Abstract
Elderberries are being increasingly produced and consumed in North America for their edible and medicinal flowers and fruits. The American elderberry (Sambucus nigra subsp. canadensis) is native to, and most often cultivated in North America. The European elderberry (S. nigra subsp. nigra) has been developed into an economically-important horticultural crop in Europe, but most European cultivars do not perform well in the midwestern USA. The genotype S. nigra subsp. nigra ‘Marge’ is an open-pollinated seedling of S. nigra subsp. nigra ‘Haschberg’, which is one of the most popular elderberry cultivars grown in Europe. In a four-year study (one establishment year followed by 3 production years; 2008–2011) at three Missouri (USA) locations, ‘Marge’ significantly out-performed and out-yielded eight American elderberry genotypes within the same replicated field plots. Across 3 production years at all three sites, ‘Marge’ achieved budbreak later, flowered earlier, suffered less Eriophyid mite damage, was taller, produced larger berries, and yielded significantly greater amounts of fruit compared with all eight American elderberry genotypes in the study. At one site, ‘Marge’ produced three times the yield (1.89 kg/plant) compared with the next highest-producing American elderberry genotype (0.65 kg/plant). It is an exceptionally robust and drought-resistant elderberry. The phenotypic attributes of ‘Marge’ are similar to that of European elderberry except that it performs exceptionally well in the midwestern USA. DNA marker results, along with phenological and morphological characteristics, indicate that ‘Marge’ is a European elderberry (S. nigra subsp. nigra). As with most European genotypes, ‘Marge’ does not fruit on first-year wood, and will therefore require a different pruning regimen compared with American elderberry for success in North American production. We do not yet know how ‘Marge’ will perform outside the midwestern USA, but it is so productive, unique, and mite resistant, that it merits introduction as a cultivar.
Keywords: Sambucus, fruit, yield, cultivar, genotype, DNA
INTRODUCTION
Elderberries are increasingly produced and consumed in North America. Both flowers and fruits are used to produce wines, jams, jellies, colorants, and a variety of dietary supplements (Charlebois, 2010; Mohebalian, 2012). While the majority of elderberry products marketed in North America are produced in Europe from the European elderberry (Sambucus nigra L. subsp. nigra; syn. S. nigra L.), cultivation and processing of American elderberry [S. nigra L. subsp. canadensis (L.) Bolli; syn. S. canadensis L.] is increasing (Finn et al., 2008; Thomas et al., 2013). More research has been published on the medicinal attributes of the European subspecies contrasted with the North American (e.g., Barak et al., 2002; Roschek et al., 2009; Veberic et al., 2009; Zakay-Rones et al., 1995). Thus, some processing firms prefer fruit from the European subspecies, even if cultivated in North America.
The Elderberry Improvement Program at University of Missouri and Missouri State University has been growing and studying the cultivation of elderberry since 1997. During that period, numerous accessions of European elderberry have been screened in non-replicated plots at multiple sites for evaluation, including ‘Samyl’, ‘Samdal’, ‘Sampo’, ‘Korsør’, and ‘Haschberg’. In nearly all cases, the European elderberries did not thrive and eventually weakened or died, while neighboring American elderberries thrived. It is not clear why most European elderberries have not performed well in Missouri, but we believe it may be more of a summer heat-stress issue rather than winter hardiness. The genotype ‘Marge’ is an exception among European genotypes, and a very promising elderberry selection for Missouri and the midwestern USA.
‘Marge’ was selected by and named after Marge Millican (born 1927), who is affiliated with Wyldewood Cellars Winery, Mulvane, Kansas, USA. She previously released the ‘Wyldewood’ American elderberry cultivar in 2010 (Byers et al., 2010). ‘Marge’ is a seedling progeny of an open-pollinated ‘Haschberg’ European elderberry. The ‘Haschberg’ mother plant was obtained from a commercial elderberry planting near Tattendorf, Austria in 2001, and eventually grown at Wyldewood Cellars Winery, Mulvane, Kansas. Open-pollinated fruits and seeds were harvested from these ‘Haschberg’ plants, with resulting seedlings planted in the field for evaluation. The ‘Marge’ genotype was selected in 2004 as showing exceptional vigor and producing very large berries (Figs. 1 and 2). Propagules were provided to the University of Missouri and Missouri State University for evaluation in 2006. ‘Marge’ immediately showed outstanding vigor, pest resistance, and was noted for its very large berries; promising enough to be included in a multi-location genotype evaluation that was established in Missouri in 2008 (Thomas et al., 2015a).
