Abstract
MicroRNAs (miRNAs) are significant regulators of human hematopoietic stem cells (HSC), and their deregulation contributes to hematological malignancies. Myelodysplastic syndromes (MDS) represent a spectrum of hematological disorders characterized by dysfunctional HSC, ineffective blood cell production, progressive marrow failure, and an increased risk of developing acute myeloid leukemia (AML). Although miRNAs have been primarily studied in AML, only recently have similar studies been performed on MDS. In this review, we describe the normal function and expression of miRNAs in human HSC, and describe mounting evidence that deregulation of miRNAs contributes to the pathogenesis of MDS.
Keywords: microRNA, Myelodysplastic syndrome, microarray
INTRODUCTION TO MDS
Myelodysplastic syndromes (MDS) are heterogeneous hematopoietic stem cell (HSC) disorders characterized by persistent cytopenias due to ineffective hematopoiesis and dysplasia of marrow cells. As the disease progresses, patients may develop bone marrow (BM) failure. MDS patients are also at an elevated risk of transforming to acute myeloid leukemia (AML) [1]. Epidemiological studies have identified several environmental and occupational risk factors, however, the best-known predictor for de novo MDS is old age [2, 3]. Secondary MDS mainly occurs following chemotherapy (e.g., alkylating agents and topoisomerase inhibitors) and radiotherapy for unrelated cancers [3]. Despite the heterogeneous clinical presentation and variable outcome of MDS, many patients share underlying pathological features. These include peripheral blood cytopenias affecting one or more blood lineages (e.g., erythrocytes, granulocytes, monocytes, and/or megakaryocytes), and normal/hypercellular marrows with morphological dysplasia [4]. As per the diagnostic criteria, blasts account for less than 30% of the marrow.
The first line of treatment for low-risk MDS patients is supportive care and transfusions, but may also include growth factor treatment or immunosuppressive therapy. For advanced disease or high-risk MDS, patients are treated with immunosuppressive, epigenetic modifying drugs, chemotherapy, and/or undergo stem cell transplantation [5]. Despite recent progress in therapeutic options and the potential cure by stem cell transplantations, most MDS patients are not suitable candidates for transplantation, exhibit treatment-related toxicities, and/or can become refractory to treatment [6].
Classification of MDS
The most current classification of MDS, which was established by the World Health Organization (WHO), comprises eight subtypes based on biological, genetic, and morphological features [7]. As compared to previous iterations of MDS classification schemes, the current version of the WHO classification criteria distinguishes the cellular lineages involved and incorporates cytogenetic alterations. The WHO subtypes of MDS include refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), refractory anemia with multilineage dysplasia (RCMD), refractory anemia with multilineage dysplasia and ringed sideroblasts (RCMD-RS), refractory anemia with excess blasts-1 (RAEB-1), refractory anemia with excess blasts-2 (RAEB-2), myelodysplastic syndrome unclassified (MDS-U), and MDS associated with isolated deletion of chromosome 5q (del(5q); or 5q- syndrome). A distinct category encompasses the myelodysplastic/myeloproliferative neoplasms including chronic myelomonocytic leukemia (CMML).
Risk stratification is also used to guide clinicians for predicting outcome and selecting the appropriate therapy. Risk assessment and prognosis is defined by the International Prognostic Scoring System (IPSS) [8], and has been divided into four risk categories: low-risk (median survival of 97 months), intermediate-1 risk (INT-1; median survival of 63 months), intermediate-2 risk (INT-2; median survival of 26 months), and high-risk (median survival of 11 months). For patients with low-risk MDS, treatments are designed to improve quality of life and minimize transfusions, while for those with higher risk disease, the therapy is aimed at altering the natural progression of the disease and delaying transformation to AML.
WHO and IPSS both rely on the number and type of cytopenias to assign classification or risk, respectively. Unlike the WHO criteria, the IPSS system also utilizes cytogenetic information to calculate risk. Unbalanced cytogenetic alterations are detected in 40–50% of MDS patients, and submegabase alterations are observed in another ~20% of patients [9–12]. Common genetic alterations, such as deletion of chr 5q (del(5q)), monosomy 7 (mono 7), trisomy 8 (tri 8), and del(20q), are useful predictors of disease risk and serve as guides for treatment strategy [13]. For example, sole deletion of chr 5q is associated with a favorable prognosis and patients respond to Lenalidomide [14], while del(17p) is associated with transformation to AML and poor prognosis [15]. Despite the strong correlation between specific cytogenetic alterations and disease outcome, a large proportion of MDS patients have no detectable cytogenetic alterations. In these cases, other predictors of disease progression are needed.
Cellular Basis of MDS
The mechanism underlying the initiation and progression of MDS is poorly understood. Despite this gap in knowledge, MDS is widely accepted to originate in primitive hematopoietic stem cells (HSC) that have acquired mutations [16]. The cell of origin has been immunophenotypically described, and has been shown to reside within the CD34+CD38−Thy1+ HSC fraction [17–19]. Early stages of MDS include the accumulation of dysfunctional hematopoietic stem/progenitor cells (HSPC), likely accounting for the observed ineffective hematopoiesis and eventual marrow failure. As the disease progresses, the abnormal HSPC can acquire additional genetic alterations which can result in overt leukemia. Collectively, the cell intrinsic defects of HSPC contribute to the pathological features of MDS, which may lead to the acquisition of additional mutations that contribute to leukemic transformation.
Ineffective hematopoiesis of the normal HSC compartment is also evident and is attributed to niche competition or paracrine factors from the MDS-derived cells. In support of this interpretation, dysplastic cells are detectable in the cytogenetically normal and abnormal proportions of cells co-occupying the marrow of an MDS patient [20, 21]. Terminally differentiated cells from MDS-derived clones exhibit increased basal apoptosis and clearance from the circulation [22]. It has been demonstrated that the enhanced death of hematopoietic cells from MDS patients is due to hyperactivated extrinsic and intrinsic apoptotic signaling. For example, progenitors from the marrows of MDS patients overexpress CD95 and TRAIL death receptors, both of which mediate the caspase-dependent apoptotic pathway [23–25]. Several apoptosis-inducing ligands, such as TNFα, Fas ligand, and TRAIL are elevated in early stage/low-risk MDS marrow [24, 26–28]. In 5q- syndrome patients, the macrocytic anemia is attributed to a p53-dependent apoptosis program within the erythroid progenitor compartment [29–32]. Other indicators of an imbalance in apoptotic signals include altered expression of anti-and pro-apoptotic Bcl-2 family members. In RA/RARS CD34+ marrow cells, a direct correlation between apoptosis and expression of pro-apoptosis proteins, Bax and Bad, was observed. Conversely, these patients exhibit reduced expression of anti-apoptotic proteins, Bcl-2 and Bcl-XL, indicating a shift toward apoptosis [33–35]. Despite peripheral blood cytopenias, MDS patients paradoxically have a normal or hypercellular marrow and few immature blasts. Once the blast counts exceed 20% of the marrow, the outcome is poor as the patients will rapidly transform to AML [36]. The mechanisms by which patients progress from MDS to overt AML are still under intense investigation. However, as with de novo AML, AML secondary to MDS exhibits similar key pathological cellular features, such as a differentiation block, high proliferation rates, and increased cell survival. Part of the transition from MDS to AML is thought to result from a shift from pro- to anti-apoptotic signals. For example, marrow cells from low-risk MDS have higher expression of Bax and Bad. In contrast, high-risk MDS and AML patients exhibit downregulation of Bax and Bad and a concomitant increase in Bcl-2 and Bcl-xL [37]. Knowledge gained from identifying genetic networks that contribute to the transformation of MDS to AML will provide hope for new therapeutic strategies to delay or prevent progression to overt leukemia, and extend life expectancy.
