Abstract
Human immunodeficiency virus type 1 (HIV-1) has a small, multifunctional genome that encodes a relatively large and complex proteome. The virus has adopted specialized post-transcriptional control mechanisms to maximize its coding capacity while economically maintaining the information stored in cis-acting replication sequences. The conserved features of the 5′ untranslated region of all viral transcripts suggest they are poor substrates for cap-dependent ribosome scanning and provide a compelling rationale for internal initiation of translation. This article summarizes key experimental results of studies that have evaluated HIV-1 translation initiation. A model is discussed in which cap-dependent and cap-independent initiation mechanisms of HIV-1 co-exist to ensure viral protein production in the context of 1) structured replication motifs that inhibit ribosome scanning, and 2) alterations in host translation machinery in response to HIV-1 infection or other cellular stresses. We discuss key issues that remain to be understood and suggest parameters to validate internal initiation activity in HIV-1 and other retroviruses.
Keywords: Retrovirus translational control, unspliced RNA, 5′ untranslated region, ribosome scanning, internal ribosome entry site
INTRODUCTION
Retrovirus Primary Transcription Product Can Function as Precursor mRNA, mRNA, or Viral Genomic RNA
Retroviral genomes are multifunctional RNAs that utilize virally encoded reverse transcriptase to replicate genomic RNA through a proviral DNA intermediate [81]. The provirus becomes permanently integrated into the host cell chromosome and is expressed like a cellular gene by the host cell transcription, RNA processing, and translation machinery. The primary retroviral transcript interacts with the cellular RNA processing machinery to become capped and polyadenylated. One fraction of the retroviral transcript behaves like a typical cellular pre-mRNA in that the spliceosome is engaged and the intron is removed. As a consequence, it is expected that the exon junction complex is deposited on spliced retroviral transcripts and functions as it does on cellular mRNAs to promote a pioneer round of translation, followed by nuclear export and finally, steady state translation in the cytoplasm [20, 28, 33, 50, 57, 86]. Another fraction of pre-mRNA achieves nuclear export and translational utilization independently of splicing commitment and this activity is trans-activated by the essential HIV Rev protein. Rev interacts with the Rev response element (RRE) within unspliced and incompletely spliced viral RNAs and solicits interaction with the CRM1 nuclear export receptor [35, 65]. In the cytoplasm, the unspliced transcript plays a dual role as mRNA template for translation and as genomic RNA that is packaged into assembling virions [17]. Experiments with metabolic inhibitors to address the relationship between HIV-1 RNA packaging and translation have determined that unspliced HIV-1 RNA does not segregate into separate pools for translation and packaging, as has been identified for murine leukemia virus [16, 43, 54]. Lack of translation is not a prerequisite to qualify HIV-1 unspliced RNA for packaging into progeny virions [16]. Instead, unspliced HIV-1 RNA functions interchangeably as an mRNA template for translation and as genomic RNA that is packaged [16]. A similar conclusion was reached for Rous sarcoma virus (RSV) in a study that evaluated the relationship between translation-dependent nonsense mediated decay and RNA packaging [48].
