Abstract
Objective
To assess the validity of White’s classification, including the role of chronic hypertension, in a contemporary diabetic population.
Methods
We performed a retrospective cohort study of all singleton pregnancies with preexisting diabetes mellitus from 2008 to 2013. Adverse outcomes were compared across classes B, C, D and vascular disease (R, F, H) and further stratified by the presence or absence of chronic hypertension. Outcomes examined were a composite perinatal outcome (stillbirth, neonatal death, shoulder dystocia, birth injury, seizures, requiring chest compressions or intubation at delivery, blood pressure support), small for gestational age (SGA), large for gestational age (LGA), macrosomia, shoulder dystocia, preterm delivery <37 weeks, preeclampsia, and cesarean delivery.
Results
Of the 475 patients, the 1980 White’s classification was significantly associated with SGA, LGA, macrosomia, preterm delivery, preeclampsia, and cesarean (p ≤ 0.01). Within each White’s class based on age or time since diagnosis alone, hypertension was significantly associated with a higher incidence of preeclampsia in class B (16% without hypertension versus 32% with hypertension, p < 0.01) and C (22% vs. 40%, p = 0.04), SGA in C (4.7% vs. 21%, p < 0.01), preterm delivery in B (25% vs. 46%, p < 0.01) and C (35% vs. 58%, p = 0.01), and the composite neonatal outcome in B (7.9% vs. 17%, p = 0.03). The incidence of adverse outcomes in classes B and C with hypertension resembles the incidence of adverse outcomes in those with diabetes one class higher.
Conclusion
The 1980 White’s classification system, taking into consideration the presence of chronic hypertension, remains a useful system for counseling pregestational diabetic women regarding adverse pregnancy outcomes.
INTRODUCTION
The White’s classification was developed by Priscilla White in 1949 to estimate the risks of “perinatal wastage” in pregnancies complicated by diabetes.1 She concluded that pregnancy complications could be predicted by maternal factors such as disease duration, age of onset, and the presence or absence of vascular diseases such as “transitory” hypertension, retinopathy, nephropathy, or heart disease. In the 1980 revision, the classification system was revised to upstage those with chronic hypertension to class D regardless of their age at diagnosis or duration of disease (Box 1).2
Box 1. The White Classification of Diabetes in Pregnancy: A) Initial (1949) Version and B) Final (1980) Version.
A. 1949 (1)
Class A: Diagnosis of diabetes made on a glucose tolerance test, which deviates but slightly from the normal
Class B: Duration less than 10 y and Onset age 20 y or older and No vascular disease
Class C: Duration 10–19 y or Onset 10–19 y of age or Minimal vascular disease (eg, retinal arteriosclerosis or calcified leg vessels)
Class D: Duration 20 y or longer or Onset younger than 10 y of age or More evidence of vascular disease, eg, retinitis, transitory albuminuria, or transitory hypertension
Class E: Calcified pelvic arteries on X-ray
Class F: Nephritis
B. 1980 (13)
Gestational diabetes: Abnormal glucose tolerance test, but euglycemia maintained by diet alone; diet alone insufficient, insulin required
Class A: Diet alone, any duration or onset age
Class B: Onset age 20 y or older and duration less than 10 y
Class C: Onset age 10–19 y or duration 10–19 y
Class D: Onset age younger than 10 y, duration over 20 y, background retinopathy, or hypertension (not preeclampsia)
Class R: Proliferative retinopathy or vitreous hemorrhage
Class F: Nephropathy with over 500 mg/d proteinuria
Class RF: Criteria for both Classes R and F coexist
Class H: Arteriosclerotic heart disease clinically evident
Class T: Prior renal transplantation
Reprinted from Sacks DA, Metzger BE. Classification of diabetes in pregnancy: time to reassess the alphabet. Obstet Gynecol 2013;121:345–8.
In Sacks et al, this Box cites the following sources: Data from White P. Pregnancy complicating diabetes. Am J Med 1949;7:609–16 and Hare JW, White P. Gestational diabetes and the White classification. Diabetes Care 1980;3:394.
The authors of this article obtained permission to use Hare JW, White P. Gestational diabetes and the White classification. Diabetes Care 1980;3:394. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association.