Molecular Basis of MDS
The most common cytogenetic alterations are unbalanced chromosomal amplifications and deletions, and new insights have been made toward the elucidation of how these large regions contribute to the pathogenesis of MDS at a molecular level. Although deletions of chr 5q span several megabase pairs, two distinct commonly deleted regions (CDR) were identified. A proximal CDR at 5q31 is associated with more aggressive forms of MDS and AML, and several genes located at this band have been implicated in aspects of MDS and AML pathogenesis. In contrast, the distal CDR, which involves band q33 and contains 40 protein-coding genes and three miRNAs, is associated with indolent forms of MDS [4, 13]. Among the protein coding genes, ribosomal protein S14 (RPS14) has been demonstrated to be the causal gene for erythroid hypoplasia in 5q- syndrome, as is supported by a knockout mouse that includes deletion of the RPS14 locus (among 8 other neighboring loci within the deleted segment) [29]. These mice exhibited macrocytic anemia, ineffective erythropoiesis, and increased apoptosis of erythroid progenitors. The erythroid defect could be reversed when the mice were crossed with p53 knockout mice, suggesting that the anemia results from apoptotic pathways imposed by RPS14-mediated induction of p53. Recently, two miRNAs, miR-145 and miR-146a, located near 5q33, have been shown to be downregulated in HSPC in MDS patients with del(5q). Knockdown of these miRNAs using a miRNA decoy approach in mouse HSPCs contributes to other features of 5q- syndrome, such as dysplastic megakaryocytes, thrombocytosis, and evolution to AML [38, 39]. Conversely, overexpression of miR-145 or miR-146a in mouse HSPC or human AML cell lines results in impaired growth and increased apoptosis [39, 40]. Consistent with the phenotype of the miR-145/miR-146a decoy mice, overexpression of the miR-146a mRNA target, TRAF6, encoding an adaptor protein with E3 ubiquitin ligase activity, also induces marrow failure or AML in a mouse model [38].
Somatic nucleotide substitutions resulting in nonsense and missense mutations have recently been identified in MDS patients. Deletions or inactivating mutations of c-Cbl, a negative regulator of receptor tyrosine kinase, have been demonstrated to induce growth factor-independent proliferation and to be associated with poor survival [41, 42]. Epigenetic changes have also been implicated in the pathogenesis and treatment of MDS [43–45]. Whole genome scanning for DNA methylation patterns in MDS patients revealed a global hypomethylation signature, which is associated with rapid transformation to AML and poor prognosis [46]. In support of these observations, TET2, a putative epigenetic regulator, which normally converts 5-methylcytosine to 5-hydroxymethylcytosine, is frequently deleted or inactivated in MDS and MPN patients and results in defective conversion of 5-methylcytosine [47]. The contribution of TET2 inactivation to MDS has been verified by the rapid onset of myeloproliferative and MDS features in TET2 knockout mice [48–50]. Other examples of epigenetic regulators implicated in MDS include ASXL1, a polycomb-associated gene involved in chromatin remodeling that acquires mutations in high-risk MDS and AML patients [51, 52].
Insufficient knowledge about the basic biology of MDS continues to impede effective treatment strategies. For some cellular defects identified in MDS patients, the genetic and/or epigenetic causes is known (e.g., TET2 inactivation) and future therapies might be on the horizon; however, for others the molecular basis is not yet identified (e.g., regulation of pro- and anti-apoptotic signals in MDS HSPC). It is possible that deregulation of miRNAs, which directly or indirectly impact multiple genes, is a mechanism that contributes to the unexplained molecular and cellular defects associated with MDS.
BIOGENESIS AND FUNCTIONS OF MIRNA
Recent and encouraging advances, including the discovery of deregulated miRNAs in MDS, have improved our understanding and provided novel potential clinical targets for MDS. miRNAs are short endogenous RNA molecules found in most eukaryotic cells [53]. miRNAs function by binding to target mRNA transcripts at complementary sequences and inducing gene-specific mRNA degradation and/or protein translation inhibition [54]. Some miRNA genes are intragenic and others are expressed from independent promoters by RNA Polymerase II [55, 56]. The miRNA transcript contains a 5’ capped end and a 3’ polyadenylated tail [57]. Once the transcript is spliced, it is referred to as a primary miRNA (pri-miRNA). The RNase III–type enzyme, Drosha, processes these pri-miRNAs, yielding hairpin precursors (pre-miRNAs). Pre-miRNA hairpins are then exported to the cytoplasm by Exportin-5 to be further processed into 19–25 nucleotide miRNA duplex structures by the RNase III protein Dicer [58]. Although either strand of the duplex may function as the miRNA, only one strand is thought to be incorporated into the RNA-induced silencing complex (RISC) [53]. This complex is the site of interaction for the miRNA and its mRNA target. The specificity of each miRNA is attributed to the seed sequence, which includes nucleotide position 2–8 of the mature miRNA. The seed sequence binds with perfect complementarity within target mRNAs. If the miRNA and its target have perfect nucleotide complimentarity across the entire miRNA, the target mRNA will be degraded [59]. In contrast, imperfect nucleotide complementarity (except for the seed region) results in translation repression of the target protein.
MIRNA IN HUMAN HEMATOPOIETIC STEM CELLS
Hematopoiesis is a tightly regulated process of maintenance, lineage commitment, proliferation, differentiation, and survival of HSCs and their progenies [60]. A network of key cytokines and transcription factors has been shown to concordantly control hematopoiesis; however, an imbalance of this process is associated with hematological malignancies. Recently, accumulating evidence suggests that miRNAs are of key importance in normal and malignant hematopoiesis [61, 62]. Using a combination of miRNA microarray platforms and next generation sequencing approaches, select miRNAs have been demonstrated to be specifically expressed in hematopoietic tissues. Many reviews now exist that describe the role of miRNAs in mouse hematopoiesis; by comparison, much less is known about the role of miRNAs in human HSC and hematopoietic differentiation.
Different cell populations in human BM have been utilized to identify miRNAs relevant to human HSC function. In one example, CD34+cells, which consist of stem and progenitor cells, were isolated from BM and mobilized blood and used to estimate the abundance of miRNAs based on fluorescent intensity of microarray probes [63]. The reported miRNAs abundantly expressed in CD34+ cells include, but not limited to, Let-7b, miR-10a, miR-25, miR-26 family, miR-128a, miR-146, miR-155, miR-181a, miR-222, and miR-223. A second study examined the relative differences in miRNA expression between more primitive and differentiated subsets of CD34+ cells: CD34+CD38− and CD34+CD38+ cells [64]. It was shown that miR-127, miR-365, miR-452, and miR-520h were highly expressed and miR-129, miR-19a, miR-142-3p, and miR-181a were most underexpressed in the more primitive CD34+CD38−HSC [64]. The miR-125 family has also been implicated in HSC function. Ooi et al. (2011) identified increased expression of miR-125b in lin−CD34+CD38−CD90+CD45RA− HSC and lin-CD34+ CD38−CD90−CD45RA− multipotent progenitors as compared to myeloid progenitor populations (lin−CD34+CD38−CD123+CD45RA−[CMP] and lin−CD34+CD38−CD123+CD45RA+ [GMP])[65]. miR-125 is thought to protect HSC from cell death, as expression of miR-125a in mouse HSC has been shown to reduce apoptosis through targeting Bak1 in vivo [66]. Differences in miRNA expression between umbilical cord (UC) and adult BM CD34+ cells were also investigated [67]. Thirteen miRNAs were differentially expressed between UC and BM CD34+ cells: miR-548d, miR-203, miR-1, miR-34a, miR-545, let-7b, miR-429, miR-214, miR-195, miR-520h, miR-518a, miR-519d, and miR-517c.
