Abstract
Levels of eukaryotic initiation factor 4E (eIF4E) are frequently elevated in human cancers and in some instances have been associated with poor prognosis and outcome. Here we utilize transgenic and allograft breast cancer models to demonstrate that increased mammalian target of rapamycin (mTOR) signalling can be a significant contributor to breast cancer progression in vivo. Suppressing mTOR activity, as well as levels and activity of the downstream translation regulators, eIF4E and eIF4A, delayed breast cancer progression, onset of associated pulmonary metastasis in vivo and breast cancer cell invasion and migration in vitro. Translation of vascular endothelial growth factor (VEGF), matrix metallopeptidase 9 (MMP9) and cyclin D1 mRNAs, which encode products associated with the metastatic phenotype, is inhibited upon eIF4E suppression. Our results indicate that the mTOR/eIF4F axis is an important contributor to tumor maintenance and progression programs in breast cancer. Targeting this pathway may be of therapeutic benefit.
Keywords: eIF4E, eIF4A, PyMT mouse model, breast cancer, pulmonary metastasis
INTRODUCTION
Recruitment of ribosomes to mRNA templates is generally the rate-limiting step of translation initiation and is mediated by eukaryotic initiation factor (eIF) 4F—a heterotrimeric complex consisting of: (1) eIF4E, the cap-binding protein responsible for binding to mRNA cap structures; (2) eIF4A, an RNA helicase required to unwind local secondary structure and facilitate access of the 43S ribosomal complex to mRNA templates; and (3) eIF4G, a large scaffolding protein. The availability of the eIF4F complex is regulated by mammalian target of rapamycin (mTOR) through phosphorylation of eIF4E-binding partners, called 4E-BP (there are three 4E-BPs with the best characterized being 4E-BP1). Increased signalling flux through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway leads to an mTOR-dependent phosphorylation of 4E-BP1, which in turn augments its dissociation from eIF4E and stimulates eIF4F complex formation.1 The PI3K/Akt pathway also regulates the availability of eIF4A for eIF4F complex assembly.2
In human cancers, including breast malignancies, the PI3K/Akt/mTOR signalling axis is frequently deregulated as a consequence of genetic alterations or epigenetic changes that result in constitutive pathway activation. A prominent consequence of elevated PI3K/Akt/mTOR activity is deregulated translational control—a feature implicated in tumor initiation and maintenance in a number of in vitro and in vivo settings.3 – 5 There is thus considerable interest in targeting this pathway in cancer, either by inhibiting mTOR (using rapalogs or mTOR-kinase inhibitors (KIs)) or PI3K/mTOR activity (using PI3K/mTOR-KIs), the expectation being that tumors bearing lesions in the PI3K pathway have acquired dependency on its activity to support their growth and survival programs.6 Some of the therapeutics effects of rapalogs and PI3K/mTOR-KIs appear to be a consequence of inhibitory effects on the translational regulatory node in tumor cells as elevated eIF4E levels can bypass tumor cell sensitivity to mTOR inhibition in vivo7 and is a genetic modifier of PI3K/mTOR-KI sensitivity in vitro in human mammary epithelial cells.8 MYC amplification also leads to PI3K-independent breast cancer cell survival and resistance to PI3K inhibitors9 and can overcome the proliferative block induced by PI3K/TOR-KIs.10 eIF4E, eIF4AI and eIF4G are transcriptional targets of MYC and may be contributing to the MYC phenotype in these drug-resistant settings.11 – 13
Perturbing eIF4F activity, either as a consequence of elevated eIF4E levels or hyperphosphorylation of 4E-BP1, is associated with, and thought to promote, breast cancer cell maintenance and progression through translational deregulation of a number of oncogenic drivers and inhibitors.14,15 In human tumors, the phosphorylation status of 4E-BP116 and eIF4E/4E-BP levels17 have been reported to be associated with malignant progression and adverse progression in breast cancer. Ectopic overexpression of 4E-BP1 mutant,15,18 or suppression of eIF4E expression,19,20 both of which repress cap-dependent translation initiation, significantly decreases cell proliferation and survival of breast tumor cells. eIF4E is a genetic modifier of anti-HER2-directed therapies, such as the FDA (Food and Drug Administration)-approved trastuzumab antibody, in breast cancer, with high levels capable of rescuing growth inhibition in vitro and associated with drug resistance in xenograft models.21 Therapeutic strategies targeting eIF4E by antisense inhibition,22 or with a small-molecule inhibitor that removes the eIF4A helicase from the eIF4F complex,23 delays growth of breast cancer cells in xenograft models. Here we utilize the well-characterized MMTV-PyMT transgenic model, where breast neoplasia is induced by restricted expression of polyoma virus middle T antigen in the mammary epithelium,24 to explore the therapeutic potential of inhibiting eIF4F activity. Our results suggest a rationale for targeting eIF4F to curtail breast cancer maintenance and progression.
RESULTS
Reductions in Tsc2 expression cooperate with oncogenic signalling in breast cancer metastasis
Given the association between increased PI3K/Akt/mTOR activity and breast cancer (see Introduction), we wished to determine in a genetically controlled setting if increased mTOR signalling flux (and thus potentially increased eIF4F levels) could affect tumor onset and maintenance in this disease in vivo. Our model consisted of intercrossing two transgenic mouse models. First, we used the MMTV-PyMT breast cancer model, which not only morphologically recapitulates the progression of human breast cancer from hyperplasia to carcinoma, but also leads to pulmonary metastasis ~8 weeks following tumor onset.24,25 The similarity of this model with the human disease is exemplified by the loss of steroid hormone receptors and β1-integrin associated with increased ErbB2 and cyclin D1 expression in late-stage disease.26 We placed the MMTV-PyMT transgene onto the C57Bl/6 background as this has been shown to significantly delay tumor onset.27,28
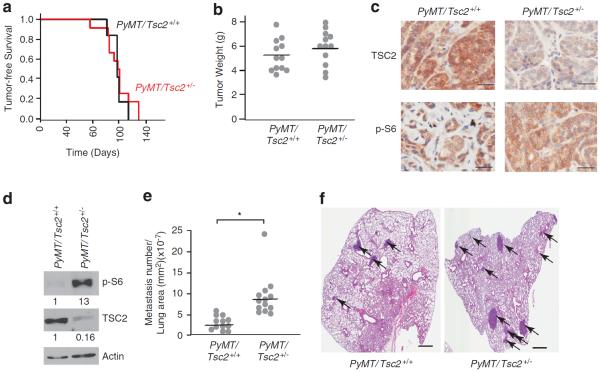