In stark contrast to today’s diabetic population, the White’s classification was based largely on women with type 1 diabetes1. The increasing rates of childhood obesity and the overall increase in obesity in the population has resulted in more women of reproductive age with type 2 diabetes. A recent study over an 8 year period revealed a 21.1% and 30.5% increase in prevalence of type 1 and 2 diabetes, respectively in children.3–6 With changes in the demographics and type of diabetes in reproductive-age women, it is useful to reassess the utility of White’s classification.7
The practice of upstaging the class of diabetes to category D in the presence of hypertension has not been widely accepted.8 This may be due to the widespread coexisting diagnosis of hypertension in type 2 diabetics, such that practitioners do not consider hypertension a complication of diabetes.
Due to changes in both the obstetric population and alterations in the uses of the White’s class system, we aimed to evaluate the validity of White’s classification in predicting adverse pregnancy outcomes in a modern cohort and to assess the effects of hypertension on perinatal outcomes within each White class.
MATERIALS AND METHODS
This was a retrospective cohort study of all singleton pregnancies delivered from 2008–2013 at the University of Alabama at Birmingham with a diagnosis of pregestational diabetes. Institutional review board approval was obtained.
Patients were identified from the obstetric automated record system9 and a diagnosis of pregestational diabetes was confirmed on review of medical records. Only patients who reported a history of diabetes prior to pregnancy were considered to have pregestational diabetes; patients diagnosed by glucose tolerance testing during pregnancy were considered to have gestational diabetes and excluded from this analysis regardless of gestational age at diagnosis. Trained personnel reviewed electronic medical records to abstract pertinent information using standardized data collection forms. Maternal data included age, ethnicity, insurance status, prepregnancy body mass index (BMI), comorbid conditions, age of diabetes diagnosis, type of diabetes, use of prepregnancy hypoglycemic medications, baseline laboratory values (hemoglobin A1c, urine protein and creatinine, 24-hour urine), obstetric history, delivery details, selected maternal complications, and perinatal outcomes. Patients were excluded for late prenatal care (first ultrasound after 26 weeks), any fetal anomaly, and major maternal comorbidities unrelated to diabetes other than chronic hypertension (e.g. systemic lupus erythematosus, HIV, and hepatitis). For this analysis, patients were excluded if information related to their diabetes diagnosis (age or year of diagnosis) was not available. Maternal-Fetal Medicine specialists supervised the care of all patients. As per institutional protocol, diabetic medications (oral hypoglycemic or insulin) were adjusted to maintain goal blood sugars of <95 mg/dL fasting and <120 mg/dL 2-hours post-prandial.3 Ultrasounds for fluid and growth were routinely performed every 4 weeks beginning at 28 weeks, and antenatal testing (once or twice weekly) was started at 32–34 weeks. Delivery was scheduled for between 39–40 weeks, or earlier if evidence of poor diabetic control or positive fetal lung maturity testing.
Patients were classified according to the 1980-revised White’s class to create Tables 1–3. Each class was determined by maternal age at diagnosis and disease duration, additionally upstaging class B and C patients with chronic hypertension to class D. Women were considered to have proliferative retinopathy if documented as having “proliferative retinopathy” and background retinopathy if documented as “background” or “benign.” In cases where a woman was documented to have unclassified “retinopathy”, we assumed proliferative retinopathy since documentation otherwise would have most likely not occurred. Classes F, R, RF, and H were combined to create a “Vascular” class due to the small number of patients representing each of these groups.
Table 1.
