Abstract
Cotreatment of rats with nontoxic doses of ranitidine (RAN) and lipopolysaccharide (LPS) causes liver injury, and this drug-inflammation interaction might be a model for idiosyncratic adverse drug responses in humans. Both polymorphonuclear neutrophils (PMNs) and the hemostatic system have been shown to be important in the injury. We tested the hypothesis that PMNs cause liver injury by interacting with the hemostatic system and producing subsequent hypoxia. In rats cotreated with LPS/RAN, PMN depletion by anti-PMN serum reduced fibrin deposition and hypoxia in the liver. PMN depletion also reduced the plasma concentration of active plasminogen activator inhibitor-1 (PAI-1), a major down-regulator of the fibrinolytic system. This suggests that PMNs promote fibrin deposition by increasing PAI-1 concentration. PMNs were activated in the livers of LPS/RAN-cotreated rats as evidenced by increased staining for hypochlorous acid-modified proteins generated by the myeloperoxidase-hydrogen peroxide-chloride system of activated phagocytes. Antiserum against the PMN adhesion molecule CD18 protected against LPS/RAN-induced liver injury. Because CD18 is important for PMN transmigration and activation, these results suggest that PMN activation is required for the liver injury. Furthermore, anti-CD18 serum reduced biomarkers of hemostasis and hypoxia, suggesting the necessity for PMN activation in the interaction between PMNs and the hemostatic system/hypoxia. Liver injury, liver fibrin, and plasma PAI-1 concentration were also reduced by eglin C, an inhibitor of proteases released by activated PMNs. In summary, PMNs are activated in LPS/RAN-cotreated rats and participate in the liver injury in part by contributing to hemostasis and hypoxia.
In rats, cotreatment with lipopolysaccharide (LPS) and ranitidine (RAN) causes liver injury resembling hepatotoxic idiosyncratic adverse drug responses that RAN causes in humans (Luyendyk et al., 2003). Both polymorphonuclear neutrophils (PMNs) and the hemostatic system are important in LPS/RAN-induced liver injury (Luyendyk et al., 2004, 2005). Hemostasis-associated fibrin deposition probably contributes to injury in this model by causing liver hypoxia (Luyendyk et al., 2005).
The hemostatic system is tightly regulated by the interplay between the coagulation and fibrinolytic systems (Lasne et al., 2006). Tissue factor is the principal initiator of the coagulation system, a complex cascade that ultimately generates active thrombin. Thrombin cleaves circulating fibrinogen into fibrin monomers, which upon cross-linking and polymerization can form obstructive clots in blood vessels. Plasminogen activators (PAs), including urokinase and tissue-specific PA, are important proteolytic activators of plasmin, which cleaves and dissolves cross-linked fibrin. The activity of PAs is inhibited by plasminogen activator inhibitor-1 (PAI-1) (Padró et al., 1997; Keller et al., 2006).
PMNs usually require transmigration across the endothelial barrier and subsequent activation to kill pathogens or injure tissues (Springer, 1995). These cytotoxic effects are mediated in part by release of reactive oxygen species and/or granular proteases (Jaeschke et al., 1996). PMN-derived proteases such as elastase and cathepsin G kill hepatocytes directly in vitro (Ho et al., 1996; Hill and Roth, 1998). Moreover, the killing of hepatocytes by PMN-derived proteases is potentiated by hypoxia (Luyendyk et al., 2005).
In the LPS/RAN model of hepatotoxic drug-inflammation interaction, PMNs accumulate in liver, but how they participate in the pathogenesis and their relationship to the hemostatic system are unknown. Here, we tested the hypothesis that hepatic PMNs are activated in the livers of LPS/RAN-cotreated rats and contribute to liver injury by releasing proteases and interacting with the hemostatic system.
Materials and Methods
Materials
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Two lots of LPS derived from Escherichia coli serotype O55:B5 (catalog no. L-2880) with activities of 6.6 × 106 EU/mg (lot no. 51K4115) and 13 × 106 EU/mg (lot no. 43K4112) were used for these studies. These activities were determined using a QCL Chromogenic Limulus amoebocyte lysate endpoint assay purchased from Cambrex Bio Science, Inc. (Baltimore, MD).
Animals
Male, Sprague-Dawley rats [Crl:CD (SD)IGS BR; Charles River Breeding Laboratories, Portage, MI] weighing 250 to 350 g were fed standard chow (rodent chow/Tek 8640; Harlan Teklad, Madison, WI) and allowed access to water ad libitum. They were allowed to acclimate for 1 week in a 12-h light/dark cycle before use.
Experimental Protocol
Rats fasted for 24 h were given 2.5 × 106 EU/kg LPS (lot 43K4112) or its saline vehicle (Veh) i.v. at 5 ml/kg, and food was then returned. For the 6-h CD18 antiserum study, 44.4 × 106 EU/kg LPS (lot 51K4115) or its vehicle at 2 ml/kg was given. These two LPS doses from different lots render approximately the same degree of hepatocellular injury in RAN-treated rats as marked by serum alanine aminotransferase (ALT) activity. Two hours after administration of LPS, 30 mg/kg RAN or its vehicle [sterile phosphate-buffered saline (PBS)] was administered (i.v.). RAN solution was administered at 2 ml/kg at a rate of approximately 0.15 ml/min. Two, 3, or 6 h after RAN administration, rats were anesthetized with sodium pentobarbital (75 mg/kg i.p.). Plasma was collected by drawing 2 ml of blood from the vena cava into a syringe containing sodium citrate (final concentration, 0.38%). Another portion of blood was collected and allowed to clot at room temperature; serum was prepared from this fraction and stored at −20°C until use. Representative slices (3–4 mm thick) of the ventral portion of the left lateral liver lobe were collected and fixed in 10% (v/v) neutral buffered formalin. For immunohistochemical analysis, a portion of the left medial lobe of the liver was flash-frozen in isopentane cooled by liquid nitrogen.
PMN Depletion, CD18 Neutralization, and Eglin C Treatment
PMN depletion was accomplished by administration of a PMN antiserum before treatment with LPS/RAN. Rats were given 0.25 ml of either control normal rabbit serum (CS) or rabbit anti-rat PMN antiserum (NAS) (Intercell Technologies, Jupiter, FL) diluted 1:1 in sterile saline (0.5 ml/rat i.v.) 18 h before administration of LPS. A previous study in which anti-PMN serum was administered to rats demonstrated a selective and complete depletion of PMNs (Snipes et al., 1995). Rats were killed 2 and 6 h after treatment with RAN, and citrated plasma was collected. Total blood leukocytes were quantified using a Unopette white blood cell determination kit (BD Biosciences, San Jose, CA) and a hemocytometer. To determine the number of PMNs in blood, slides were prepared from whole blood and stained using the Hema 3 staining system (Fisher Diagnostics, Middletown, VA), and differential counting was performed.