To establish a setting of activated mTOR signalling, we took advantage of Tsc2+/− mice. The tuberous sclerosis complex, a genetic disorder characterized by hamartomas in different organs, is the result of mutations in either one of two tumor-suppressor genes, Tsc1 and Tsc2. TSC1 and TSC2 form a complex that integrate different signalling cues to control mTOR activity.6,29 TSC2 contains a GTPase Activating Protein domain that stimulates the GTPase of Rheb (which in its GTP-bound form is an activator of mTOR Complex I). Although Tsc1 or Tsc2 mutations are rare in sporadic human cancers,30 human tumors show reduced levels of TSC1 or TSC2,31 – 33 including breast cancer.34 We asked if mTOR signalling flux could be limiting for either primary tumor or metastasis onset in the MMTV-PyMT model by crossing Tsc2+/− and MMTV-PyMT mice. PyMT/Tsc2+/+ and PyMT/Tsc2+/− females showed a similar tumor onset time (median of 121 – 123 days; Figure 1a), with no significant differences in overall tumor burden (Figure 1b) or growth (Supplementary Figure S1). We noted increased mTOR signalling flux in primary PyMT/Tsc2+/− tumors compared with PyMT/Tsc2+/+ tumors as measured by elevated phospho-S6 levels (Figures 1c and d, Supplementary Figure S2). Primary PyMT/Tsc2+/− tumors also showed reduced TSC2 levels relative to PyMT/Tsc2+/+ tumors (Figures 1c and d), with levels below the 50% decrease expected for monoallelic expression. This was not a consequence of reduction to homozygosity by the mutant allele, as the wild-type Tsc2 allele was retained in all PyMT/Tsc2+/− tumor samples (Supplementary Figure S3). We attribute the almost complete loss of Tsc2 expression to epigenetic changes that might further dampen TSC2 production—an event that may favor tumorigenic progression, but this hypothesis will require more direct testing. Histopathological analysis of PyMT/Tsc2+/+- and PyMT/Tsc2+/−-derived tumors indicated that both genotypes develop multifocal adenocarcinomas with similar appearance, size and properties (necrosis, cellular atypia, mitotic apoptotic index; data not shown). However, we noted a threefold increase in the number of pulmonary metastasis in PyMT/Tsc2+/− mice 8 weeks following appearance of primary tumors compared with PyMT/Tsc2+/+ mice (Figure 1e). Most of the lung lesions were glandular/papillary in nature (data not shown), and lesions from PyMT/Tsc2+/− mice were generally larger than those from PyMT/Tsc2+/+ mice, suggesting an earlier onset time (Figure 1f). These results suggest that whereas mTOR activation does not appear limiting for primary tumor initiation and burden, reduced Tsc2 expression cooperates with PyMT to increase pulmonary metastasis in this model.
Figure 1.
Loss of Tsc2 in PyMT mice increases pulmonary metastasis without affecting primary tumor burden. (a) Kaplan–Meier plot illustrating tumor-free survival of PyMT/Tsc2+/+ (n = 12) and PyMT/Tsc2+/− (n = 12) mice. (b) Tumor burden of PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice determined 8 weeks after initial appearance. For each genotype, n = 12. (c) Immunohistochemical staining for phospho-S6 and TSC2 in primary breast tumors from PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice. Bar, 50 μm. (d) Western blot analysis of phospho-S6 and TSC2 levels in breast tumors from PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice. Quantitation of the relative intensity of the bands is provided below each panel and was compiled from three different experiments. (e) Comparison of pulmonary metastases in PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice. Metastases were counted in all lobes and normalized to the lung area. For each genotype, n = 12; *P<0.001 as determined by Welsh's t-test. (f) A representative hematoxylin and eosin (H&E)-stained lung section from a PyMT/Tsc2+/+ and a PyMT/Tsc2+/− mouse. Arrows indicate metastatic lesions. Bar, 1 mm.
Inhibition of mTOR suppresses PyMT-induced breast cancer development and pulmonary metastasis
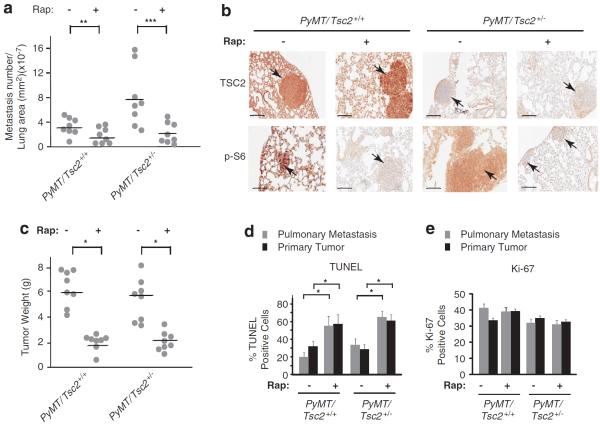
We next asked if the increased metastatic burden observed in PyMT/Tsc2+/− mice upon Tsc2 loss was a consequence of increased mTOR signalling flux. Treatment of tumor-bearing mice with rapamycin (RAP) caused a reduction in lung metastasis in both PyMT/Tsc2+/+ and PyMT/Tsc2+/− RAP-treated mice (Figure 2a). Pulmonary tissues and metastases showed reduced p-S6 staining, indicating that RAP was effectively inhibiting mTOR flux in the target tissues (Figures 2b and Supplementary Figure S4). RAP also reduced primary tumor burden regardless of genotype (Figure 2c). This appeared to be a consequence of increased cell death (Figures 2d and Supplementary Figure S5) in both the primary tumors and pulmonary metastasis. In contrast, RAP appeared to exert little effect on tumor cell proliferation (Figures 2e and Supplementary Figure S5). Whereas these results define a critical role for mTOR signalling in breast cancer maintenance in this model, they do not allow us to determine if the reduction in pulmonary metastasis observed with RAP is a direct consequence of the effects on the metastatic program or secondary to inhibition of primary tumor maintenance.
Figure 2.
Inhibiting mTOR signalling reduces primary tumor burden and lung metastasis in PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice. (a) Pulmonary metastatic burden in PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice. Pulmonary metastases were counted in all lobes and normalized to the lung area; n = 8; **P = 0.05; ***P<0.01. (b) Immunohistochemical analysis of TSC2 and p-S6 expression levels in lungs from PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice. Bar, 0.2 mm. (c) Tumor weight in PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice treated with RAP (n = 8) or vehicle (n = 8). *P<0.001 (d) Quantitation of TUNEL-positive cells from pulmonary metastasis and primary tumors from PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice treated with vehicle or RAP. A total of three tumors were analyzed. *P<0.005; error bars denote s.e.m. (e) Quantitation of Ki-67-positive cells from pulmonary metastasis and primary tumors from PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice treated with vehicle or RAP. A total of three tumors were analyzed. Error bars denote s.e.m.