MIRNA IN HEMATOPOIETIC MALIGNANCIES
Given the significance of miRNAs during hematopoiesis, it is not surprising that expression of miRNAs is also perturbed in hematological malignancies. Abnormal miRNA expression has been associated with cytogenetic alterations in hematopoietic malignancies. The earliest evidence of altered miRNA expression due to a cytogenetic alteration comes from work in chronic lymphocytic leukemia (CLL). Two miRNAs, miR-15a and miR-16-1, were identified as tumor suppressors at 13q14, a region commonly deleted in CLL [68]. Deletion and down-regulation of miR-15a and miR-16-1 results in derepression of Bcl-2, indicating their tumor suppressor potential in the pathogenesis of CLL [69]. Interestingly, inspection of human genomes revealed that approximately half of all miRNA genes are located at fragile sites and regions of copy number alteration associated with cancer [39, 70]. This observation was confirmed by expression profiling of human cancers, and revealed that miRNAs are significantly deregulated and can reproducibly classify and distinguish human cancers [71]. Corroborative studies by Mi et al. (2007) described only 4 miRNAs that distinguished ALL and AML [72]. They found increased miR-128a/b, and decreased Let-7b and miR-223, in ALL as compared to AML. Minimal number of miRNAs have also been identified that discriminate AML subtypes: miR-126/126* is associated with core binding factor (CBF) mutations; miR-224, miR-368, and miR-382 are associated with acute promyelocytic leukemia (APL) fusions; and, miR-17–92 polycistron is associated with mixed lineage leukemia (MLL)-fusions [73, 74]. In young AML patients, a distinct miRNA signature was associated with risk and survival. In this study, miR-124, miR-128-1, miR-194, miR-219-5p, miR-220a and miR-320 levels were positively correlated to risk, while miR-181a/b levels were inversely correlated to risk [75]. Balanced chromosomal translocations have also been shown to impact expression levels of miRNA. In one example, chromosomal translocation t(15;17) in APL correlates with increased expression levels of miR-382, miR-134, miR-376a, miR-127, miR-299, and miR-323 [76]. In AML subtypes with t(8;21) and inv(16), miRNAs Let-7b and Let-7c were decreased [76]. Moreover, certain miRNAs are associated with or directly regulated by key leukemia-associated molecular alterations. For example, expression of FLT3-ITD in AML is associated with inducing the expression of miR-155 [77]. A mutant version of NPM1 is associated with increased expression of miR-10, and decreased expression of miR-127, miR-193, and miR-299-5p [76]. Similarly, mutant CEBPα correlates with miR-335 and miR-181a expression [76].
MIRNA IN MDS
As compared to miRNA-related studies performed in AML, unfortunately, far fewer investigations have been carried out to study miRNAs in MDS. The hypothesis that deregulation of miRNAs can contribute to MDS is supported by global miRNA expression analysis on large MDS patient cohorts. These key studies are described below and summarized in Tables 1 and 2, and in Fig. (1).
Table 1.
miRNAs Differentially Expressed Between MDS and Control.
| Study | Cell Source |
Experimental Method |
miRNAs Upregulated Compared to Control |
mRNAs Downregulated Compared to Control |
Experimental Group |
Control |
|---|---|---|---|---|---|---|
| Sokol | BM MNC |
microarray | miR-222, miR-10a | miR-146a, miR-150, Let-7e | Low or INT-1 | Normal |
| Erdogan | BM MNC |
microarray | miR-10b, miR-365, miR-558, miR-636 | miR-23a, miR103, miR- 126, miR-140, miR-150, miR-342, miR-378, miR- 483, miR-632, miR-639 |
Low | Normal |
| Hussein | BM MNC |
qRT-PCR | miR150 | 5q- syndrome | Normal | |
| Pons | BM MNC |
qRT-PCR | miR-17-3p, miR-17-5p, miR-18a, miR-10a, miR-10b, miR-15a, miR-16, miR-222, miR- 155, miR-181a, miR-21, miR-126 |
Multiple Risk Groups |
Normal | |
| Pons | PB | qRT-PCR | miR-17-3p, miR-17-5p, miR-18a, miR-15, miR-21, miR-142-3p |
Multiple Risk Groups |
Normal | |
| Merkerova | CD34+ | microarray | miR-299-3p, miR-299-5p, miR-323-3p, miR-329, miR-370, miR-409-3p, miR-431, miR-432, miR-494, miR-654-5p, miR-665 |
miR-196a*, miR-423-5p, miR-525-5p, miR-507, miR-583, miR-940, miR- 1284, miR-1305 |
Multiple Risk Groups |
Normal |
| Hussein | BM MNC |
Low density qPCR array |
miR-96, miR-339, miR- 182, miR-93, miR-25, miR- 106b, miR-196b, miR-550, miR-148a, miR-29a |
MDS with or without del(7) |
Normal | |
| Starczynowski | BM MNC |
qRT-PCR | miR-145, miR146a | del(5q) | Normal and MDS dip(5q) |
|
| Votavova | CD34+ | qRT-PCR | miR146a | del(5q) | Normal | |
| Votavova | CD34+ | High density qPCR array |
miR-34a, miR-148a, miR-199b, miR-451, miR-486, miR-125a, miR-151, miR-199a, miR-10a, miR-10b, miR-125b, miR-29c, miR-130a, miR-24, miR-126, miR-335, miR-99b |
miR-128b, miR-95, miR- 213, miR-520c |
5q- syndrome | Normal |
| Beck | BM MNC |
Sequencing | miR-376a-2, miR-376a-1, miR-376c, miR- 144, miR-374b*, miR-374a*, miR-126*, miR-17, miR-106a*, miR-10b, miR-10a*, miR-20a, miR-598*, miR-30d, miR-20b* |
RA | Normal | |
| Beck | BM MNC |
Sequencing | miR-127, miR-10b, miR-134, miR-155, miR-125b-2, miR-144, miR-1974**, miR- 1973**, miR-17, miR-20a, miR-139, miR- 30d, miR-216b, miR-19b-2*, miR19b-1 |
RAEB2 | Normal |
BM, bone marrow; MNC, mononuclear cells; PB, peripheral blood; Low, low-risk; INT, intermediate risk; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RCMD, refractory cytopenia with multilineage dysplasia
Table 2.
miRNAs Differentially Expressed Between MDS Subtypes.
| Study | Cell Source |
Experimental Method |
miRNAs Upregulated Compared to Control |
mRNAs Downregulated Compared to Control |
Experimental Group |
Control |
|---|---|---|---|---|---|---|
| Pons | BM MNC |
qRT-PCR | miR-15a | High Risk | Low Risk | |
| Pons | PB | qRT-PCR | miR-16 | High Risk | Low Risk | |
| Sokol | BM MNC |
microarray | miR-181a/b/c/d, miR-221, miR-376b, miR-125b, miR-155, miR-130 |
miR-486-5p | High Risk | Low Risk |
| Hussein | BM MNC |
Low density qPCR array |
miR-383 | miR-143, miR-378, miR-93, miR-25, miR-106b, miR- 196b,miR-550 |
RA/RCMD Trisomy 8 |
RA/RCMD Normal Karyotype |
| Hussein | BM MNC |
Low density qPCR array |
miR-1/miR-133a–cluster, miR-133b/miR- 206-cluster, miR-199a/miR-214-cluster, miR-125a, miR-342, miR-452, miR-497 |
del(5q) | RA/RCMD Normal Karyotype |
|
| Starczynowski | BM MNC |
qRT-PCR | miR-145, miR146a | del(5q) | Normal and MDS dip(5q) |
|
| Bousquet | BM MNC |
qRT-PCR | miR-125b-1 | with t(2:11)(p21:q23) |
without t(2:11)(p21:q23) |
|
| Beck | BM MNC |
Sequencing | miR-451, miR-98*, miR-29a*, miR-29c*, miR-29b-2*, miR-29b-1*, miR-16-1-*, miR-195*, miR-191*, miR-26-a-2*, miR- 181b-2, miR-181-b-1, miR-23b, miR101- 1*, miR-221* |
RA | RAEB2 |
BM, bone marrow; MNC, mononuclear cells; PB, peripheral blood; Low, low-risk; INT, intermediate risk; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RCMD, refractory cytopenia with multilineage dysplasia
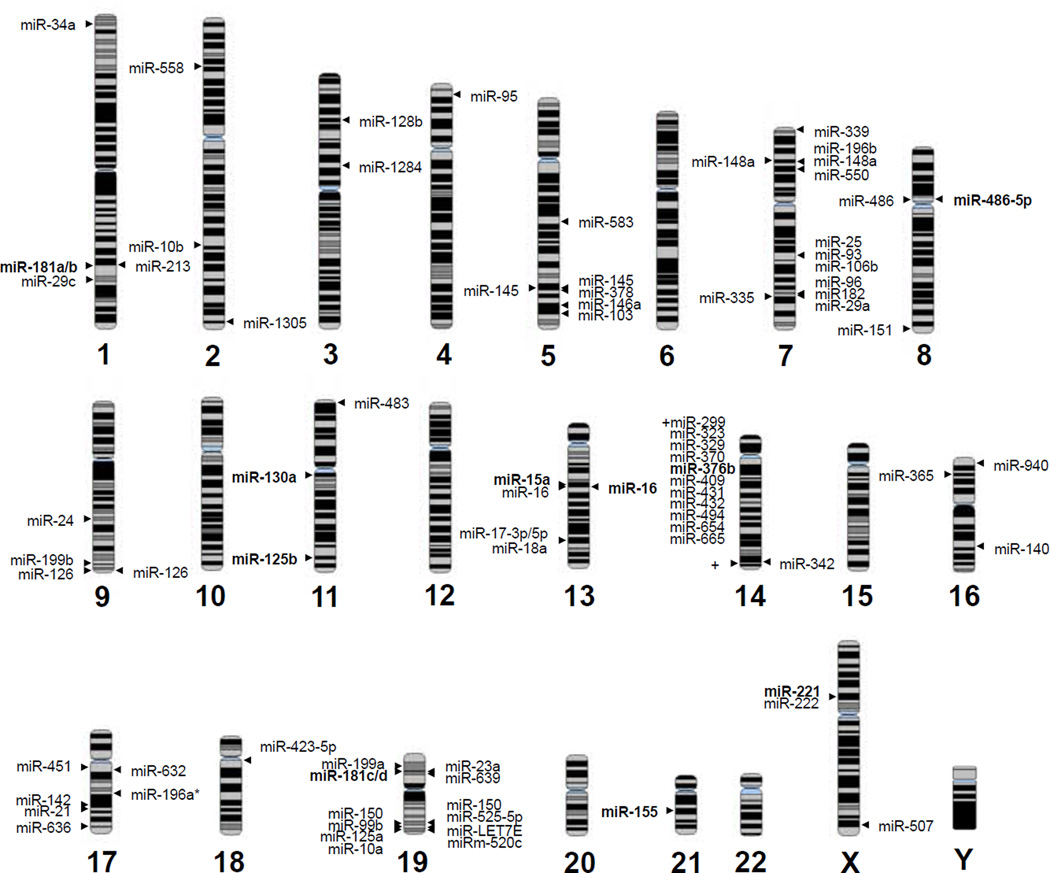
Fig 1. Chromosome position of miRNAs implicated in MDS.