Demographic and Clinical Characteristics of Women by 1980 White’s Classification Revision
| Total N = 475 | B (n = 128) | C (n = 86) | D (n = 208) | Vascular (n = 53) | p |
|---|---|---|---|---|---|
| Age | 31 ± 5.2 | 25 ± 5.4 | 30 ± 6.2 | 28 ± 4.7 | <0.01 |
| Ethnicity | |||||
| African American | 68 (53) | 48 (56) | 132 (64) | 40 (76) | *<0.01 |
| Caucasian | 31 (24) | 34 (40) | 68 (33) | 12 (23) | |
| Hispanic | 24 (19) | 3 (3.5) | 6 (2.9) | 1 (1.9) | |
| Other | 5 (3.9) | 1 (1.2) | 2 (0.96) | 0 (0) | |
| Insurance | |||||
| Self-pay | 15 (12) | 6 (7) | 9 (4.3) | 3 (5.7) | *0.15 |
| Private | 37 (29) | 18 (21) | 59 (28) | 13 (25) | |
| Government | 76 (59) | 62 (72) | 140 (67) | 37 (70) | |
| Tobacco use | 28 (22) | 18 (21) | 51 (25) | 16 (30) | 0.60 |
| Prepregnancy BMI (kg/m2) | 33 ± 6.4 | 32 ± 9 | 37 ± 11 | 34 ± 8 | <0.01 |
| - Underweight | 1 (0.85) | 1 (1.2) | 1 (0.52) | 0 (0) | *<0.01 |
| - Normal | 7 (5.9) | 22 (27) | 21 (11) | 8 (16) | |
| - Overweight | 30 (26) | 17 (21) | 26 (14) | 11 (22) | |
| - Obese | 80 (68) | 43 (52) | 145 (75) | 32 (63) | |
| Diabetes (DM) Type | |||||
| DM Type I | 7 (5.5) | 41 (48) | 66 (32) | 33 (62.26) | *<0.01 |
| DM Type 2 | 120 (94) | 44 (51) | 137 (66) | 20 (38) | |
| DM type Undocumented | 1 (0.78) | 1 (1.2) | 4 (1.9) | 0 (0) | |
| Nulliparous | 29 (23) | 42 (49) | 90 (43) | 21 (40) | <0.01 |
| Prior preterm delivery | 28 (22) | 24 (28) | 62 (30) | 22 (42) | 0.06 |
| Prior cesarean surgery | 40 (31) | 23 (27) | 59 (28) | 19 (36) | 0.65 |
| 1st HgA1c (%) | 7.5 ± 1.9 | 7.8 ± 1.8 | 8.09 ± 2.3 | 9.5 ± 2.6 | <0.01 |
| cHTN | 0 (0) | 0 (0) | 165 (79) | 34 (64) | <0.01 |
| Hypoglycemic/Insulin regimen prior to pregnancy | 90 (70) | 75 (87) | 191 (92) | 49 (93) | <0.01 |
Data are mean ± standard deviation or n (%) unless otherwise specified..
The p-values for ethnicity, diabetes type, BMI type, and insurance type represent the comparison of each respective demographic/clinical characteristic as a whole.
Table 3.
Maternal Outcomes by the 1980 White’s Classification Revision
| B (n=128) | C (n=86) | D (n=208) | Vascular (n=53) | p* | |
|---|---|---|---|---|---|
| Preeclampsia | 21 (16) | 19 (22) | 79 (38) | 37 (70) | <0.01 |
| Any Cesarean | 65 (51) | 43 (50) | 133 (64) | 34 (64) | <0.01 |
| Primary Cesarean | 31 (24) | 23 (27) | 81 (39) | 17 (33) | 0.01 |
Data are n (%) unless otherwise specified..
Chi-squared test for trend
To evaluate the predictive value of the type of diabetes and vascular complications on pregnancy outcomes, we stratified each diabetes type by those with and without vascular complications. Women with any retinopathy, nephropathy, heart disease, or a combination were considered to have a vascular complication.
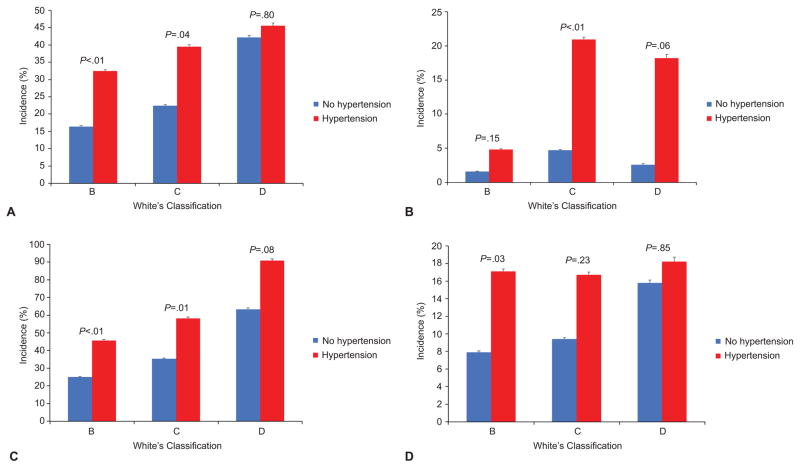
In order to assess the effects of chronic hypertension on pregnancy outcomes, we also classified women according to only maternal age at diagnosis and disease duration classifying those with end organ damage (retinopathy, nephropathy, heart disease) in the vascular category only. The effect of stratifying each class by the presence or absence of chronic hypertension was then analyzed, as shown in Figure 1.
