Abstract
The challenges posed by acute brain injury (ABI) involve the management of the initial insult in addition to downstream inflammation, edema, and ischemia that can result in secondary brain injury (SBI). SBI is often subclinical, but can be detected through physiologic changes. These changes serve as a surrogate for tissue injury/cell death and are captured by parameters measured by various monitors that measure intracranial pressure (ICP), cerebral blood flow (CBF), brain tissue oxygenation (PbtO2), cerebral metabolism, and electrocortical activity. In the ideal setting, multimodality monitoring (MMM) integrates these neurological monitoring parameters with traditional hemodynamic monitoring and the physical exam, presenting the information needed to clinicians who can intervene before irreversible damage occurs. There are now consensus guidelines on the utilization of MMM, and there continue to be new advances and questions regarding its use. In this review, we examine these recommendations, recent evidence for MMM, and future directions for MMM.
Keywords: Multimodal monitoring, Intracranial pressure, Microdialysis, Brain metabolism, Brain tissue oxygenation, Cerebral blood flow
Introduction
Despite the medical and surgical interventions available for acute brain injury (ABI), the initial insult often times has already left an irreversible impact on the brain. Subsequently, the early detection and prevention of secondary brain injury (SBI) has become the primary focus of neurocritical care.
Historically, the neurologic exam and neuroimaging have driven therapeutic interventions in the neurointensive care unit (NICU). However, these changes may be nonspecific or may reflect SBI that is already complete. Consequently, the use of physiologic markers has become a powerful tool in the NICU to detect and treat early signs of SBI before irreversible injury occurs.
The goal of examining continuous physiologic endpoints with multimodality monitoring (MMM) is to capture the multifaceted and dynamic nature of brain injury. There is no single best or complete monitor or absolute physiologic cutoffs that can be generalized to every ABI patient. However, certain threshold recommendations have been placed for these devices (see Table 1). Originally derived from the traumatic brain injury (TBI) literature, MMM has become utilized across different etiologies of severe ABI. This review will discuss the current consensus guidelines, new evidence for MMM, and the limitations and difficulties of data integration for real-time patient care.
Table 1.
Invasive MMM monitors
| ICP/CPP | Brain oxygenation | CBF | Brain metabolism | |
|---|---|---|---|---|
| Devices | Parenchymal vs ventricular |
|
Thermal diffusion probe | Microdialysis |
| Parameter | ICP < 20 mmHg; CPP > 60 mm-Hg |
|
>20 cm3/100 g/min |
|
| Interpretation | Indirect surrogate for CBF | Indirect surrogate for CBF and oxygen demand
|
Direct regional measurement of CBF | Brain energy crisis |
ICP intracranial pressure, CPP cerebral perfusion pressure, PbtO2 parenchymal brain tissue oxygen, SjvO2 jugular bulb venous oxygen saturation, CBF cerebral blood flow
Intracranial Pressure
The intracranial pressure (ICP) monitor is historically the most widely used brain monitoring tool in ABI as higher ICPs had been shown to be predictive of mortality [1–3]. Subsequently, the Brain Trauma Foundation (BTF) and MMM consensus guidelines put forth recommendations for ICP monitoring in TBI and ABI patients, respectively. Indications for ICP monitoring include a Glasgow coma scale (GCS) of 8 or less, concerning neuroimaging features for ICP crises, and patients receiving active treatment for presumed elevated ICP [4, 5••]. Although normal CT brain findings have low incidence of ICP elevation in TBI, a subset population of TBI patients with age over 40, systolic blood pressure under 90 mmHg, and clinical evidence of posturing require ICP monitoring regardless of CT findings [2, 4]. Evidence for improved outcomes with continuous ICP monitoring has been varied [6, 7••, 8, 9] leading to subsequent variability of ICP monitoring practices amongst various NICU centers [10, 11]. The most recent MMM guidelines have subsequently been formed to provide expert consensus on the use of these monitoring modalities and a more congruent practice amongst difference centers.
Currently recommended device options include parenchymal ICP monitors and extraventricular catheters (EVD) [5••]. Both require invasive placement with minimal risk of infection and bleeding when placed by experienced operators [12]. EVDs are the preferred device in the presence of hydrocephalus due to the ability of cerebrospinal fluid (CSF) diversion [13]. It may confer benefits with lower rates of refractory ICP and improved outcomes over parenchymal monitors [14] along with the added ability of administering intrathecal thrombolytics in intraventricular hemorrhage [15, 16]. The benefit of parenchymal ICP monitoring is the relative ease of placement in addition to providing continuous ICP monitoring whereas EVD pressure transduction is performed momentarily when the drain system is closed. Limitations of parenchymal probes include its inability to be recalibrated, having value drifts with time, durability issues, and susceptibility to compartmentalization of ICP across dura mater, particularly in space-occupying lesions [17, 18]. Consequently, recommendations are to place probes ipsilateral to space-occupying lesions. Despite these differences, both probes provide reliable and comparable measurements of ICP [19] with normal ICP ranging between 7 and 15 mmHg in a supine adult. Although there is a lack of data supporting an absolute threshold for critically elevated ICP, the typical treatment thresholds have been ICPs greater than 20–25 mmHg per the BTF and MMM guidelines [4, 5••]. Refractory elevations of ICP >20 mmHg to aggressive treatment have been shown to be a strong predictor of mortality, particularly within the first 48 h. However, it is unclear whether elevated ICP burden itself translates into poor cognitive outcomes in survivors [3, 20].
The BEST:TRIP trial revealed that an ICP treatment protocol based on a treatment threshold of >20 mmHg (compared to a treatment protocol based on clinical exam/imaging alone) may not be beneficial in overall outcome [7••]. However, this does not speak against the utility of ICP monitoring as the trial concurrently showed more efficient and targeted treatment in the ICP monitoring group. Rather, it has brought to light that a solely ICP-driven approach to prevention of SBI may oversimplify the complexity of ABI. ICP has been utilized as a tool to monitor cerebral perfusion pressure (CPP), which has often been seen as a surrogate for cerebral blood flow (CBF). However, observational data has revealed that in comparison to perfusion CT (the current gold standard for CBF), CBF is better predicted by MMM rather than ICP or CPP alone [21•].