For the CD18 neutralization study, rabbit anti-CD18 serum raised against rat CD18-specific peptide (Ac-CLKFDKGPFQKN-amide) was obtained from New England Peptide (Gardner, MA). The anti-CD18 serum or normal rabbit serum was diluted 1:1 (v/v) in sterile saline and administered (0.5 ml/rat i.v.) immediately after LPS injection. Rats were killed 6 h after RAN treatment for evaluation of serum ALT activity, plasma PAI-1 concentration, liver PMN accumulation, and fibrin. Rats were killed 3 h after RAN for hypoxia and PMN activation evaluation. For the eglin C study, eglin C (provided by Novartis Pharm AG, Basel, Switzerland) was given at 8 mg/kg i.v. immediately after RAN and 2 and 4 h later. Rats were killed 6 h after RAN treatment.
Hepatotoxicity Assessment
Hepatic parenchymal cell injury was estimated as an increase in serum ALT activity. ALT activity was determined spectrophotometrically using Infinity-ALT reagent from Thermo Electron Corporation (Louisville, CO). Previous studies in this LPS/RAN model have shown that serum ALT activity reflects histopathologic evidence of hepatocellular necrosis (Luyendyk et al., 2003).
Fibrin Immunohistochemistry and Quantification
Fibrin immunohistochemistry and quantification were performed as described previously (Copple et al., 2002). This protocol solubilizes all fibrinogen and fibrin except for cross-linked fibrin (Schnitt et al., 1993). Therefore, only cross-linked fibrin stains in sections of liver. The positive area fraction refers to the fraction of the area of the microscopic field that was positive for staining of fibrin.
Evaluation of Coagulation System Activation and Plasma PAI-1
Plasma fibrinogen was determined from thrombin clotting time of diluted samples using a fibrometer and a commercially available kit (B4233) from Dade Behring Inc. (Deerfield, IL). Plasma thrombin-antithrombin (TAT) concentration was determined by an enzyme-linked immunosorbent assay (ELISA) using a kit from Dade Behring Inc. (catalog no. OWMG15). Total plasma PAI-1 concentration was evaluated using a commercially available ELISA purchased from American Diagnostica Inc. (Greenwich, CT). This ELISA measures PAI-1 in active, inactive, and plasminogen activator/PAI-1 complexed forms. The concentration of functionally active PAI-1 in plasma was assessed using a commercially available ELISA kit purchased from Molecular Innovations Inc. (Southfield, MI).
Evaluation of Liver Hypoxia
Liver hypoxia was evaluated by immunohistochemical staining for pimonidazole (PIM) adducts. PIM is a 2-nitroimidazole marker of hypoxia and has been used to identify regions of hypoxia in liver (Arteel et al., 1995, 1998). Unless otherwise noted, rats were given 120 mg/kg hypoxyprobe-1 (PIM hydrochloride; Chemicon International, Temecula, CA) i.p. 2 h before they were killed. PIM adduct immunostaining was performed as described previously (Copple et al., 2004). Quantification of immunostaining was performed using Scion Image Beta 4.0.2 software (Scion Corporation, Frederick, MD). Background was set to be the average pixel intensity in periportal regions of Veh/Veh-treated livers (i.e., an area where no hypoxia occurs) (Arteel et al., 1998). An increase in positive immunostaining for PIM-modified proteins indicates hypoxia in the liver tissue.
Evaluation of Liver PMNs and PMN Activation
PMNs accumulated in liver were visualized by immunohistochemical staining and quantified as described previously (Yee et al., 2003). PMN activation was measured by staining of hypochlorous acid (HOCl)-protein adducts in liver. The monoclonal antibody (clone 2D10G9, subtype IgG2bk) is specific for HOCl-modified epitopes generated in vivo (Malle et al., 1997) and in vitro (Malle et al., 1995) and does not cross-react with other oxidative protein modifications. Frozen sections of liver were fixed in 4% (v/v) formalin for 10 min at room temperature with gentle rocking. The slides were washed three times for 5 min each with PBS, then blocked for 1 h at room temperature with 3% goat serum in PBS. Antibody (diluted 1:1 in 3% goat serum) was added and incubated for 2 h at room temperature with gentle rocking. The slides were washed three times for 5 min each with PBS. Alexa Fluor 488-labeled goat anti-mouse secondary antibody (Invitrogen, Carlsbad, CA) diluted 1:500 in 3% goat serum was applied, and the slides were incubated for 3 h at room temperature. After washing three times for 5 min each with PBS, they were examined microscopically. Staining was quantified as for fibrin staining described above and presented as positive area fraction. The integrated density within areas of positive staining was calculated as the sum of the products of pixel gray intensity and pixel numbers with that intensity.
Evaluation of PMN Protease Effects on Fibrinogen and PAI-1 in Vitro
Normal rat plasma was incubated with a mixture of elastase and cathepsin G or vehicle (PBS) at two different conditions: elastase (0.04 U/ml)/cathepsin G (4 μg/ml) and elastase (8 U/ml)/cathepsin G (4 μg/ml). Elastase activity was determined using the colorometric PMN elastase substrate, MeOSuc-Ala-Ala-Pro-Val-pNA (Calbiochem, San Diego, CA). One unit of PMN elastase activity was defined as the amount of enzyme required to cause a change of absorbance of 1.0 at 410 nm in 10 min at 37°C. After 2 and 6 h of incubation, fibrinogen was measured by fibrometer as described above. Purified PAI-1 purchased from American Diagnostica Inc., was incubated with elastase and cathepsin G under the same conditions, and the reaction was stopped by adding anti-trypsin (40 nM) after 2 or 6 h. The total PAI-1 level was measured by PAI-1 ELISA as described above. For the SDS-PAGE analysis, purified fibrinogen (250 μg/ml) was incubated with PBS, thrombin (0.1 U/ml), elastase (8 U/ml)/cathepsin G (4 μg/ml), or elastase (0.04 U/ml)/cathepsin G (4 μg/ml). The reaction was stopped by adding 1% SDS and 2% 2-mercaptoethanol after 2 or 6 h. The resulting protein products were separated on 4 to 12% gradient SDS-PAGE and visualized with silver staining (Bio-Rad, Hercules, CA).
Statistical Analysis
For the PMN depletion and in vitro studies, two-way ANOVA was applied with time and treatment as the two factors. For the CD18 neutralization and eglin C studies, two-way ANOVA was used with LPS/RAN and drug (anti-CD18 serum or eglin C) as the two factors. For the ALT data from the eglin C study, a Student’s t test was applied. Tukey’s test was used as post hoc analysis for ANOVA. The criterion for statistical significance for all studies was p < 0.05.