Fig. 1.
Mature ‘Marge’ elderberry shrub showing upright non-suckering habit and early, sporadic bloom, growing among less vigorous American elderberries in Jefferson City, Missouri; photo taken 22 May 2011.
Fig. 2.
Ripe fruit of ‘Marge’ elderberry harvested near Mt. Vernon, Missouri; photo taken 8 Sept. 2010.
MATERIALS AND METHODS
Field Trials
The European elderberry genotype ‘Marge’ was evaluated within a large, multi-location study that included eight American elderberry genotypes (Thomas et al., 2015a); ‘Marge’ was the only European genotype evaluated. The study was conducted at three sites in Missouri, USA: University of Missouri’s Southwest Research Center near Mt. Vernon (lat. 37.0710°N, long. 93.8795°W, alt. 378 m), Missouri State University’s State Fruit Experiment Station near Mountain Grove (lat. 37.1559°N, long. 92.2644°W, alt. 434 m), and Lincoln University’s Carver Farm at Jefferson City (lat. 38.5299°N, long. 92.1383°W, alt. 175 m). The three sites are a minimum of 140 km apart. The soils at Mt. Vernon and Mountain Grove were relatively similar silt loams with fragipans at 45–90 cm; whereas, the soil at Jefferson City was a deeper, more alluvial silt loam with no fragipan. The Jefferson City site might have been considered better for elderberry production based on soil type; however, soil test results from that site indicated a superficially poorer soil with low organic matter and low cation exchange capacity (Thomas et al., 2015a). The plantings were established at each of the three sites in July 2008 by rooting cuttings from our own mother plants. Additional orchard and experimental details, including sites, soils, environmental conditions, genotypes used, field layout, orchard management, and experimental design are provided in Thomas et al. (2015a). Within the larger genotype evaluation of American elderberry described therein, four additional plots of ‘Marge’ were randomly interspersed among the plots at all three sites. With the ‘Marge’ plots, each planting included nine elderberry genotypes, each planted in four-plant plots with four replications per site, totaling 36 plots and 144 plants per site. Data on phenology, plant morphology, pest resistance, fruit yields, berry size, and basic juice chemistry were collected at all three sites over three growing seasons (2009–2011), as in Thomas et al. (2015a).
DNA Marker Analysis
A PCR-based target region amplification polymorphism (TRAP) genotyping technique (Hu and Vick, 2003) was utilized to assess the genetic diversity and relationship of 16 elderberry accessions across two subspecies (Johnson et al., 2008). Some of the elderberry genotypes evaluated were not included in the current field production study. DNA samples were prepared from 100 mg young leaf bud tissue and extracted with the DNeasy Plant Mini Kit (Qiagen, Valencia, CA) according to manufacturer instructions. DNA purification and concentration were monitored and determined spectrophotometrically by BioPhotometer 6131 (Eppendorf-Netheler-Hinz BmbH Hamburg, Germany). DNA concentrations were adjusted to 10 ng/μl for PCR amplification.