Figure 1.
A comparison of select adverse outcomes across the White Classification, B D, in the presence and absence of chronic hypertension. Preeclampsia (A), small for gestational age (B), preterm delivery (C), and composite neonatal outcome (D). The P-values above each diabetes class compare the incidence of the selected adverse outcome in the presence and absence of chronic hypertension. The error bars represent the 95% confidence interval of the incidence for each adverse outcome.
The primary perinatal outcome was a composite of stillbirth, neonatal death, shoulder dystocia, birth injury (brachial plexus injury, fracture), neonatal seizures, blood pressure support, or a high level of resuscitation in the delivery room (CPR or intubation). Stillbirth was also considered separately as a primary outcome. Secondary perinatal outcomes considered were shoulder dystocia, small for gestational age (SGA, <10th percentile),10 large for gestational age (LGA, >90th percentile),10 macrosomia (birth weight >4000 g), and preterm delivery (delivery <37 weeks). Outcomes that were a direct marker of an adverse outcome were considered a primary outcome. The secondary outcomes were surrogate markers chosen based on outcomes that were associated but did not always result in a poor outcome.
Maternal outcomes considered included preeclampsia and cesarean delivery. Cesarean delivery was considered as both any cesarean (primary or repeat) and primary cesarean delivery only. Preeclampsia was documented by the admitting physician, delivering physician, or both. According to institutional protocol, preeclampsia is diagnosed with two blood pressure readings ≥140/90 at rest 4 hours apart with either proteinuria (≥1+ dipstick, protein-creatinine ratio ≥0.3 or ≥300-mg of protein on 24-hour urine) or blood pressure criteria and evidence of end-organ involvement including abnormal preeclampsia laboratory values.
Maternal characteristics were compared with descriptive and univariable statistics using analysis of variance for continuous variables and chi-square tests for categorical variables as appropriate. Rates of maternal and neonatal outcomes across the White’s classifications were compared using a chi-square test for trend. All analyses were completed using Stata SE, version 13 (College Station, Texas). A p-value <0.05 was chosen to represent statistical significance.
RESULTS
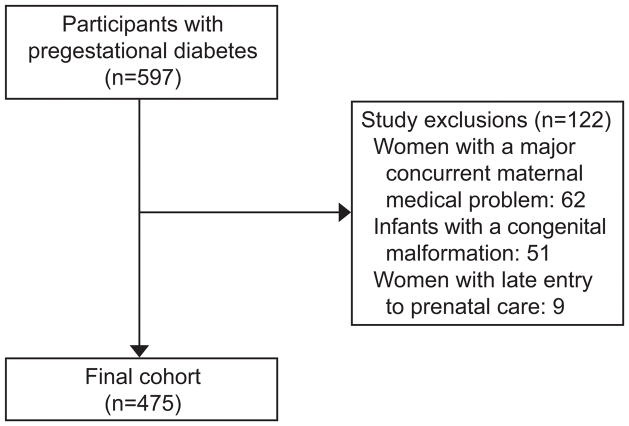
Of the 597 patients identified with a diagnosis of pregestational diabetes, 475 were included in the study (62 women were excluded due to a major concurrent maternal medical problem, 51 had an infant with a congenital malformation, and 9 had late entry to prenatal care) (Figure 2). Of the 475 patients, classified by the 1980 White’s revision categorizing women with chronic hypertension as D in Tables 1–3, 128 (27%) were classified as B, 86 (18%) as C, 208 (44%) as D, and 53 (11%) as vascular (Classes F, R, RF, and H). The proportion of African American race, type 1 diabetes, nulliparous patients, and patients on prepregnancy diabetic medications increased across each class (Table 1). The mean BMI in each class was 33±6.4 kg/m2 in B, 32±9 in C, 37±11 in D, and 34±8 kg/m2 in the Vascular Class. Type of insurance, tobacco use, prior preterm delivery and prior cesarean did not vary by White’s classification.