Results
PMN Depletion Studies in the LPS/RAN Model
To assess the role of PMNs in hemostasis and hepatocellular injury, PMN numbers were reduced before LPS/RAN cotreatment. NAS or its CS was administered 16 h before LPS treatment. Circulating total leukocytes and PMNs and liver PMNs were evaluated at 2 and 6 h after RAN treatment. As shown previously (Luyendyk et al., 2005), the antiserum markedly reduced blood PMNs at both 2 and 6 h (Table 1). The number of total blood leukocytes was also reduced by NAS treatment, although this loss can be explained largely by the decreased numbers of PMNs. NAS treatment also significantly reduced liver PMN accumulation caused by LPS/RAN treatment at both 2 and 6 h (data not shown). In addition, the antiserum produced a qualitative change in liver PMNs, causing a shift toward the hepatic accumulation of immature PMNs (i.e., band cells).
TABLE 1. Circulating leukocytes after PMN antiserum treatment in LPS/RAN-treated rats.
Depletion of PMNs in LPS/RAN-treated rats was accomplished as in Fig 1. Circulating total leukocytes and PMNs were measured at 2 and 6 h after LPS/RAN treatment as described under Materials and Methods.
| Time after RAN | Treatment | Leukocytes | PMNs |
|---|---|---|---|
| no./μl | |||
| 2 h | CS/Veh/Veh | 4012 + 457 | 776 + 277 |
| CS/LPS/RAN | 4640 + 628 | 2482 + 661* | |
| NAS/LPS/RAN | 1560 + 181*† | 75 + 17*† | |
| 6 h | CS/Veh/Veh | 4138 + 819 | 497 + 153 |
| CS/LPS/RAN | 4236 + 591 | 2594 + 559 | |
| NAS/LPS/RAN | 1812 + 368*† | 344 + 121† | |
Significantly different from CS/Veh/Veh group at the same time.
Significantly different from CS/LPS/RAN group at the same time. p < 0.05, n = 4 to 7.
NAS treatment alone did not cause serum ALT activity to increase either at 2 or 6 h (Fig. 1). Serum ALT activity was unaffected by LPS/RAN treatment at 2 h after RAN administration but was significantly elevated by 6 h. Treatment with the antiserum significantly decreased ALT activity in the serum of LPS/RAN-treated rats at 6 h after RAN, confirming previous results (Luyendyk et al., 2005).
Fig. 1.
Effects of PMN depletion on hepatotoxicity induced by LPS/RAN. NAS or CS was administered 16 h before LPS or its vehicle. Two hours after LPS treatment, RAN or its vehicle was administered. Serum ALT activity was measured 2 and 6 h after administration of RAN or its vehicle. *, significantly different from the respective group without LPS/RAN at the same time. #, significantly different from the respective group without CS at the same time. a, significantly different from the same treatment group at 2 h. p < 0.05, n = 4 to 7.
The effect of PMN depletion on coagulation system activation was also assessed. CS/LPS/RAN treatment caused a significant increase in liver fibrin compared with CS/Veh/Veh control both at 2 and 6 h (Fig. 2). NAS pretreatment did not affect fibrin deposition at 2 h after RAN but significantly reduced it at 6 h. Activation of the coagulation system leads to a decrease in plasma fibrinogen concentration because this protein is cleaved to form insoluble fibrin clots. At both 2 and 6 h, LPS/RAN treatment decreased plasma fibrinogen concentration and increased plasma TAT concentration, a biomarker of coagulation system activation (Fig. 3A). This suggests early and persistent activation of the coagulation system. In PMN-depleted rats, there was a more modest decrease in plasma fibrinogen (Fig. 3A), but the elevation in TAT was unaffected (Fig. 3B).
Fig. 2.
Effects of PMN depletion on liver fibrin after LPS/RAN treatment. PMN depletion of LPS/RAN-treated rats was accomplished as in Fig. 1. Fibrin staining was performed 2 and 6 h after treatment with RAN or its vehicle. *, significantly different from CS/Veh/Veh group at the same time. #, significantly different from CS/LPS/RAN group at the same time. p < 0.05, n = 4 to 7.
Fig. 3.
Effects of PMN depletion on coagulation system activation after LPS/RAN treatment. PMN depletion of LPS/RAN-treated rats was accomplished as in Fig. 1. Plasma fibrinogen and TAT concentrations were measured 2 and 6 h after RAN or vehicle treatment. *, significantly different from CS/Veh/Veh group at the same time. #, significantly different from CS/LPS/RAN group at the same time. p < 0.05, n = 4 to 7.
The observation that depletion of PMNs partially prevented the loss of fibrinogen (Fig. 3A) suggested that PMNs contribute to fibrinogen consumption in this model. One mechanism by which this could occur is that proteases, such as elastase and cathepsin G, released by activated PMNs cleave fibrinogen directly. We tested this possibility by exposing fibrinogen to a combination of elastase and cathepsin G in vitro. Incubation of fibrinogen with PMN elastase/cathepsin G reduced fibrinogen concentration after 2 or 6 h (Fig. 4A). These results suggest that PMN proteases cleave and inactivate fibrinogen. SDS-PAGE of fibrinogen produced three predominant bands identified by silver staining, with molecular masses of approximately 62, 53, and 47 kDa (Fig. 4B, lanes 1 and 2). These are probably the Aα (62 kDa), Bβ (53 kDa), and γ (47 kDa) chains of fibrinogen as described by Gorkun et al. (1997). There was also a minor band, which is probably the smaller Aα chain lacking a C-terminal fragment. Thrombin treatment reduced the size of the smaller Aα and Bβ chains (Fig. 4B, lanes 3–5). This is probably due to the release of fibrin peptides A and B, respectively (Weisel et al., 1993; Gorkun et al., 1994). Elastase (0.04 U/ml) and cathepsin G (4 μg/ml) treatment caused the degradation of the smaller Aα chain and the formation of degradation products of smaller size (Fig. 4B, lanes 8 and 9). Increasing the concentration of elastase in the mixture resulted in the degradation of Bβ, γ, and the smaller size Aα chain and the formation of degradation products of smaller size (Fig. 4B, lanes 6 and 7).
Fig. 4.