Barriers Posed Against Efficient Translation of All HIV-1 Transcripts
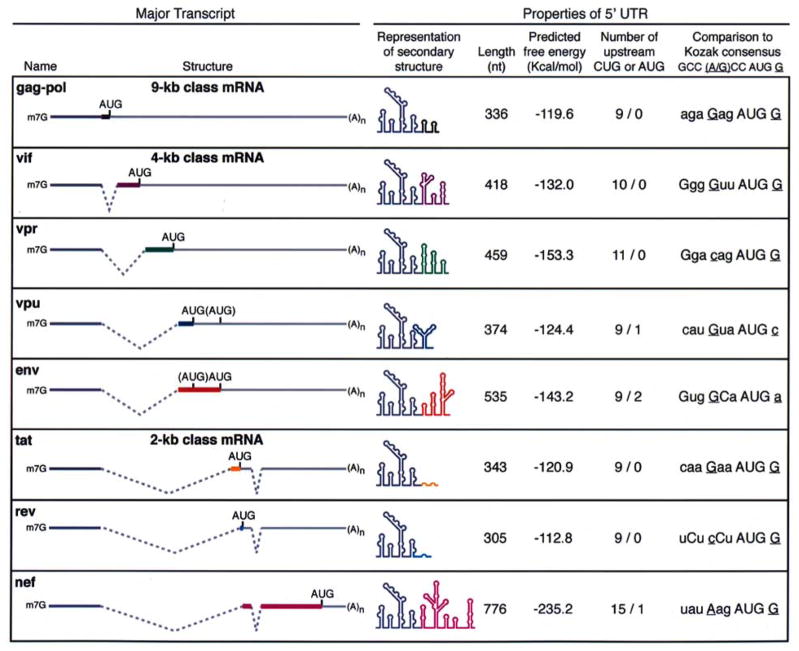
While translation and packaging are not mutually exclusive processes for HIV-1, the packaging signal and other structural motifs in the 5′ untranslated regions (UTRs) have been demonstrated to inhibit ribosome scanning and translation initiation [29, 55, 59]. The 5′ UTR is the most conserved region in HIV-1 [7] and contains several cis-acting replication sequences, including the Tat trans-activation response element (TAR), poly(A) signal, primer binding site, dimerization signal, splice donor site and the packaging signal. Some of the same structural motifs are maintained in spliced HIV-1 transcripts. HIV-1 encodes over 30 distinct spliced transcripts as a result of alternative splicing of the primary transcription product [66, 73]. All of the spliced transcripts and the unspliced gag mRNA share the same 289 nt 5′ noncoding exon (Fig. 1). Because this 5′ UTR contains several of the highly conserved structured replication motifs, ribosome scanning of the spliced HIV-1 mRNAs is likewise expected to be inefficient. In addition, ligation to various distal exons produces a collection of 5′ UTRs that are ~350 to 775 nt in length and contain degenerate Kozak consensus sequences and upstream AUG or CUG initiator codons (Fig. 1).
Fig. 1. Organization and features of the 5′ untranslated regions of the major HIV-1 transcripts.
Structure of the predominant transcript for each open reading frame is shown from among the ~30 alternatively spliced transcripts that are detectable by RT-PCR [66]. Colored lines highlight sequences that comprise the various 5′ UTRs. The blue line depicts the first noncoding exon, which is 289 nts in length and is present in all HIV-1 transcripts. The black line denotes the additional 47 nts that are maintained in the unspliced transcript, which is mRNA template for translation to Gag and Gag-Pol protein and is genomic RNA that is packaged into progeny virions. The gray lines represent coding regions and dashed lines denote introns. AUG indicates the translation initiation codon. Also indicated is the number of CUG and AUG codons that are present upstream of actual initiation codon. Translation to Vpu and Env is achieved from the same transcript. The vpu AUG is subject to leaky scanning and scanning may continue until recognition of the downstream env AUG. The unused AUG in this bicistronic transcript is labeled in parenthesis. Depiction of predicted secondary structure of replication motifs is adapted from [7]; the motifs include the trans-activation responsive sequence; primer binding site; dimer initiation site; 5′ splice site; and core RNA packaging signal. Alternatively spliced exons, which are depicted in color, may induce variations in the folding of the upstream region, which are not shown. Free energy predictions were calculated using Mfold [51, 87]. Deviations from Kozak consensus are indicated in lowercase letters; underlined nts indicate those most critical for efficient cap-dependent translation initiation.
CUG can be recognized as a non-AUG initiator codon [34] and the recognition of an upstream CUG or AUG typically decreases initiation at downstream initiator codon [23]. The efficiency of non-AUG initiation can be modulated by the context surrounding the codon, the secondary structure of the transcript, and in response to changes in the growth status of the cells. The observation that the efficiency of non-AUG initiation in growth-regulatory mRNAs changes in response to changes in cellular growth conditions indicates that the scanning preinitiation complex can be regulated to affect the recognition of such a non-AUG codon [34].