Figure 2.
The flow diagram illustrates the difference between the initial 597 participants and the final cohort group of 475 participants.
The increasing risks of composite perinatal outcome and stillbirth in the class D and vascular compared to B and C was not statistically significant (p = 0.07 and p = 0.08, respectively) (Table 2). Rates of SGA increased across the advancing White class groups (B to Vascular, p < 0.01) while the inverse was true for LGA (p < 0.01) and macrosomia (p < 0.01), which occurred more frequently in class B. Although the incidence of fetal overgrowth decreased from the B to vascular classes, the decreased incidence of shoulder dystocia did not reach statistical significance. A significant effect of advancing White’s class on preterm delivery (p < 0.01) was observed.
Table 2.
Neonatal Outcomes by the 1980 White’s Classification Revision
| B (n=128) | C (n=86) | D (n=208) | Vascular (n=53) | p* | |
|---|---|---|---|---|---|
| Composite Neonatal Outcome | 19 (15) | 13 (15) | 46 (23) | 11 (22) | 0.07 |
| Stillbirth | 2 (1.6) | 2 (2.4) | 9 (4.6) | 3 (6) | 0.08 |
| SGA (<10th Percentile) | 2 (1.6) | 4 (4.7) | 19 (9.2) | 9 (17) | <0.01 |
| LGA (>90th Percentile) | 44 (34) | 24 (28) | 44 (21) | 4 (7.6) | <0.01 |
| Macrosomia (>4000 g) | 33 (26) | 19 (22) | 34 (16) | 2 (3.8) | <0.01 |
| Shoulder Dystocia | 9 (7.0) | 5 (5.8) | 10 (4.8) | 0 | 0.08 |
| Preterm Delivery <37 weeks | 30 (24) | 29 (34) | 103 (52) | 34 (68) | <0.01 |
Data are n (%) unless otherwise specified.
Chi-squared test for trend
Table 3 depicts maternal outcomes by White’s classification. A trend (p < 0.01) of increasing rates of preeclampsia and any cesarean (primary and repeat) occurred with increasing White’s class (B to Vascular).
A statistically significant trend (p < 0.02) of increasing SGA, preterm delivery, and preeclampsia was observed in those with a vascular complication, which occurred more frequently in type 2 diabetics (Table 4). Conversely, rates of LGA (p = 0.02) and macrosomia (p = 0.04) increased among those without a vascular complication, occurring more commonly in type 1 diabetics. Additionally, the presence or absence of vascular disease appeared to be more predictive of adverse outcomes than the type of diabetes.
Table 4.
Adverse Outcomes by Diabetes Type and the Presence/Absence of Vascular Disease
| No Vascular Complication (n = 404) | Vascular Complication (n = 64) | ||||
|---|---|---|---|---|---|
| Type 1 (n = 107) | Type 2 (n = 297) | Type 1 (n = 40) | Type 2 (n = 24) | p* | |
| Composite Neonatal Outcome | 12 (11) | 38 (13) | 8 (21) | 6 (25) | 0.12 |
| Stillbirth | 3 (2.8) | 21 (7.1) | 4 (10) | 2 (8.3) | 0.78 |
| SGA (<10th Percentile) | 5 (4.7) | 16 (5.4) | 4 (10) | 7 (29) | 0.02 |
| LGA (>90th Percentile) | 37 (35) | 74 (25) | 3 (7.5) | 1 (4.2) | 0.02 |
| Macrosomia (>4000 g) | 30 (28) | 55 (19) | 1 (2.5) | 1 (4.2) | 0.04 |
| Shoulder Dystocia | 8 (7.5) | 15 (5.1) | 1 (2.5) | 0 (0) | 0.48 |
| Preterm Delivery <37 weeks | 55 (51) | 112 (38) | 26 (65) | 17 (71) | <0.01 |
| Preeclampsia | 38 (36) | 73 (25) | 25 (63) | 19 (79) | <0.01 |
| Any Cesarean | 59 (55) | 171 (58) | 26 (65) | 18 (75) | 0.23 |
| Primary Cesarean | 36 (43) | 93 (42) | 16 (53) | 7 (54) | 0.23 |
Data are n (%) unless otherwise specified.
