Abstract
Background
Despite the known links between weakness and early mortality, what remains to be fully understood is the extent to which strength preservation is associated with protection from cardiometabolic diseases such as diabetes.
Purpose
The purposes of this study were to determine the association between muscle strength and diabetes among adults, and to identify age- and sex-specific thresholds of low strength for detection of risk.
Methods
A population-representative sample of 4,066 individuals, aged 20–85 years, was included from the combined 2011–2012 National Health and Nutrition Examination Survey datasets. Strength was assessed using a hand-held dynamometer, and the single largest reading from either hand was normalized to body mass. A logistic regression model was used to assess the association between normalized grip strength and risk of diabetes, as determined by hemoglobin A1c (HbA1c) levels (≥6.5% [≥48 mmol/mol]), while controlling for sociodemographic characteristics, anthropometric measures, and television viewing time.
Results
For every 0.05 decrement in normalized strength, there was a 1.26 times increased adjusted odds for diabetes in men and women. Women were at lower odds of having diabetes (OR: 0.49; 95% CI: 0.29–0.82), whereas age, waist circumference and lower income were inversely associated. Optimal sex- and age-specific weakness thresholds to detect diabetes were 0.56, 0.50, and 0.45 for men, and 0.42, 0.38, and 0.33 for women, for ages 20–39 years, 40–59 years, and 60–80 years.
Conclusions and Clinical Relevance
We present thresholds of strength that can be incorporated into a clinical setting for identifying adults that are at risk for developing diabetes, and that might benefit from lifestyle interventions to reduce risk.
Introduction
Worldwide, there are more than 380 million people with diabetes, and the associated economic burden has reached nearly $550 billion in the U.S. alone [1]. Age-related declines in physical function and morphological health further contribute to exaggerated risk at the individual level; and yet, increases in the incidence of diagnosed diabetes combined with declining mortality or increased life-expectancy have led to an acceleration of lifetime risk and more years spent with diabetes at the population level [2]. Early screening and promotion efforts for healthy aging among higher-risk populations are thus vital to reduce incidence and preventable comorbidities, as well as to curtail the escalating healthcare burden associated with diabetes.
Of particular relevance to both, there is increasingly compelling evidence to highlight the importance of muscular strength as a protective factor for health across populations. Perhaps the quintessential example of this is represented by the growing body of survival studies that demonstrates an independent association between muscle weakness and early, cardiovascular- and all-cause mortality [3–9]. The contribution of muscle atrophy and weakness on progression of secondary complications with aging and/or disease (e.g., frailty, mobility-disability, etc.) is equally unequivocal and recent national efforts to identify cut points or thresholds for weakness among older adults [9–11] will aid clinicians to screen individuals with greatest risk.
What remains to be fully understood is the extent to which strength preservation is associated with protection from cardiometabolic diseases such as diabetes, and moreover, whether age- and sex-specific cut points for strength can be established for risk stratification. Senechal and colleagues [12] have shown that low strength is independently associated with an increased odds of the metabolic syndrome in middle-aged and older men, and were able to identify cut points for low normalized strength that best predicted increased risk. Two very recent studies from the Baltimore Longitudinal Study of Aging (BLSA) have demonstrated that greater adiposity [13] and chronic hyperglycemia [14] (i.e., two hallmark features of diabetes) are associated with persistently lower muscle quality and strength, respectively, and that these secondary consequences may be mediated by neurological factors such as neuropathy. We and others have shown an independent, inverse association between low strength and cardiometabolic risk clustering even among adolescents [15–17]-reiterating the need for early and improved clinical screening strategies across populations. Therefore, the purposes of this study were to examine the independent association between handgrip strength capacity and diabetes in a large, nationally-representative sample, and to explore potential age- and sex-specific thresholds of weakness, for optimal risk categorization.
Research Design and Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a program of studies designed to assess the health and nutritional status of adults and children in the United States. The NHANES 2011–2012 survey was specifically chosen based on the wealth of relevant information pertaining to markers of cardiometabolic health for diabetes and insulin resistance (IR), and direct measures of muscle strength capacity. Of the 5,319 participants of the NHANES 2011–2012 who were 20 years and older, 4,066 had (1) complete demographic and anthropometric data; (2) valid strength data from a handgrip dynamometer; (3) the necessary blood samples obtained for non-fasting glycohemoglobin determination, and (4) had valid questionnaire data pertaining to daily and weekly physical activities and sedentary behaviors. Ethical approval was obtained through the National Center for Health Statistics (NCHS) Research Ethics Review Board (Protocol #2011–17), and subsequent approval for secondary data analyses was not required. All procedures followed were in accordance with the ethical standards of the NCHS Research Ethics Review Board, and with the Helsinki Declaration of 1975, as revised in 2013, and informed consent was obtained from all patients included in the study.
Demographic and Anthropometric Factors
Socio-demographic characteristics were all assessed by self-report during the in-home interview. Age was used as a continuous variable. Race/ethnicity was categorized as: (1) non-Hispanic white, (2) non-Hispanic black, (3) Mexican American or other Hispanic, and (4) Other-including multi-racial. Education was categorized as: (1) less than high school graduate, (2) high school graduate/general educational development (GED) or equivalent, and/or some college or Associate’s degree (e.g., Associate of Arts or Associate of Science), and (3) college graduate or above. Annual household income was categorized as: (1) ≤ $24,999, (2) $25,000–$54,999, (3) $55,000–$74,999, and (4) ≥$75,000.