Effects of PMN proteases on plasma fibrinogen in vitro. A, elastase and cathepsin G or their vehicle (PBS) was incubated with plasma fibrinogen as described under Materials and Methods. Fibrinogen was measured using a fibrometer 2 and 6 h later. *, significantly different from PBS group. #, significantly different from elastase (0.04 U/ml)/cathepsin G (4 μg/ml) treatment. p <0.05, n = 4. B, thrombin, elastase/cathepsin G (4 μg/ml), or their vehicle (PBS) was incubated with purified fibrinogen, and the protein products were analyzed by SDS-PAGE. Lanes 1 and 2, fibrinogen + PBS, 2 and 6 h, respectively; lanes 3 to 5, fibrinogen + thrombin, 2 h; lanes 6 and 7, fibrinogen + elastase (8 U/ml)/cathepsin G (4 μg/ml), 2 and 6 h, respectively; lanes 8 and 9, fibrinogen + elastase (0.04 U/ml)/cathepsin G (4 μg/ml), 2 and 6 h, respectively. Molecular mass was marked at the right margin (kilodaltons).
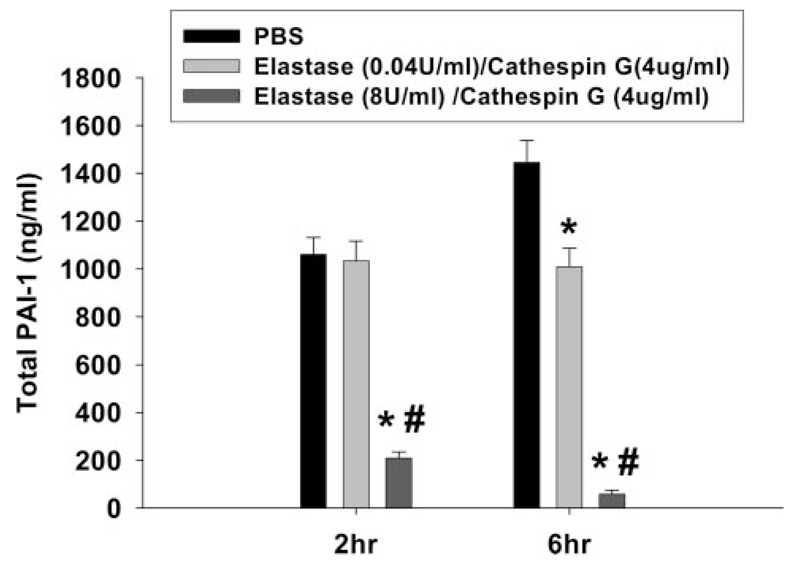
The inhibition of fibrin deposition in liver by NAS at 6 h without associated changes in coagulation biomarkers led us to explore effects on fibrinolysis. PAI-1 is the major endogenous down-regulator of the fibrinolytic system, which lyses insoluble fibrin clots and thereby controls the degree of fibrin deposition. Total PAI-1 includes active PAI-1, inactive PAI-1, and PAI-1 complexed with PA. LPS/RAN treatment caused a significant increase in the plasma concentrations of both total and active PAI-1 at 2 and 6 h after RAN (Fig. 5). PMN depletion did not affect total plasma PAI-1 concentration at 6 h but increased it at 2 h (Fig. 5A). On the other hand, PMN depletion did not significantly affect active PAI-1 concentration at 2 h but decreased it at 6 h (Fig. 5B). The increase in total plasma PAI-1 concentration with PMN depletion suggested that PMN-derived proteases might degrade PAI-1. Indeed, incubation of purified PAI-1 with elastase/cathepsin G reduced total PAI-1 concentration (Fig. 6).
Fig. 5.
Effects of PMN depletion on plasma PAI-1 concentration after LPS/RAN treatment. PMN depletion of LPS/RAN-treated rats was accomplished as in Fig. 1. Plasma total PAI-1 (A) and active PAI-1 (B) were measured 2 and 6 h after LPS/RAN treatment as described under Materials and Methods. *, significantly different from CS/Veh/Veh group at the same time. #, significantly different from CS/LPS/RAN group at the same time. a, significantly different from the same treatment group at 2 h. p < 0.05, n = 4–7.
Fig. 6.
Effects of PMN proteases on PAI-1 concentration in vitro. Elastase and cathepsin G or their vehicle (PBS) was incubated with purified PAI-1 as described under Materials and Methods. Total PAI-1 concentration was measured by ELISA 2 and 6 h later. *, significantly different from vehicle group. #, significantly different from elastase (0.04 U/ml)/cathepsin G treatment. p < 0.05, n = 4.
Because fibrin deposition and hemostasis can lead to tissue hypoxia, immunohistochemistry of PIM-bound protein was used as a marker for liver hypoxia. LPS/RAN treatment caused a significant increase in PIM-protein adduct staining at 2 h after RAN, and this was prevented by NAS treatment (Fig. 7). Hypoxia staining in LPS/RAN-treated livers tended to be panlobular, compared with the necrotic foci that were predominantly midzonal.
Fig. 7.
Effect of PMN depletion on liver hypoxia after LPS/RAN treatment. PMN depletion of LPS/RAN-treated rats was accomplished as in Fig. 1. For PIM-protein adduct staining, PIM was administered to rats 1 h after RAN, and the rats were killed at 2 h after RAN. *, significantly different from CS/Veh/Veh group. #, significantly different from CS/LPS/RAN group. p < 0.05, n = 4 to 7.
HOCl-Protein Adduct Staining
Activation of PMNs is required for many of their damaging effects on host tissue. The potent oxidant HOCl, generated in vivo by the myeloperoxidase-hydrogen peroxide-chloride system of activated phagocytes, reacts with proteins to form chloramines that can be used as a biomarker for PMN activation. Neither LPS alone nor RAN alone affected HOCl-protein adduct staining (Fig. 8, B, C, and E). LPS/RAN cotreatment increased the staining, indicating the activation of PMNs in LPS/RAN-treated livers (Fig. 8, D and E). The HOCl-protein epitopes were predominantly within the midzonal areas of liver lobules, which was where the necrotic foci occurred in livers of LPS/RAN-cotreated rats. This increase was apparent 3 to 6 h after RAN but not at 2 h (Fig. 8F).
Fig. 8.
PMN activation after LPS/RAN treatment. Rats were treated with LPS or vehicle 2 h before RAN or its vehicle. HOCl-protein adduct staining was performed 3 h after treatment with RAN or vehicle (A–E). The representative photomicrographs for individual treatment groups are presented in gray scale. A, Veh/Veh; B, Veh/RAN; C, LPS/Veh; D, LPS/RAN; E, quantification of staining for all four treatment groups. *, significantly different from the respective group without LPS. #, significantly different from the respective group without RAN. F, rats were treated with LPS/RAN or Veh/Veh as described in Fig. 1. HOCl-modified protein staining was performed 2, 3, 4, and 6 h after LPS/RAN treatment. The Veh/Veh-treated animals were killed at 2 h after RAN. *, significantly different from Veh/Veh group. p < 0.05, n = 5 to 7.
