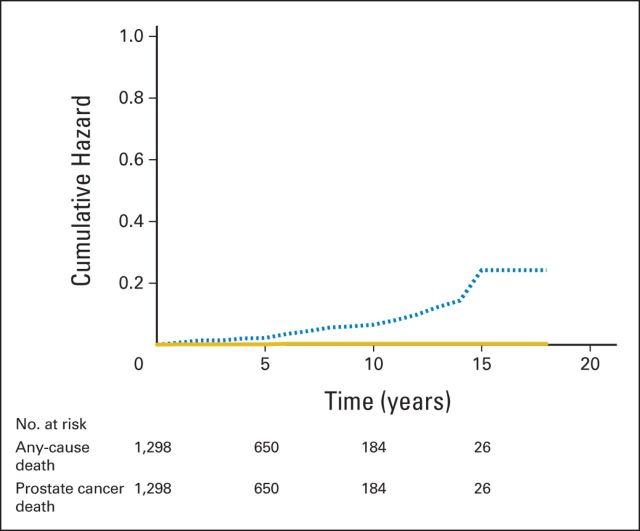
Two deaths (0.15%) were a result of prostate cancer; both occurred in men who had a diagnosis of VLR disease (Table 2). One patient died 16 years after enrollment, with a cancer clonally distinct from his original cancer,15 and the other died within 15 months of diagnosis, with follow-up elsewhere after he received a recommendation of surveillance in a consultation at Johns Hopkins. Although the patient who received this recommendation did not receive follow-up in our program, the event was included because he would have been enrolled had he chosen to be monitored at Johns Hopkins. The cumulative hazard ratio (HR) of nonprostate cancer–to–prostate cancer mortality or metastases was 24:1 (Fig 2). Two lymph node metastases and one distant metastasis occurred in three men, which led to a total of five men (0.40%) who experienced metastasis or death as a result of prostate cancer (Table 2). The overall, cancer-specific, and metastasis-free survival rates were 93%, 99.9%, and 99.4%, respectively, at 10 years and 69%, 99.9%, and 99.4%, respectively, at 15 years.
Fig 2.
Cumulative hazard of death as a result of any cause (dashed line) and prostate cancer death or metastasis (solid line).
Secondary Outcomes: Reclassification and Curative Intervention
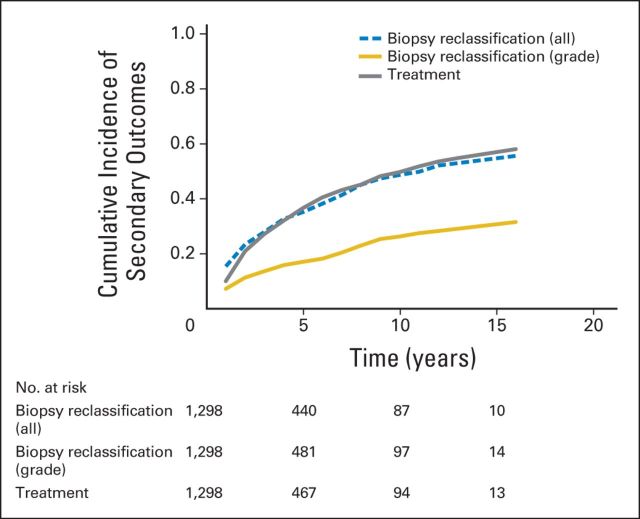
In 467 men (36%), disease was reclassified during biopsy at a median of 2.0 years (range, 0.3 to 16.0 years) after enrollment; 233 involved grade reclassification, and 234 involved volume reclassification (Table 2, Fig 1). At 5, 10, and 15 years after the start of surveillance, the cumulative incidence of any biopsy reclassification was 35%, 49%, and 56%, respectively, and the cumulative incidence of grade reclassification was 17%, 26%, and 31%, respectively (Fig 3).
Fig 3.
Cumulative incidence of secondary outcomes: treatment (gray line), grade reclassification (gold line), and reclassification by grade or cancer extent (dashed blue line).