Weight was measured using a digital Toledo scale (Mettler-Toledo International, Inc., Columbus, OH), and participants wore only underwear gown and foam slippers. Height was measured using a fixed stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Standard categories were applied to determine if each participant was normal weight (18.5–24.9), overweight (25–29.9), or obese (≥ 30). Individuals with BMI <18.5 kg/m2 were excluded, due to the known association between underweight status and diabetes risk in older adults [18]. Waist circumference was measured to the nearest 0.1 cm at the level of the iliac crest, and used in the analyses as a continuous variable.
Cardiometabolic Parameters
Participants were tested on routine cardiometabolic parameters. Resting systolic and diastolic blood pressures were measured three to four times with a mercury sphygmomanometer by trained staff. Non-fasting measures of total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, glucose and insulin concentrations were measured. Non-fasting serum measures of glycohemoglobin (%) were included as a diagnostic test for diabetes, which reflects average plasma glucose for the previous ~3 months. For a subset of individuals, fasting measures were obtained for plasma glucose, and insulin, as well as a 2-hour oral glucose tolerance test (OGTT). For fasting glucose and insulin, the homeostasis model assessment (HOMA) was calculated according to the formula: [Insulin (I)0 (μU/ml) × Glucose (G)0 (mmol/l)]/22.5. For the OGTT, eligible participants were administered a 75 g (or a calibrated dose for participants weighing <94 pounds) glucose load (Trutol) OGTT and a blood sample was drawn 2 hours later. Glucose was measured in plasma by a hexokinase method using a Roche/Hitachi 911 Analyzer and Roche Modular P Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN). The interassay coefficient of variation ranged from 0.8% to 2.6%.
Subjects were classified with/without diabetes or IR, on the basis of laboratory data from fasting and non-fasting plasma. The primary analyses were based on elevated non-fasting HbA1c (≥6.5% [≥48 mmol/mol]) [19], as this provided the largest sample size. A separate analysis was completed on the smaller subset of individuals with fasting plasma measures for which diabetes or IR was designated on the combined basis of (1) elevated fasting glucose (≥ 126 mg/dL), (2) elevated 2-hour glucose during the OGTT (≥ 200 mg/dL) and/or (3) a HOMA score of ≥5.9, as validated against hyperinsulinemic-euglycemic clamp by Tam and colleagues.[20] Diabetes was not categorized into type 1 or 2. Therefore, participants with diabetes that had been diagnosed at age 30 years or younger, and/or that were being treated with only insulin alone were excluded, as they were considered likely to have type 1 diabetes.
Exposure Variable
Grip Strength
Strength was assessed using a hydraulic handgrip dynamometer (Takei Digital Grip Strength Dynamometer, Model T.K.K.5401). Detailed descriptions of the protocol are provided in the NHANES Muscle Strength/Grip Test Procedure Manual [21]. Briefly, a trained examiner explained and demonstrated the protocol to the participant, then adjusted the grip size of the dynamometer to the participant’s hand size, and asked the participant to squeeze the dynamometer for a practice trial. Thereafter, the participant was randomly assigned to start the test with his/her dominant or non-dominant hand, and was asked to squeeze the dynamometer as hard as possible, exhaling while squeezing. The test was then repeated for the opposite hand. Each hand was tested three times, alternating hands between trials with a 60-second rest between measurements on the same hand. The grip test was performed in the standing position unless the participant was physically limited. Participants were excluded from this component if they were unable to hold the dynamometer and perform strength testing with both hands. Participants who had surgery on either hand or wrist in the last three months were not tested on that particular hand. Since there is substantial covariance between strength capacity and body mass, and moreover that the link between muscle strength and both physical function and chronic health is mediated by the proportion of strength relative to body mass, grip strength was normalized as strength per body mass (i.e., ).
Potential Covariates
Sedentary Behavior
Sedentary behavior was determined during the 2011–2012 NHANES cohort through questionnaires (variable name prefix PAQ), which was based on the World Health Organization Global Physical Activity Questionnaire (GPAQ) [22, 23]. For this study, sedentary behavior was analyzed in two ways, including (1) participants’ answers to a general question about total combined sitting time per day (i.e., in hours) during work, at home, transportation, reading, playing cards, using a computer, etc.; and (2) participants’ self-reported television or video viewing time per day, during the past 30 days. For television or video viewing time, participants were asked to provide answers as relevant to the following categories (a) <1 hour, (b) 1 hour, (c) 2 hours, (d) 3 hours, (e) 4 hours, (f) ≥5 hours, or (g) does not watch TV or videos (which we combined with “< 1 hour” for the analyses). For both questions, all data from participants that refused to answer or did not know the answer were coded as missing.