miRNAs listed to the left of the chromosome indicate overexpressed miRNAs in MDS patients as compared to normal controls. miRNAs listed to the right of the chromosome indicate downregulated miRNAs in MDS patients as compared to normal controls. Bolded miRNAs indicate miRNAs overexpressed or downregulated in high-risk MDS as compared to low-risk MDS. Certain miRNAs are duplicated if reported to be up and down regulated in MDS patients.
MDS Versus Controls
Diagnosis and prognosis of MDS is most challenging for patients categorized as low- and INT-1 risk, therefore miRNA bio-markers in this cohort of MDS patients will be beneficial. One of the largest studies to examine differential miRNA expression in low- or INT-1 risk MDS patients was performed by Sokol et al. (2011) [78]. Of the 44 patients examined, 32 were classified as low-risk or INT-1, and as expected, the majority (21/32) exhibited normal cytogenetics. Using a miRNA array approach, the expression profile from BM mononuclear cells was examined in the 44 MDS patients and 17 control samples. A 13 miRNAs signature was identified that distinguished MDS patients from age-matched controls. Notably, high levels of miR-222, miR-10a, and low levels of miR-146a, miR-150, and Let-7e were reported (Table 1, Fig. (1)). Several of these miRNAs have been implicated in regulating normal and malignant hematopoiesis. For instance, miR-146a has been demonstrated to be important in HSPC self-renewal and lineage commitment [40, 79, 80], in part by regulating its targets TRAF6, IRAK1, and/or TLR4 [38, 39, 81, 82]. The Let-7 family has been shown to target several key factors in tumorigenesis, such as RAS and MYC [83, 84]. miR-222 and miR-10a modulate erythroid and megakaryocytic maturation [85, 86]. These data suggested that dysregulation of these miRNAs form a network in the pathogenesis (dyshematopoiesis) and clinical manifestation (cytopenia) of MDS.
Erdogan and colleagues also examined primarily low-risk MDS patients [87]. Using mononuclear BM cells, miRNA expression was examined by Agilent miRNA arrays from 10 MDS patients and 10 controls, as part of the initial discovery set (Table 1). Further validation of classifier miRNAs was performed on 24 MDS and 25 control BM samples collected from formalin-fixed paraffinembedded clot sections. This study identified 13 miRNAs from the initial discovery set that were differentially expressed between MDS and normal samples, of which, 8 were statistically significant in the validation group (Table 1 and Fig. (1)). Expression of miR-103, miR-140, miR-150, miR-342, miR-378, miR-483, miR-632, and miR-636 demonstrated the highest discriminatory power between low-risk MDS patients and control samples. Despite similar patient cohorts, unfortunately only two common miRNA (miR-150 and miR-10) were identified in the Sokol and Erdogan studies (Table 1).
In another retrospective study, 52 MDS patients, 11 secondary AML patients derived from MDS, and 20 controls were evaluated [88]. BM samples were collected at diagnosis, and miR-221, miR-222, miR-150, and miR-155 expression was evaluated by quantitative real-time (qRT) PCR. miR-150 was significantly increased in 5q- syndrome samples as compared to controls (Table 1), and also correlated with a favorable karyotype. In contrast, miR-221 did not significantly differ between MDS and control samples. However, down-regulation of miR-221 was observed in secondary-AML as compared to MDS, suggesting a role in the progression of the disease. Furthermore, transcript levels of MYB and p27, which are predicted targets of miR-150 and miR-221, respectively, were inversely correlated with their corresponding targeting miRNA. Although forced expression of miR-155 in murine HSCs results in MDS/MPN phenotype in vivo, this report did not detect abnormal expression of miR-155 in the MDS samples.
In another report, fresh mononuclear cells from MDS patients or controls were used to assay 25 preselected miRNA by qRT-PCR [92]. Of the miRNAs examined, 12 were found to be increased in BM MDS samples as compared to controls: miR-17-3p, miR-17-5p, miR-18a, miR-10a, miR-10b, miR-15a, miR-16, miR-222, miR-155, miR-181a, miR-21, and miR-126 (Table 1 and Fig. (1)). Among them, 6 miRNA were elevated in PB MDS patients versus controls: miR-17-3p, miR-17-5p, miR-18a, miR-15, miR-21, and miR-142-3p. miR-142-3p was increased in PB MDS, but not in BM MDS samples. As mentioned above, miR-17–92 polycistron has been shown to be oncogenic and deregulated in other hematological malignancies; therefore, elevated levels of miR-17–92 family members might play a role in the progression of MDS to AML. Two miRNAs, miR-181a and miR-222, are significantly increased at the early-stages of MDS compared to controls, and then increase further as the disease progresses. This result suggests a biomarker potential of miR-181a and miR-222 for the diagnosis and prognosis for MDS and secondary AML.
Since the CD34+ population is implicated as containing the abnormal HSCs in MDS, the information drawn from such studies may yield more knowledge about intrinsic HSC defects. In a study by Dostalova Merkerova et al. (2011), miRNAs were isolated from CD34+ BM cells from 43 MDS patients and 9 healthy donors [93]. The patient cohort consisted of RA/RARS (n = 5), RCMD (n = 6), del(5q) (n = 6), RAEB (n = 17) and secondary AML (n = 9). As compared to normal controls, 22 miRNAs were differentially expressed across all the MDS subtypes using an Illumina array platform (Table 1 and Fig. (1)). Of which, 11 miRNA were upregulated (miR-299-3p, miR-299-5p, miR-323-3p, miR-329, miR-370, miR-409-3p, miR-431, miR-432, miR-494, miR-654-5p, and miR-665) and 8 were downregulated (miR-196a*, miR-423-5p, miR-525-5p, miR-507, miR-583, miR-940, miR-1284, and miR-1305). As with the other reports, hierarchical clustering of 22 differentially expressed miRNAs distinguished MDS patients from the normal controls.