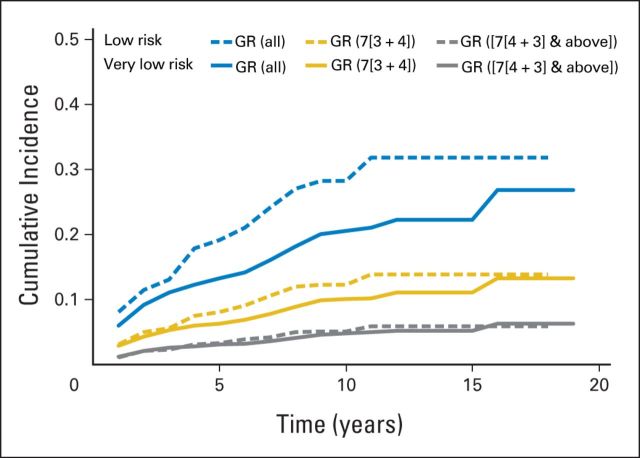
Because cancer grade is the most significant factor for the prediction of long-term cancer-specific survival,16 we additionally evaluated grade reclassification in men with VLR and LR disease. For men with VLR cancer, respective cumulative incidences of grade reclassification at 5, 10, and 15 years were 13%, 21%, and 22%, respectively; reclassification rates to a Gleason score of 3 + 4 at 5, 10, and 15 years were 6%, 10%, and 11%, respectively; and reclassification rates to a Gleason score of 4 + 3 or greater at 5, 10, and 15 years were 3%, 5%, and 5%, respectively (Fig 4). For men with LR prostate cancer, the respective cumulative incidences of any grade reclassification at 5, 10, and 15 years were 19%, 28%, and 31%; reclassification rates to a Gleason score of 3 + 4 at 5, 10, and 15 years were 8%, 12%, and 14%, respectively, and reclassification rates to a Gleason score of 4 + 3 at 5, 10, and 15 years were 3%, 5%, and 5.9%, respectively (Fig 4).
Fig 4.
Cumulative incidence of Gleason grade reclassification (GR) for men with very-low-risk prostate cancer (solid lines) and low-risk prostate cancer (dashed lines): all GR (blue), GR to 3 + 4 (gold), and GR to 4 + 3 and greater (gray).
Treatment was recommended in the event of reclassification during biopsy. For those treated, intervention occurred at a median of 3.0 years (range, 0.75 to 16.0 years) after enrollment. One hundred nine men elected treatment in the absence of biopsy reclassification, whereas 361 men underwent treatment on the basis of biopsy reclassification (Fig 1). The cumulative incidence of treatment at 5, 10, and 15 years after the start of surveillance was 37%, 50%, and 57%, respectively (Fig 3). For the overall cohort, the median time during surveillance that was free of intervention was 8.5 years (range, 0.01 to 18.0 years).
In multivariable analysis, after adjustment for the year of diagnosis, diagnostic factors associated with grade reclassification were older age (HR, 1.03 for each additional year; 95% CI, 1.01 to 1.06), PSAD (HR, 1.21 per 0.1 unit increase; 95% CI, 1.12 to 1.46), and greater number of positive biopsy cores (HR, 1.47 for each additional positive core; 95% CI, 1.26 to 1.69). Factors associated with intervention were PSAD (HR, 1.38 per 0.1 unit increase; 95% CI, 1.22 to 1.56) and a greater number of positive biopsy cores (HR, 1.35 for one additional positive core; 95% CI, 1.19 to 1.53).
Biochemical Outcomes After Intervention
Of 471 men who underwent intervention during the study, 287 (61%) received at least 1 year of post-treatment follow-up for assessment of BCR: 138 (48%) underwent radical prostatectomy, and 149 (52%) underwent radiation therapy. Those who underwent surgery were significantly younger (median age, 63.0 v 67.5 years; P < .001) and were treated at a date closer to diagnosis (median interval to treatment, 2.2 v 2.5 years; P = .02) than those who underwent radiation. The surgery and radiation groups were similar with respect to PSA levels, PSAD, number of positive biopsy cores, maximum percentage of core involvement with cancer, proportion reclassified by grade (ie, Gleason score ≥ 7) before treatment, and median follow-up time (3.8 years). Of 287 treated patients with sufficient follow-up, 23 (8%) experienced BCR: 11 (8%) from the surgery group and 12 (8%) from the radiation group. KM analysis revealed that survival that was free of BCR was not significantly different on the basis of treatment modality (P = .90).