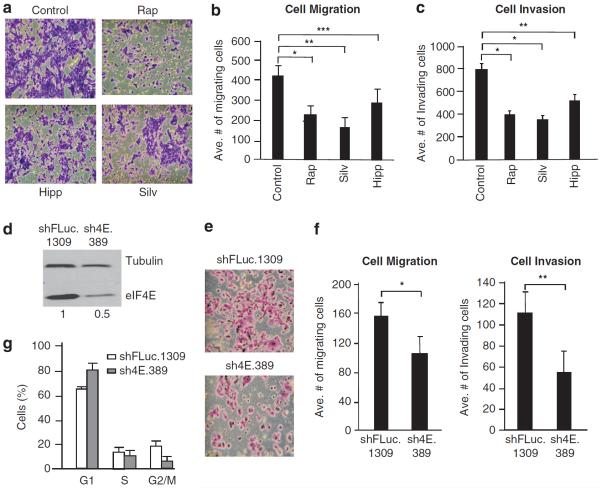
Therefore, to more directly assess the consequences of mTORC1 inhibition on features of the metastatic program, we turned to the murine TM15 metastatic breast cancer cell line—a HER2-driven model dependent on activation of the HER3/PI3K axis.35 We also specifically targeted the downstream mTOR effector, eIF4F, using silvestrol and hippuristanol, the two small-molecule inhibitors that block its eIF4A-driven helicase activity.36,37 Both compounds inhibit translation in a dose-dependent manner in TM15 cells (Supplementary Figure S6A) at concentrations significantly lower than those required to induce substantial cell death (Supplementary Figure S6B). We therefore assessed the effects of silvestrol, hippuristanol and RAP on two properties of TM15 cells that relate to the metastatic process—cellular migration and invasion. When TM15 cells were exposed to 20 nM RAP, or to the half-maximal inhibitory concentration of silvestrol or hippuristanol required for inhibition of translation, cell migration and invasion were significantly impaired (Figures 3a–c), indicating that reductions in mTORC1 or eIF4A/eIF4F helicase activity reduce metastatic potential in this setting.
Figure 3.
Suppressing eIF4F activity decreases invasion and migration of TM15 cells. (a) Representative crystal violet staining of migrating and invading TM15 cells exposed to 20 nm RAP, 40 nm silvestrol or 40 nm hippuristanol. (b) Quantitation of TM15 cell migration. Data representing three different experiments with five fields counted per experiment. Bars represent s.e.m. *P<0.01; **P<0.001; ***P<0.05. (c) Quantitation of TM15 cell invasion. Data representing three different experiments with five fields counted per experiment. Bars represent s.e.m. *P<0.001; **P<0.01. (d) Immunoblot analysis of DOX-treated sh4E.389- and shFLuc.1309-infected TM15 cells. The relative quantitation of the relative eIF4E levels is provided below the blot and is from three independent experiments. (e) Representative crystal violet staining of migrating and invading DOX-treated sh4E.389- or shFLuc.1309-infected TM15 cells. (f) Quantitation of cell migration (left panel) and Matrigel invasion (right panel) of DOX-treated sh4E.389- and shFLuc.1309-infected TM15 cells. Data represent three independent experiments with five fields counted per experiment. Bars signify s.e.m. * P = 0.08; **P<0.005. (g) Cell cycle analysis of shFLuc.1309- and sh4E.389-infected TM15 cells; n = 3; Bars represent s.e.m.
To determine if suppression of eIF4E levels could phenocopy these results, we took advantage of an inducible RNA interference (RNAi) strategy to engineer a potent small hairpin RNA (shRNA) targeting eIF4E (sh4E.389) or a neutral control shRNA to firefly luciferase (shFLuc.1309) into a doxycycline (DOX)-inducible retroviral vector. Suppression of eIF4E in TM15 cells (Figure 3d) was also sufficient to impair cell migration and invasion (Figures 3e and f). As previously reported,19,38 knockdown of eIF4E caused a prolongation of the G1 cell cycle phase (Figure 3g). Similar results were obtained using a second, independent shRNA to eIF4E, sh4E.610 (Supplementary Figure S7A–C). Taken together, these data suggest that inhibition of translation initiation can significantly affect the metastatic program.
To assess the consequences of targeting translation initiation in vivo on TM15 cell tumor growth and metastatic potential, we injected sh4E.389- and shFLuc.1309-infected cells into the fat pads of female BALB/c nude mice. When tumors were palpable, shRNAmir expression was activated by DOX administration. As observed in the PyMT-driven breast cancer model treated with RAP (Figure 2), a significant inhibitory effect on primary tumor growth (Figure 4a) and pulmonary metastatic burden (Figure 4b) was detected upon in vivo suppression of eIF4E. In some cases in DOX-treated mice, staining for green fluorescent protein (GFP) and knockdown of eIF4E was not homogenous throughout the tumor area but appeared focal in nature (Figure 4c and Supplementary Figure S8). This likely reflects corruption of the rtTA/TRE-inducible system in the tumor setting, as has been previously documented,39 and may underestimate the effect of eIF4E suppression in this setting. Apoptosis was elevated in primary tumors and metastatic lesions in which eIF4E expression was suppressed (Figure 4d), whereas no effect on Ki-67 staining upon eIF4E suppression was noted (Figure 4e). The increased apoptosis apparent in the metastatic lesions is suggestive of a direct effect on cellular viability upon eIF4E suppression.
Figure 4.
eIF4E suppression decreases breast tumor and lung metastasis burden by increased apoptosis in vivo. (a) sh4E.389 and shFLuc.1309-infected TM15 cells were injected into fat pads of nude mice and treated with DOX upon tumor onset. Tumor weights were documented at 40 days after detection by palpation. *P<0.005; **P = 0.02. (b) Pulmonary metastasis was quantitated 40 days after appearance of primary tumors. *P<0.001; **P = 0.02. (c) Representative immunohistological staining of eIF4E and GFP in a breast tumor (entire section) and lung metastatic (arrow) lesion of a DOX-treated mouse with either sh4E.389-infected or shFLuc.1309-infected TM15 cells. Bar, 0.2 mm. (d) Quantitation of TUNEL-positive cells of pulmonary metastasis and primary tumors from allografts of sh4E.389- and shFLuc.1309-infected TM15 cells; n = 3; *P<0.005. (e) Quantitation of Ki-67-positive cells from pulmonary metastasis and primary tumors from allografts of sh4E.389- and shFLuc.1309-infected TM15 cells; n = 3.
Suppression of eIF4E affects the metastasis cascade
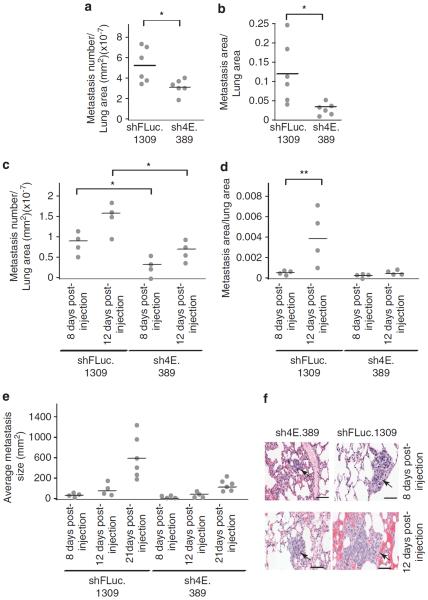
In a separate set of experiments aimed at removing the constraints of primary tumor growth on cell extravasation, we injected shRNAmir-TM15 cells intravenously into recipient mice to allow tumor cell dissemination and seeding. Here also, eIF4E suppression resulted in reduced metastatic burden (Figures 5a and b). To assess if these differences could be observed early in the colonization process, we analyzed lungs at different time points following injection of tumor cells. It was clear that eIF4E suppression led to both a decrease in the number of cells colonizing the lungs (Figure 5c) and a reduction in growth rate of those lesions that did colonize relative to shFLuc.1309-infected controls (Figures 5d–f). These results suggest that eIF4E suppression can interfere with the metastasis cascade-processes distinct from the effects documented on primary tumor burden above.