Although treatment of elevated ICP continues to be a cornerstone of NICU care, future focus should be on features and trends of ICP rather than absolute threshold values. Additional components of ICP monitoring including optimal CPP and ICP waveform morphology [22] may hold the key to individualized treatment goals and may even predict ICP crises before they occur [23].
Non-Invasive Intracranial Pressure Monitoring
Non-invasive ICP monitoring techniques have been studied in recent years to provide an alternative means of monitoring when there is a medical contraindication or high threshold for invasive monitoring (coagulopathy). Most published techniques have included transcranial Dopplers (TCD), EEG, pupillometry, and ultrasound measurement of optic nerve sheath diameter (ONSD). However, reproducibility, inexperience, and device testing limitations have hindered the translation of many of these devices to widespread clinical practice. To date, there have been limited studies revealing equal accuracy between non-invasive ICP monitors to invasive ones in TBI literature, let alone all other ABI [24].
Perhaps the most researched non-invasive ICP monitor involves the use of TCD and pulsatility index (PI). By examining the systolic and diastolic velocities within each cardiac cycle, these velocity changes provide the PI value and can allow for ICP trends to be tracked. PI > 1.6 has been associated with higher ICP values and worse outcomes [25, 26]. However, evidence has been limited to translate this non-invasive bedside technique to be used as real-time ICP monitoring reliable for treatment [27, 28]. Similar to ICP monitoring, assimilating high PI to low CPP oversimplifies the flow velocity changes that are reliant on multiple different physiologic parameters other than mere ICP.
Opthalmologic techniques have become increasingly utilized in the NICU for non-invasive ICP monitoring adjuncts. The increasing popularity of the use of ultrasound in point of care critical care medicine has been reflected in the NICU with optic nerve sheath diameter (ONSD) measurements. The optic nerve sheath, contiguous with brain dura and containing CSF communicating with cerebral subarachnoid components, can be used as a means of indirectly detecting increased ICP. By measuring the anterior part of the optic nerve, specifically 3 mm behind the globe, ONSD can be measured via ultrasound with 5-mm ONSD roughly translating to an ICP of 20 [24, 29, 30]. Inter/intraobserver variability does not appear to be a limiting factor in this technology [31, 32]. However, ONSD variation is dependent on individual factors such as age and underlying pathology; this has made it difficult to create an absolute ONSD cutoff value for ICP crises. Another ophthalmologic approach that is being studied to indirectly assess ICP has been the pupillometer. The automated Neurological Pupil index (NPi) reflects pupillary reactivity. Values range from 0 to 5 with values <3 being associated with poor outcome and trend with elevated ICP [33]. While NPI may not be superior to the clinical exam in determining a state associated with rising ICP nor replace invasive ICP monitoring, there may be an opportunity for quality improvement in practice when examination across shifts need standardization.
Cerebral Perfusion Pressure and Autoregulation
CPP has been defined as a pressure gradient for blood flow to the brain and is derived by MAP–ICP. Requiring both ICP and MAP monitoring, pressure transducer placement of both devices should ideally be placed at the same level (tragus) to prevent an overestimation of true CPP; however, this practice has varied widely amongst neurocritical care centers [34]. This variability in the measurement of CPP makes it difficult to translate research findings to clinical practice and vice versa. However, a target CPP of 60 mmHg is recommended by the BTF guidelines due to retrospective evidence of worse outcomes at levels below this threshold [35, 36]. These thresholds are typically achieved via MAP augmentation and ICP optimization in efforts to maintain CBF [37]. CPP has long been seen as a surrogate marker of CBF as CBF = CPP/CVR (cerebrovascular resistance). However, ABI often leads to a loss of autoregulatory mechanisms including CVR. These effects can be seen up to 2 weeks following TBI [38].
Loss of intrinsic CNS protection to blood pressure put the patient at risk of SBI via ischemia with hypotensive episodes and conversely, elevated ICP and hyperemia with MAP augmentation. Furthermore, attaining the goal of higher CPPs put patients at risk for systemic complications (ARDS) and poorer outcomes [39, 40]. Recent evidence has suggested that current CPP goals >60 mmHg may reflect an oversimplification of ABI with findings that at optimal CPP and ICP ranges (>60 and <20 mmHg, respectively), cerebral hypoxia can still be present [41]. This has resulted in the increasingly studied idea of an optimal CPP and the role of autoregulation measurement in its determination.
Impaired vascular reactivity and loss of intracranial compliance will result in ICP changes driven by MAP fluctuations, a concept reflected in the index of pressure reactivity (PRx) [42]. Simply put, a positive PRx reflects a positive linear correlation between changes in MAP and revealing an impaired autoregulatory state. This has been associated with poor outcome following TBI particularly within the first 48 h [42]. Active work in this area is ongoing to define the limitations of defining optimal CPP (CPPopt) using PRx and understanding the conditions that influence its interpretation [43].
CPP and PRx are used in specialized centers in conjunction with brain oxygenation and microdialysis as a means to understand individual patient states [44]. In the future, it is hoped that the validation of optimal CPP determination and integration with MMM will spare patients from the detriments of passive pressure changes in the autoregulation impaired brain. The concept of CPPopt is to identify a narrow range of CPPs within a patient’s recent real-time data that reflects the state in which there was optimal cerebral vasoreactivity (lowest PRx) [45]. In retrospective studies, improved outcomes have been seen when CPP approximated CPPopt with evidence of higher mortality when CPP was below CPPopt (additionally, increased survival but worse outcome was seen in cases where CPP was elevated above CPPopt) [37, 43, 46, 47]. This advent in autoregulation-based CPP target optimization could simplify some of the heterogeneity seen in ABI and may yield new individualized neuroprotective strategies.
Brain Oxygenation
Oxygenation is vital to the maintenance of cellular homeostasis, and neuronal integrity and its values can be used as a marker for tissue at risk for secondary injury. The advent of invasive parenchymal catheter brain tissue oxygen (PbtO2) monitoring and jugular bulb venous oxygen saturation (SjvO2) monitoring has allowed for continuous real-time evaluation of the balance of brain tissue oxygenation providing more meaningful treatment targets for CPP and triggers for systemic evaluation. BTF guidelines have recommended placement of brain oxygenation monitoring when hyperventilatory strategies are employed after TBI with more recent MMM guidelines recommending its placement in patients at risk for ischemia [4, 5••].