DISCUSSION
Prostate cancer is a heterogeneous disease with risk that varies according to host and tumor characteristics that have not been fully elucidated. Incontrovertible evidence demonstrates that early detection and effective treatment are associated with reduced prostate cancer mortality for some men with unfavorable cancers17,18 and that the number of men in whom a diagnosis is needed to prevent a death as a result of prostate cancer is substantial.17 Therefore, overtreatment of LR disease is common and problematic, especially among older men for whom treatment is not likely to improve health outcomes.18 With concurrent goals of reducing overtreatment and identifying lethal tumors while curable, AS has evolved into a well-accepted management strategy for appropriately selected men.
In men with favorable-risk prostate cancer, we found that the risk of prostate cancer progression to a lethal phenotype is low for a decade after diagnosis. Although five men (0.4%) died as a result of prostate cancer or developed metastatic disease during follow-up, this should be tempered by the exclusion of men from AS and recommended curative intervention on the basis of criteria that overestimate the risk of harm without treatment (ie, biopsy findings). In addition to restricting enrollment to those patients with the lowest-risk classifications, our surveillance protocol has stressed annual biopsy procedures for most men to ensure that higher-grade cancers are not missed during follow-up. Although prostate biopsy is itself associated with morbidity, advances in magnetic resonance imaging and molecular techniques will hopefully render frequent biopsy procedures unnecessary in the near future. Indeed, our criteria for inclusion, monitoring, and intervention may be overly conservative, and relaxation of these criteria could achieve similar outcomes.
Our study was conceived when resistance to monitoring men with a diagnosis of prostate cancer, regardless of grade, was substantial. Therefore, our intents were to demonstrate the safety of this approach for carefully selected men and to identify markers of a lethal phenotype that might lead to wider inclusion in AS. The expansion of AS during the past decade and the sharing of institutional data sets will likely help delineate factors associated with varying outcomes19,20 and will allow patients to play a greater role in selecting the strategy that best suits their individual preferences.
Our results with respect to cancer-specific mortality and development of metastatic disease would be comparable to those of men who have cancers with Gleason scores of 6 at the time of radical prostatectomy, in whom the 15- and 20-year probability of prostate cancer death was 0.2% at ages 60 to 69 years.16 Our intermediate outcomes of freedom from biopsy reclassification and treatment-free survival were generally consistent with those of Welty et al,21 who reported freedom from biopsy reclassification and treatment-free survival rates of 40% and 60%, respectively, at 5 years. Consistent with others, we found no differences in the rates of BCR when we compared men who underwent surgery and radiation after a period of surveillance.8
Biopsy grade reclassification is the most common trigger for curative intervention among men who receive surveillance. PSAD and the number of positive biopsy cores at diagnosis—both indicators of cancer volume—and older age were associated with grade reclassification, whereas PSAD and the number of positive biopsy cores were associated with curative intervention. One unique aspect of our report is the competing-risks analysis of grade reclassification in a large cohort of patients who underwent intensive surveillance biopsy procedures during follow-up. Our analysis revealed that biopsy reclassification to a prognostic grade group of 3 or higher (ie, Gleason score 4 + 3 or greater),22 which is associated with cancer-specific death,16 was 5% to 6% at 15 years for men with VLR or LR disease. This finding may explain the rarity of metastatic disease or prostate cancer death in our cohort.
Other investigators have reported favorable intermediate outcomes in men who receive AS.6 One other prospective AS study had comparable long-term follow-up; at 10 years, 206 (21%) men remained at risk8 compared with 184 (14%) men in the current study. By using less restrictive criteria for enrollment and a less-intensive monitoring protocol, Klotz et al8 reported that 3% of 993 men with favorable- or intermediate-risk prostate cancer either died as a result of the disease or developed metastatic disease at a median follow-up of 6 years. At 10 and 15 years, overall survival rates were 80% and 62%, respectively, compared with 93% and 69%, respectively, in the current study. Fifteen years after enrollment, survival free of treatment was 55% in the cohort of Klotz et al8 compared with 37% in the current study. Therefore, tradeoffs were observed in the attempt to balance overtreatment of prostate cancer with the small, but present, risk of underestimation of cancer lethality. In the PIVOT (Prostate Intervention Versus Observation) trial, 3% of men with favorable-risk disease who elected watchful waiting, compared with AS, died as a result of prostate cancer at a median follow-up of 10 years after diagnosis.23 Given the similarity in cancer-specific outcomes between the watchful-waiting arm of PIVOT and the AS outcomes in the report of Klotz et al,8,23 one could conclude that, in the absence of curative intervention, outcomes may depend more on the selection of candidates for noncurative care than on the intensity of monitoring.