Figure 5.
eIF4E suppression reduces both pulmonary colonization and metastasis growth. (a) eIF4E suppression decreases the number of pulmonary metastatic lesions following injection of sh4E.389- and shFLuc.1309-infected TM15 cells into tail vein of nude mice. The number of metastatic lesions was determined as described in the Materials and methods. *P<0.005. (b) eIF4E suppression reduces pulmonary metastatic burden. Inducible sh4E.389- and shFLuc.1309-infected TM15 cells were injected into tail vein of DOX-treated nude mice and metastatic burden determined as described in the Materials and methods. *P<0.001. (c–e) DOX-inducible sh4E.389- and shFLuc.1309-infected TM15 cells were injected into tail vein of DOX-treated nude mice and metastatic number (c), burden (d) and average size (e) determined at 8 and 12 days. *P<0.05; **P<0.001. (f) Representative hematoxylin and eosin (H&E)-stained lung sections from DOX-treated nude mice injected with inducible sh4E.389- and shFLuc.1309-infected TM15 cells. Arrows highlight a metastatic lesion. Bar, 50 μm.
Suppression of eIF4E diminishes translation of mRNAs whose products have been implicated in metastasis
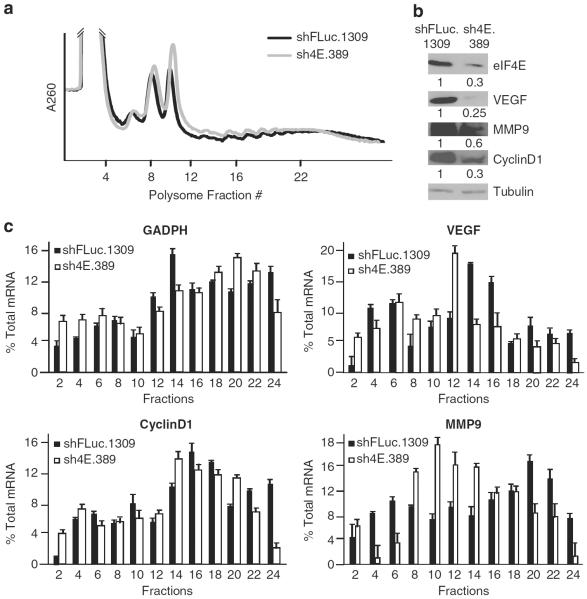
To gain insight into the molecular basis of eIF4E suppression on TM15 tumor cell growth and metastatic potential, we determined if the translation of growth- and metastasis-regulatory mRNAs was affected. Global polysome profiles from TM15 cells were not dramatically affected upon eIF4E suppression (Figure 6a). However, we noticed a reduction in the levels of proteins encoded by transcripts previously shown to be eIF4E dependent and implicated in the oncogenic and metastatic processes (that is, vascular endothelial growth factor (VEGF)), invasion through the extracellular matrix (that is, degrading enzymes such as matrix metallopeptidase 9 (MMP9)), and cell cycle progression (cyclin D1)40 (Figure 6b). To determine if reduced translation activity was contributing to the diminished protein levels, we documented the distribution of VEGF, MMP9 and cyclin D1 mRNAs in polysomes from sh4E.389- and shFLuc.1309-infected TM15 cells (Figure 6c). Shifts from heavy to lighter polysomes of VEGF, MMP9 and cyclin D1 mRNAs in sh4E.389-infected as compared with shFLuc.1309-infected TM15 cells were noted (Figure 6c). In contrast, the distribution of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA, a transcript whose expression is not altered upon suppression of eIF4E activity (data not shown), showed little difference among polysomes from sh4E.389- or shFLuc.1309-infected cells (Figure 6c). The subtle but consistent shifts from heavier to lighter polysomes indicate that whereas translation of the target mRNAs is being affected, additional gene regulatory mechanisms not queried here may also contribute to the decrease in VEGF, MMP9 and cyclin D1 protein levels observed upon eIF4E knockdown. Taken together, these results demonstrate that eIF4E suppression in TM15 cells dampens translation of several key mRNAs encoding proteins implicated in the metastatic process.
Figure 6.
eIF4E suppression decreases translation of metastasis-related mRNAs. (a) Representative polysome profiles of DOX-treated sh4E.389- or shFLuc.1309-infected TM15 cells are shown. (b) Western blot showing relative levels of eIF4E, and the putative targets, VEGF, MMP9 and cyclin D1, in DOX-treated sh4E.389- or shFLuc.1309-infected TM15 cells. Quantitation of the relative protein levels is provided from two independent biological replicates. (c) Polysome distribution of the indicated mRNAs in DOX-treated sh4E.389- or shFLuc.1309-infectedTM15 cells. mRNA distribution was determined by quantitative reverse-transcription–PCR (qRT–PCR) and the amount of mRNA in each fraction plotted as the percentage of total mRNA (n = 3).
Deregulated eIF4E expression affects invasion and migration in human breast cancer cells
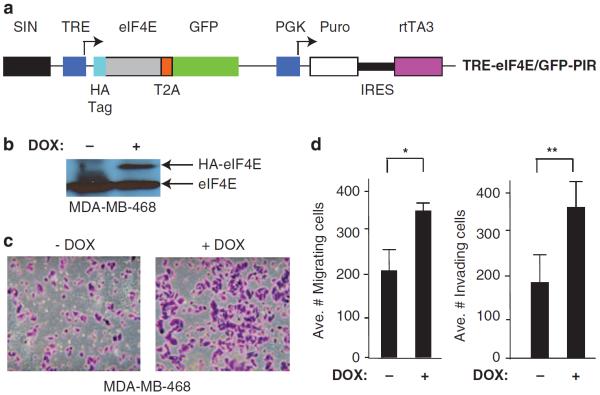
To determine if our results could be extended to the human setting, we undertook several complementary approaches to modulate eIF4E levels in breast cancer cells and link this to invasion and migration. As overexpression of eIF4E in breast cancer has been linked to poorer survival,41 we assessed if overexpression of eIF4E in cells could increase their metastatic potential (Figure 7). For these purposes, we chose to ectopically overexpress eIF4E in the weakly metastatic, HER2-negative MDA-MB-468 cell line using a DOX-inducible `All-In-One' retroviral vector (Figures 7a and b).42 Indeed, elevated eIF4E levels were associated with increased migration and invasion in this setting (Figures 7c and d).