There are two commercially available invasive probes measuring oxygen content: the Licox system (Integra neurosciences) and the Neurovent-PTO system (Raumedic). These probes provide regional, yet continuous monitoring via a Clark-type membrane electrode or a quenching process of fluorescence, which measures the oxygen content present within the adjacent white matter. Studies have revealed the safety and efficacy of these catheters with the mechanical caveats that values are dependent on patient temperature, location/depth, and calibration time [48].
Normal ranges of PbtO2 (Licox) have been documented as 20–35 mmHg [49], with the most recent MMM guidelines suggesting a treatment threshold of 20 mmHg [5••]. Morbidity, mortality, and extracellular evidence of metabolic crises have been associated with lower PbtO2 levels, particularly levels below 10 mmHg [50, 51]. Additionally, PbtO2 has been utilized as a target for CPP-driven therapy with evidence for improved long-term outcomes in both TBI and SAH [52, 53] (see Fig. 1). However, low PbtO2 is not specific for simple perfusion failure. Factors such as CO2, O2, and hypermetabolic states (fever, shivering, seizures) can all alter PbtO2 [54, 55]. PbtO2 is a product of CBF and arteriovenous tension of O2, indicating brain oxygenation relies on both adequate oxygen supply (perfusion/oxygenation) and extraction (diffusion) [56]. Consequently, PbtO2 monitoring can provide information on two fronts: (1) it can assess for adequate oxygenation delivery when targeting optimal CPP ranges, and (2) it can reveal non-perfusion-related brain hypoxia when CPP is at goal [54]. Similar to the other invasive probes, values will reflect the regional area in which the probe is placed. Based on the underlying etiology of ABI, the preferred location of probe placement will vary [57].
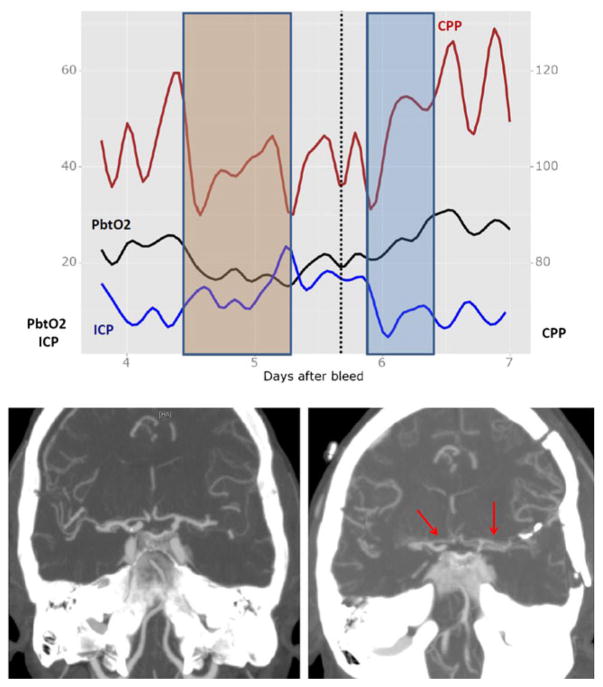
Fig. 1.
MMM in SAH patient with vasospasm. Pt is a 66-year-old woman with a Hunt Hess 4, modified Fischer scale 3 aneurysmal subarachnoid hemorrhage status post left MCA aneurysm clipping. Patient had right frontal MMM bundle placed on post bleed day 3. On post bleed day 4, patient had evidence of low PbtO2 levels to 13 with mild elevation of ICP to low 20s and subsequent drop in CPP to 90s (orange box in top figure). No evidence of seizure, infection, anemia, or systemic hypoxia. Patient received CTA head on day 5 (signified by dotted line) revealing vasospasm in bilateral A1 and M1 segments (bottom figure; a baseline CTA; b CTA on day 5). Patient received hyperosmolar therapy for elevated ICP and received pressor support with norepinephrine to goal CPP >100 with normalization of PbtO2 (blue box in top figure)
Jugular bulb venous oxygen saturation (SjvO2), unlike PbtO2 monitoring, provides information on global cerebral utilization of oxygen. Catheter placement is performed in the dominant internal jugular vein (when possible) and advanced superiorly into the jugular bulb [58]. Normal values range between 55 and 75 %. Values below 55 % signify increased oxygen extraction and tissue at risk for ischemia [59] and have been associated with poor outcomes especially when there is a failure to improve values with CBF-directed treatment [60, 61]. Cerebral compensatory mechanism for either decreased oxygen delivery or increased demand is an increase in oxygen extraction. However, once this compensatory mechanism is exhausted, ischemia is encountered and is represented by lower values of SjvO2. On the other end of the spectrum, SjvO2 above 75 % may signify hyperemia, decreased metabolic demand, or even cell death [62]. Despite its ability to represent global cerebral oxygenation, its accuracy and safety is limited in comparison to PbtO2 monitoring [63]. Furthermore, similar to PbtO2 monitoring, the primary limitation lies in its nonspecific nature. However, its usefulness lies in its trends’ ability to trigger suspicion of a subclinical state change.
Treatment strategies have focused on optimizing a twofold approach to brain oxygenation: delivery and demand. Efforts to optimize CPP, oxygenation delivery in addition to identifying, and treating hypermetabolic demand states such as fevers and seizures have been the current goals behind PbtO2 and SjvO2 optimization. By incorporating both regional (PbtO2) and global (SjvO2) monitoring with more traditional ICP/CPP monitoring, there is promise for improved outcomes over traditional methods alone [64].