Given the current evidence, AS appears to be an underused approach in the management of favorable-risk prostate cancer.5 Although evidence suggests that an increasing number of men are initially treated with AS,7 the large variability in use of this approach depends more on the physician practice patterns than on characteristics of cancer.24 Alignment of evidence and practice will require improved communication with patients about cancer risk and greater confidence on the part of physicians that a nonlethal phenotype is being monitored.
Although the strengths of this study include its prospective nature and strictly defined criteria for enrollment and monitoring, a number of limitations should be mentioned. First, given the long natural history of prostate cancer, follow-up in this single-arm study was incomplete. Therefore, although our reported rates at 15 years were based on small numbers of patients who remained at risk, it is important that our 10-year rates of metastasis—considered a valid end point—were low and had fairly narrow confidence intervals. Second, our population of primarily white men lacked ethnic diversity, and these results may not be applicable to other ethnicities. For example, African Americans may have prostate cancers with a biology that is more aggressive than that observed in white men.25 Third, we were restrictive in the recommendation of surveillance to individuals, and we included no men with a biopsy Gleason score of greater than 6. Therefore, we cannot comment on the safety of surveillance in these men or in those with more extensive low-grade disease who we excluded from surveillance.
In conclusion, our data suggest that, for men with favorable-risk prostate cancer, the paradigm of immediate intervention must be replaced by one of immediate contemplation—a thoughtful assessment of prognostic risk, life expectancy, and the relative risks and benefits of available management options considered in the context of personal preferences.
Glossary Terms
- active surveillance:
approach to management of suspected or proven malignancy thought to pose a low risk of progression in the short to intermediate term. Tumors are observed closely with blood tests, imaging, and/or serial biopsy, and intervention is undertaken if or when evidence of tumor growth or progression is found.
- biochemical failure:
increase in prostate-specific antigen level after prostate cancer is treated but meets the criteria for progression. Examples are the American Society for Radiation Oncology definition, which is three consecutive increases after radiotherapy; the Phoenix definition, which is an increase of 2.0 ng/mL greater than the nadir after radiotherapy; or the American Urological Association definition, which is a level of 0.2 ng/mL and increasing after prostatectomy.
- competing risks:
events that prevent an event of interest from occurring. “Competing risks are said to be present when a patient is at risk of more than one mutually exclusive event, such as death from different causes, and the occurrence of one of these will prevent any other event from ever happening” (Hinchliffe S, presentation at the University of Leicester, 2012).
- cumulative incidence:
statistical measure of an event of interest (eg, relapse, death, second malignant neoplasm, a specific disease) that occurs in a specified time period in the population at risk. It is calculated as the number of new cases of the event of interest divided by the total population at risk.
- disease-specific survival rate:
percentage of people in a study who have not died from a specific disease since diagnosis or treatment. Patients who died as a result of some other cause are not counted.
- Gleason score:
pathologic description of the grade of prostate cancer made on the basis of the degree of abnormality in the glandular architecture. Gleason patterns 3, 4, and 5 denote low, intermediate, and high levels of histologic abnormality and tumor aggressiveness, respectively. The score is used to assign primary and secondary numbers on the basis of the most common and second-most common patterns identified.
- prospectively defined:
study design (eg, study objectives, outcome measures, analytical methods, analysis plan) specified and documented before the study is conducted. Prospective definition of the study design and analysis plan is critical to produce level-1 evidence for clinical use of a biomarker, as defined by Simon et al (Simon RM, et al: J Natl Cancer Inst 101:1446-1452, 2009).