Effects of CD18 Neutralization on Hepatotoxicity, PMNs, and Hemostasis
CD18 is an adhesion molecule expressed on PMN plasma membranes that is important for the transmigration and subsequent activation of these cells (Springer, 1994). Based on staining for HOCl-modified epitopes (Fig. 8), it was clear that PMNs were activated in livers of LPS/RAN-treated rats. To investigate the role of PMN activation in the LPS/RAN model, anti-CD18 serum was administered immediately after LPS, and various biomarkers were evaluated 6 h after RAN. The anti-CD18 serum did not reduce the number of circulating PMNs after LPS/RAN treatment (data not shown). LPS/RAN caused an increase in PMNs accumulated in liver (Fig. 9A). Anti-CD18 serum alone caused an increase in the number of PMNs in livers of Veh/Veh-treated rats but did not affect hepatic PMN accumulation after LPS/RAN treatment. Anti-CD18 serum reduced staining for HOCl-modified epitopes at 3 h after RAN (Fig. 9B) and protected from LPS/RAN-induced hepatotoxicity, as reflected by reduction of serum ALT activity (Fig. 9C). In addition, anti-CD18 serum decreased liver fibrin at 6 h after RAN treatment (Fig. 9D).
Fig. 9.
Effects of CD18 neutralization on biomarkers of hemostasis, PMNs and hepatotoxicity in LPS/RAN-cotreated rats. Rabbit anti-CD18 serum or normal serum was administered at the same time as LPS or its vehicle. RAN or its vehicle was administered 2 h after LPS treatment. A, liver PMN accumulation; C, serum ALT activity; D, liver fibrin; E, plasma total PAI-1; and F, plasma active PAI-1 were evaluated 6 h after RAN or its vehicle. B, HOCL-protein adduct staining was evaluated 3 h after RAN or its vehicle. *, significantly different from the respective group without LPS/RAN. #, significantly different from the respective group without anti-CD18 serum. p < 0.05, n = 4 to 7.
Consistent with the PMN depletion results above, anti-CD18 serum did not affect the increase in total PAI-1 in plasma but reduced active PAI-1 concentration (Fig. 9, E and F). Moreover, treatment with anti-CD18 serum prevented the LPS/RAN-induced liver hypoxia (Fig. 10).
Fig. 10.
Effects of CD18 neutralization on liver hypoxia after LPS/RAN treatment. CD18 neutralization in LPS/RAN-treated rats was accomplished as described in Fig. 9. PIM probe was administered 1 h after RAN treatment, and PIM-protein adduct staining was performed 3 h after RAN treatment. *, significantly different from the respective group without LPS/RAN. #, significantly different from the respective group without anti-CD18 serum. p < 0.05, n = 4 to 7.
Effects of Eglin C on Serum ALT Activity and Hemostasis
Eglin C is a PMN protease inhibitor that inhibits PMN elastase and cathepsin G but does not inhibit thrombin (Braun et al., 1987; Renesto et al., 1990). It was used to investigate the contribution of PMN proteases to LPS/RAN induced hepatotoxicity and alterations in hemostasis. Eglin C administration significantly reduced serum ALT activity, indicating attenuation of hepatocellular injury (Fig. 11). Eglin C also reduced plasma active PAI-1 concentration, liver fibrin, and fibrinogen consumption after LPS/RAN treatment (Fig. 12, A, B, and D). It did not affect the LPS/RAN-induced increase in plasma TAT concentration (Fig. 12C).
Fig. 11.
Effects of PMN-protease inhibitor on hepatotoxicity after LPS/RAN treatment. Rats were treated with LPS/RAN as described in Fig. 1. Eglin C or its vehicle was administered 0, 2, and 4 h after RAN treatment. Serum ALT activity was evaluated 6 h after RAN treatment. #, significantly different from Veh group. p < 0.05, n = 11 to 12.
Fig. 12.
Effects of PMN protease inhibitor on markers of hemostatic system activation after LPS/RAN treatment. Rats were treated with LPS/RAN or Veh/Veh as described in Fig. 1. Eglin C or its vehicle was administered 0, 2, and 4 h after RAN treatment. Liver fibrin (B) and plasma active PAI-1 (A), TAT (C), and fibrinogen (D) concentrations were evaluated 6 h after RAN treatment. *, significantly different from the respective group without LPS/RAN. #, significantly different from the respective group without eglin c. p < 0.05, n = 5 to 12.
Discussion
In LPS/RAN-treated rats, hepatocellular injury begins 2 to 3 h after RAN administration and progresses through 6 h, at which time serum ALT activity is maximal (Luyendyk et al., 2003). Confirming previous results (Luyendyk et al., 2005), PMN depletion with NAS dramatically reduced serum ALT activity 6 h after RAN, suggesting an important role for PMNs in the liver injury. PMNs could contribute to this response by a variety of mechanisms. One possibility is that PMNs promote coagulation and fibrin deposition in liver, leading to hypoxia. Fibrin deposition occurs before injury (2–3 h) in liver sinusoids of LPS/RAN-cotreated rats (Luyendyk et al., 2004). PMN depletion decreased fibrin deposition at 6 h after LPS/RAN treatment (Fig. 2) but not at 2 h. This result suggests that PMN-dependent fibrin deposition contributes to the progression but not to the initiation of hepatocellular injury in LPS/RAN-treated rats. To investigate how PMNs contribute to fibrin deposition in the LPS/RAN model, coagulation system activation and PAI-1 were evaluated. PMN depletion attenuated the decrease in plasma fibrinogen caused by LPS/RAN (Fig. 3A) but did not influence the elevation in plasma TAT concentration (Fig. 3B). Because TAT concentration reflects generation of active thrombin, this result suggests that PMNs do not contribute to LPS/RAN-induced coagulation but are capable of thrombin-independent cleavage of fibrinogen. Indeed, a combination of PMN elastase and cathepsin G cleaved fibrinogen in vitro in a manner different from thrombin (Fig. 4). Furthermore, this was demonstrated by the fact that eglin C reduced the consumption of fibrinogen caused by LPS/RAN treatment but did not affect plasma TAT concentration (Fig. 12, C and D). Taken together, these results suggest that PMNs contribute to fibrin deposition in this model but not via activating the coagulation cascade.