Statistical Analysis
All statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC) and R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria) with survey package [24]. NHANES employs a multistage sampling design. Sample weights were used to adjust for oversampling, survey nonresponse, and post-stratification. Further, we took into account subsample weights since we conducted analysis on persons with non-fasting glucose measure. These weights were used to produce the unbiased estimates. To obtain correct variance estimation, information on strata and primary sampling unit (PSU) were also utilized. Descriptive characteristics are provided as means, standard errors, and percentages. Differences in these characteristics across age categories were tested using linear regression (proc surveyreg) and logistic regression (proc surveylogistic) for continuous and categorical variables respectively, after creating appropriate categories and dummy coding for each. A similar strategy was used to test differences for outcomes between men and women across equivalent age categories. To assess the odds of diabetes or IR in the entire sample, we utilized the multivariate logistic regression modeling approach with the forward and backward selection procedure. Known risk factors, including gender, age, waist circumference, race, sedentary behavior, normalized strength, were adjusted in the model. Education, income, quadratic of age, quadratic of income, quadratic of sedentary behavior, interaction of gender and race, interaction of gender and normalized strength were determined if included in the final through the forward and backward selection procedure. The logistic regression model with the highest Akaike information criterion (AIC) was retained as the final model. The performance of the final logistic regression model was evaluated through a receiver operating characteristic (ROC) curve, which was created by plotting the true positive rate (TPR) against the false positive rate (FPR) at various threshold settings. The calculation of TPR and FPR were also properly accounted for sample weights, strata, and PSU.
Threshold Analyses
We also investigated individual strength thresholds across subgroups stratified by gender and age category (20–40 years, 40–60 years, and 60–80 years). Within each group, the predictive probabilities of all subjects were calculated. A non-parametric curve with local polynomials fitted with 4 degrees of freedom on normalized strength vs logit of predictive probabilities was obtained (svysmooth in R survey package). This non-parametric smoothing curve depicts the relationship between decreased normalized strength and increased risk of diabetes (HbA1c≥6.5% [≥48 mmol/mol]). We then calculated the lower and upper tertiles of predictive probabilities (33.3% and 66.7% percentiles) of risk for diabetes, and the corresponding normalized strength values were determined as the screening thresholds.
Results
Descriptive data are presented as means, standard errors, and percentages across age categories in Table 1. In both men and women, prevalence of abdominal obesity and most cardiometabolic risk factors were significantly higher across age categories. Diabetes prevalence, according to elevated HbA1c (≥6.5% [≥48 mmol/mol]), was higher with increasing age and was 3.2%, 13.2%, 23.7% for men and 3.1%, 11.1%, 19.0% for women, for ages 20–39 years, 40–59 years, and 60–80 years old respectively. Diabetes or IR prevalence, according to elevated fasting glucose (≥126 md/dL), 2-hour glucose (≥200 md/dL), and/or HOMA-IR (≥5.9), was also higher with increasing age and was 14.9%, 27.7%, 39.5% for men and 14.7%, 21.5%, 34.3% for women, for ages 20–39 years, 40–59 years, and 60–80 years old respectively. There were no significant differences in diabetes or IR between men and women at any age category, except for a lower prevalence of the combined elevated fasting glucose, 2-hour glucose, and/or HOMA-IR designation among middle-aged women (ages 40–59 years) (OR: 0.52; 95% CI: 0.36–0.75; p<0.001). Across all age categories, men were stronger than women, in both absolute and normalized grip strength capacity (all p<0.001); however, daily television viewing time did not differ between men and women for any age category. For both men and women, there were significantly lower grip strengths across higher ages, and significantly greater television viewing time in men (all p<0.001). Among women, the oldest age category (ages 60–80 years) had significantly greater daily television viewing time than both the youngest women (20–39 years) and middle aged women (40–59 years) (both p<0.01).
Table 1.
Demographic and cardiometabolic characteristics of the study population by sex and age category.
| Men
|
Women
|
|||||
|---|---|---|---|---|---|---|