Non-Invasive Brain Oxygenation
Near-infrared spectroscopy (NIRS) calculates the concentration of a particular chromophore based on the amount of light attenuation between a NIR light source and receiver. The chromophore most widely evaluated in the scope of neuroanesthesia and brain injury is oxygenated hemoglobin of cerebral tissue (rSO2). In the operating room setting of vascular and cardiothoracic surgery, NIRS has shown utility in detecting time critical global perfusion changes. The critical changes leading to SBI in the adult neurocritical care setting can result from much more subtle and regional perfusion changes. In ABI patients with often impaired baseline rSO2 levels, threshold rSO2 values <60 % have been studied to avoid ischemia and poor outcomes [65]. However, studies have not yet validated these thresholds. A second important limitation of NIRS has been the existence of intracranial pathology such as subdural hemorrhage. The transmission path of the NIR light, while enhanced in newer technology, is still quite superficial, and in the presence of a large subdural hemorrhage or scalp swelling, can lead to unreliable measurements of brain oxygenation. This continues to be an active and hopeful area of investigation given the advantage of its noninvasiveness.
Cerebral Blood Flow
Seen as the most direct marker for fuel delivery, neuroimaging modalities have been the gold standard of quantifying CBF. However, limitations of providing momentary insight into CBF at a particular timepoint have limited its use. Subsequently, the potential of continuous CBF monitoring has been promising in clinical practice. Continuous CBF monitoring has been approximated using transcranial Doppler (TCD) and achieved, albeit for a focal region of brain, with invasive thermal diffusion catheter.
TCD noninvasively measures blood flow velocity by emitting and receiving high-frequency energy. The change in frequency reflects velocity and direction of CBF [66]. It has become a mainstay in detecting vasospasm following SAH [67]. Its utility is the greatest when evaluating the anterior circulation, particularly the MCA and ICA arteries [68], and has a high degree of correlation with angiography when using a peak mean flow velocity threshold of >200 cm/s [67]. Although evidence regarding posterior circulation velocity thresholds has been conflicting, recent guidelines have recommended 85 cm/s as a threshold for vasospasm detection [5••]. Importantly, a high flow velocity can be a reflection of decreased vessel diameter (vasospasm) or increased blood volume (hyperemia). The Lindegaard ratio (LR) between the middle cerebral artery and external carotid artery velocities can help differentiate between vasospasm and hyperemia. LR values greater than 3 have been accurate in differentiating clinical and radiographic vasospasm from hyperemia [69, 70] (see Table 2). However, this data cannot be used in isolation as not all vasospasm leads to ischemic injury, and a significant proportion of ischemic injury can occur with (normal) TCD velocities <120 cm/s [71]. Environmental and technologic limitations usually prevent continuous TCD monitoring, and variability can be introduced by different operators.
Table 2.
Non-invasive MMM monitors
| Devices | Suggested parameters | Representation | |
|---|---|---|---|
| ICP |
|
|
|
| Oxygenation | NIRS | >60 % | |
| Cerebral Blood flow | TCD | <200 cm/s for anterior circulation <85 cm/s for posterior circulation LR < 3 |
Vasospasm |
ONSD optic nerve sheath diameter, TCD PI transcranial Doppler pulsatility index, NPi Neurological Pupil index, PI pulsatility index, NIRS near-infrared spectroscopy, LR Lindegaard ratio
Transcranial color-coded duplex sonography (TCCS) is another form of ultrasound-derived blood flow velocity detection. This technique visualizes arteries with angle-corrected flow velocities that may increase the yield of vessels previously thought to be difficult to insonate with conventional TCD (ICA and ACA) and may yield better correlation to angiographic vasospasm than TCD alone [72].
Invasive parenchymal thermal diffusion probes allow for continuous regional CBF monitoring, have been reported to have low rates of infection and hemorrhage [73], and have been validated by Xenon perfusion neuroimaging [74]. The most commonly utilized commercial CBF catheter is the QFlow catheter (Hemedex) which introduces heat in subcortical white matter at a depth of 20 mm and calculates the rate of temperature dissipation from measurement at a set distance, revealing local CBF (mL/100 g/min) [75]. The same limitation of a focal monitor in ABI exists for CBF probes: its utility depends on proximity to the penumbra of interest. Although CBF levels below 20 cm3/100 g/min are associated with ischemia burden and vasospasm detection [76], treatment thresholds for values of CBF with these probes have not been well established. However, CBF extremes along with its trends can be useful as an adjunctive monitor to other modalities and may provide feedback for therapeutic interventions.
Electroencephalography
Continuous electroencephalography (cEEG) for the early detection of seizure continues to be the cornerstone of its utility, due to the high incidence of subclinical seizures in the ICU [77, 78]. Delayed detection and treatment results in a significant decrease in the abortive efficacy of anti-epileptic drugs [79]. Prolonged seizures can result in increased ICP, increased metabolic demand, and excitotoxicity. Therefore, indications for cEEG monitoring after SBI are unexplained or prolonged altered consciousness [5••].
cEEG is increasingly utilized as a continuous and regional monitor of cerebral ischemia. EEG changes including attenuation and slowing, while nonspecific, can be indicative of decreasing CBF. Quantitative analysis of EEG (qEEG) via fast fourier transform provides an objective and processed depiction of raw EEG data. Alpha/delta ratio, power, and relative alpha variability can be utilized in the detection of delayed cerebral ischemia (DCI) following subarachnoid hemorrhage (SAH) [80, 81]. While theoretical, qEEG variables could provide feedback while targeting optimal CPP goals. However, in the absence of evidence, this cannot be recommended.
Intracortical depth electrodes detect seizures and cortical spreading depression that cannot be detected on scalp EEG [82, 83]. Depth seizures may be common after brain injury based on cohort studies and may be reflective of severe injury leading to worse outcomes [82]. However, the effect on outcome when treating such seizures is unclear. The placement of the depth electrode has a similar safety profile to other MMM invasive devices [84] and may potentially be utilized to carry out quantitative analysis for a more sensitive means to detect vasospasm [85]. Larger studies have yet to evaluate the full potential of the depth electrode. At a minimum, different EEG modalities provide both global and regional perspectives that can serve a valuable adjunctive role in interpreting focal physiologic data from other MMM techniques. There is promise in the high spatial and temporal resolution for aiding in treatment decisions and prognostication in all forms of ABI.