Four fixed primers and four arbitrary primers used in this study were synthesized by Applied Biosystems (Foster City, CA). Three fixed primer and four arbitrary primer sequences were designed and provided by Jinguo Hu, USDA-ARS Plant Germplasm Introduction and Testing Research Unit, Pullman Washington. Fixed Primer TeloRA was designed against the conserved Arabidopsis thaliana L. telomere-repeat sequence; QHF6H12L against a sunflower (Helianthus annuus L.) expressed sequence tag annotated as the BEL1-like homeobox 1 protein (BLH1) of Arabidopsis thaliana; WRKY6-1a against the sequence of transcription factors WRKY6 involved in plant defenses; and FLAVONOID designed according to sequence of Pelargonium × hortorum flavonoid 3′-hydroxylase mRNS (GenBank accession No. AF315465) by Primer3 software (Rozen and Skaletsky, 2000). In this study each fixed primer was combined with four arbitrary primers, each labeled with a different fluorescent dye.
PCR was conducted in a 12-μl reaction mixture consisting of 20 ng DNA sample, 1.5 μl 10x reaction buffer, 1.5 μl 25 mM MgCl2, 0.6 μl 10 mM dNTPs, 0.8 μl 1 pmol 5’ labeled arbitrary primer, 0.8 μl 13 pmol fixed primer, and 0.3 μl Qiagen Taq DNA polymerase. The PCR cycles were performed using an Eppendorf Mastercycler programmed for DNA denaturation at 94°C for 45 s, 40°C for 45 s, and 72°C for 1 min, followed by 35 cycles of 94°C for 45 s, 53°C for 45 s, and 72°C for 1 min. After 35 cycles the extension temperature of 72°C was held for 7 min. Upon completing the PCR cycles, the product was diluted 10× and analyzed by an ABI 3730 DNA sequencer (Applied Biosystems, Foster City, CA). The TRAP amplification provided more than one thousand polymorphic markers that were used for cluster analysis aided by the NTSYSpc software, version 2.2 (Exeter Software, Setauket, NY).
Statistical Analysis
The field experiment was established and analyzed as a completely randomized design at each of the three locations. The experimental unit was the entire four-plant plot; however, all production data are presented on a single-plant basis. Data were analyzed using the general linear model procedure (SAS Institute, Cary, N.C.), with means separated by the least significant difference method at P ≤ 0.05. The analyses were similar to those used in Thomas et al. (2015a), however for this study, the additional data from the interspersed ‘Marge’ plots were included, and the statistical analyses revised.
RESULTS AND DISCUSSION
‘Marge’ grew and performed exceptionally well at all three Missouri sites during the establishment year (2008) and the 3 study years (2009–2011). For many horticultural factors, ‘Marge’ performed significantly differently compared with the American elderberry genotypes in the study (Table 1). This table includes some data excerpted from Thomas et al. (2015a) because the ‘Marge’ plots were integrated among the plots used in that study. ‘Marge’ broke bud later and flowered earlier than all American genotypes, including ‘York’ which is known for late budbreak and early flowering in Missouri (Thomas et al., 2015a). However, fruit harvest occurred at about the same time as most American genotypes, and significantly later than ‘York’. ‘Marge’ is a vigorous, tall plant, and was significantly taller (178 cm) than all American genotypes in the study. One of ‘Marge’s’ outstanding characteristics is its near-complete resistance to Eriophyid mites [most likely Phyllocoptes wisconsinensis Keifer (Warmund and Amrine, 2015)]. These mites are a serious pest in many American elderberry orchards; other Eriophyid mite species have also been reported in Europe on European elderberry (e.g., Vaněčkova-Skuhravá, 1996). For this study, mites were allowed to develop without intervention in order to assess potential mite resistance or susceptibility among genotypes. While most neighboring American elderberry plots suffered various levels of mite damage, ‘Marge’ plots at all three sites remained virtually unaffected. And while ‘Marge’ did incur some damage from Japanese beetle (Popillia japonica Newman), it was significantly less affected than several of the American elderberry genotypes.
Table 1.