An alternative mechanism by which PMNs could contribute to deposition of fibrin is through inhibiting fibrinolysis by increasing active PAI-1. PMN lysosomal proteases cathepsin B, D, and G increased PAI-1 activity in the medium of human umbilical vein endothelial cells by cleaving PAI-1 from the extracellular matrix (Pintucci et al., 1992, 1993; Kimura and Yokoi-Hayashi, 1996). Consistent with this, PMN depletion reduced active PAI-1 in the plasma at 6 h in LPS/RAN-cotreated rats, although it did not affect it at 2 h (Fig. 5B). This suggests that PMNs contribute to fibrin deposition after 2 h by activating PAI-1 and thereby inhibiting fibrinolysis. In contrast, PMN depletion did not affect total plasma PAI-1 concentration at 6 h, but it did increase total plasma PAI-1 concentration at 2 h. The mechanism by which PMN depletion increases total PAI-1 concentration is unknown. PMN-derived proteases such as elastase can cleave and inactivate PAI-1 (Wu et al., 1995), which has also been shown here (Fig. 6). However, this is unlikely in vivo at 2 h in the LPS/RAN model because PMNs are not activated to release granular products at that time (Fig. 8). Interestingly, internalization of urokinase PA-PAI-1 complexes by immune cells can occur through urokinase PA receptors (Conese et al., 1995; Nykjaer et al., 1997); this might explain why PMN depletion increased total PAI-1 but had no effect on active PAI-1 at the earlier time (i.e., 2 h).
One explanation for the different effects of PMNs on active and total PAI-1 is that PMNs are not activated to release proteases during earlier times but begin to degranulate thereafter. In this regard, the shedding of PAI-1 from endothelial matrix caused by PMN proteases might play a more dominate role at later times (i.e., 6 h). This is consistent with the results showing that formation of HOCl-modified epitopes, a marker of PMN degranulation/activation, did not increase until 3 h after LPS/RAN treatment (Fig. 8), suggesting that PMNs did not start the transmigration and activation process until after 2 h.
In the LPS/RAN model, PMN accumulation in liver is caused largely by LPS (Luyendyk et al., 2005). However, LPS exposure alone did not lead to activation of the accumulated PMNs (Fig. 8A). RAN did cause activation of the PMNs that accumulated in liver after LPS treatment, as reflected by an increase in HOCl-protein adduct formation. Anti-CD18 serum did not alter PMN accumulation but reduced PMN activation in liver (Fig. 9, A and B), suggesting that the early, LPS-induced sinusoidal accumulation of PMNs does not require CD18, whereas their activation is CD18-dependent. In addition, anti-CD18 serum markedly reduced hepatocellular injury (Fig. 9A), suggesting that PMN transmigration/activation is required for LPS/RAN-induced liver injury.
The anti-CD18 serum and NAS had similar effects on the hemostatic system. Anti-CD18 serum reduced fibrin deposition 6 h after LPS/RAN treatment (Fig. 9D), suggesting that activation of PMNs is required for their effect on hemostasis. Moreover, PMN proteases seem to be important in this regard because eglin C also reduced the deposition of fibrin (Fig. 12B). This promotion of fibrin deposition by activated PMNs is most probably mediated by PAI-1 because both anti-CD18 serum and eglin C reduced the elevation in active PAI-1 concentration in plasma. In addition, the reduction of active PAI-1 by eglin C treatment supports the findings of others (Pintucci et al., 1993; Kimura and Yokoi-Hayashi, 1996) that PMN proteases can cleave PAI-1 from endothelial matrix and increase the concentration of active PAI-1 in plasma.
There are several reports showing that hypoxia can cause hepatocyte death in vitro (Nagatomi et al., 1997; Lluis et al., 2005). In the LPS/RAN model, agents that reduce hypoxia also reduce hepatocellular injury. These include heparin (Luyendyk et al., 2005), PMN antiserum (Fig. 7), and CD18 antiserum (Fig. 8). Accordingly, it seems likely that hypoxia caused in part by sinusoidal fibrin deposition that reduces blood flow plays a role in inducing hepatocellular injury in this model. PMNs could also contribute to tissue hypoxia. In LPS/RAN-treated rats, PMN depletion reduced PIM staining at 2 h after LPS/RAN treatment, suggesting that PMNs contribute to early development of liver hypoxia. This could occur by several mechanisms. One possibility is that accumulated PMNs could clog sinusoidal vessels and impair microvascular blood flow. Furthermore, PMN-derived factors could impair the balance between vasodilators and vasoconstrictors, causing constriction of sinusoids and subsequent hypoxia. For example, PMN-derived proteases reduce vasodilatory prostacyclin production both in vitro and in vivo (Weksler et al., 1989; Harada et al., 1997; Okajima et al., 2004). Myeloperoxidase, abundantly present in PMNs (5% of total cell protein content) and released by PMNs during the oxidative burst, could also oxidize nitric oxide and thereby impair vascular relaxation (Eiserich et al., 2002). This early contribution of PMNs to hypoxia did not depend on sinusoidal fibrin deposition because PMN deletion in LPS/RAN-treated rats did not affect liver fibrin at 2 h after RAN (Fig. 2). Furthermore, PMNs accumulated in livers are not activated at 2 h (Fig. 8), suggesting that enhancement of hypoxia by PMNs at this time does not require their activation and might be mediated directly by plugging sinusoids.
LPS-RAN cotreatment caused a greater degree of tissue hypoxia than LPS given alone (Luyendyk et al., 2004), although these two treatments had a similar effect on PMN accumulation (Luyendyk et al., 2005). It is possible that RAN cotreatment causes PMNs to adhere more firmly to sinusoidal endothelium and/or to undergo a shape change that results in reduced sinusoidal blood flow and consequent hypoxia. Interestingly, the early tissue hypoxia and PMN accumulation were both panlobular in distribution, whereas the hepatocellular necrosis that followed was midzonal. The HOCl adduct staining also tended to be midzonal. These results suggest that the early hypoxia might be necessary but insufficient to cause the liver injury without additional factors such as PMN activation.
The contribution of PMNs to liver hypoxia at later times might occur by a different mechanism. Anti-CD18 serum reduced liver hypoxia at 3 h, suggesting that the contribution of PMNs to liver hypoxia after 2 h requires PMN transmigration and/or activation. Because sinusoidal fibrin deposition seems to be important for liver hypoxia occurring at 3 h after LPS/RAN treatment (Luyendyk et al., 2005), PMNs might contribute to liver hypoxia after 2 h by promoting sinusoidal fibrin deposition.
Among the products released by activated PMNs, cathepsin G and elastase are important mediators of hepatic parenchymal cell killing caused by PMNs in vitro (Ho et al., 1996). These proteases also have been implicated in models of liver injury in vivo such as ischemia-reperfusion (Okajima et al., 2004) and LPS-induced liver dysfunction (Ishii et al., 2002). Interestingly, killing of hepatocytes in vitro by neutrophil elastase is enhanced by a hypoxic environment (Luyendyk et al., 2005). The time course over which hepatocyte killing occurred during hypoxia in vitro is consistent with the development of liver injury in LPS/RAN-treated rats. Taken together with the evidence that fibrin deposition and hypoxia are critical to liver injury in LPS/RAN-treated rats, it is likely that PMNs contribute to the pathogenesis by releasing proteases that kill hepatocytes in an environment made hypoxic by fibrin deposition. Indeed, eglin C attenuated the hepatocellular injury caused by LPS/RAN treatment. Thus, PMN proteases could contribute to liver injury by direct killing of hepatocytes and indirectly by promoting fibrin deposition and hypoxia, thereby increasing hepatocellular susceptibility to injury.