Cerebral Metabolism
Cerebral microdialysis (MD) analyses substrates within extracellular fluid of regional subcortical white matter to provide real-time monitoring of brain chemistry and detect energy crisis. Artificial CSF dialysate is exposed through a MD catheter with a semipermeable membrane, allowing molecules of a certain size or smaller (usually 20,000 Da) to equilibrate down its concentration gradient. Perilesional placement is recommended for focal injuries. While controversy exists regarding ideal placement for more diffuse injuries such as TBI, right frontal placement is recommended to favor less eloquent brain regions [5••].
The extracellular substrates typically measured are lactate, pyruvate, glucose, glutamate, and glycerol. Trends and combined change patterns have become the focus of its utility [86]. Glutamate is an excitatory neurotransmitter associated with injury and inflammatory cascade responses. Glycerol is a lipid-rich component of neurons and is a marker of CNS cellular breakdown. Its elevation is commonly associated with irreversible cellular death/ischemia.
Lactate, pyruvate, and the lactate to pyruvate ratio (LPR) are commonly interpreted as straightforward chemical markers of hypoxia or ischemia, which many utilize as targets for perfusion optimization. LPR of >40 and >25 have both been reported as thresholds of metabolic distress, and observational studies have suggested LPR >25 in the first 72 h is associated with poor outcome in TBI [87, 88]. Whether these thresholds represent outcome-modifying targets is unproven. Mitochondrial dysfunction appears to play a significant role in LPR derangement; LPR and lactate levels can be elevated in the absence of hypoxia or ischemia [89]. Care must be taken to recognize this complexity in the interpretation of suboptimal LPR, and look to adjunctive modalities to confirm perfusion failure (e.g., CBF) in targeting interventions (e.g., optimizing CPP) [89, 90, 91•].
MMM Bioinformatics Integration Systems
The advent and continued development of MMM has created a plethora of data with the potential of driving therapeutic interventions. A temporal, integrative analytic approach to multiple components of neuromonitoring is necessary to identify subclinical events in order to intervene in a timely fashion. However, the sheer quantity of data and cognitive complexity required to translate MMM data into a timely treatment regimen can be out of reach of the typical neuroICU workflow. An integration system relying on bioinformatics would enhance the ability to dynamically treat the complex multivariable disease which is SBI.
The key steps for integrating these multiple parameters are data acquisition, integration, processing, and visualization in a single, user friendly interface [92]. Current efforts towards accomplishing this are necessarily individualized to practice environments and include bedside integration systems or centralized database solutions (see Fig. 1).
Conclusion
Strides must be taken to utilize the myriad of moving physiologic variables in a centralized, real-time montage in order to create an information interface that can influence real-time treatment decisions. However, these treatment strategies and future trial designs must step away from a “one size fits all” treatment threshold utilizing a single component of MMM. Rather individual modes of MMM that can reveal cerebral and cerebrovascular physiology should integrate information from multiple modalities to reveal the patient-specific “injury profile” and vascular compliance in order to formulate an optimal treatment plan. It is not the device itself that will improve outcomes, but early recognition of these injury patterns and timely administration of individually tailored treatments.
Acknowledgments
Soojin Park reports grants from National Institutes of Health K01-ES026833.
Funding/Support SP is supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number K01ES026833. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This article is part of the Topical Collection on Critical Care
Conflict of Interest David Roh declares that he has no conflict of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Authors’ Contributions Roh: Acquisition of data, analysis and interpretation, draft of the manuscript, and critical revision of the manuscript for important intellectual content.
Park: Critical revision of the manuscript for important intellectual content.
Contributor Information
David Roh, Email: dr2753@cumc.columbia.edu.
Soojin Park, Email: sp3291@cumc.columbia.edu.
References
Papers of particular interest, published recently in the past 3 years, have been highlighted as:
• Of importance
• Of major importance
- 1.Marmarou A, et al. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. Spec Suppl. 1991;75:S59–66. [Google Scholar]
- 2.Narayan RK, et al. Intracranial pressure: to monitor or not to monitor? A review of our experience with severe head injury. J Neurosurg. 1982;56:650–9. doi: 10.3171/jns.1982.56.5.0650. [DOI] [PubMed] [Google Scholar]
- 3.Badri S, et al. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012;38:1800–9. doi: 10.1007/s00134-012-2655-4. [DOI] [PubMed] [Google Scholar]
- 4.Brain Trauma Foundation, American Association of Neurological Surgeons & Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 5••.Le Roux P, et al. The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a list of recommendations and additional conclusions: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21(Suppl 2):S282–96. doi: 10.1007/s12028-014-0077-6. International, collaborative consensus statement on the use of physiologic bedside brain monitoring in ABI. Multidisciplinary experts in the field reviewed current literature on multiple MMM techniques in order to create evidence-based or expert-based practice guidelines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremer OL, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit Care Med. 2005;33:2207–13. doi: 10.1097/01.ccm.0000181300.99078.b5. [DOI] [PubMed] [Google Scholar]