Phenology, plant height, pest, yield, and juice characteristics from nine elderberry genotypes grown at three Missouri (USA) sites, 2009–2011; some data excerpted from Thomas et al. (2015a) for comparison, with statistical analysis revised.
| Genotype | Budbreakz | Full bloomz |
Peak harvestz |
Plant height (cm) |
Eriophyid mitey |
Japanese beetley |
Berry weight (mg) |
Yield per plant (kg) |
Cymes per plant |
Cyme weight (g) |
pH | Soluble solids (°Brix) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marge | 76.5 ax | 156 d | 228 cd | 178 a | 1.0 e | 1.2 d | 176 a | 1.12 a | 72 a | 21 b | 4.57 e | 10.2 ab |
| Bob Gordon | 63.0 cde | 170 b | 223 d | 122 bc | 2.5 c | 1.2 d | 88 cd | 0.38 bc | 21 b | 37 ab | 4.68 de | 10.4 ab |
| Dallas | 65.3 bc | 169 b | 230 c | 119 bcd | 1.9 d | 1.3 d | 95 c | 0.40 bc | 11 b | 43 ab | 4.86 bc | 9.8 b |
| Ocoee | 64.9 cd | 172 b | 243 a | 114 cd | 2.0 d | 2.7 ab | 84 cd | 0.48 bc | 12 b | 55 a | 4.63 de | 10.0 b |
| Ozark | 61.2 e | 169 b | 230 c | 119 bcd | 2.8 bc | 2.2 c | 86 cd | 0.60 b | 21 b | 43 ab | 4.71 cde | 11.3 a |
| Ozone | 64.0 cde | 170 b | 233 bc | 124 b | 3.1 ab | 2.2 c | 76 d | 0.28 c | 13 b | 35 ab | 4.78 bcd | 10.1 b |
| Sperandio | 64.0 cde | 170 b | 233 bc | 109 d | 3.5 a | 2.6 b | 86 cd | 0.31 c | 7 b | 41 ab | 5.04 a | 7.9 c |
| Wyldewood | 61.6 de | 177 a | 236 b | 117 bcd | 3.1 ab | 3.0 a | 83 cd | 0.28 c | 11 b | 32 ab | 4.92 ab | 8.3 c |
| York | 68.5 b | 161 c | 217 e | 119 bcd | 2.8 bc | 1.3 d | 139 b | 0.37 bc | 21 b | 37 ab | 4.75 bcd | 7.9 c |
Days from 1 January.
Based on 1–5 scale, where 1 = no incidence and 5 = severe incidence.
Means within columns with the same letters are not significantly different according to the least significant difference test (P ≤ 0.05).
The ‘Marge’ berries were exceptionally large, averaging 176 mg; significantly larger than ‘York’ (139 mg) which is known for large berries, and much larger than the other American genotypes (76–95 mg). Across the three sites and 3 years of this study, ‘Marge’ significantly out-yielded all other elderberry genotypes, in most cases by two to three times the amount of fruit. Parallel to this is a significantly higher number of fruiting cymes. However, as can be seen (Table 1), the individual cyme size on ‘Marge’ was numerically the smallest among all genotypes, although not significantly smaller than most. These data indicate that while ‘Marge’ produces very high yields, the fruit is borne on more numerous but individually smaller fruiting cymes. This factor must be considered in terms of harvest labor – the increased yield and larger berries versus potential higher labor requirements to harvest more numerous but smaller cymes.
Juice characteristics of ‘Marge’ berries in the study were excellent. Assuming that low pH and high soluble solids levels are desirable in elderberry, ‘Marge’ juice might be considered superior with a significantly lower pH and higher soluble solids levels compared with most American genotypes. Additionally, separate laboratory studies of the same fruit found higher levels of several polyphenols in ‘Marge’ compared with most American elderberry genotypes (Thomas et al., 2015b).
Details on fruit yields from each of the three sites are provided (Table 2). ‘Marge’ yielded triple that of most other genotypes at Mt. Vernon and two to three times higher than most genotypes at Mountain Grove. At Jefferson City, ‘Marge’ had statistically similar yields compared to four genotypes, but out-yielded four other genotypes. This unexpectedly lower yield for ‘Marge’ at Jefferson City may have been due to bird predation of the larger, juicer berries as no bird protection was deployed.
Table 2.