In conclusion, PMNs and the hemostatic system are key players in the pathogenesis of liver injury in LPS/RAN-cotreated rats. A working hypothesis of their involvement is presented in Fig. 13. PMN accumulation in liver in this model is mediated by LPS and is insufficient to damage hepatocytes. Activation of hepatic PMNs by RAN occurs around the time liver injury begins and is critical for the progression of hepatocellular injury. Moreover, the protection afforded by eglin C suggests that proteases released during PMN activation are important. These proteases may injure hepatocytes directly, but they also participate in liver injury progression by promoting hemostasis, in part by activating antifibrinolytic PAI-1. PMNs also contribute to the liver hypoxia at a time before PMN activation is evident. In addition, during injury progression, PMNs probably enhance tissue hypoxia by promoting hemostasis in this model of idiosyncratic adverse drug reaction.
Fig. 13.
Interaction of PMNs with the hemostatic system in the LPS/RAN model of liver injury: working hypothesis. LPS causes modest coagulation system activation and PAI-1 production, leading to fibrin deposition and hypoxia (Luyendyk et al., 2004). RAN augments fibrin deposition by enhancing coagulation system activation and PAI-1 concentration. LPS also causes PMN accumulation in liver, and RAN activates these cells, which release toxic PMN proteases (Fig. 8). The early hypoxia (2 h) is PMN-dependent (Fig. 7), whereas later hypoxia is enhanced by hemostasis. Hypoxia acts synergistically with PMN proteases to kill hepatocytes (Luyendyk et al., 2005). In addition to its direct killing of hepatocytes, PMN proteases increase active PAI-1 production and subsequent fibrin deposition (Fig. 12).
ABBREVIATIONS
- LPS
lipopolysaccharide
- RAN
ranitidine
- PMN
polymorphonuclear neutrophil
- PA
plasminogen activator
- PAI-1
plasminogen activator inhibitor-1
- Veh
vehicle
- ALT
alanine aminotransferase
- PBS
phosphate-buffered saline
- CS
control normal rabbit serum
- NAS
rabbit anti-rat PMN antiserum
- TAT
thrombin-antithrombin
- ELISA
enzyme-linked immunosorbent assay
- PIM
pimonidazole
- HOCl
hypochlorous acid
- PAGE
polyacrylamide gel electrophoresis
- ANOVA
analysis of variance
Footnotes
This work was supported by the National Institutes of Health (Grant DK061315) and by the Austrian Science Fund (Grants FWF P17013 and P19074-B05).
References
- Arteel GE, Thurman RG, Raleigh JA. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem. 1998;253:743–750. doi: 10.1046/j.1432-1327.1998.2530743.x. [DOI] [PubMed] [Google Scholar]
- Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer. 1995;72:889–895. doi: 10.1038/bjc.1995.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun NJ, Bodmer JL, Virca GD, Metz-Virca G, Maschler R, Bieth JG, Schnebli HP. Kinetic studies on the interaction of eglin c with human leukocyte elastase and cathepsin G. Biol Chem Hoppe Seyler. 1987;368:299–308. doi: 10.1515/bchm3.1987.368.1.299. [DOI] [PubMed] [Google Scholar]
- Conese M, Nykjaer A, Petersen CM, Cremona O, Pardi R, Andreasen PA, Gliemann J, Christensen EI, Blasi F. alpha-2 Macroglobulin receptor/Ldl receptor-related protein (Lrp)-dependent internalization of the urokinase receptor. J Cell Biol. 1995;131:1609–1622. doi: 10.1083/jcb.131.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple BL, Banes A, Ganey PE, Roth RA. Endothelial cell injury and fibrin deposition in rat liver after monocrotaline exposure. Toxicol Sci. 2002;65:309–318. doi: 10.1093/toxsci/65.2.309. [DOI] [PubMed] [Google Scholar]
- Copple BL, Rondelli CM, Maddox JF, Hoglen NC, Ganey PE, Roth RA. Modes of cell death in rat liver after monocrotaline exposure. Toxicol Sci. 2004;77:172–182. doi: 10.1093/toxsci/kfh011. [DOI] [PubMed] [Google Scholar]
- Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- Gorkun OV, Veklich YI, Medved LV, Henschen AH, Weisel JW. Role of the alpha C domains of fibrin in clot formation. Biochemistry. 1994;33:6986–6997. doi: 10.1021/bi00188a031. [DOI] [PubMed] [Google Scholar]
- Gorkun OV, Veklich YI, Weisel JW, Lord ST. The conversion of fibrinogen to fibrin: recombinant fibrinogen typifies plasma fibrinogen. Blood. 1997;89:4407–4414. [PubMed] [Google Scholar]
- Harada N, Okajima K, Murakami K, Uchiba M, Tanaka K, Okabe H, Takatsuki K. Leukocyte depletion and ONO-5046, a specific inhibitor of granulocyte elastase, prevent a stress-induced decrease in gastric prostaglandin I2 in rats. Biochem Biophys Res Commun. 1997;231:52–55. doi: 10.1006/bbrc.1996.6041. [DOI] [PubMed] [Google Scholar]
- Hill DA, Roth RA. α-Naphthylisothiocyanate causes neutrophils to release factors that are cytotoxic to hepatocytes. Toxicol Appl Pharmacol. 1998;148:169–175. doi: 10.1006/taap.1997.8314. [DOI] [PubMed] [Google Scholar]
- Ho JS, Buchweitz JP, Roth RA, Ganey PE. Identification of factors from rat neutrophils responsible for cytotoxicity to isolated hepatocytes. J Leukoc Biol. 1996;59:716–724. doi: 10.1002/jlb.59.5.716. [DOI] [PubMed] [Google Scholar]
- Ishii K, Ito Y, Katagiri H, Matsumoto Y, Kakita A, Majima M. Neutrophil elastase inhibitor attenuates lipopolysaccharide-induced hepatic microvascular dysfunction in mice. Shock. 2002;18:163–168. doi: 10.1097/00024382-200208000-00013. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Smith CW, Clemens MG, Ganey PE, Roth RA. Mechanisms of inflammatory liver injury: adhesion molecules and cytotoxicity of neutrophils. Toxicol Appl Pharmacol. 1996;139:213–226. doi: 10.1006/taap.1996.0160. [DOI] [PubMed] [Google Scholar]