Figure 7.
eIF4E overexpression stimulates invasion and migration of human breast cancer cells. (a) Schematic representation of the TRE-eIF4E/GFP-PIR `All-In-One' expression vector used to ectopically induce expression of eIF4E. TRE, Tet-responsive promoter; T2A, Thosea asigna virus 2A translational cleavage site; IRES, EMCV internal ribosome entry site. (b) Immunoblot analysis of MDA-MB-468 cells infected with TRE-eIF4E/GFP-PIR vector and expanded in the presence or absence of DOX. Extracts were prepared and blotted 3 days after induction of eIF4E expression. (c) Representative crystal violet staining of migrating MDA-MB-468 cells infected with TRE-eIF4E/GFP-PIR and maintained in the presence or absence of DOX. (d) Quantitation of cell migration (left panel) and Matrigel invasion (right panel) of MDA-MB-468 cells infected with TRE-eIF4E/GFP-PIR and maintained in the presence or absence of DOX. Data represent three independent experiments with five fields counted per experiment. Bars represent s.e.m. *P = 0.01; **P<0.001.
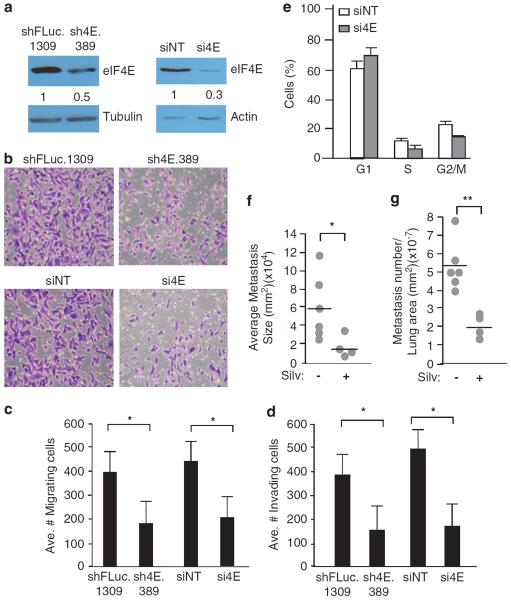
Next, we used RNAi to suppress eIF4E expression in the highly metastatic, HER2-negative MDA-MB-231 cell line (Figure 8a). Knockdown of eIF4E by shRNAs or small interfering iRNAs reduced by 2.5-fold the migration and invasion of MDA-MB-231 cells, relative to shFLuc.1309-infected cells (Figures 8b and c). Associated with eIF4E suppression in MDA-MB-231 cells was a prolongation of the G1 cell cycle phase (Figure 8d).
Figure 8.
eIF4E suppression impairs invasion and migration of human breast cancer cells. (a) Immunoblot analysis of MLP/sh4E.389- and MLP/shFLuc.1309-infected MDA-MB-231 cells (left) and MDA-MB-231 cells transfected with either siNT (non-targeting) or siRNAs against eIF4E (si4E) (right). Quantitation of the relative eIF4E levels is provided below the blot and is from three independent experiments. (b) Representative crystal violet staining of migrating MDA-MB-231 cells in which eIF4E was suppressed by RNAi using shRNAs (top) or siRNAs (bottom). (c, d) Quantitation of cell migration (c) and Matrigel invasion (d) of MDA-MB-231 cells in which eIF4E was suppressed by RNAi. Data represent three independent experiments with five fields counted per experiment. Bars represent s.e.m. *P<0.005. (e) Cell cycle analysis of MDA-MB-231 cells infected with retroviruses expressing the indicated shRNAs, or transfected with the indicated siRNAs; n = 3; Bars represent s.e.m. (f, g) MDA-MB-231 cells (106 cells) were injected into the tail vein of 8–10-week-old nude mice and 2 weeks later treated with vehicle or silvestrol (0.2 mg/kg) daily for 4 weeks, at which point lungs were harvested. The average metastatic size (f) and number (g) are presented. *P = 0.05; **P = 0.001.
We also injected MDA-MB-231 cells into the tail vein of nude mice and assessed the effects on dissemination and seeding upon suppression of eIF4F activity with silvestrol. Mice receiving silvestrol showed a significant decrease in the number and size of metastatic lesions (Figures 8e and f). These results phenocopy those obtained with TM15 cells upon suppression of eIF4E by RNAi (Figure 5), making it unlikely that the former results were cell line specific. Taken together, these results indicate that altering eIF4E levels can significantly affect the migration and invasion potential of human breast cancer cells.
DISCUSSION
Herein, we utilize powerful transgenic mouse models and RNAi to validate a link between deregulated mTOR/eIF4E signalling and primary breast cancer maintenance and metastasis in vivo. Loss of Tsc2, and hence increased mTOR signalling, in mice harboring the PyMT mutation had little effect on primary tumor onset, burden or histology, but did affect metastatic burden (Figure 1). In both PyMT/Tsc2+/+ and PyMT/Tsc2+/− mice, inhibiting mTOR signalling with RAP exerted effects on primary tumor development and pulmonary metastasis (Figure 2). PyMT/Tsc2+/+ tumors likely respond to RAP because the PyMT transgene activates PI3K signalling—an event necessary for stimulation of survival signalling pathways in these tumors.43 In this regard, it will be interesting to assess if Tsc2 loss increases pulmonary metastasis in the mutant PyMT (Y315/322F) mouse, a transgenic strain where PyMT-mediated PI3K activation is reduced ~75% and where this diminished signalling is associated with a reduced penetrance of pulmonary metastasis.43 The increase in metastatic burden seen in PyMT/Tsc2+/− mice would suggest that mTOR signalling is limiting for, or another Tsc2 effector contributes to, this process. The differential effect of RAP versus genetic reduction of Tsc2 on primary tumor burden (Figures 1b and 2c) may be due to reduced Tsc2 levels affecting only mTORC1 activity, whereas long-term treatment with RAP inhibits mTORC1, mTORC2 assembly and Akt/protein kinase B signalling, as previously reported.44 RAP and eIF4E suppression exerted direct effects on metastatic cell survival (Figures 2d and 4d) as assessed by increased apoptosis detected in pulmonary metastasis in treated animals.