- 7••.Chesnut RM, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–81. doi: 10.1056/NEJMoa1207363. Multicenter randomized controlled trial of 324 severe TBI patients comparing ICP treatment protocols utilizing parenchymal ICP monitoring compared to imaging and clinical examination alone as treatment triggers. No difference between two groups at 3- and 6-month follow-up in outcome and functional status. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber LM, Chiu Y-L, Carney N, Härtl R, Ghajar J. Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg. 2013;119:1583–90. doi: 10.3171/2013.8.JNS13276. [DOI] [PubMed] [Google Scholar]
- 9.Alali AS, et al. Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma. 2013;30:1737–46. doi: 10.1089/neu.2012.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talving P, et al. Intracranial pressure monitoring in severe head injury: compliance with Brain Trauma Foundation guidelines and effect on outcomes: a prospective study. J Neurosurg. 2013;119:1248–54. doi: 10.3171/2013.7.JNS122255. [DOI] [PubMed] [Google Scholar]
- 11.Olson DM, Batjer HH, Abdulkadir K, Hall CE. Measuring and monitoring ICP in Neurocritical Care: results from a national practice survey. Neurocrit Care. 2014;20:15–20. doi: 10.1007/s12028-013-9847-9. [DOI] [PubMed] [Google Scholar]
- 12.Bauer DF, Razdan SN, Bartolucci AA, Markert JM. Meta-analysis of hemorrhagic complications from ventriculostomy placement by neurosurgeons. Neurosurgery. 2011;69:255–60. doi: 10.1227/NEU.0b013e31821a45ba. [DOI] [PubMed] [Google Scholar]
- 13.Helbok R, Olson DM, Le Roux PD, Vespa P. Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. Intracranial pressure and cerebral perfusion pressure monitoring in non-TBI patients: special considerations. Neurocrit Care. 2014;21(Suppl 2):S85–94. doi: 10.1007/s12028-014-0040-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, et al. External ventricular drains versus intraparenchymal intracranial pressure monitors in traumatic brain injury: a prospective observational study. World Neurosurg. 2015;83:794–800. doi: 10.1016/j.wneu.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Webb AJS, et al. Resolution of intraventricular hemorrhage varies by ventricular region and dose of intraventricular thrombolytic: the Clot Lysis: Evaluating Accelerated Resolution of IVH (CLEAR IVH) program. Stroke J Cereb Circ. 2012;43:1666–8. doi: 10.1161/STROKEAHA.112.650523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziai WC, et al. Occurrence and impact of intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Crit Care Med. 2012;40:1601–8. doi: 10.1097/CCM.0b013e318241e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahuquillo J, Poca MA, Arribas M, Garnacho A, Rubio E. Interhemispheric supratentorial intracranial pressure gradients in head-injured patients: are they clinically important? J Neurosurg. 1999;90:16–26. doi: 10.3171/jns.1999.90.1.0016. [DOI] [PubMed] [Google Scholar]
- 18.Bekar A, et al. Risk factors and complications of intracranial pressure monitoring with a fiberoptic device. J Clin Neurosci Off J Neurosurg Soc Australas. 2009;16:236–40. doi: 10.1016/j.jocn.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Lescot T, et al. In vivo accuracy of two intraparenchymal intracranial pressure monitors. Intensive Care Med. 2011;37:875–9. doi: 10.1007/s00134-011-2182-8. [DOI] [PubMed] [Google Scholar]
- 20.Treggiari MM, Schutz N, Yanez ND, Romand J-A. Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: a systematic review. Neurocrit Care. 2007;6:104–12. doi: 10.1007/s12028-007-0012-1. [DOI] [PubMed] [Google Scholar]
- 21•.Bouzat P, et al. Accuracy of brain multimodal monitoring to detect cerebral hypoperfusion after traumatic brain injury*. Crit Care Med. 2015;43:445–52. doi: 10.1097/CCM.0000000000000720. Prospective observational study on 27 severe TBI patients with MMM and its ability to predict low cerebral blood flow measured via perfusion CT. Combining ICP, PbtO2, and microdialysis provides more accurate detection of cerebral hypoperfusion than ICP monitoring alone. [DOI] [PubMed] [Google Scholar]
- 22.Scalzo F, Bergsneider M, Vespa PM, Martin NA, Hu X. Intracranial pressure signal morphology: real-time tracking. IEEE Pulse. 2012;3:49–52. doi: 10.1109/MPUL.2011.2181024. [DOI] [PubMed] [Google Scholar]
- 23.Güiza F, Depreitere B, Piper I, Van den Berghe G, Meyfroidt G. Novel methods to predict increased intracranial pressure during intensive care and long-term neurologic outcome after traumatic brain injury: development and validation in a multicenter dataset. Crit Care Med. 2013;41:554–64. doi: 10.1097/CCM.0b013e3182742d0a. [DOI] [PubMed] [Google Scholar]
- 24.Kristiansson H, et al. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25:372–85. doi: 10.1097/ANA.0b013e31829795ce. [DOI] [PubMed] [Google Scholar]
- 25.Moreno JA, et al. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg Focus. 2000;8:e8. doi: 10.3171/foc.2000.8.1.1702. [DOI] [PubMed] [Google Scholar]
- 26.Bellner J, et al. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP) Surg Neurol. 2004;62:45–51. doi: 10.1016/j.surneu.2003.12.007. discussion 51. [DOI] [PubMed] [Google Scholar]
- 27.Zweifel C, et al. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012;71:853–61. doi: 10.1227/NEU.0b013e3182675b42. [DOI] [PubMed] [Google Scholar]
- 28.Behrens A, et al. Transcranial Doppler pulsatility index: not an accurate method to assess intracranial pressure. Neurosurgery. 2010;66:1050–7. doi: 10.1227/01.NEU.0000369519.35932.F2. [DOI] [PubMed] [Google Scholar]
- 29.Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506–15. doi: 10.1007/s12028-011-9606-8. [DOI] [PubMed] [Google Scholar]
- 30.Cammarata G, et al. Ocular ultrasound to detect intracranial hypertension in trauma patients. J Trauma. 2011;71:779–81. doi: 10.1097/TA.0b013e3182220673. [DOI] [PubMed] [Google Scholar]
- 31.Bäuerle J, Lochner P, Kaps M, Nedelmann M. Intra- and interobsever reliability of sonographic assessment of the optic nerve sheath diameter in healthy adults. J Neuroimaging Off J Am Soc Neuroimaging. 2012;22:42–5. doi: 10.1111/j.1552-6569.2010.00546.x. [DOI] [PubMed] [Google Scholar]
- 32.Ballantyne SA, O’Neill G, Hamilton R, Hollman AS. Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur J Ultrasound Off J Eur Fed Soc Ultrasound Med Biol. 2002;15:145–9. doi: 10.1016/s0929-8266(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 33.Chen JW, et al. Pupillary reactivity as an early indicator of increased intracranial pressure: the introduction of the Neurological Pupil index. Surg Neurol Int. 2011;2:82. doi: 10.4103/2152-7806.82248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosty JA, et al. Brief report: a comparison of clinical and research practices in measuring cerebral perfusion pressure: a literature review and practitioner survey. Anesth Analg. 2013;117:694–8. doi: 10.1213/ANE.0b013e31829cc765. [DOI] [PubMed] [Google Scholar]