| Age 20–39.9 n=648 |
Age 40–59.9 n=592 |
Age 60–80 n=552 |
Age 20–39.9 n=646 |
Age 40–59.9 n=610 |
Age 60–80 n=550 |
|
| Age, years | 29.17 (0.43) | 49.56 (0.38) | 69.00 (0.39) | 29.26 (0.43) | 49.61 (0.18) | 70.00 (0.34) |
| Body Mass Index (BMI), kg/m2 | 27.83 (0.36) | 29.37 (0.25)† | 28.65 (0.36) | 28.63 (0.30)* | 29.64 (0.34)† | 29.31 (0.41) |
| Obesity (BMI >30), % | 30.3 | 35.7† | 32.6 | 35.1* | 45.2*† | 43.2*§ |
| Waist Circumference, cm | 95.92 (0.97) | 103.69 (0.67) | 105.74 (0.94) | 93.45 (0.79) | 97.62 (0.61) | 99.19 (0.92) |
| Abdominal Obesity (sex-specific WC), % | 30.1 | 44.0† | 51.2§‡ | 54.2* | 71.1*† | 76.0*§‡ |
| Self-Reported TV Viewing, hours/week | 2.20 (0.10) | 2.46 (0.08)† | 3.16 (0.08)§‡ | 2.33 (0.14) | 2.35 (0.09) | 3.10 (0.08)§‡ |
| Grip Strength, kg | 50.51 (0.38) | 47.30 (0.59)† | 40.17 (0.63)§‡ | 31.92 (0.23)* | 30.38 (0.28)*† | 24.89 (0.24)*§‡ |
| Normalized Grip Strength (relative to body mass) | 0.60 (0.01) | 0.53 (0.01)† | 0.47 (0.01)§‡ | 0.44 (0.01)* | 0.41 (0.01)*† | 0.35 (0.01)*§‡ |
| Glycohemoglobin, % | 5.37 (0.03) | 5.75 (0.07)† | 6.01 (0.07)§‡ | 5.31 (0.03) | 5.70 (0.04)† | 5.92 (0.05)§‡ |
| a Diabetes, % | 3.2 | 13.2† | 23.7§‡ | 3.1 | 11.1† | 19.0§‡ |
| Glucose, mg/dL | 99.05 (1.12)* | 111.69 (2.66)*† | 116.56 (2.53)§‡ | 95.39 (0.75) | 104.07 (1.69)† | 114.61 (3.20)§‡ |
| Insulin, μU/mL | 12.82 (0.59) | 15.22 (1.47)† | 13.64 (0.78) | 12.84 (0.50) | 13.05 (0.82) | 12.90 (0.35) |
| HOMA | 3.31 (0.19) | 4.52 (0.54)*† | 4.24 (0.45)§ | 3.21 (0.14) | 3.59 (0.27) | 3.93 (0.20)§ |
| 2-Hour OGTT, mg/dL | 98.43 (1.51) | 119.33 (4.50)*† | 130.65 (6.92)§‡ | 102.48 (2.11) | 112.28 (2.32)† | 141.75 (4.89)*§‡ |
| b Diabetes or IR, % | 14.9 | 27.7*† | 39.5§‡ | 14.7 | 21.5† | 34.3§‡ |
| Triglycerides, mg/dL | 121.52 (5.47)* | 154.34 (6.99)*†‡ | 126.12(8.46) | 105.69 (4.93) | 120.02 (7.01)† | 132.07 (6.11)§‡ |
| Total Cholesterol, mg/dL | 182.37 (1.66) | 200.15 (1.85)†‡ | 180.86 (1.41) | 182.57 (1.35) | 207.14 (1.90)*† | 204.64 (2.45)*§ |
| HDL-Cholesterol, mg/dL | 47.41 (0.52)*§ | 46.45 (0.62)*‡ | 50.47 (1.13)* | 55.64 (0.94)§ | 58.38 (1.23) | 59.33 (1.19) |
| LDL-Cholesterol, md/dL | 112.60 (1.81)*§ | 120.92 (2.50)†‡ | 105.19 (2.56) | 107.72 (1.11) | 125.37 (1.85)*†‡ | 118.57 (1.86)*§ |
| Systolic Blood Pressure, mmHg | 118.54 (0.53)* | 124.25 (0.97)*† | 131.23 (1.01)§‡ | 111.00 (0.77) | 119.33 (0.70)† | 134.89 (1.27)*§‡ |
| Diastolic Blood Pressure, mmHg | 72.22 (0.59)* | 77.20 (0.59)*†‡ | 71.20 (0.49) | 69.90 (0.74) | 73.78 (0.47)†‡ | 70.90 (0.88) |
Abreviations: BMI-body mass index; WC-waist circumference; TV-televion; GHB-glycohemoglobin; HOMA-Homeostasis Model of Assessment; IR-HOMA-Insulin Resistant; OGTT-oral glucose tolerance test; HDL-high density lipoprotein; LDL-low density lipoprotein
Diabetes (GHB ≥ 6.5%)
Diabetes or HOMA-IR (Glucose ≥ 126 md/dL or OGTT ≥ 200 mg/dL or HOMA ≥ 5.9)
Significant difference between men and women in equivalent age category: Denoted as group with higher risk.
Significant difference between ages 20–39.9 years and 40–59.9 years (p<0.01): Denoted as group with higher risk.
Significant difference between ages 40–59.9 years and 60–80 years (p<0.01):Denoted as group with higher risk.
Significant difference between ages 20–49.9 years and 60–80 years (p<0.01): Denoted as group with higher risk.
In both men and women, normalized grip strength was inversely correlated with HbA1c (r=−0.26 and −0.27; p<0.001), fasted glucose (r=−0.22 and −0.27; p<0.001), 2-hour glucose (r=−0.29 and −0.26; p<0.001), and HOMA (r=−0.19 and −0.34; p<0.001). In the sex-stratified univariate analyses, lower grip strength was strongly associated with increased odds of diabetes (HbA1c ≥ 6.5% [≥48 mmol/mol]) in adults, such that for every 0.05 decrement of normalized strength, there was a 1.51 and 1.83 increased odds (p<0.001) for men and women respectively.
In the adjusted model (Table 2), women were at lower odds of having diabetes than men, whereas non-Hispanic blacks, Mexican Americans or other Hispanics, and other races or multi-racial were at significantly higher odds (reference category: Non-Hispanic White; all p<0.001). Moreover, age, waist circumference and lower income were each inversely associated with diabetes; however, self-reported television viewing time was not a significant predictor. The final adjusted model with the best AIC value had an area under the ROC of 0.85 (Figure 1). Even after adjustment for all other model predictors, lower grip strength was still strongly associated with diabetes, such that for every 0.05 decrement of normalized strength, there was a 1.26 increased odds (95% CI: 1.07–1.45). Stratifying by sex in the fully adjusted models revealed no differences in the association between strength and diabetes between men and women (data not shown).