Pathways that impinge on translational control have been previously targeted in breast cancer cell lines. Jiang et al.45 generated a mutant of 4E-BP1 whose binding to eIF4E could not be reversed by mTOR. When expressed in the human breast cancer cell line, MCF7, this mutant inhibited proliferation.45 An innovative approach in which the eIF4E-binding segment of eIF4G1 was stabilized by an α-helical inducer and fused to the cell-permeable TAT peptide also inhibited cap-dependent translation and triggered apoptosis in MCF-7 cells.46 Dowling et al.47 have shown that translation can be suppressed in MCF-7 cells with metformin, an activator of AMP-activated protein kinase that regulates cellular energy metabolism and mTOR activity. In vivo, metformin is effective as adjuvant therapy against xenograft models of breast cancer,48 but whether this is related to a translational block is not known. The mTOR inhibitors, RAP and PP242, have shown effects on cell cycle progression in breast cancer cells in vitro—a response that correlates with the presence of PI3K mutations.49 We have also previously reported that silvestrol, a small-molecule inhibitor of eIF4A, is effective at curtailing tumor maintenance in vivo in a xenograft model of breast cancer cells.23 eIF4E suppression by RNAi in breast cancer cells has also been shown to reduce foci formation, enhance chemosensitivity, reduce cell migration and delay primary tumor onset in nude mice.19,20 Here we extend these studies by demonstrating that eIF4F inhibition: (1) suppresses mouse and human breast cancer cell migration and invasion, (2) affects the metastasis cascade in vivo (Figures 5 and 8e–f) and (3) we identify mRNA targets whose translation is suppressed upon eIF4E knockdown in TM15 cells and implicated in the metastatic program (Figure 6).
Both MDA-MB-231 and MDA-MB-468 cells have been extensively analyzed for their eIF4F status.15 MDA-MB-231 cells express more eIF4F than MDA-MB-468 cells and are more resistant to apoptosis induced by serum starvation,15 a feature consistent with MDA-MB-231 being the more metastatic line.42 Introduction of eIF4E confers upon human mammary epithelial cells the ability to form anchorage-dependent colonies on plastic substrates as well as anchorage-independent growth in soft agar.15 In our hands, ectopic overexpression of eIF4E in human MDA-MB-468 breast cells also increased their migration and invasion properties (Figure 7). In addition to elevated eIF4E levels, altered expression levels of a number of other translation factors or regulators (for example, PKR, eIF3a, eIF3e, eIF4G and 4E-BPs) have been documented in human breast cancer.50 The complexity of the situation is highlighted by the fact that inflammatory breast cancer shows elevated levels of eIF4GI—a feature postulated to reprogram the translational apparatus for increased internal initiation events that drive inflammatory breast cancer cell survival and tumor emboli formation.
With respect to metastasis, less is known about the role that deregulated translation may play in supporting this process. Differences in eIF4E levels have been correlated with invasion potential in vivo among a number of different cell lines in lung seeding and renal capsule invasion assays.51 As well, inhibition of Mnk-directed phosphorylation of eIF4E reduced metastasis in a B16 melanoma model.52 In a murine ovarian cancer model, ascites tumor burden was significantly reduced by use of 4EBP-based peptides that blocked interaction of eIF4E with eIF4G.53 By utilizing TM15 cells and RNAi-mediated suppression of eIF4E, we were able to document direct effects on the metastatic properties of cell invasion and migration, as well as distinguish between effects of suppressing translation initiation on this process from other RAP-inhibited processes (Figure 3). TM15 cells are dependent on the HER3/PI3K axis for their survival, and inhibiting eIF4F function significantly dampened the metastatic program in vitro (Figure 3)—likely through decreasing production of regulators involved in this process (Figure 6). The requirement of eIF4F for ribosome recruitment is not equivalent among mRNAs, with a subset of mRNA species being more responsive to alterations in eIF4F levels, some of which also play roles in cell growth, survival, angiogenesis and tissue remodelling.54 Taken together, our results suggest that inhibiting eIF4F/eIF4A activity in breast cancer may have therapeutic potential in affecting not only primary tumor maintenance but also the metastasis program.
MATERIALS AND METHODS
Cell culture
Murine TM15 cells were isolated from a breast cancer that arose in a MMTVCre/floxneoNeuNT mouse55 and cultured in Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 5 μg/ml insulin, 10 ng/ml epidermal growth factor, 1 μg/ml hydrocortisone and 35 μg/ml bovine pituitary extract at 37 °C and 5% CO2. TM15 Subclone 10, of high metastatic potential and having elevated p-Akt2 relative to less metastatic clones, was used in the studies presented herein.56 The MDA-MB-231 and MDA-MB-468 human breast cancer cell lines were purchased from ATCC (Manassas, VA, USA) and cultured in L15 (Leibovitz) medium with 10% fetal bovine serum at 37 °C in CO2-free environment.
Mouse studies
See Supplementary Information
Immunohistochemistry
Tissue sections were placed in 10 mm citric acid buffer (pH 6.0) and subjected to antigen retrieval by boiling for 15 min after deparaffinization and rehydration. After incubation with blocking buffer UltraVBlock (Anti-Rabbit HRP/DAB Detection Kit, Abcam, Cambridge, MA, USA) for 1 h and 3% hydrogen peroxide for 10 min, rabbit primary antibody against eIF4E (Cell Signalling, Danvers, MA, USA, no. 9742 1:50), GFP (Cell Signalling, no. 2555, 1:800), p-S6 ribosomal protein (Cell Signalling, no. 4858, 1:75), TSC2 (Novus Biological, Littleton, CO, USA, no. NB100-80856, 1:50) and Ki-67 (NeoMarkers, Kalamazoo, MI, USA, no. RM-9106, 1:200) were applied overnight at 4 °C followed by incubation with biotinylated goat anti-rabbit IgG and streptavadin peroxidase (Anti-Rabbit HRP/DAB Detection Kit, Abcam) for 30 min each. Sections were washed three times with Tris-buffered saline (0.1 m Tri-HCl7.5, 0.15 m NaCl) after each step. Signal was visualized using DAB chromogen and chromogen substrate at room temperature for 5 min. Sections were counterstained with hematoxylin, dehydrated, and mounted with permount. Signals were quantitated using the Aperio ImageScope (Aperio, Vista, CA, USA) analysis program. Apoptosis was detected in tumor and lung tissues with the TUNEL (TdT-mediated dUTP nick end labelling) kit according to the manufacturer's recommendations (Roche Applied Science, Laval, Quebec, Canada).
RNAi and retroviral transduction
See Supplementary Information.
Cell cycle analysis
Cells (106 cells/ml) were fixed in 75% ethanol solution for 1 h at 4 °C and stained with propidium iodide (Sigma, Oakville, Ontario, Canada; 50 μg/ml propidium iodide, 3.8 mm sodium citrate and 500 μg/ml RNase A) for 3 h at 4 °C. Cells were then analyzed for DNA content using a FACScan flow cytometer (BD Biosciences, Mississauga, ON, Canada).
Cell invasion and migration assays
Cell invasion and migration potential was measured using an in vitro chamber assay.57 Approximately 105 cells in 0.5 ml serum-free Dulbecco's modified Eagle's medium were added to the wells of 8 μm pore membrane chambers (BD Biosciences, no. 353097), either coated with (for invasion assays) or without (for migration assays) Matrigel (Transwells; BD Biosciences). The lower chambers contained 0.8 ml Dulbecco's modified Eagle's medium/10% fetal bovine serum that served as chemoattractant. For compound treatment, these were added at the indicated concentrations after cells had been seeded. For DOX induction of sh4E.389, cells were pretreated with DOX for 2 days, and then plated for invasion/migration assays, during which time the presence of DOX was maintained. Cells were allowed to migrate or invade over the course of 24 h. Cells that had not penetrated the filters were removed by swabbing with cotton swabs. Chambers were fixed in 10% neutral buffered formalin for 20 min, stained in 0.5% crystal violet for 20 min, rinsed with phosphate-buffered saline after each step, and examined using a bright-field microscope. Values for invasion and migration were obtained by counting cells in five fields per membrane (× 20 objective) and are the average of three independent experiments.