- prostate-specific antigen (PSA):
protein produced by cells of the prostate gland. The blood level of PSA is used as a tumor marker for men who may be suspected of having prostate cancer. Most physicians consider 0 to 4.0 ng/mL the normal range. Levels of 4 to 10 and 10 to 20 ng/mL are considered slightly elevated and moderately elevated, respectively. PSA levels must be considered along with other test results to make a firm diagnosis of prostate cancer.
- PSA decrease and/or increase:
relative difference between the nadir and/or zenith of the PSA during the study period and the baseline value.
- risk-based screening:
Screening for long-term and late cancer and cancer treatment–related effects that encompasses health risks related to the patient (eg, age at treatment, attained age, sex, ethnicity, genetics, health behaviors) and to the cancer (histology; involved sites; specific treatment like surgery, radiation, chemotherapy, hematopoietic cell transplantation, transfusion).
Footnotes
See accompanying editorial on page 3365
Processed as a Rapid Communication manuscript.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Presented in part at the American Urological Association Annual Meeting, New Orleans, LA, May 15-19, 2015.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey J. Tosoian, Jonathan I. Epstein, Bruce J. Trock, H. Ballentine Carter
Collection and assembly of data: Mufaddal Mamawala, Patricia Landis, Sacha Wolf, Bruce J. Trock, H. Ballentine Carter
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jeffrey J. Tosoian
No relationship to disclose
Mufaddal Mamawala
No relationship to disclose
Jonathan I. Epstein
Consulting or Advisory Role: Metamark Genetics, MDxHealth, Dianon Systems, DAKO
Patricia Landis
No relationship to disclose
Sacha Wolf
No relationship to disclose
Bruce J. Trock
Honoraria: Roche
Consulting or Advisory Role: Champions Oncology, SonaCare Medical
Research Funding: Myriad Genetics
Expert Testimony: Rochon Genova, Rothwell Figg
Travel, Accommodations, Expenses: Roche
H. Ballentine Carter
No relationship to disclose
REFERENCES
- 1.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA Force USPST. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 3.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: A review of current evidence. JAMA. 2014;311:1143–1149. doi: 10.1001/jama.2014.2085. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman KE, Niu J, Shen Y, et al. Physician variation in management of low-risk prostate cancer: A population-based cohort study. JAMA Intern Med. 2014;174:1450–1459. doi: 10.1001/jamainternmed.2014.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dall'Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: A systematic review of the literature. Eur Urol. 2012;62:976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 7.Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67:44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–277. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 9.Ganz PA, Barry JM, Burke W, et al. National Institutes of Health State-of-the-Science Conference: Role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med. 2012;156:591–595. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 11.Carroll PR, Parsons JK, Andriole G, et al. Prostate cancer early detection, version 1.2014. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2014;12:1211–1219. doi: 10.6004/jnccn.2014.0120. [DOI] [PubMed] [Google Scholar]
- 12.Umbehr MH, Platz EA, Peskoe SB, et al. Serum prostate-specific antigen (PSA) concentration is positively associated with rate of disease reclassification on subsequent active surveillance prostate biopsy in men with low PSA density. BJU Int. 2014;113:561–567. doi: 10.1111/bju.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Southern DA, Faris PD, Brant R, et al. Kaplan-Meier methods yielded misleading results in competing risk scenarios. J Clin Epidemiol. 2006;59:1110–1114. doi: 10.1016/j.jclinepi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. National Death Index. http://www.cdc.gov/nchs/ndi.htm.
- 15.Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123:4918–4922. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–875. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–2035. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: The PRIAS study. Eur Urol. 2013;63:597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Newcomb LF, Brooks JD, Carroll PR, et al. Canary Prostate Active Surveillance Study: Design of a multi-institutional active surveillance cohort and biorepository. Urology. 2010;75:407–413. doi: 10.1016/j.urology.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welty CJ, Cowan JE, Nguyen H, et al. Extended follow-up and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015;193:807–811. doi: 10.1016/j.juro.2014.09.094. [DOI] [PubMed] [Google Scholar]
- 22.Pierorazio PM, Walsh PC, Partin AW, et al. Prognostic Gleason grade grouping: Data based on the modified Gleason scoring system. BJU Int. 2013;111:753–760. doi: 10.1111/j.1464-410X.2012.11611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundi D, Ross AE, Humphreys EB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: Should active surveillance still be an option for them? J Clin Oncol. 2013;31:2991–2997. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]