Table 2.
Multiple logistic regression models for independent predictors of diabetes in adults.
| Model Predictor(s) | Estimate | SE | Odds Ratio | 95% CIs | Pr > ChiSq | |
|---|---|---|---|---|---|---|
|
|
||||||
| Diabetes (GHB ≥ 6.5%) | ||||||
| Sex (Reference: Males) | −0.71 | 0.26 | 0.49 | 0.29–0.82 | 0.01 | |
| Age (years) | 0.18 | 0.02 | 1.20 | 1.20–1.26 | <0.001 | |
| Waist Circumference | 0.04 | 0.01 | 1.04 | 1.02–1.06 | <0.001 | |
| Race/ethnicity (Ref: Non-Hispanic White) | ||||||
| Non-Hispanic black | 1.01 | 0.23 | 2.75 | 1.78–4.24 | <0.001 | |
| Hispanic or Mexican American | 1.02 | 0.21 | 2.77 | 1.83–4.18 | <0.001 | |
| Other Race or Multi-Racial | 1.42 | 0.22 | 4.11 | 2.67–6.32 | <0.001 | |
| Annual Household Income (Ref: ≥$75,000) | ||||||
| < $24,999 | 0.76 | 0.23 | 2.13 | 1.35–3.35 | <0.001 | |
| $25,000 – $54,999 | 0.18 | 0.20 | 1.19 | 0.80–1.77 | 0.38 | |
| $55,000–$74,999 | 0.59 | 0.24 | 1.82 | 1.12–2.93 | 0.01 | |
| Self-Reported Television Viewing Time (hrs) | 0.04 | 0.04 | 1.04 | 0.96–1.13 | 0.38 | |
| Relative Grip Strength | −4.61 | 1.39 | 0.79* | 0.69–0.91** | <0.001 | |
| Age*Age | 0.00 | 0.00 | 0.99 | 0.99–1.00 | <0.001 | |
OR for every 0.05 (5%) incremental increase in relative grip strength.
95% CIs for every 0.05 (5%) incremental increase in relative grip strength.
Figure 1.
Receiver operating characteristic (ROC) curve for the final, best model for the prediction of diabetes (i.e., HBA1c ≥ 6.5% [≥48 mmol/mol]). Area under the ROC curve=0.85.
In the smaller subset of individuals with fasted serum, results were similar albeit attenuated, such that for every 0.05 decrement of normalized strength, there was a 1.18 increased odds of diabetes (95% CI: 1.05–1.33), even after adjustment for all other predictors.
Threshold Analysis
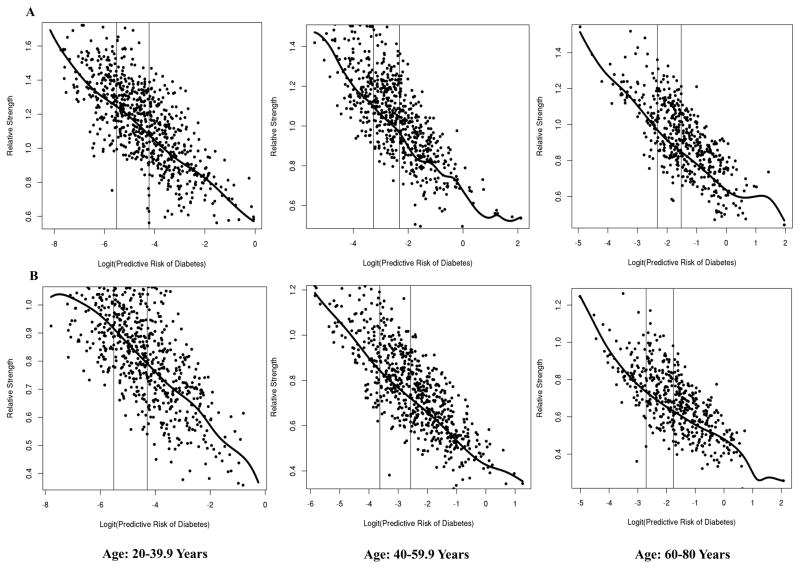
Thresholds were determined as the corresponding normalized strength at the lower and upper tertiles of predictive probabilities for risk of diabetes, per sex and age categories, through fitting non-parametric local polynomials curves. Age- and sex-specific thresholds for high strength and low strength are provided in Table 3, with corresponding risk percentages for diabetes (according to HbA1c≥6.5% [≥48 mmol/mol]). These thresholds may therefore be used to categorize individuals into three categories of risk (i.e., low, medium, and high risk) on the combined basis of sex, age, body mass, and combined grip strength capacity. Figure 2 illustrates the non-parametric curves between the logit of predicted risk for diabetes (x-axis) and normalized strength (y-axis), with age- and sex-specific low and high strength thresholds represented with vertical bars corresponding lower and upper tertiles of predicted risks.
Table 3.