Experimental metastasis assay
shRNAmir-infected TM15 cells (2.5 × 105) were injected subcutaneously into the fat pad of 6–8-week-old female BALB/c nude mice. Upon tumor onset, mice were supplied with DOX (1 mg/ml) in the drinking water (+5% sucrose) to induce shRNAmir synthesis. Mice were killed when tumors had reached a maximum size of 2.5 cm3. Breast tumors and lung tissues were excised and processed for histological analysis as described above.
To monitor the ability of cells to home to the lungs in vivo, TM15 cells (2.5 × 105) were injected into the lateral tail vein of 8 – 10-week-old female BALB/c nude mice predosed with DOX 1 day before injection. Mice were kept on DOX for 4, 8, 12 or 21 days, at which point lungs were harvested and assessed for the presence of metastatic lesions. Nude mice were used as TM15 cells were generated from a mouse of mixed background.
Polysome analysis and quantitative reverse-transcription-PCR
For polysome profiling, TM15 cells infected with DOX-inducible shRNAs targeting eIF4E (sh4E.389) or FLuc (shFLuc.1309) were treated with DOX (2 μg/ml) for 3 days, washed in phosphate-buffered saline and lysed in hypotonic solution (5 mm Tris-HCl7.5, 2.5 mm MgCl2, 1.5 mm KCl, 2 mm DTT, 1% Triton X-100, 0.5% sodium deoxycholate and 100 μg/ml cycloheximide). Lysates were loaded onto 10–50% sucrose gradients and centrifuged in an SW40 rotor at 35 000 r.p.m. for 2 h. Fractions were collected and A260 monitored using an ISCO UA-6 UV/VIS detector (Teledyne Isco, Lincoln, NE, USA). RNA was extracted from the fractions using TRIzol according to the manufacturer's instructions (Invitrogen, Burlington, ON, Canada). The amount of MMP9, VEGF, cyclin D1 and GAPDH mRNA was determined by quantitative reverse-transcriptase PCR according to the manufacturer's instructions (Bio-Rad, Hercules, CA, USA).
Data analysis
For statistical analysis, unpaired Student t-test with Welch correction was performed using GraphPad InStat version 3.10 (San Diego, CA, USA).
Supplementary Material
ACKNOWLEDGEMENTS
WJM is supported by the Canadian Institutes of Health Research (MOP-89791) and a CRC Chair in Molecular Oncology. JAP, Jr thanks the National Institutes of Health (GM-073855) for research support. This work was supported by the Canadian Institutes of Health Research (MOP-106530) and the Canadian Cancer Society Research Institute (CCSRI no. 17099) to JP.
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
REFERENCES
- 1.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 2.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 3.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 4.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 5.Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–486. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 7.Wendel HG, Malina A, Zhao Z, Zender L, Kogan SC, Cordon-Cardo C, et al. Determinants of sensitivity and resistance to rapamycin-chemotherapy drug combinations in vivo. Cancer Res. 2006;66:7639–7646. doi: 10.1158/0008-5472.CAN-06-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilic N, Utermark T, Widlund HR, Roberts TM. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc Natl Acad Sci USA. 2011;108:E699–E708. doi: 10.1073/pnas.1108237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011;17:1116–1120. doi: 10.1038/nm.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muellner MK, Uras IZ, Gapp BV, Kerzendorfer C, Smida M, Lechtermann H, et al. A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat Chem Biol. 2011;7:787–793. doi: 10.1038/nchembio.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RM, Branda J, Johnston KA, Polymenis M, Gadd M, Rustgi A, et al. An essential E box in the promoter of the gene encoding the mRNA cap-binding protein (eukaryotic initiation factor 4E) is a target for activation by c-myc. Mol Cell Biol. 1996;16:4754–4764. doi: 10.1128/mcb.16.9.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenwald IB. Upregulated expression of the genes encoding translation initiation factors eIF-4E and eIF-2alpha in transformed cells. Cancer Lett. 1996;102:113–123. doi: 10.1016/0304-3835(96)04171-7. [DOI] [PubMed] [Google Scholar]
- 13.Lin CJ, Cencic R, Mills JR, Robert F, Pelletier J. c-Myc and eIF4F are components of a feedforward loop that links transcription and translation. Cancer Res. 2008;68:5326–5334. doi: 10.1158/0008-5472.CAN-07-5876. [DOI] [PubMed] [Google Scholar]
- 14.Larsson O, Li S, Issaenko OA, Avdulov S, Peterson M, Smith K, et al. Eukaryotic translation initiation factor 4E induced progression of primary human mammary epithelial cells along the cancer pathway is associated with targeted translational deregulation of oncogenic drivers and inhibitors. Cancer Res. 2007;67:6814–6824. doi: 10.1158/0008-5472.CAN-07-0752. [DOI] [PubMed] [Google Scholar]
- 15.Avdulov S, Li S, Michalek V, Burrichter D, Peterson M, Perlman DM, et al. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell. 2004;5:553–563. doi: 10.1016/j.ccr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Armengol G, Rojo F, Castellvi J, Iglesias C, Cuatrecasas M, Pons B, et al. 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 17.Coleman LJ, Peter MB, Teall TJ, Brannan RA, Hanby AM, Honarpisheh H, et al. Combined analysis of eIF4E and 4E-binding protein expression predicts breast cancer survival and estimates eIF4E activity. Br J Cancer. 2009;100:1393–1399. doi: 10.1038/sj.bjc.6605044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pons B, Peg V, Vazquez-Sanchez MA, Lopez-Vicente L, Argelaguet E, Coch L, et al. The effect of p-4E-BP1 and p-eIF4E on cell proliferation in a breast cancer model. Int J Oncol. 2011;39:1337–1345. doi: 10.3892/ijo.2011.1118. [DOI] [PubMed] [Google Scholar]
- 19.Zhou FF, Yan M, Guo GF, Wang F, Qiu HJ, Zheng FM, et al. Knockdown of eIF4E suppresses cell growth and migration, enhances chemosensitivity and correlates with increase in Bax/Bcl-2 ratio in triple-negative breast cancer cells. Med Oncol. 2011;28:1302–1307. doi: 10.1007/s12032-010-9630-0. [DOI] [PubMed] [Google Scholar]
- 20.Dong K, Wang R, Wang X, Lin F, Shen JJ, Gao P, et al. Tumor-specific RNAi targeting eIF4E suppresses tumor growth, induces apoptosis and enhances cisplatin cytotoxicity in human breast carcinoma cells. Breast Cancer Res Treat. 2009;113:443–456. doi: 10.1007/s10549-008-9956-x. [DOI] [PubMed] [Google Scholar]