Risk Stratification and WHO Classification
As described above, miRNA signatures can effectively discriminate MDS from control marrow samples. Thus, it is not surprising that certain miRNAs are also associated with MDS subtypes and risk groups. For example, Dostalova Merkerova and colleagues grouped patients into 5 common MDS categories [93]. Hierarchical clustering of 45 differentially expressed miRNAs distinguished 5q-syndrome, low-risk MDS (RA, RARS, and RCMD), high-risk MDS (RAEB−1/−2), and AML [93]. When applying IPSS risk stratification, miR-15a levels in BM samples were correlated to high-risk MDS, while miR-16 levels in PB were inversely associated with high-risk MDS [92] (Table 2). In a similar study, a signature consisting of 10 miRNAs was able to discriminate low- versus high-risk MDS patients [78] (Table 2). Of which, 9 miRNAs (miR-181a/b/c/d, miR-221, miR-376b, miR-125b, miR-155, and miR-130) were up-regulated and one miRNA (miR-486-5p) was down-regulated in higher risk (INT-2 and high-risk) MDS versus lower risk (low-risk and INT-1) MDS. In support of a pathological role of miR-155, a mouse model in which miR-155 is overexpressed in HSPC, resulted in expansion of granulocyte-monocyte progenitors resembling a myeloproliferative disease [94]. One explanation for this phenotype is due to abnormal regulation of myeloid transcription factors PU.1, C/EBPβ and CSF1R by miR-155 [94]. Notably, in this study 4 miR-181 family members fall into the MDS-associated signature, and correlate with overall survival. This result is consistent with another report that showed miR-181 levels increasing in advanced MDS and sAML [92]. Taken together, the miR-181 family expression may be a useful diagnostic marker for MDS risk stratification, as well as an indicator of MDS to AML transformation.
Unbalanced Cytogenetics: Chromosomes 5, 7, and 8
The most common cytogenetic alterations in MDS are deletions of chr 5q and 7, and trisomy chr 8. In one of the original miRNA expression studies in MDS, miRNAs were collected from unsorted BM cells from 24 MDS patients and 3 control samples and evaluated by a low-density real-time PCR-based array[95]. Cluster analysis revealed distinct miRNA signatures from those with cytogenetic abnormalities compared to those with normal karyotype even within a single MDS subtype. For instance, RA/RCMD with a normal karyotype expressed 7 unique miRNAs as compared to RA/RCMD with trisomy 8, all of which are downregulated in trisomy 8 group (Table 2). Among the 8 miRNAs that reside on chr 8, only miR-383 level increased with trisomy 8 in the RA/RCMD patients (Table 2). This result indicated that gains of chr 8 did not accordingly increase the level of most miRNAs within this chromosome, except for miR-383. Loss of the entire chr 7 (del(7)) is another common cytogenetic alteration. Thirteen miRNAs are encoded by chr 7, most of which were down-regulated in MDS patients with del (7) (Table 2). A pathological role of these miRNAs is supported by the observation that these same miRNAs are also downregulated in MDS with diploid chr 7.
The majority of miRNA expression studies have focused on miRNAs residing on chr 5q. We have previously examined all annotated miRNAs residing with the del(5q) region in MDS patients for abnormal expression patterns [38]. Of the 13 miRNAs that are found on chr 5q, expression of miR-145 and miR-146a was significantly reduced in BM cells from del(5q) MDS patients as compared to controls and MDS patients diploid at chr 5q [38] (Table 2). Three independent groups have also reported that miR-145 and/or miR-146a are reduced in CD34+ or whole marrow cells from MDS patients [96–98], however differences in expression of these miRNAs were not reported by Boultwood et al. (2007) and Hussein et al. (2010) [95, 99] (Table 2). The first evidence that genetic modification of miRNAs in HSPC can induce MDS-like features was shown in mice with reduced levels of miR-145 and miR-146a [38]. Knockdown of miR-145 and miR-146a using a miRNA decoy approach in mouse HSPC resulted hematological abnormalities including elevated platelets, mild neutropenia, megakaryocytic dysplasia, and a long-latency myeloid leukemia. Germline deletion of the miR-146a locus has also recently been described, and results in immune dysfunction and hematological malignancies [79, 80, 100]. Knockout of miR-146a results in an early onset of myeloid expansion in the marrow, and progression to more aggressive diseases such as lymphomas, marrow failure, and myeloid leukemia. miR-146a knockout mice do not show evidence of elevated platelets suggesting that loss of miR-145 may contribute to thrombocytosis associated with del(5q) MDS patients. A more in-depth analysis to dissect the precise roles of miR-145 and miR-146a is required.
Global analyses of deregulated miRNAs in 5q- syndrome patients have also been performed utilizing high-density Taqman arrays (Table 1). In one report, 21 differentially expressed miRNAs were detected in 5q- syndrome patients (n = 7) as compared to normal (n = 5) CD34+ BM samples [96]. Of these, 17 miRNAs were found to be overexpressed in del(5q) CD34+ cells, while 4 were found to be underexpressed. As expected, most of these miRNAs did not reside on chr 5q. Most strikingly, miR-34a (1p36.22) was overexpressed in del(5q) patient samples by 12-fold. The role of miR-34a in MDS may be partly explained by its previously described function, which includes mediating apoptosis by directly inhibiting expression of BCL2 [101]. Alternatively, miR-34a expression may be a marker of stressed HSPC given that miR-34a has been shown to be a direct pro-apoptotic target of p53 [102]. In addition, Votavova et al. (2010) identified overexpression of miR-223 (Xq12), miR-199a (19p13), and miR-335 (7q32.2). Lower expression of miR-128b (3p22.3) and miR-520c (19q13.42) were also observed in 5q- syndrome patients. In accordance with two other studies, increased expression of miR-199a, miR-125a/b, and miR-10a/b was detected in del(5q) MDS samples [95, 103]. Differentially expressed miRNAs also mapped to or adjacent to commonly deleted regions on chr 5q. While miR-143, miR-145, and miR-378 showed non-significant differences, miR-146a was significantly reduced in 5q- syndrome patient. Paradoxically, miR-146a was upregulated in circulating granulocytes of del(5q) MDS patients, while miR-143 was downregulated. Overall, it is not surprising that few overlapping differentially expressed miRNAs in del(5q) MDS patients were identified between the Votavova and Heussein studies as different cellular populations were used as a source of RNA. Nevertheless, deregulated miRNAs in del(5q) MDS patients, whether they reside on chr 5q or not, are widely reported.
Translocations: t(2;11)(p21;q23)
Balanced chromosomal alterations in MDS patients are rare. One reported chromosomal translocation in MDS is t(2;11) (p21;q23) [104, 105]. Notably, the t(2;11)(p21;q23) translocation does not result in a novel gene fusion. To clarify the mechanism of t(2;11)(p21;q23) in MDS/AML pathogenesis, Bousquet, et al. (2008) studied 19 AML or MDS patients with t(2;11)(p21;q23), and found a striking elevation of miR-125b-1 compared to AML and MDS patients without t(2;11)(p21;q23) and control group [103] (Table 2). Given that miR-125b-1 is located near the breakpoint region of chromosome 11, elevation of miR-125b-1 provides evidence that miRNA dysregulation is more frequent at fragile sites within the genome. In vitro, ectopic miR-125b-1 significantly blocked myeloid differentiation of leukemic cells as well as CD34+ primary cells. This study indicated that the leukemogenic potential of t(2;11)(p21;q23) is mediated by miR-125b-1. In support of this finding, miR-125a and miR-125b belong to the miR-125 family and have identical seed regions. Interestingly both miRNAs are preferentially enriched in human HSCs as compared to committed hematopoietic BM cells, and confer a competitive advantage to engrafting human hematopoietic cells in mice [106]. Similar findings were reported in mice; overexpression of miR-125b resulted in a survival advantage of immature HSCs, myeloproliferative disorder and progression to a fatal myeloid leukemia in mice [65, 66, 106].