- 35.Andrews PJD, et al. Predicting recovery in patients suffering from traumatic brain injury by using admission variables and physiological data: a comparison between decision tree analysis and logistic regression. J Neurosurg. 2002;97:326–36. doi: 10.3171/jns.2002.97.2.0326. [DOI] [PubMed] [Google Scholar]
- 36.Juul N, Morris GF, Marshall SB, Marshall LF. Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J Neurosurg. 2000;92:1–6. doi: 10.3171/jns.2000.92.1.0001. [DOI] [PubMed] [Google Scholar]
- 37.Johnson U, Nilsson P, Ronne-Engström E, Howells T, Enblad P. Favorable outcome in traumatic brain injury patients with impaired cerebral pressure autoregulation when treated at low cerebral perfusion pressure levels. Neurosurgery. 2011;68:714–21. doi: 10.1227/NEU.0b013e3182077313. discussion 721–722. [DOI] [PubMed] [Google Scholar]
- 38.Sviri GE, Aaslid R, Douville CM, Moore A, Newell DW. Time course for autoregulation recovery following severe traumatic brain injury. J Neurosurg. 2009;111:695–700. doi: 10.3171/2008.10.17686. [DOI] [PubMed] [Google Scholar]
- 39.Contant CF, Valadka AB, Gopinath SP, Hannay HJ, Robertson CS. Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg. 2001;95:560–8. doi: 10.3171/jns.2001.95.4.0560. [DOI] [PubMed] [Google Scholar]
- 40.Robertson CS, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27:2086–95. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson EA, et al. Cerebral perfusion pressure and intracranial pressure are not surrogates for brain tissue oxygenation in traumatic brain injury. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2012;123:1255–60. doi: 10.1016/j.clinph.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 42.Czosnyka M, et al. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–7. doi: 10.1097/00006123-199707000-00005. discussion 17–19. [DOI] [PubMed] [Google Scholar]
- 43.Aries MJH, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–63. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- 44.Ko S-B, et al. Multimodality monitoring for cerebral perfusion pressure optimization in comatose patients with intracerebral hemorrhage. Stroke J Cereb Circ. 2011;42:3087–92. doi: 10.1161/STROKEAHA.111.623165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steiner LA, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–8. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Depreitere B, et al. Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg. 2014;120:1451–7. doi: 10.3171/2014.3.JNS131500. [DOI] [PubMed] [Google Scholar]
- 47.Balestreri M, et al. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit Care. 2006;4:8–13. doi: 10.1385/NCC:4:1:008. [DOI] [PubMed] [Google Scholar]
- 48.Stewart C, et al. The new Licox combined brain tissue oxygen and brain temperature monitor: assessment of in vitro accuracy and clinical experience in severe traumatic brain injury. Neurosurgery. 2008;63:1159–64. doi: 10.1227/01.NEU.0000333265.19131.7C. discussion 1164–1165. [DOI] [PubMed] [Google Scholar]
- 49.Pennings FA, Schuurman PR, van den Munckhof P, Bouma GJ. Brain tissue oxygen pressure monitoring in awake patients during functional neurosurgery: the assessment of normal values. J Neurotrauma. 2008;25:1173–7. doi: 10.1089/neu.2007.0402. [DOI] [PubMed] [Google Scholar]
- 50.Maloney-Wilensky E, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med. 2009;37:2057–63. doi: 10.1097/CCM.0b013e3181a009f8. [DOI] [PubMed] [Google Scholar]
- 51.Hlatky R, Valadka AB, Goodman JC, Contant CF, Robertson CS. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J Neurotrauma. 2004;21:894–906. doi: 10.1089/0897715041526195. [DOI] [PubMed] [Google Scholar]
- 52.Bohman L-E, et al. Medical management of compromised brain oxygen in patients with severe traumatic brain injury. Neurocrit Care. 2011;14:361–9. doi: 10.1007/s12028-011-9526-7. [DOI] [PubMed] [Google Scholar]
- 53.Bohman L-E, et al. Response of brain oxygen to therapy correlates with long-term outcome after subarachnoid hemorrhage. Neurocrit Care. 2013;19:320–8. doi: 10.1007/s12028-013-9890-6. [DOI] [PubMed] [Google Scholar]
- 54.Stiefel MF, et al. Conventional neurocritical care and cerebral oxygenation after traumatic brain injury. J Neurosurg. 2006;105:568–75. doi: 10.3171/jns.2006.105.4.568. [DOI] [PubMed] [Google Scholar]
- 55.Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke J Cereb Circ. 2007;38:981–6. doi: 10.1161/01.STR.0000257964.65743.99. [DOI] [PubMed] [Google Scholar]
- 56.Rosenthal G, et al. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008;36:1917–24. doi: 10.1097/CCM.0b013e3181743d77. [DOI] [PubMed] [Google Scholar]
- 57.Ulrich CT, et al. Occurrence of vasospasm and infarction in relation to a focal monitoring sensor in patients after SAH: placing a bet when placing a probe? PLoS ONE. 2013;8:e62754. doi: 10.1371/journal.pone.0062754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gok F, Kilicaslan A, Yosunkaya A. Ultrasound-guided jugular bulb catheterisation in the intensive care unit. Anaesth Intensive Care. 2014;42:523–4. [PubMed] [Google Scholar]
- 59.Sheinberg M, et al. Continuous monitoring of jugular venous oxygen saturation in head-injured patients. J Neurosurg. 1992;76:212–7. doi: 10.3171/jns.1992.76.2.0212. [DOI] [PubMed] [Google Scholar]
- 60.Gopinath SP, et al. Jugular venous desaturation and outcome after head injury. J Neurol Neurosurg Psychiatry. 1994;57:717–23. doi: 10.1136/jnnp.57.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le Roux PD, Newell DW, Lam AM, Grady MS, Winn HR. Cerebral arteriovenous oxygen difference: a predictor of cerebral infarction and outcome in patients with severe head injury. J Neurosurg. 1997;87:1–8. doi: 10.3171/jns.1997.87.1.0001. [DOI] [PubMed] [Google Scholar]
- 62.Cormio M, Valadka AB, Robertson CS. Elevated jugular venous oxygen saturation after severe head injury. J Neurosurg. 1999;90:9–15. doi: 10.3171/jns.1999.90.1.0009. [DOI] [PubMed] [Google Scholar]