The conservation of a relatively long 5′ UTR that harbors complex structural motifs and upstream initiator codons implies that both the unspliced gag mRNA and the alternatively spliced HIV-1 transcripts are poor substrates for ribosome scanning and efficient cap-dependent translation. Because the replication strategy of this virus family necessitates conservation of a long and highly structured 5′ UTR, the ribosome scanning and initiation of cap-dependent translation is expected to be inefficient for retroviruses in general.
HIV-1 UTRs May Mediate Alternative Initiation Strategies
An interesting possibility is that the variant 5′ UTRs created by alternative splicing induce unique RNA conformations that serve to affect differential modulation of HIV-1 translation initiation. An example of this paradigm is provided by experiments with ornithine decarboxylase (ODC) mRNA, which have identified two alternatively spliced ODC RNAs that use different translation initiation strategies [69]. The ODC gene product is a rate limiting enzyme for polyamine biosynthesis. The utilization of an internal initiation mechanism ensures that this short-lived enzyme (t½, 10–20 min) is synthesized at a peak level during G2/M phase. One ODC mRNA isoform contains a 273 nt 5′ UTR that poses structural barriers to efficient ribosome scanning to the downstream initiator codon. The 5′ UTR of the other isoform contains an additional 30 nt and folds into an internal ribosome entry site (IRES) that supports internal initiation. This IRES is efficient during G2/M, but is inefficient during the other phases of the cell cycle [68]. These findings illuminate the possibility that unique features of the 5′ UTRs of alternatively spliced retrovirus transcripts may choreograph alternative initiation strategies. In particular, alternative 5′ UTRs of one or more HIV-1 transcripts may contain an IRES that facilitates the synthesis of the respective viral gene products in a cell cycle-dependent manner.
Global Cap-Dependent Translation Initiation is Suppressed During Cellular Stress
Conditions including virus infection, hypoxia, progression to apoptosis and cell cycle arrest compromise global mRNA translation [42, 76, 77, 85]. Initiation of apoptosis triggers caspase-induced cleavage of eIF4G scaffold protein, which produces a global decrease in cap-dependent translation [15]. During the G2/M phases of the cell cycle, eIF4E dephosphorylation blocks formation of the eIF4F complex and reduces the magnitude of cap-dependent translation [11]. IRES-containing cellular mRNAs figure prominently under these conditions in controlling cell growth and cell cycle progression [67, 70].
While global cellular translation is suppressed by as much as 80% upon experimental induction of apoptosis by treatment with etoposide [85], translation of selected mRNAs is sustained. For example, translation of the cellular inhibitor of apoptosis protein 1 (cIAP-1) transcript is sustained. The cIAP-1 transcript contains an 1100 nt 5′ UTR with IRES activity that is activated during G2 arrest and progression to apoptosis [85]. The ability to sustain or even increase translation during G2 arrest and progression to apoptosis is consistent with previous analysis of replication of HIV-1 in T cells. The multi-functional HIV-1 accessory protein viral protein R (Vpr) induces G2 arrest and drives progression to apoptosis (reviewed in another issue of this journal) [3]. Studies of Goh et al. [32] identified that HIV-1 transcription is increased and Gag production remains robust during Vpr-induced G2 arrest. These observations suggest that HIV-1 protein synthesis is not hindered by a global reduction in cap-dependent translation. An interesting possibility is that HIV-1 sustains translation during G2 arrest by adopting an alternative initiation strategy such as cap-independent internal initiation.