- Keller TT, van der Sluijs KF, de Kruif MD, Gerdes VEA, Meijers JCM, Florquin S, van der Poll T, van Gorp ECM, Brandjes DPM, Buller HR, et al. Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ Res. 2006;99:1261–1269. doi: 10.1161/01.RES.0000250834.29108.1a. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yokoi-Hayashi K. Polymorphonuclear leukocyte lysosomal proteases, cathepsins B and D affect the fibrinolytic system in human umbilical vein endothelial cells. Biochim Biophys Acta. 1996;1310:1–4. doi: 10.1016/0167-4889(95)00161-1. [DOI] [PubMed] [Google Scholar]
- Lasne D, Jude B, Susen S. From normal to pathological hemostasis. Can J Anaesth. 2006;53:S2–11. doi: 10.1007/BF03022247. [DOI] [PubMed] [Google Scholar]
- Lluis JM, Morales A, Blasco C, Colell A, Mari M, Garcia-Ruiz C, Fernandez-Checa JC. Critical role of mitochondrial glutathione in the survival of hepatocytes during hypoxia. J Biol Chem. 2005;280:3224–3232. doi: 10.1074/jbc.M408244200. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Maddox JF, Cosma GN, Ganey PE, Cockerell GL, Roth RA. Ranitidine treatment during a modest inflammatory response precipitates idio-syncrasy-like liver injury in rats. J Pharmacol Exp Ther. 2003;307:9–16. doi: 10.1124/jpet.103.054288. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Maddox JF, Green CD, Ganey PE, Roth RA. Role of hepatic fibrin in idiosyncrasy-like liver injury from lipopolysaccharide-ranitidine coexposure in rats. Hepatology. 2004;40:1342–1351. doi: 10.1002/hep.20492. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Shaw PJ, Green CD, Maddox JF, Ganey PE, Roth RA. Coagulation-mediated hypoxia and neutrophil-dependent hepatic injury in rats given lipopolysaccharide and ranitidine. J Pharmacol Exp Ther. 2005;314:1023–1031. doi: 10.1124/jpet.105.087981. [DOI] [PubMed] [Google Scholar]
- Malle E, Hazell L, Stocker R, Sattler W, Esterbauer H, Waeg G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995;15:982–989. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- Malle E, Woenckhaus C, Waeg G, Esterbauer H, Grone EF, Grone HJ. Immunological evidence for hypochlorite-modified proteins in human kidney. Am J Pathol. 1997;150:603–615. [PMC free article] [PubMed] [Google Scholar]
- Nagatomi A, Sakaida I, Matsumura Y, Okita K. Cytoprotection by glycine against hypoxia-induced injury in cultured hepatocytes. Liver. 1997;17:57–62. doi: 10.1111/j.1600-0676.1997.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Conese M, Christensen EI, Olson D, Cremona O, Gliemann J, Blasi F. Recycling of the urokinase receptor upon internalization of the uPA: serpin complexes. EMBO J. 1997;16:2610–2620. doi: 10.1093/emboj/16.10.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima K, Harada N, Uchiba M, Mori M. Neutrophil elastase contributes to the development of ischemia-reperfusion-induced liver injury by decreasing endothelial production of prostacyclin in rats. Am J Physiol. 2004;287:G1116–G1123. doi: 10.1152/ajpgi.00061.2004. [DOI] [PubMed] [Google Scholar]
- Padró T, Steins M, Li CX, Mesters RM, Hammel D, Scheld HH, Kienast J. Comparative analysis of plasminogen activator inhibitor-1 expression in different types of atherosclerotic lesions in coronary arteries from human heart explants. Cardiovasc Res. 1997;36:28–36. doi: 10.1016/s0008-6363(97)00144-2. [DOI] [PubMed] [Google Scholar]
- Pintucci G, Iacoviello L, Amore C, Evangelista V, Cerletti C, Donati MB. Cathepsin G, a polymorphonuclear cell protease, affects the fibrinolytic system by releasing PAI-1 from endothelial cells and platelets. Ann N Y Acad Sci. 1992;667:286–288. doi: 10.1111/j.1749-6632.1992.tb51628.x. [DOI] [PubMed] [Google Scholar]
- Pintucci G, Iacoviello L, Castelli MP, Amore C, Evangelista V, Cerletti C, Donati MB. Cathepsin G–induced release of PAI-1 in the culture medium of endothelial cells: a new thrombogenic role for polymorphonuclear leukocytes? J Lab Clin Med. 1993;122:69–79. [PubMed] [Google Scholar]
- Renesto P, Ferrer-Lopez P, Chignard M. Interference of recombinant eglin C, a proteinase inhibitor extracted from leeches, with neutrophil-mediated platelet activation. Lab Invest. 1990;62:409–416. [PubMed] [Google Scholar]
- Schnitt SJ, Stillman IE, Owings DV, Kishimoto C, Dvorak HF, Abelmann WH. Myocardial fibrin deposition in experimental viral myocarditis that progresses to dilated cardiomyopathy. Circ Res. 1993;72:914–920. doi: 10.1161/01.res.72.4.914. [DOI] [PubMed] [Google Scholar]
- Snipes MB, Barnett AL, Harkema JR, Hotchkiss JA, Rebar AH, Reddick LJ. Specific biological effects of an anti-rat PMN antiserum intraperitoneally infected into f344/n rats. Vet Clin Pathol. 1995;24:11–17. doi: 10.1111/j.1939-165x.1995.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Veklich Y, Gorkun O. The sequence of cleavage of fibrinopeptides from fibrinogen is important for protofibril formation and enhancement of lateral aggregation in fibrin clots. J Mol Biol. 1993;232:285–297. doi: 10.1006/jmbi.1993.1382. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Jaffe EA, Brower MS, Cole OF. Human leukocyte cathepsin G and elastase specifically suppress thrombin-induced prostacyclin production in human endothelial cells. Blood. 1989;74:1627–1634. [PubMed] [Google Scholar]
- Wu K, Urano T, Ihara H, Takada Y, Fujie M, Shikimori M, Hashimoto K, Takada A. The cleavage and inactivation of plasminogen activator inhibitor type 1 by neutrophil elastase: the evaluation of its physiologic relevance in fibrinolysis. Blood. 1995;86:1056–1061. [PubMed] [Google Scholar]
- Yee SB, Hanumegowda UM, Hotchkiss JA, Ganey PE, Roth RA. Role of neutrophils in the synergistic liver injury from monocrotaline and bacterial lipopolysaccharide exposure. Toxicol Sci. 2003;72:43–56. doi: 10.1093/toxsci/kfg019. [DOI] [PubMed] [Google Scholar]