Single plant fruit yields from nine elderberry genotypes grown at three Missouri sites, averaged over 3 years (2009–2011).
| Genotype | Mean single plant yield (kg/plant)
|
||
|---|---|---|---|
| Mt. Vernon | Mtn. Grove | Jefferson City | |
| Marge | 1.89 | 0.63 | 0.69 |
| Bob Gordon | 0.62 | 0.15 | 0.39 |
| Dallas | 0.65 | 0.12 | 0.45 |
| Ocoee | 0.33 | 0.44 | 0.78 |
| Ozark | 0.56 | 0.48 | 0.82 |
| Ozone | 0.23 | 0.28 | 0.35 |
| Spernadio | 0.23 | 0.27 | 0.51 |
| Wyldewood | 0.24 | 0.15 | 0.51 |
| York | 0.63 | 0.10 | 0.33 |
LSD = 0.24
Because ‘Marge’ is an open-pollinated seedling originating in Kansas (USA), we cannot be certain that it was pollinated by a European parent; however, it is most likely a self-pollinated ‘Haschberg’. This self-pollination hypothesis is supported by the fact that European elderberry blooms about three weeks earlier than the American subspecies when grown in the Midwest, and that no other European genotypes were growing in the vicinity of the mother plant. Morphologically, in the field, ‘Marge’ behaves similarly to other European cultivars that we have grown, except that it is exceptionally robust whereas nearly all other European cultivars, including ‘Haschberg’, have performed poorly in our trials. Foremost among these European characteristics are the late budbreak and early bloom time of ‘Marge’, along with its larger fruit size (Table 1). Additional morphological characteristics that were not quantified in this study include our observation that ‘Marge’ does not fruit on the current season’s growth, similar to most European elderberries, and unlike the American subspecies. Furthermore, its growth habit, even after several years, remains a relatively dense multi-stemmed shrub with very little tendency to sucker as does American elderberry. This non-suckering habit is exploited in European elderberry horticulture in that many plants are pruned to a single trunk that is coppiced annually at ≈1 m above soil level.
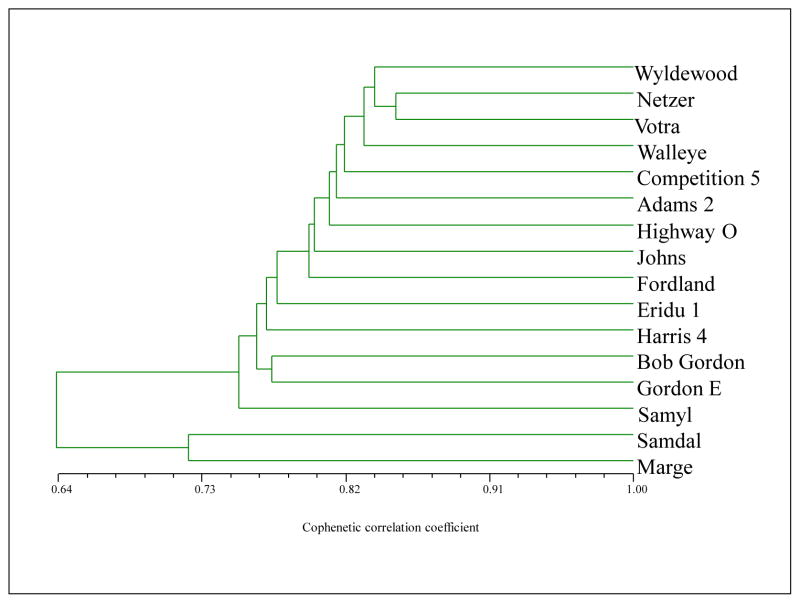
TRAP markers clearly distinguished the two elderberry subspecies (Fig. 3). In this dendogram, ‘Marge’ is strongly clustered with ‘Samdal’, a European elderberry cultivar. The American genotypes are also clustered together and are clearly genetically distinct from ‘Marge’ and ‘Samdal’. The position of ‘Samyl’ in the dendogram suggests that it may be intermediate between European and American elderberry, but it is a known European elderberry (Kaack, 1989) and is also closely clustered with ‘Marge’. In terms of genetic diversity reviewed by the DNA marker data, the American group had a lower level of variation with the cophenetic correlation coefficients of similarity larger than 77%. Less can be concluded with the European genotypes as only three were studied; however, the results clearly show that ‘Marge’ is most closely related to the other European elderberry genotypes. While the TRAP analysis herein was not exhaustive, these DNA marker results, combined with the phenological and morphological factors described above underscore our assertion that ‘Marge’ is indeed S. nigra subsp. nigra.