Thus, in addition to features of the 5′ UTR that pose barriers to ribosome scanning, another challenge to retroviral protein synthesis would be crippling of the host cell translation machinery as a consequence of virus-induced cell cycle arrest or other cellular stress. Together these hurdles stand against the generation of protein products from both the viral unspliced pre-mRNA and the collection of alternatively spliced transcripts. The need to sustain translation in the face of barriers to ribosome scanning and deleterious virus-host interactions implies that retroviruses can adopt an alternative translation initiation strategy. The following sections of this article discuss the major translation initiation mechanism in eukaryotic cells in relation to an alternative translation initiation strategy of internal ribosome entry. The final section of the article summarizes findings that have addressed whether or not internal initiation is a bona fide control mechanism of importance to HIV-1 replication.
Cap Recognition and Ribosome Scanning Characterize Translation Initiation on Typical Cellular mRNAs
Translational control regulates expression of 30% of the eukaryotic proteome and plays a pivotal role in the cellular response to stress and other changes in cellular growth conditions [53]. Initiation is the rate-limiting step in protein synthesis and the primary target for translational control [30]. The prevailing model for translation initiation describes the strategy used by the majority of cellular mRNAs. The ribosome scanning model prescribes that efficiently scanned mRNAs are monocistronic, contain a m7G(5′)ppp(5′)N cap structure at their 5′ termini, and have UTRs that are <100 nt in length and are relatively unstructured [45, 46]. A conserved sequence adjacent to the initiation codon, which is designated the Kozak consensus (GCC (A/G)CC AUG G), promotes efficient recognition of a particular AUG [47]. The 5′ cap mediates interaction with the multicomponent eIF4F preinitiation complex, which recruits the small ribosomal subunit [53].
Briefly, the eIF4F complex is composed of the eIF4E cap binding protein, the eIF4G modular scaffolding protein, and the eIF4A RNA helicase [53]. eIF4G acts as a scaffold to connect eIF4A and eIF3 with eIF4E that is bound to the 5′ cap. eIF4A provides RNA helicase activity to remodel the 5′ UTR RNA and present an open single stranded template for the small ribosomal subunit to scan. Thus, eIF4F activates the mRNA template for interaction with the 43S ribosome complex, which consists of the 40S ribosomal subunit, eIF1A, eIF3, and eIF2-GTP bound to an initiator tRNA. The charged small ribosomal subunit scans in the 5′ to 3′ direction until an initiator codon embedded in a Kozak consensus (Fig. 1) is recognized. The 60S ribosomal subunit joins the small ribosomal subunit and the elongation of the polypeptide chain proceeds.
Modulation of ribosome scanning is a distinguishing feature of at least one HIV-1 transcript. As depicted in Fig. 1, Vpu and Env are translated from the same mRNA with initiation occurring at separate AUG initiation codons. Initiation at the downstream AUG to produce Env is reliant on leaky scanning of the upstream vpu AUG by the 43S ribosomal subunit [74]. When the context of the Vpu AUG is mutated match a strong Kozak consensus and reduce readthrough, Env translation is abolished [74]. On the other hand, a higher level of Env translation is achieved upon mutation of the vpu AUG [72]. Thus, an important mechanism to achieve balanced expression of these gene products is regulation of translation initiation by leaky scanning.
Internal Initiation is Necessary for Translation of Picornavirus mRNAs
An alternative to initiation by ribosome scanning was identified initially in the Picornaviridae virus family, which includes poliovirus and encephalomyocarditis virus (EMCV) [41, 61]. In distinction from cap-dependent mRNA templates, the mRNA of picornaviruses lacks the 5′ m7G cap and also contains long 5′ UTRs of up to ~750 nt in length that form stable secondary structures and contain oligopyrimidine tracts and multiple cryptic AUG or CUG codons positioned upstream of the authentic initiation codon [27]. As described above, these features are deleterious to initiation by a ribosome scanning mechanism. Here, internal recognition of the in-frame start codon at an IRES is responsible for successful recruitment of the small ribosome subunit [27]. Members of the Picornaviridae are completely dependent on the IRES to initiate viral protein synthesis [19, 41, 61]. IRES is a structural motif that interacts with IRES transacting factors (ITAF) such as Lupus antigen (La) [44, 52] and polyprimidine tract binding protein [12, 36] and is recognized by a modified preinitiation complex [62]. The IRES promotes recruitment of the charged small ribosomal subunit independently of cap-binding [37].