Next Generation Sequencing of miRNAs
Besides microarray based assays for large-scale profiling of miRNAs, next generation sequencing also provides an alternative way to investigate miRNA expression levels and identify unique miRNA variants. Applying next-generation sequencing technology, Beck et al. (2011) used fresh BM cells from low- and high-risk MDS patients and controls [107]. As compared to control samples, the expression of 15 miRNAs was increased in low-risk MDS (Table 1 and 2). Interestingly, half of the miRNAs identified were miRNA* (miRNA star). When exclusively examining the high-risk MDS patients, expression of 15 miRNAs were found increased as compared to control samples. Interestingly, miR-10b, miR-155, and miR-125b-2 were also identified by expression profiling studies described above. As indicated, one advantage of sequencing based techniques is the ability to identify novel/unreported miRNAs. In this study, at least 14 novel miRNAs were documented. By applying an integrative bioinformatics analysis of exon and miRNA expression profiling, miRNA target prediction algorithms, and transcription factor target predictions, the authors deduced the biological functions potentially altered by the miRNAs. One of the top enriched biological functions in low-risk MDS patients is the cell death pathway, which is in agreement with the underlying defects in low-risk MDS. In addition, low- and high-risk MDS samples exhibited defects in the cell cycle pathways; however, defects in G1 cell cycle genes were associated with low-risk MDS, while G2/M cell cycle genes were associated with high-risk MDS. Interestingly, they observed increased abundance of piRNA, tRNA and rRNA in high-risk MDS as compared to low-risk MDS or controls. One interpretation of this finding is that cells from high-risk disease are proliferating more rapidly and therefore require increased rate of protein synthesis. Previously, high tRNA content has been observed in solid tumor tissues, reflecting rapid proliferation of tumor cells.
CLINICAL AND FUTURE IMPLICATIONS
Diagnosis, prognosis, and management of MDS can potentially be improved by assigning specific miRNAs as biomarkers. The promise of miRNAs in the clinic has been realized in part by miRNA expression profile studies that successfully distinguish different cancer types. In contrast, protein coding genes did not differentiate the same cancer sample set [71]. As another advantage, miRNAs exhibit better stability during RNA preparations allowing for a more reproducible analysis [108]. Delivery of miRNA mimics or antagomiRs as therapeutic agents for hematological malignancies is a future goal. In contrast to targeted inhibition or activation of a single gene, administration of a miRNA mimics or antagomiRs can regulate multiple genes, and potentially enhance the desired effect.
As described above, unique miRNA expression profiles have been identified and can be used to discriminate between different MDS subtypes. Most approaches rely on mononuclear cells isolated during a marrow aspiration or from peripheral blood. Given the enhanced stability of miRNAs, isolation and analysis of miRNAs from paraffin-embedded sections may also be feasible, as demonstrated for a small cohort of MDS patients [87]. Notwithstanding, to gain from the potential benefits of miRNAs in the clinic, additional retrospective and prospective analyses are necessary to convincingly correlate miRNA expression profiles to outcome, prognosis, and treatment response in MDS patients. For investigations aimed at uncovering the molecular and cellular basis of MDS, selecting relevant HSC populations is ideal, but not necessary for diagnostic and prognostic measures.
miRNAs are also being studied for their potential indications in response to therapies for MDS. Lenalidomide is the most effective therapy for del(5q) MDS patients [14]. Low-risk MDS patients, other than ones with del(5q), also appear to respond favorably to Lenalidomide treatment [109]. The mechanism of Lenalidomide action in MDS is not known; however, CDC25C and PP2A, cell cycle genes located on chr 5q, have been reported to contribute to the Lenalidomide response [110]. In addition, miR-145 and miR-146a, two miRNAs expressed lower in del(5q) MDS patients, are derepressed after Lenalidomide treatment. Re-expression of miR-145 and miR-146a also correlates with erythroid responses and cytogenetic remission in one small trial [97]. This study does not distinguish whether the increased expression of miR-145 and miR-146a following Lenalidomide treatment is due to re-expression from the intact arm of chr 5q in the diseased cells, or if expression correlates with the re-emergence of normal cells in the marrow of treated patients. Independent evidence suggests that Lenalidomide treatment of CD34+ cells from patients with MDS resulted in induction of miR-143 and miR-145 expression, but not miR-146a or miR-146b. As with the above study, Lenalidomide-mediated re-expression of miR-143 and miR-145 in vitro correlated with clinical response to Lenalidomide in patients. In contrast, non-responders in the del(5q) group did not exhibit upregulation of miR-145 following in vitro treatment with Lenalidomide. Overall, these studies provide promise for a role of miRNAs as markers for drug response.
In general, scientific evidence supporting a role of miRNAs in the pathogenesis of MDS is becoming increasingly clear, particularly in the last few years. miRNA expression profiling, genetic models, and functional analyses have yielded select miRNAs that are implicated in MDS. It is the hope that future studies will clarify which miRNAs might serve as biomarkers in MDS, and will generate additional mouse models with altered miRNA function to aid in our understanding of their role in the biology of MDS.
Acknowledgments
Support was provided by Cincinnati Children’s Hospital Medical Center Foundation, Department of Defense/US Army Medical Research and Material Command for Bone Marrow Failure (BM100015), and American Society of Hematology Junior Faculty Scholar Award to D.T.S.
REFERENCES
- 1.Scott BL, Deeg HJ. Myelodysplastic syndromes. Annu Rev Med. 2011;61:345–358. doi: 10.1146/annurev.med.051308.132852. [DOI] [PubMed] [Google Scholar]
- 2.Aul C, Giagounidis A, Germing U. Epidemiological features of myelodysplastic syndromes: results from regional cancer surveys and hospital-based statistics. Int J Hematol. 2001;73:405–410. doi: 10.1007/BF02994001. [DOI] [PubMed] [Google Scholar]
- 3.Sekeres MA. The epidemiology of myelodysplastic syndromes. Hematol Oncol Clin North Am. 2011;24:287–294. doi: 10.1016/j.hoc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–1885. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg PL. Current therapeutic approaches for patients with myelodysplastic syndromes. Br J Haematol. 2010;150:131–143. doi: 10.1111/j.1365-2141.2010.08226.x. [DOI] [PubMed] [Google Scholar]
- 6.Sekeres MA. Are we nearer to curing patients with MDS? Best Pract Res Clin Haematol. 2010;23:481–487. doi: 10.1016/j.beha.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Vardiman JW, Thiele J, Arber DA, Brunning RD, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 8.Cazzola M, Malcovati L. Prognostic classification and risk assessment in myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010;24:459–468. doi: 10.1016/j.hoc.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Gondek LP, Tiu R, O’Keefe CL, et al. Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood. 2008;111:1534–1542. doi: 10.1182/blood-2007-05-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamedali A, aken J, Twine NA, et al. Prevalence and prognostic significance of allelic imbalance by single-nucleotide polymorphism analysis in low-risk myelodysplastic syndromes. Blood. 2007;110:3365–3373. doi: 10.1182/blood-2007-03-079673. [DOI] [PubMed] [Google Scholar]
- 11.Starczynowski DT, Vercauteren S, Sung S, et al. Prevalence and prognostic significance of allelic imbalance by single-nucleotide polymorphism analysis in low-risk myelodysplastic syndromes. Leuk Res. 2010 doi: 10.1182/blood-2007-03-079673. [DOI] [PubMed] [Google Scholar]
- 12.Starczynowski DT, Vercauteren S, Telenius A, et al. High-resolution whole genome tiling path array CGH analysis of CD34+ cells from patients with low-risk myelodysplastic syndromes reveals cryptic copy number alterations and predicts overall and leukemia-free survival. Blood. 2008;112:3412–3424. doi: 10.1182/blood-2007-11-122028. [DOI] [PubMed] [Google Scholar]