Chi-squared test for trend
To evaluate the effect of chronic hypertension on White’s classification, patients were classified using the 1949 White’s classification version by maternal age at diagnosis and disease duration, and then each class was further stratified by the presence or absence of chronic hypertension. Within each class, women with hypertension had a higher incidence of the outcomes of interest (adverse composite perinatal outcome, SGA, preterm delivery, and preeclampsia) than women in the same class without hypertension (Figure 2). These differences were statistically significant for White’s classes B and C for both preeclampsia (B - 16% without hypertension versus 32% with hypertension and C - 22% vs. 40%) and preterm delivery (B - 25% vs. 46% and C - 35% vs. 58%) whereas outcomes were similar in class D for women with and without chronic hypertension. A significant difference in the rate of adverse neonatal composite outcomes emerged in White’s class B only (7.9% vs. 17%), and SGA was statistically significant in only Class C (4.7% vs. 21%). The effect of chronic hypertension was null in Class D.
DISCUSSION
In this contemporary cohort, using the 1980 revision of White’s classification system, which utilizes chronic hypertension to reclassify women with classes B & C as Class D, assigned class was significantly associated with adverse perinatal outcomes including fetal growth disorders, preeclampsia, and cesarean delivery. Interestingly, the incidence of LGA and macrosomia decreased as the class increased towards the vascular categories whereas the incidence of SGA increased. Though probably underpowered to show significance, an increasing incidence in both outcomes of the composite perinatal outcome and stillbirth as well as a decreasing incidence of shoulder dystocia was observed across each White’s class. Furthermore, the presence of vascular disease, being more distinctive than diabetes type, was significantly associated with SGA, preterm delivery, and preeclampsia. Conversely, the incidence of LGA and macrosomia was decreased in those with vascular disease. Our findings revealed that within each class of diabetes defined based on maternal age at diagnosis and disease duration, hypertension is associated with a significantly increased incidence of SGA, preeclampsia, preterm delivery, and composite perinatal outcome. Although White’s classification was associated with adverse outcomes; the presence of hypertension appeared to have a significant effect on the occurrence of adverse outcomes, validating the 1980 revision.
Multiple studies have evaluated the validity of White’s classification, including Pederson et al, who set out to improve the methods for identifying diabetic pregnancies at risk for adverse outcomes.8,11–12 They created a new system by identifying four “prognostically bad signs of pregnancy” which included clinical pvelonephritis, precoma and severe acidosis, toxemia (i.e. preeclampsia or eclampsia), and maternal neglectors (i.e. late entry to prenatal care, poor social circumstances, etc). They concluded that his classification system, when combined with the White’s class, improved the prediction of adverse outcomes.11
In 1987 Diamond et al examined both Pederson’s prognostically bad signs of pregnancy and White’s classification system.12 In this retrospective cohort of 199 diabetic pregnancies, they found that both White’s classification & Pederson’s prognostic factors were associated with perinatal death, neonatal respiratory morbidity, and birth weight. However, as this cohort was composed of women from 1977–1983, these findings do not necessarily apply to a contemporary diabetic cohort.
Cormier et al also examined White’s classification system in a contemporary obstetric cohort of 114 gestational and 82 pregestational diabetics. In this study, women with hypertension were not assigned a D class. They concluded that the presence of vascular disease (including hypertension) was more predictive of adverse outcome than White’s classification based on disease duration and maternal age of onset alone.8 This is consistent with our findings that chronic hypertension results in worsened outcomes; however, they did not examine the results according to the 1980 White’s classification revision with all women with chronic hypertension classified as D.
One of our study’s strengths is in the detailed clinical information collected by clinicians trained in chart abstraction. Confirmation of both the exposure (pregestational diabetes) and the perinatal outcome minimized misclassification bias. Additionally, we had a relatively large sample size of pregestational diabetics compared to prior studies, enabling us to stratify by White’s classification and examine uncommon outcomes.