Fig. 3.
Dendogram showing genetic relationships among 16 American and European elderberry genotypes as determined by Target Region Amplification Polymorphism (TRAP) markers. Among the 16 genotypes evaluated, ‘Marge’ is most closely related to the European cultivars ‘Samdal’ and ‘Samyl’ (the remaining genotypes in the dendogram are American). Collection and provenance information for most of these American elderberry genotypes are found in Finn et al. (2008) and Thomas and Byers (2000).
Additional horticultural attributes derived from several years’ experience growing ‘Marge’ in both experimental and on-farm orchards include that it is exceptionally drought resistant whereas most American elderberries are not. Further, weed control may be easier with ‘Marge’ because of its dense, non-suckering, habit. Mulch, cultivation, or herbicides can be used to manage weeds with ‘Marge’ much more easily than with American elderberries which sucker prolifically.
However, because ‘Marge’ grows and produces fruit quite differently from American elderberry, some potential drawbacks to ‘Marge’ cultivation should be considered: Many North American producers of American elderberry have adopted a pruning management system of annually pruning their plants to the ground in winter. In addition to significantly reducing pruning labor costs, this practice usually results in more uniform flowering and fruiting at branch terminals, and production of fewer but larger fruiting cymes that are easier and less costly to harvest (Thomas et al., 2009). Because it does not fruit on current season’s growth, such a pruning regimen is not feasible with ‘Marge’, and more laborious annual pruning may be required. Additionally, the harvest period for ‘Marge’ is generally longer than with American elderberry that is annually pruned to the ground. Combined with the observation that fruiting cymes in ‘Marge’ are produced throughout the entire aspect of the plant (including the interior of the shrub) rather than mostly at branch terminals as in American elderberry, harvest labor requirements may be higher with ‘Marge’. These potentially higher management costs of ‘Marge’ production must be weighed against the potential for significantly greater production of larger, high-quality berries on vigorous plants that are pest- and drought-resistant.
We feel that ‘Marge’ merits release as a named elderberry cultivar because of its exceptional vigor, mite and drought resistance, high yields of very large berries, and excellent juice characteristics shown consistently in scientific trials as well as in private orchards. ‘Marge’ is already available through a variety of commercial nurseries and other sources in the marketplace, and has been released by Marge Millican for public use and distribution without remuneration.
The authors believe that ‘Marge’ has excellent potential for horticultural production in the midwestern USA, however we acknowledge that we do not know the ecological consequences (if any) of widely planting the European ‘Marge’ into areas where it is not native. We do not know its ability or propensity to escape into the wild (the large juicy berries are very attractive to birds and other wildlife) or to displace or hybridize with native American elderberries in a given region. Therefore, until more is learned about the behavior of ‘Marge’ in such settings, some prudence should be considered as to where it is planted, and the nearby environment should possibly be monitored to determine if damage to native ecosystems is possible due to escape or cross-pollination.
Acknowledgments
The assistance of Jinguo Hu, Gwo Yuh Liu, Ruifen Li, Jaime Piñero, and Theresa Blank is gratefully acknowledged. This work was partially funded through the Center for Agroforestry, University of Missouri under cooperative agreements with the USDA-ARS Dale Bumpers Small Farm Research Center, Booneville, AR. This publication was also made possible by Grant Number P50AT006273 from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, USDA, or the National Institutes of Health.