- 63.Gupta AK, et al. Measuring brain tissue oxygenation compared with jugular venous oxygen saturation for monitoring cerebral oxygenation after traumatic brain injury. Anesth Analg. 1999;88:549–53. doi: 10.1097/00000539-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 64.Stiefel MF, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103:805–11. doi: 10.3171/jns.2005.103.5.0805. [DOI] [PubMed] [Google Scholar]
- 65.Davies DJ, et al. Near-infrared spectroscopy in the monitoring of adult traumatic brain injury: a review. J Neurotrauma. 2015;32:933–41. doi: 10.1089/neu.2014.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller C, Armonda R. Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring. Monitoring of cerebral blood flow and ischemia in the critically ill. Neurocrit Care. 2014;21(Suppl 2):S121–8. doi: 10.1007/s12028-014-0021-9. [DOI] [PubMed] [Google Scholar]
- 67.Vora, Suarez-Almazor, Steinke, Martin, Findlay Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44:1237–1247. discussion 1247–1248. [PubMed] [Google Scholar]
- 68.Suarez JI, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30:1348–55. doi: 10.1097/00003246-200206000-00035. [DOI] [PubMed] [Google Scholar]
- 69.Gonzalez NR, Boscardin WJ, Glenn T, Vinuela F, Martin NA. Vasospasm probability index: a combination of transcranial doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107:1101–12. doi: 10.3171/JNS-07/12/1101. [DOI] [PubMed] [Google Scholar]
- 70.Nakae R, Yokota H, Yoshida D, Teramoto A. Transcranial Doppler ultrasonography for diagnosis of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: mean blood flow velocity ratio of the ipsilateral and contralateral middle cerebral arteries. Neurosurgery. 2011;69:876–83. doi: 10.1227/NEU.0b013e318222dc4c. discussion 883. [DOI] [PubMed] [Google Scholar]
- 71.Carrera E, et al. Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2009;65:316–23. doi: 10.1227/01.NEU.0000349209.69973.88. discussion 323–324. [DOI] [PubMed] [Google Scholar]
- 72.Proust F, et al. Usefulness of transcranial color-coded sonography in the diagnosis of cerebral vasospasm. Stroke J Cereb Circ. 1999;30:1091–8. doi: 10.1161/01.str.30.5.1091. [DOI] [PubMed] [Google Scholar]
- 73.Sioutos PJ, et al. Continuous regional cerebral cortical blood flow monitoring in head-injured patients. Neurosurgery. 1995;36:943–9. doi: 10.1227/00006123-199505000-00009. discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 74.Vajkoczy P, et al. Continuous monitoring of regional cerebral blood flow: experimental and clinical validation of a novel thermal diffusion microprobe. J Neurosurg. 2000;93:265–74. doi: 10.3171/jns.2000.93.2.0265. [DOI] [PubMed] [Google Scholar]
- 75.Bhatia A, Gupta AK. Neuromonitoring in the intensive care unit. I. Intracranial pressure and cerebral blood flow monitoring. Intensive Care Med. 2007;33:1263–71. doi: 10.1007/s00134-007-0678-z. [DOI] [PubMed] [Google Scholar]
- 76.Vajkoczy P, Horn P, Thome C, Munch E, Schmiedek P. Regional cerebral blood flow monitoring in the diagnosis of delayed ischemia following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;98:1227–34. doi: 10.3171/jns.2003.98.6.1227. [DOI] [PubMed] [Google Scholar]
- 77.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 78.DeLorenzo RJ, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833–40. doi: 10.1111/j.1528-1157.1998.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 79.Chen JWY, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246–56. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 80.Vespa PM, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103:607–15. doi: 10.1016/s0013-4694(97)00071-0. [DOI] [PubMed] [Google Scholar]
- 81.Claassen J, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2004;115:2699–710. doi: 10.1016/j.clinph.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 82.Claassen J, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol. 2013;74:53–64. doi: 10.1002/ana.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Claassen J, Vespa P. Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring. Electrophysiologic monitoring in acute brain injury. Neurocrit Care. 2014;21(Suppl 2):S129–47. doi: 10.1007/s12028-014-0049-x. [DOI] [PubMed] [Google Scholar]
- 84.Stuart RM, et al. Intracranial multimodal monitoring for acute brain injury: a single institution review of current practices. Neurocrit Care. 2010;12:188–98. doi: 10.1007/s12028-010-9330-9. [DOI] [PubMed] [Google Scholar]
- 85.Stuart RM, et al. Intracortical EEG for the detection of vasospasm in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2010;13:355–8. doi: 10.1007/s12028-010-9414-6. [DOI] [PubMed] [Google Scholar]
- 86.Reinstrup P, et al. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery. 2000;47:701–9. doi: 10.1097/00006123-200009000-00035. discussion 709–710. [DOI] [PubMed] [Google Scholar]
- 87.Timofeev I, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain J Neurol. 2011;134:484–94. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- 88.Hutchinson P, O’Phelan K. Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. International multidisciplinary consensus conference on multimodality monitoring: cerebral metabolism. Neurocrit Care. 2014;21(Suppl 2):S148–58. doi: 10.1007/s12028-014-0035-3. [DOI] [PubMed] [Google Scholar]
- 89.Vespa PM, et al. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit Care Med. 2007;35:1153–60. doi: 10.1097/01.CCM.0000259466.66310.4F. [DOI] [PubMed] [Google Scholar]
- 90.Vespa P, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2005;25:763–74. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91•.Sala N, et al. Cerebral extracellular lactate increase is predominantly nonischemic in patients with severe traumatic brain injury. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33:1815–22. doi: 10.1038/jcbfm.2013.142. Prospective observational study of 24 patients with severe TBI with MMM and perfusion CT. Elevations in lactate were found primarily in non-hypoxic periods suggesting glycolytic pathway to elevated lactate formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt JM, De Georgia M. Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. Multimodality monitoring: informatics, integration data display and analysis. Neurocrit Care. 2014;21(Suppl 2):S229–38. doi: 10.1007/s12028-014-0037-1. [DOI] [PubMed] [Google Scholar]