- 13.Bejar R, Ebert BL. The genetic basis of myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010;24:295–315. doi: 10.1016/j.hoc.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 14.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 15.Soenen V, Preudhomme C, Roumier C, et al. 17p Deletion in acute myeloid leukemia and myelodysplastic syndrome. Analysis of breakpoints and deleted segments by fluorescence in situ. Blood. 1998;91:1008–1015. [PubMed] [Google Scholar]
- 16.Corey SJ, Minden MD, Barber DL, et al. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–129. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson L, Astrand-Grundstrom I, Arvidsson I, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96:2012–2021. [PubMed] [Google Scholar]
- 18.Nilsson L, Eden P, Olsson E, et al. The molecular signature of MDS stem cells supports a stem-cell origin of 5q myelodysplastic syndromes. Blood. 2007;110:3005–3014. doi: 10.1182/blood-2007-03-079368. [DOI] [PubMed] [Google Scholar]
- 19.Tiu R, Gondek L, O’Keefe C, et al. Clonality of the stem cell compartment during evolution of myelodysplastic syndromes and other bone marrow failure syndromes. Leukemia. 2007;21:1648–1657. doi: 10.1038/sj.leu.2404757. [DOI] [PubMed] [Google Scholar]
- 20.Hast R, Eriksson M, Widell S, et al. Neutrophil dysplasia is not a specific feature of the abnormal chromosomal clone in myelodysplastic syndromes. Leuk Res. 1999;23:579–584. doi: 10.1016/s0145-2126(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 21.Bigoni R, Cuneo A, Milani R, et al. Multilineage involvement in the 5q- syndrome: a fluorescent in situ hybridization study on bone marrow smears. Haematologica. 2001;86:375–381. [PubMed] [Google Scholar]
- 22.Emura I. High incidence of apoptosis in peripheral blood of myelodysplastic syndrome patients determined by Papanicolaoustained preparations. Lab Hematol. 2003;9:42–46. [PubMed] [Google Scholar]
- 23.Gyan E, Frisan E, Beyne-Rauzy O, et al. Spontaneous and Fas-induced apoptosis of low-grade MDS erythroid precursors involves the endoplasmic reticulum. Leukemia. 2008;22:1864–1873. doi: 10.1038/leu.2008.172. [DOI] [PubMed] [Google Scholar]
- 24.Kerbauy DB, Deeg HJ. Apoptosis and antiapoptotic mechanisms in the progression of myelodysplastic syndrome. Exp Hematol. 2007;35:1739–1746. doi: 10.1016/j.exphem.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Economopoulou C, Pappa V, Kontsioti F, et al. Analysis of apoptosis regulatory genes expression in the bone marrow (BM) of adult de novo myelodysplastic syndromes (MDS) Leuk Res. 2008;32:61–69. doi: 10.1016/j.leukres.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Stifter G, Heiss S, Gastl G, et al. Over-expression of tumor necrosis factor-alpha in bone marrow biopsies from patients with myelodysplastic syndromes: relationship to anemia and prognosis. Eur J Haematol. 2005;75:485–491. doi: 10.1111/j.1600-0609.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 27.Gersuk GM, Beckham C, Loken MR, et al. A role for tumour necrosis factor-alpha, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol. 1998;103:176–188. doi: 10.1046/j.1365-2141.1998.00933.x. [DOI] [PubMed] [Google Scholar]
- 28.Gersuk GM, Lee JW, Beckham CA, et al. Fas (CD95) receptor and Fas-ligand expression in bone marrow cells from patients with myelodysplastic syndrome. Blood. 1996;88:1122–1123. [PubMed] [Google Scholar]
- 29.Barlow JL, Drynan LF, Hewett DR, et al. A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q- syndrome. Nat Med. 2010;16:59–66. doi: 10.1038/nm.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutt S, Narla A, Lin K, et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117:2567–2576. doi: 10.1182/blood-2010-07-295238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGowan KA, Pang WW, Bhardwaj R, et al. Reduced ribosomal protein gene dosage and p53 activation in low-risk myelodysplastic syndrome. Blood. 2011;118:3622–3633. doi: 10.1182/blood-2010-11-318584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellagatti A, Marafioti T, Paterson JC, et al. Induction of p53 and up-regulation of the p53 pathway in the human 5q- syndrome. Blood. 2010;115:2721–2723. doi: 10.1182/blood-2009-12-259705. [DOI] [PubMed] [Google Scholar]
- 33.Parker JE, Mufti GJ, Rasool F, et al. The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood. 2000;96:3932–3938. [PubMed] [Google Scholar]
- 34.Rajapaksa R, Ginzton N, Rott LS, et al. Altered oncoprotein expression and apoptosis in myelodysplastic syndrome marrow cells. Blood. 1996;88:4275–4287. [PubMed] [Google Scholar]
- 35.Boudard D, Vasselon C, Bertheas MF, et al. Expression and prognostic significance of Bcl-2 family proteins in myelodysplastic syndromes. Am J Hematol. 2002;70:115–125. doi: 10.1002/ajh.10108. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 37.Parker JE, Fishlock KL, Mijovic A, et al. ‘Low-risk’ myelodysplastic syndrome is associated with excessive apoptosis and an increased ratio of pro- versus anti-apoptotic bcl-2-related proteins. Br J Haematol. 1998;103:1075–1082. doi: 10.1046/j.1365-2141.1998.01114.x. [DOI] [PubMed] [Google Scholar]
- 38.Starczynowski DT, Kuchenbauer F, Argiropoulos B, et al. Identification of miR-145 and miR-146a as mediators of the 5q-syndrome phenotype. Nat Med. 2010;16:49–58. doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- 39.Starczynowski DT, Morin R, McPherson A, et al. Genome-wide identification of human microRNAs located in leukemia-associated genomic alterations. Blood. 2011;117:595–607. doi: 10.1182/blood-2010-03-277012. [DOI] [PubMed] [Google Scholar]
- 40.Starczynowski DT, Kuchenbauer F, Wegrzyn J, et al. MicroRNA-146a disrupts hematopoietic differentiation and survival. Exp Hematol. 2010;39:167–178. doi: 10.1016/j.exphem.2010.09.011. e4. [DOI] [PubMed] [Google Scholar]
- 41.Dunbar AJ, Gondek LP, O’Keefe CL, et al. 250K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res. 2008;68:10349–10357. doi: 10.1158/0008-5472.CAN-08-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathinam C, Thien CB, Flavell RA, et al. Myeloid leukemia development in c-Cbl RING finger mutant mice is dependent on FLT3 signaling. Cancer Cell. 2010;18:341–352. doi: 10.1016/j.ccr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Issa JP. Epigenetic changes in the myelodysplastic syndrome. Hematol Oncol Clin North Am. 2010;24:317–330. doi: 10.1016/j.hoc.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szmigielska-Kaplon A, Robak T. Hypomethylating Agents in the Treatment of Myelodysplastic Syndromes and Myeloid Leukemia. Curr Cancer Drug Targets. 2011 doi: 10.2174/156800911796798940. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Quintas-Cardama A, Santos FP, Garcia-Manero G. Histone deacetylase inhibitors for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Leukemia. 2010;25:226–235. doi: 10.1038/leu.2010.276. [DOI] [PubMed] [Google Scholar]
- 46.Jiang Y, Dunbar A, Gondek LP, et al. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009;113:1315–1325. doi: 10.1182/blood-2008-06-163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 48.Moran-Crusio K, Reavie L, Shih A, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quivoron C, Couronne L, Della Valle V, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Cai X, Cai C, et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011 doi: 10.1182/blood-2010-12-325241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gelsi-Boyer V, Trouplin V, Adelaide J, et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145:788–800. doi: 10.1111/j.1365-2141.2009.07697.x. [DOI] [PubMed] [Google Scholar]
- 52.Gelsi-Boyer V, Trouplin V, Roquain J, et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia. Br J Haematol. 2010;151:365–375. doi: 10.1111/j.1365-2141.2010.08381.x. [DOI] [PubMed] [Google Scholar]
- 53.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 54.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 55.Hinske LC, Galante PA, Kuo WP, Ohno-Machado L. A potential role for intragenic miRNAs on their hosts’ interactome. BMC Genomics. 2010;11:533. doi: 10.1186/1471-2164-11-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y, Kim M, Han J, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 59.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 60.Orkin SH, Zon LI. SnapShot: hematopoiesis. Cell. 2008;132:712. doi: 10.1016/j.cell.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Havelange V, Garzon R. MicroRNAs: emerging key regulators of hematopoiesis. Am J Hematol. 2010;85:935–942. doi: 10.1002/ajh.21863. [DOI] [PubMed] [Google Scholar]