Literature Cited
- Barak V, Birkenfeld S, Halperin T, Kalickman I. The effect of herbal remedies on the production of human inflammatory and anti-inflammatory cytokines. Isr Med Assoc J. 2002;4(11 Suppl):919–922. [PubMed] [Google Scholar]
- Byers PL, Thomas AL, Millican M. ‘Wyldewood’ elderberry. HortScience. 2010;45:32–313. [Google Scholar]
- Charlebois D, Byers PL, Finn CE, Thomas AL. Elderberry: botany, horticulture, potential. In: Janick J, editor. Horticulture Reviews. Vol. 37. Wiley-Blackwell; New Jersey: 2010. pp. 213–280. [Google Scholar]
- Finn CE, Thomas AL, Byers PL, Serçe S. Evaluation of American (Sambucus canadensis) and European (S. nigra) elderberry genotypes grown in diverse environments and implications for cultivar development. HortScience. 2008;43(5):1385–1391. [Google Scholar]
- Hu J, Vick BA. Target region amplification polymorphism: a novel marker technique for plant genotyping. Plant Molecular Biology Reporter. 2003;21:289–294. [Google Scholar]
- Johnson HY, Byers PL, Hu J, Thomas AL, Tesfaye S. Assessment of genetic diversity among elderberry (Sambucus sp.) species, cultivars, and wild selections by TRAP technique. HortScience. 2008;43(4):1137–1138. abstr. [Google Scholar]
- Kaack K. New varieties of elderberry (Sambucus nigra L.) Tidsskr Planteavl (Danish J Plant Soil Science) 1989;93:59–65. [Google Scholar]
- Mohebalian PM, Cernusca MM, Aguilar FF. Discovering niche markets for elderberry juice in the United States. HortTechnology. 2012;22:556–566. [Google Scholar]
- Roschek B, Jr, Fink RC, McMichael MD, Li D, Alberte RS. Elderberry flavonoids bind to prevent H1N1 infection in vitro. Phytochemistry. 2009;70:1255–1261. doi: 10.1016/j.phytochem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Thomas AL, Byers PL. Multi-locational elderberry cultivar and management study. Proc Missouri Small Fruit Conf. 2000;20:37–40. Southwest Missouri State Univ., Springfield, MO. [Google Scholar]
- Thomas AL, Byers PL, Ellersieck MR. Productivity and characteristics of American elderberry in response to various pruning methods. HortScience. 2009;44(3):671–677. [Google Scholar]
- Thomas AL, Perkins-Veazie P, Byers PL, Finn CE, Lee J. A comparison of fruit characteristics among diverse elderberry genotypes grown in Missouri and Oregon. J Berry Research. 2013;3:159–168. doi: 10.3233/JBR-130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AL, Byers PL, Avery JD, Jr, Kaps M, Gu S. Horticultural performance of eight American elderberry genotypes at three Missouri locations. Acta Hort. 2015a;1061:237–244. doi: 10.17660/ActaHortic.2015.1061.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AL, Byers PL, Gu S, Avery JD, Jr, Kaps M, Datta A, Fernando L, Grossi P, Rottinghaus GE. Occurrence of polyphenols, organic acids, and sugars among diverse elderberry genotypes grown in three Missouri (USA) locations. Acta Hort. 2015b;1061:147–154. doi: 10.17660/ActaHortic.2015.1061.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaněčkova-Skuhravá I. Life cycles of five eriophyid mites species (Eriophyoidea, Acari) developing on trees and shrubs. J Appl Entomol. 1996;120:513–517. [Google Scholar]
- Veberic R, Jakopic J, Stampar F, Schmitzer V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009;114:511–515. [Google Scholar]
- Warmund MR, Amrine JW., Jr Eriophyid mites inhabiting American elderberry. Acta Hort. 2015;1061:155–160. [Google Scholar]
- Zakay-Rones Z, Varsano N, Zlotnik M, Manor O, Regev L, Schlesinger M, Mumcuoglu M. Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. J Altern Complementary Med. 1995;1:361–369. doi: 10.1089/acm.1995.1.361. [DOI] [PubMed] [Google Scholar]