Age- and sex-specific thresholds for high strength and low strength, with corresponding risk percentages for diabetes (i.e., HBA1c ≥6.5% [≥48 mmol/mol]).
| Relative Strength Thresholds | Men
|
Women
|
||||
|---|---|---|---|---|---|---|
| Age: 20–39.9 | Age: 40–59.9 | Age: 60–80 | Age: 20–39.9 | Age: 40–59.9 | Age: 60–80 | |
|
|
|
|||||
| Low Risk/High Strength | 0.65 (0.4%) | 0.57 (3.7%) | 0.50 (8.9%) | 0.48 (0.4%) | 0.44 (2.6%) | 0.38 (6.2%) |
| High Risk/Low Strength | 0.56 (1.4%) | 0.45 (8.9%) | 0.45 (17.9%) | 0.42 (1.3%) | 0.38 (7.1%) | 0.33 (14.7%)) |
Figure 2.
Non-parametric local polynomial curves between the logit of predicted risk for diabetes (x-axis) and normalized strength (y-axis) among men (A) and women (B). Age-specific low- and high-strength thresholds represented with vertical bars corresponding to lower and upper tertiles of predicted risk.
Discussion
The principal finding of this study was that normalized grip strength is significantly associated with type 2 diabetes in men and women of all ages, even after controlling for known sociodemographic, anthropometric, and behavioral predictors. Specifically, for every 5% decrement in strength-to-body mass-ratio, there was a 26% increased adjusted odds of diabetes. These findings lend additional support to the growing body of literature revealing a strong link between muscle strength and reduced risk for metabolic abnormalities and cardiovascular diseases. Our results suggest that grip strength testing may be valuable as a simple screening strategy for diabetes risk detection among adults. Herein, we provide unique sex- and age-specific thresholds of strength to predict diabetes based on HbA1c levels, according to American Diabetes Association diagnostic criteria (≥6.5% [≥48 mmol/mol]) [25, 26]. Importantly, our modeling technique allowed for the identification of two strength cuttofs, and three respective risk categories, that may be incorporated into a clinical setting for screening adults that are at low-, medium-, and high risk for developing diabetes. In this study we identified highest risk for diabetes to coincide with normalized strength equal to or less than 0.56, 0.50, and 0.45 for men, and 0.42, 0.38, and 0.33 for women, for ages 20–39 years, 40–59 years, and 60–80 years. As an example, a 50 year old woman that has a body mass of 75 kg and grip strength of 26kg has a normalized strength of 0.347, and thus would be categorized as “high risk” for diabetes, i.e., below the low-strength threshold of 0.38 for those sex- and age-categories.
Such thresholds may also be useful in identifying individuals that could benefit from lifestyle interventions to improve muscular fitness and reduce risk. Indeed, among adults with and without existing risk factors, various studies have reported significantly improved insulin sensitivity and glucose tolerance with structured resistance exercise interventions [27, 28]. Since resistance exercise is known to elicit a potent insulin-sensitizing effect for hours after a single bout of training [29–31], there is some speculation about whether it is merely the repeated acute responses to habitual training that drive benefits for metabolic health, rather than any adaptive-response, per se. Regardless, progressive resistance exercise is a well-documented stimulus to induce both acute and chronic metabolic changes-attributable to decreases in hyperinsulinemia, improved insulin-stimulated glucose disposal, and enhanced insulin sensitivity [27, 32], and has recently been shown to substantially reduce risk of prospective diabetes incidence among 2 large cohorts of middle-aged and older women [33].
Conversely, muscular atrophy and weakness are closely linked with chronic hyperglycemia and diabetes, particularly with advancing age. As with all cross-sectional studies, a limitation of this investigation is the inability to disentangle the cause-effect relationship between predictors and outcomes. Whether lower relative strength capacities “cause” an elevated risk for diabetes, or if comorbid cardiometabolic abnormalities (e.g., diabetes, pulmonary disorders, etc.) or musculoskeletal conditions (e.g., carpal tunnel syndrome, diabetic cheiroarthropathy, flexor tenosynovitis, etc.) themselves, are a cause of diminished muscle function (i.e., reverse causality), is an interesting and complex topic. Moreover, we were unable to determine if other competing risks or unmeasured confounding (e.g., time spent in different categories of physical activity, or exercise participation) may have influenced the observed estimates. In their BLSA study, Kalyani et al. [14] suggested that longitudinal declines in muscle strength and quality were driven by neurological factors such as neuropathy. While there is certainly evidence to explicate the role of diabetic complications on neuromuscular function, there is also an enormous body of literature to verify the independent link between declines in physical fitness and increased diabetes risk. For individuals at elevated risk for chronic hyperglycemia and/or diabetes, these phenomena transpire as an accelerated circular cause-and-consequence of events. There is thus a dire need for more patient-specific clinical studies to identify optimal interventions that may concurrently target preservation of muscle strength capacity and achieve or maintain cardiometabolic health. Because regular exercise and drug interventions are similar in terms of their mortality benefits in the secondary prevention of heart disease, rehabilitation after stroke, and prevention of diabetes [34], a simplistic clinical directive that could dramatically reduce total healthcare burden is to continue encouraging healthy lifestyles that lead to regular participation in physical activity, preservation of muscular fitness, and healthy weight achievement/maintenance.