- 62.Schotte D, Pieters R, Den Boer ML. MicroRNAs in acute leukemia: from biological players to clinical contributors. Leukemia. 2011 doi: 10.1038/leu.2011.151. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Georgantas RW, 3rd, Hildreth R, Morisot S, et al. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci USA. 2007;104:2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao R, Sun J, Zhang L, et al. MicroRNAs play a role in the development of human hematopoietic stem cells. J Cell Biochem. 2008;104:805–817. doi: 10.1002/jcb.21668. [DOI] [PubMed] [Google Scholar]
- 65.Ooi AG, Sahoo D, Adorno M, et al. MicroRNA-125b expands hematopoietic stem cells and enriches for the lymphoid-balanced and lymphoid-biased subsets. Proc Natl Acad Sci USA. 2010;107:21505–21510. doi: 10.1073/pnas.1016218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo S, Lu J, Schlanger R, et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proc Natl Acad Sci USA. 2010;107:14229–14234. doi: 10.1073/pnas.0913574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merkerova M, Vasikova A, Belickova M, Bruchova H. MicroRNA expression profiles in umbilical cord blood cell lineages. Stem Cells Dev. 2010;19:17–26. doi: 10.1089/scd.2009.0071. [DOI] [PubMed] [Google Scholar]
- 68.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 72.Mi S, Lu J, Sun M, et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc Natl Acad Sci USA. 2007;104:19971–19976. doi: 10.1073/pnas.0709313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cammarata G, Augugliaro L, Salemi D, et al. Differential expression of specific microRNA and their targets in acute myeloid leukemia. Am J Hematol. 2010;85:331–339. doi: 10.1002/ajh.21667. [DOI] [PubMed] [Google Scholar]
- 74.Li Z, Lu J, Sun M, et al. Distinct microRNA expression profiles in acute myeloid leukemia with common translocations. Proc Natl Acad Sci USA. 2008;105:15535–15540. doi: 10.1073/pnas.0808266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marcucci G, Radmacher MD, Maharry K, et al. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- 76.Jongen-Lavrencic M, Sun SM, Dijkstra MK, et al. MicroRNA expression profiling in relation to the genetic heterogeneity of acute myeloid leukemia. Blood. 2008;111:5078–5085. doi: 10.1182/blood-2008-01-133355. [DOI] [PubMed] [Google Scholar]
- 77.Garzon R, Garofalo M, Martelli MP, et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA. 2008;105:3945–3950. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sokol L, Caceres G, Volinia S, et al. Identification of a risk dependent microRNA expression signature in myelodysplastic syndromes. Br J Haematol. 2011;153:24–32. doi: 10.1111/j.1365-2141.2011.08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao JL, Rao DS, Boldin MP, et al. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci USA. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boldin MP, Taganov KD, Rao DS, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang K, He YS, Wang XQ, et al. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011;585:854–860. doi: 10.1016/j.febslet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 84.Sampson VB, Rong NH, Han J, et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 85.Felli N, Fontana L, Pelosi E, et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci USA. 2005;102:18081–18086. doi: 10.1073/pnas.0506216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garzon R, Pichiorri F, Palumbo T, et al. MicroRNA fingerprints during human megakaryocytopoiesis. Proc Natl Acad Sci USA. 2006;103:5078–5083. doi: 10.1073/pnas.0600587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Erdogan B, Facey C, Qualtieri J, et al. Diagnostic microRNAs in myelodysplastic syndrome. Exp Hematol. 2011;39(9):915–926. doi: 10.1016/j.exphem.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Hussein K, Theophile K, Busche G, et al. Significant inverse correlation of microRNA-150/MYB and microRNA-222/p27 in myelodysplastic syndrome. Leuk Res. 2010;34:328–334. doi: 10.1016/j.leukres.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 89.Bruchova H, Yoon D, Agarwal AM, Swierczek S, Prchal JT. Erythropoiesis in polycythemia vera is hyper-proliferative and has accelerated maturation. Blood Cells Mol Dis. 2009;43:81–87. doi: 10.1016/j.bcmd.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bruchova H, Merkerova M, Prchal JT. Aberrant expression of microRNA in polycythemia vera. Haematologica. 2008;93:1009–1016. doi: 10.3324/haematol.12706. [DOI] [PubMed] [Google Scholar]
- 91.Bruchova H, Yoon D, Agarwal AM, Mendell J, Prchal JT. Regulated expression of microRNAs in normal and polycythemia vera erythropoiesis. Exp Hematol. 2007;35:1657–1667. doi: 10.1016/j.exphem.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pons A, Nomdedeu B, Navarro A, et al. Hematopoiesis-related microRNA expression in myelodysplastic syndromes. Leuk Lymphoma. 2009;50:1854–1859. doi: 10.3109/10428190903147645. [DOI] [PubMed] [Google Scholar]
- 93.Dostalova Merkerova M, Krejcik Z, Votavova H, et al. Distinctive microRNA expression profiles in CD34+ bone marrow cells from patients with myelodysplastic syndrome. Eur J Hum Genet. 2011;19:313–319. doi: 10.1038/ejhg.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Connell RM, Rao DS, Chaudhuri AA, et al. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med. 2008;205:585–594. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hussein K, Theophile K, Busche G, et al. Aberrant microRNA expression pattern in myelodysplastic bone marrow cells. Leuk Res. 2010;34:1169–1174. doi: 10.1016/j.leukres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 96.Votavova H, Grmanova M, Dostalova Merkerova M, et al. Differential expression of microRNAs in CD34+ cells of 5q-syndrome. J Hematol Oncol. 2010;4:1. doi: 10.1186/1756-8722-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oliva EN, Nobile F, Iacopino P, et al. Increases in miRNA-145 and miRNA-146a expression in patients with IPSS lower-risk myelodysplastic syndromes and del(5q) treated with lenalidomide. ASH Annual Meeting. 2010;116 Abstracts 3631. [Google Scholar]
- 98.Kumar M, Narla A, Nonami A, et al. Coordinate loss of a microRNA Mir 145 and a protein-coding gene RPS14 cooperate in the pathogenesis of 5q- syndrome. ASH Annual Meeting. 2009;114 doi: 10.1182/blood-2010-12-324715. Abstracts 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boultwood J, Pellagatti A, Cattan H, et al. Gene expression profiling of CD34+ cells in patients with the 5q- syndrome. Br J Haematol. 2007;139:578–589. doi: 10.1111/j.1365-2141.2007.06833.x. [DOI] [PubMed] [Google Scholar]
- 100.Lu LF, Boldin MP, Chaudhry A, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bousquet M, Quelen C, Rosati R, et al. Myeloid cell differentiation arrest by miR-125b-1 in myelodysplastic syndrome and acute myeloid leukemia with the t(2;11)(p21;q23) translocation. J Exp Med. 2008;205:2499–2506. doi: 10.1084/jem.20080285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gozzetti A, Tozzuoli D, Crupi R, et al. A case of acute myelogenous leukemia: myelodysplastic syndrome with t(2;11)(p21;q23) without MLL rearrangement. Cancer Genet Cytogenet. 2003;144:177–178. doi: 10.1016/s0165-4608(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto K, Nagata K, Morita Y, et al. New complex t(2;11;17)(p21;q23;q11), a variant form of t(2;11), associated with del(5)(q23q32) in myelodysplastic syndrome-derived acute myeloblastic leukemia. Cancer Genet Cytogenet. 2002;137:119–123. doi: 10.1016/s0165-4608(02)00564-2. [DOI] [PubMed] [Google Scholar]
- 106.O’Connell RM, Chaudhuri AA, Rao DS, et al. MicroRNAs enriched in hematopoietic stem cells differentially regulate long-term hematopoietic output. Proc Natl Acad Sci USA. 2010;107:14235–14240. doi: 10.1073/pnas.1009798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beck D, Ayers S, Wen J, et al. Integrative analysis of next generation sequencing for small non-coding RNAs and transcriptional regulation in Myelodysplastic Syndromes. BMC Med Genomics. 2011;4:19. doi: 10.1186/1755-8794-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung M, Schaefer A, Steiner I, et al. Robust microRNA stability in degraded RNA preparations from human tissue and cell samples. Clin Chem. 2010;56:998–1006. doi: 10.1373/clinchem.2009.141580. [DOI] [PubMed] [Google Scholar]
- 109.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 110.Wei S, Chen X, Rocha K, et al. A critical role for phosphatase haplodeficiency in the selective suppression of deletion 5q MDS by lenalidomide. Proc Natl Acad Sci USA. 2009;106:12974–12979. doi: 10.1073/pnas.0811267106. [DOI] [PMC free article] [PubMed] [Google Scholar]