Global Translation Shutdown is Induced by Picornavirus, but not HIV-1 Infection
Picornaviruses also have adapted convergent mechanisms that drastically disrupt the host capacity for cap-dependent initiation, which induce preferential IRES-mediated translation [6]. Poliovirus 2A protease causes rapid and complete shutdown of cap-dependent translation by proteolytic cleavage of eIF4G, while cleaved fragments of eIF4G1 remain competent for IRES-mediated translation initiation [2, 56]. EMCV infection does not induce cleavage of eIF4G but induces dephosphorylation of eIF4E-BP, resulting in complex formation between eIF4E and eIF4E-binding protein, which sequesters eIF4E from interaction with capped mRNA and eIF4G [5]. This suppressive activity can be mimicked by treatment with rapamycin, a drug that suppresses cap-dependent translation by shutting down the mTOR pathway [5, 31, 60]. Under conditions of normal cellular growth, mTOR phosphorylates eIF4E-BP, thereby releasing eIF4E for interaction with capped mRNAs, and providing a mechanism for the cell to bolster cap-dependent translation.
Agy and Katze investigated the possibility that HIV-1 disrupts host cell translation [1]. Using metabolic labeling of HIV-1 infected T cells, they showed that HIV-1 infection correlated with a reduction in global protein synthesis. In distinction from poliovirus, this activity correlated with a parallel reduction in global RNA level. A collection of gradient analyses of HIV-1 infected T cells determined that HIV-1 infection correlates with small, but reproducible differences in ribosome profiles [16]. These results eliminated the possibility that HIV-1 induces the rapid and dramatic global shutdown of host cell translation that is characteristic of poliovirus infection. The significance of the small change in ribosomal profile in response to HIV-1 infection remains to be determined.
Internal Initiation is Also Used by a Small but Important Minority of Cellular Transcripts
Initially, the capacity for internal initiation was postulated to be a strategy by which picornaviruses and possibly other viruses monopolize the cellular translation machinery. This view has been challenged by the discovery that selected cellular mRNAs contain an IRES [reviewed in 37]. Furthermore, in at least some cases, the capacity for internal initiation on cellular mRNAs coexists with the capacity for cap-dependent initiation and may provide a reliable mechanism to sustain de novo synthesis of critical proteins during conditions that compromise global cellular translation.
Studies of fibroblast growth factor 2 (FGF2) mRNA showed that cap-dependent and cap-independent translation initiation can occur from the same 5′ UTR [10]. The respective initiation mechanisms utilize separate start codons and produce alternative protein isoforms. Study of human FGF2 has mapped five initiation codons, four CUG and one AUG, within an alternatively translated region. The 5′-proximal CUG codon is recognized in a cap-dependent manner whereas the four distal initiation codons are recognized in the context of an IRES [10, 82]. Alternative internal initiation is stimulated by interaction with hnRNP A1, which functions as an ITAF [9]. When hnRNP A1 is depleted by RNA interference, internal initiation from the four distal initiation codons is eliminated.
The Bcl-2 associated athanogene-1 (BAG-1) 5′ UTR provides another example of a 5′ UTR that can support translation initiation by both cap-dependent and cap-independent mechanisms. In this case, IRES-mediated internal translation is stimulated by the ITAF polypyrimidine tract binding protein and poly r(C) binding protein [63]. These proteins bind and induce secondary structure changes correlated with ribosome binding [64].