The main limitation of our study is that, although we had a significant number of women with diabetes class suggestive of vascular disease (n=53), we had relatively few of each subcategory (R, F, H). Consequently, while we were able to examine the presence of any vascular disease (proliferative retinopathy, nephropathy, or heart disease) on perinatal outcomes, we were unable to explore the contribution of each specific sub-category to adverse perinatal outcomes. Also, the type of retinopathy was often not specified in our obstetric documentation diminishing our ability to distinguish benign from proliferative retinopathy. Additionally, since not all women underwent ophthalmologic examination during pregnancy, this may have led to underestimation of the association between the vascular class and adverse perinatal outcomes. Of note, since our goal was to examine White’s classification by outcomes, we did not think it was necessary to adjust the results by the baseline characteristics for this specific analysis.
Our study confirms that hypertension is a major prognostic factor and has a significant effect on adverse outcomes. This validates the 1980 revision of White’s classification, which upgrades women with class B & chronic hypertension to a class D. Indeed women with chronic hypertension who would otherwise be classified as B or C had outcomes similar to women who would be classified as D. Overall, White’s classification still provides simple and useful information for counseling women on pregnancy risks and prognosis based on information available either prior to pregnancy or at the first prenatal visit. Lastly, we believe that if the White’s class is used to counsel patients, then those with chronic hypertension should be classified as class D.
Acknowledgments
Dr. Harper is supported by K12HD001258-13, PI WW Andrews, which partially supports this work.
Footnotes
Presented as a poster at The Pregnancy Meeting, Society for Maternal-Fetal Medicine, February 2–7, 2015.
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.White P. Pregnancy complicating diabetes. Am J Med Obstet Gynecol. 1949;7:609–616. doi: 10.1016/0002-9343(49)90382-4. [DOI] [PubMed] [Google Scholar]
- 2.White P. Classification of obstetric diabetes. Am J Med Obstet Gynecol. 1978;130:228–230. doi: 10.1016/0002-9378(78)90373-3. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetricians and Gynecologist. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675–685. doi: 10.1097/00006250-200503000-00049. [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of Type 1 and Type 2 Diabetes Among Children and Adolescents From 2001 to 2009. JAMA. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31:899. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 6.Homko CJ, Reece EA. Development of early-onset type 2 diabetes in the young: implications for child bearing. Curr Diab Rep. 2003;3:313–8. doi: 10.1007/s11892-003-0023-z. [DOI] [PubMed] [Google Scholar]
- 7.Sacks DA, Metzger BE. Classification of diabetes in pregnancy: time to reassess the alphabet. Obstet Gynecol. 2013;121(2 Pt 1):345–8. doi: 10.1097/AOG.0b013e31827f09b5. [DOI] [PubMed] [Google Scholar]
- 8.Cormier CM, Martinez CA, Refuerzo JS, et al. White’s classification of diabetes in pregnancy in the 21st century: is it still valid? Am J Perinatol. 2010;27(5):349–52. doi: 10.1055/s-0029-1243307. [DOI] [PubMed] [Google Scholar]
- 9.Wirtschafter DD, Blackwell WC, Goldenberg RL, Henderson SA, Peake MN, Huddleston JF, et al. A county-wide obstetrical automated medical record system. J Med Syst. 1982 Jun;6(3):277–90. doi: 10.1007/BF00992804. [DOI] [PubMed] [Google Scholar]
- 10.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstetrics and gynecology. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen J, Pedersen LM. Prognosis of the outcome of pregnancies in diabetes: a new classification. Acta Endocrinol. 1965;50:70. doi: 10.1530/acta.0.0500070. [DOI] [PubMed] [Google Scholar]
- 12.Diamond MP, Salyer SL, Vaughn WK, Cotton R, Boehm FH. Reassessment of White’s classification and Pedersen’s prognostically bad signs of diabetic pregnancies in insulin-dependent diabetic pregnancies. Am J Obstet Gynecol. 1987;156:599–604. doi: 10.1016/0002-9378(87)90060-3. [DOI] [PubMed] [Google Scholar]
- 13.Hare JW, White P. Gestational diabetes and the White classification. Diabetes Care. 1980;3:394. [Google Scholar]