- 21.Zindy P, Berge Y, Allal B, Filleron T, Pierredon S, Cammas A, et al. Formation of the eIF4F translation-initiation complex determines sensitivity to anticancer drugs targeting the EGFR and HER2 receptors. Cancer Res. 2011;71:4068–4073. doi: 10.1158/0008-5472.CAN-11-0420. [DOI] [PubMed] [Google Scholar]
- 22.Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117:2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cencic R, Carrier M, Galicia-Vazquez G, Bordeleau ME, Sukarieh R, Bourdeau A, et al. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PLoS ONE. 2009;4:e5223. doi: 10.1371/journal.pone.0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyoma virus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, et al. Transgenic polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- 27.Lifsted T, Le Voyer T, Williams M, Muller W, Klein-Szanto A, Buetow KH, et al. Identification of inbred mouse strains harboring genetic modifiers of mammary tumor age of onset and metastatic progression. Int J Cancer. 1998;77:640–644. doi: 10.1002/(sici)1097-0215(19980812)77:4<640::aid-ijc26>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Davie SA, Maglione JE, Manner CK, Young D, Cardiff RD, MacLeod CL, et al. Effects of FVB/NJ and C57Bl/6J strain backgrounds on mammary tumor phenotype in inducible nitric oxide synthase deficient mice. Transgenic Res. 2007;16:193–201. doi: 10.1007/s11248-006-9056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Veelen W, Korsse SE, van de Laar L, Peppelenbosch MP. The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene. 2011;30:2289–2303. doi: 10.1038/onc.2010.630. [DOI] [PubMed] [Google Scholar]
- 30.Knowles MA, Hornigold N, Pitt E. Tuberous sclerosis complex (TSC) gene involvement in sporadic tumours. Biochem Soc Trans. 2003;31(Part 3):597–602. doi: 10.1042/bst0310597. [DOI] [PubMed] [Google Scholar]
- 31.Wienecke R, Guha A, Maize JC, Jr, Heideman RL, DeClue JE, Gutmann DH. Reduced TSC RNA and protein in sporadic astrocytomas and ependymomas. Ann Neurol. 1997;42:230–235. doi: 10.1002/ana.410420215. [DOI] [PubMed] [Google Scholar]
- 32.Chakraborty S, Mohiyuddin SM, Gopinath KS, Kumar A. Involvement of TSC genes and differential expression of other members of the mTOR signaling pathway in oral squamous cell carcinoma. BMC Cancer. 2008;8:163. doi: 10.1186/1471-2407-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Wang M, Wang L, Wang Y, Zhao X, Rao Q, et al. Aberrant expression of TSC2 gene in the newly diagnosed acute leukemia. Leuk Res. 2009;33:891–897. doi: 10.1016/j.leukres.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 34.Jiang WG, Sampson J, Martin TA, Lee-Jones L, Watkins G, Douglas-Jones A, et al. Tuberin and hamartin are aberrantly expressed and linked to clinical outcome in human breast cancer: the role of promoter methylation of TSC genes. Eur J Cancer. 2005;41:1628–1636. doi: 10.1016/j.ejca.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Dillon RL, Muller WJ. Distinct biological roles for the akt family in mammary tumor progression. Cancer Res. 2010;70:4260–4264. doi: 10.1158/0008-5472.CAN-10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bordeleau ME, Robert F, Gerard B, Lindqvist L, Chen SM, Wendel HG, et al. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J Clin Invest. 2008;118:2651–2660. doi: 10.1172/JCI34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bordeleau M-E, Mori A, Oberer M, Lindqvist L, Chard LS, Higa T, et al. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat Chem Biol. 2006;2:213–220. doi: 10.1038/nchembio776. [DOI] [PubMed] [Google Scholar]
- 38.Soni A, Akcakanat A, Singh G, Luyimbazi D, Zheng Y, Kim D, et al. eIF4E knockdown decreases breast cancer cell growth without activating Akt signaling. Mol Cancer Ther. 2008;7:1782–1788. doi: 10.1158/1535-7163.MCT-07-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podsypanina K, Politi K, Beverly LJ, Varmus HE. Oncogene cooperation in tumor maintenance and tumor recurrence in mouse mammary tumors induced by Myc and mutant Kras. Proc Natl Acad Sci USA. 2008;105:5242–5247. doi: 10.1073/pnas.0801197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 41.Li BD, Liu L, Dawson M, De Benedetti A. Overexpression of eukaryotic initiation factor 4E (eIF4E) in breast carcinoma. Cancer. 1997;79:2385–2390. [PubMed] [Google Scholar]
- 42.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 43.Webster MA, Hutchinson JN, Rauh MJ, Muthuswamy SK, Anton M, Tortorice CG, et al. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 45.Jiang H, Coleman J, Miskimins R, Miskimins WK. Expression of constitutively active 4EBP-1 enhances p27Kip1 expression and inhibits proliferation of MCF7 breast cancer cells. Cancer Cell Int. 2003;3:2. doi: 10.1186/1475-2867-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown CJ, Lim JJ, Leonard T, Lim HC, Chia CS, Verma CS, et al. Stabilizing the eIF4G1 alpha-helix increases its binding affinity with eIF4E: implications for peptidomimetic design strategies. J Mol Biol. 2011;405:736–753. doi: 10.1016/j.jmb.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 47.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weigelt B, Warne PH, Downward J. PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene. 2011;30:3222–3233. doi: 10.1038/onc.2011.42. [DOI] [PubMed] [Google Scholar]
- 50.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer. 2010;10:254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 51.Graff JR, Boghaert ER, De Benedetti A, Tudor DL, Zimmer CC, Chan SK, et al. Reduction of translation initiation factor 4E decreases the malignancy of ras-transformed cloned rat embryo fibroblasts. Int J Cancer. 1995;60:255–263. doi: 10.1002/ijc.2910600221. [DOI] [PubMed] [Google Scholar]
- 52.Konicek BW, Stephens JR, McNulty AM, Robichaud N, Peery RB, Dumstorf CA, et al. Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res. 2011;71:1849–1857. doi: 10.1158/0008-5472.CAN-10-3298. [DOI] [PubMed] [Google Scholar]
- 53.Ko SY, Guo H, Barengo N, Naora H. Inhibition of ovarian cancer growth by a tumor-targeting peptide that binds eukaryotic translation initiation factor 4E. Clin Cancer Res. 2009;15:4336–4347. doi: 10.1158/1078-0432.CCR-08-2924. [DOI] [PubMed] [Google Scholar]
- 54.Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–634. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- 55.Andrechek ER, Hardy WR, Siegel PM, Rudnicki MA, Cardiff RD, Muller WJ. Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc Natl Acad Sci USA. 2000;97:3444–3449. doi: 10.1073/pnas.050408497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009;69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.