Not surprisingly, nearly all research related to the influence of obesity and related cardiometabolic abnormalities to potentiate risk for secondary muscle dysfunction have been conducted within the context of an age-related phenomenon. However, the underlying changes in metabolic dysregulation leading to a disease, such as diabetes, should be regarded as a gradual continuous process throughout the lifespan rather than a discrete outcome or event. We have previously shown that even among non-obese, otherwise healthy adults, adiposity is a strong negative mediator of the relationship between muscle size and strength capacity [35]. Recent data also suggest a “lipotoxic” effect of local adipose tissue deposition on muscular function and musculoskeletal integrity [36–38]. Thus, further evaluation of the temporal sequence of these consequences is of particular importance not only for early screening efforts to reduce diabetes progression and secondary comorbidities, but also in understanding the role of obesity and diabetes on the acceleration of functional declines leading to exaggerated sedentariness and mobility disability. Moreover, future research needed to better understand the role of weakness and/or strength declines as a risk exposure for diabetes in the context of different races and ages.
Other recent efforts to calculate thresholds of weakness have used various statistical methods for defining absolute values of strength that correspond with mobility disability and early mortality among aging adults [9–11]. However, since there is substantial covariance between strength capacity and body mass, and moreover, that the link between muscle strength and both physical function and chronic health is directly mediated by the proportion of strength relative to body mass, normalization to body mass is critical to improve sensitivity of cutoff values and screening efforts. Future research is certainly needed to devise strength growth curves that may be used for the purposes of normative reference testing in clinical, academic, and community settings.
Conclusions
The purposes of this study were to examine the link between strength capacity and diabetes in a large, nationally-representative sample, and to explore thresholds of weakness, for optimal risk categorization. We found that normalized grip strength (i.e., ) was robustly associated with diabetes in adults, even after adjusting for various known covariates such as age, waist circumference, important sociodemographic variables, and sedentary behavior. Moreover, we present new, age- and sex-specific thresholds of normalized strength that could be incorporated into a clinical setting for identifying adults that are at low, intermediate, and high risk for developing diabetes. Future research is needed to create unique risk-categorization algorithms specific to other clinical and global health outcomes, as well as to examine the longitudinal trajectories of strength change as an indicator for incident cardiometabolic disease and even early mortality. Such efforts will dramatically improve the personalization of screening, stratification, and clinical decision making at the individual patient level.
Key Points.
Normalized grip strength was robustly associated with diabetes in adults, even after adjusting for various known predictors.
Sex-specific low, intermediate, and high risk categories are presented such that highest risk was represented at normalized grip strength ≤ 0.56, 0.50, and 0.45 for men, and ≤ 0.42, 0.38, and 0.33 for women, for ages 20–39 years, 40–59 years, and 60–80 years.
Grip strength measurement is a feasible strategy that can be easily incorporated into a clinical or community setting for identifying adults who are at risk for developing diabetes, and that could benefit from lifestyle interventions, such as exercise and/or weight loss to reduce risk.
Acknowledgments
Funding Sources
Dr. Peterson is funded by the National Institutes of Health (1KO1 HD074706).
Footnotes
Author Contributions:
M.P. and S.A.S. acquired research data. M.P. and P.Z. performed all analyses. M.P. wrote the manuscript. P.C., S.A.S. and K.M. reviewed/edited manuscript, and contributed to the interpretation of the results. All authors reviewed the final submitted manuscript.
Role of the Sponsors: The funders had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Disclosures
Mark Peterson he has no conflict of interest.
Peng Zhang declares that he has no conflict of interest.
Palak Choksi declares that she has no conflict of interest.
Kyriakos Markides declares that he has no conflict of interest.
Soham Al Snih declares that he has no conflict of interest.
References
- 1.Aguiree F, Brown A, Cho N, Ho N, Dahlquist D, Dodd S, Dunning T, Hirst M, Hwang C, Magliano D, et al. Basel, Switzerland: International Diabetes Federation; 2013. [Google Scholar]
- 2.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KMV, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2(11):867–74. doi: 10.1016/S2213-8587(14)70161-5. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 4.Al Snih S, Markides KS, Ray L, Ostir GV, Goodwin JS. Handgrip strength and mortality in older Mexican Americans. Journal of the American Geriatrics Society. 2002;50(7):1250–6. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz JR, Sui X, Lobelo F, Morrow JR, Jr, Jackson AW, Sjostrom M, Blair SN. Association between muscular strength and mortality in men: prospective cohort study. Bmj. 2008;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper R, Strand BH, Hardy R, Patel KV, Kuh D. Physical capability in mid-life and survival over 13 years of follow-up: British birth cohort study. Bmj. 2014;348:g2219. doi: 10.1136/bmj.g2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Jaramillo P, Cohen DD, Gomez-Arbelaez D, Bosch J, Dyal L, Yusuf S, Gerstein HC Investigators OT. Association of handgrip strength to cardiovascular mortality in pre-diabetic and diabetic patients: a subanalysis of the ORIGIN trial. International journal of cardiology. 2014;174(2):458–61. doi: 10.1016/j.ijcard.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Cooper R, Kuh D, Hardy R, Ahmad R, Sayer AA, Al Snih S, Bath PA, et al. Mortality Review G, Falcon Teams HAS. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. Bmj. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, Kenny AM, Peters KW, Ferrucci L, Guralnik JM, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69(5):576–83. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spruit MA, Sillen MJH, Groenen MTJ, Wouters EFM, Franssen FME. New normative values for handgrip strength: results from the UK Biobank. J Am Med Dir Assoc. 2013;14(10):775, e5–11. doi: 10.1016/j.jamda.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Alley DE, Shardell MD, Peters KW, McLean RR, Dam T-TL, Kenny AM, Fragala MS, Harris TB, Kiel DP, Guralnik JM, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):559–66. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senechal M, McGavock JM, Church TS, Lee DC, Earnest CP, Sui X, Blair SN. Cut points of muscle strength associated with metabolic syndrome in men. Med Sci Sports Exerc. 2014;46(8):1475–81. doi: 10.1249/MSS.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore AZ, Caturegli G, Metter EJ, Makrogiannis S, Resnick SM, Harris TB, Ferrucci L. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. Journal of the American Geriatrics Society. 2014;62(2):230–6. doi: 10.1111/jgs.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38(1):82–90. doi: 10.2337/dc14-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson MD, Saltarelli WA, Visich PS, Gordon PM. Strength capacity and cardiometabolic risk clustering in adolescents. Pediatrics. 2014;133(4):e896–903. doi: 10.1542/peds.2013-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen DD, Gomez-Arbelaez D, Camacho PA, Pinzon S, Hormiga C, Trejos-Suarez J, Duperly J, Lopez-Jaramillo P. Low muscle strength is associated with metabolic risk factors in Colombian children: the ACFIES study. PloS one. 2014;9(4):e93150. doi: 10.1371/journal.pone.0093150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artero EG, Ruiz JR, Ortega FB, Espana-Romero V, Vicente-Rodriguez G, Molnar D, Gottrand F, Gonzalez-Gross M, Breidenassel C, Moreno LA, et al. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes. 2011;12(8):704–12. doi: 10.1111/j.1399-5448.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 18.Sairenchi T, Iso H, Irie F, Fukasawa N, Ota H, Muto T. Underweight as a predictor of diabetes in older adults: a large cohort study. Diabetes care. 2008;31(3):583–4. doi: 10.2337/dc07-1390. [DOI] [PubMed] [Google Scholar]
- 19.International Expert C. Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, Davidson MB, DeFronzo R, Genuth S, et al. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes care. 2009;32(7):1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes care. 2012;35(7):1605–10. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NHANES. [Accessed May 4, 2015];Muscle Strength Procedures Manual. http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Muscle_Strength_Proc_Manual.pdf.
- 22.WHO. Global Physical Activity Questionnaire (GPAQ) Analysis Guide. http://www.who.int/chp/steps/resources/GPAQ_Analysis_Guide.pdf.
- 23.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. Journal of physical activity & health. 2009;6(6):790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 24.Complex surveys; a guide to analysis using R. Sci-Tech News. 2010;64(2):49. [Google Scholar]
- 25.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 27.van Dijk JW, Manders RJF, Tummers K, Bonomi AG, Stehouwer CDA, Hartgens F, van Loon LJC. Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. Diabetologia. 2012;55(5):1273–82. doi: 10.1007/s00125-011-2380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, Zimmet P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–36. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 29.Black LE, Swan PD, Alvar BA. Effects of intensity and volume on insulin sensitivity during acute bouts of resistance training. Journal of strength and conditioning research/National Strength & Conditioning Association. 2010;24(4):1109–16. doi: 10.1519/JSC.0b013e3181cbab6d. [DOI] [PubMed] [Google Scholar]
- 30.Yardley JE, Kenny GP, Perkins BA, Riddell MC, Balaa N, Malcolm J, Boulay P, Khandwala F, Sigal RJ. Resistance versus aerobic exercise: acute effects on glycemia in type 1 diabetes. Diabetes Care. 2013;36(3):537–42. doi: 10.2337/dc12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen E, Landstad BJ, Gundersen KT, Torjesen PA, Svebak S. Insulin sensitivity after maximal and endurance resistance training. Journal of strength and conditioning research/National Strength & Conditioning Association. 2012;26(2):327–34. doi: 10.1519/JSC.0b013e318220e70f. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of Aerobic Versus Resistance Exercise Without Caloric Restriction on Abdominal Fat, Intrahepatic Lipid, and Insulin Sensitivity in Obese Adolescent Boys A Randomized, Controlled Trial. Diabetes. 2012;61(11):2787–95. doi: 10.2337/db12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grontved A, Pan A, Mekary RA, Stampfer M, Willet WC, Manson JE, Hu FB. Muscle-strengthening and conditioning activities and risk of type 2 diabetes: A prospective study in two cohorts of US women. Plos Med. 2015 doi: 10.1371/journal.pmed.1001587. In Press( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naci H, Ioannidis JPA. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. Bmj-Brit Med J. 2013;347 doi: 10.1136/bmj.f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson MD, Liu D, Gordish-Dressman H, Hubal MJ, Pistilli E, Angelopoulos TJ, Clarkson PM, Moyna NM, Pescatello LS, Seip RL, et al. Adiposity attenuates muscle quality and the adaptive response to resistance exercise in non-obese, healthy adults. Int J Obes (Lond) 2011;35:1095–103. doi: 10.1038/ijo.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–85. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88(11):1336–44. doi: 10.2522/ptj.20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang P, Peterson MD, Su GL, Wang SC. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.113.081778. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]