Coordinate Utilization of Cap-Dependent and Cap-Independent Initiation is a Versatile Translation Strategy
High level expression of BAG-1 and selected other transcripts that can utilize internal initiation (such as c-Myc [22, 78, 79], Apaf-1 [21], Bcl-2 [75], XIAP [40], DAP5 [38]) is expected to be detrimental under normal conditions of cell growth. In at least some of these transcripts, their IRES may have evolved in response to selective pressure to maintain low levels of translation during cell growth, while sustaining or, in some cases, potently increasing protein production upon cellular stress and cycle arrest. A similar ability to respond to changes in cell growth conditions may be advantageous for HIV-1 in infected T cells, where low immunogenicity is favorable. The ability to coordinately utilize cap-dependent and cap-independent initiation could provide HIV-1 an advantageous strategy to sustain low levels of translation and consequentially virus production during cell growth, and sustain or even potently increase translation during G2 arrest.
STUDIES OF IRES-LIKE ACTIVITY IN HIV-1
A central goal of this article is to explore whether or not internal initiation has been shown to be a bona fide control mechanism of importance in HIV-1 replication. The following section summarizes experimental analyses undertaken to address internal initiation in HIV-1.
Evidence Against Internal Initiation on HIV-1 Unspliced RNA
As discussed above, compelling rationale exists in support of a role for internal ribosome entry in HIV-1 translational control. However, drawing such a conclusion from the experimental analyses has been less straightforward. At least three studies concluded that the HIV-1 unspliced gag-pol RNA does not contain an IRES [29, 55, 59]. At least two other studies have identified IRES-like sequences in the unspliced transcript, either in the 5′ UTR [13] or within the gag coding sequence [14]. The experimental approaches have included bicistronic reporter assays in transfected cells and reporter assays in extracts prepared from rabbit reticulocytes. To date, the analysis has not been extended to a study of HIV-1 provirus in infected T cells.
The bicistronic reporter assay measures protein synthesis from polycistronic reporter RNA that contains an intergenic sequence composed of the candidate IRES. The 5′ cistron provides a measure of cap-dependent translation, while the 3′ cistron provides a measure of cap-independent translation that would be mediated, in response to the candidate IRES, by internal ribosome entry. Potential alternative explanations for activity from the 3′ cistron are internal transcription from a cryptic promoter and alternative splicing that ligates the 3′ cistron in-frame with 5′ cistron sequences to create a cap-dependent form of the 3′ open reading frame [84].
Miele et al. tested a bicistronic reporter plasmid that expresses firefly luciferase in the 5′ cistron and chloramphenicol acetyltransferase (cat) in the 3′ cistron [14]. The 5′ UTR of HIV-1pSVC21 was placed in the intergenic region. Protein extracts from transfected HeLa or COS cells were standardized with respect to firefly Luciferase units and evaluated for CAT activity. The positive control IRES from poliovirus yielded 21–23% acetylation. By comparison, the HIV-1pSVC21 5′ UTR produced CAT activity equivalent to the negative control of an unrelated spacer sequence (1.3% acetylation). In addition, Miele et al. presented results of in vitro translation assays in rabbit reticulocyte lysate showing that introduction of the HIV-1pSVC21 5′ UTR to gag reporter RNA reduced protein synthesis by 75% in comparison to a UTR deletion mutant. Together, the analyses showed that the HIV-1pSVC21 5′ UTR is not a translation enhancer and instead acts to reduce translation, probably by inhibiting ribosome scanning.
Buck et al. evaluated a bicistronic reporter plasmid in which HIV-1 env was placed in the 5′ cistron, HIV-1LAI gag was placed in the 3′ cistron, and segments of the HIV-1LAI 5′ UTR were evaluated in the intergenic region [14]. Transfected COS-7 cells were analyzed for Env and Gag protein production by immunoblot or ELISA. HIV-1 Env production was barely detectable, whereas significant Gag protein was produced. Since the env coding region contains multiple AUGs and is predicted to form strong secondary structure, this sequence was interpreted to inhibit read-through activity that would yield Gag protein. Consistent with the results of Miele et al. [55], partial or complete deletion of the 5′ UTR increased Gag production by 2-fold over the parental reporter containing the intact 5′ UTR. Because the 5′ UTR did not confer Gag production, the authors concluded that the gag 5′ UTR is dispensable for Gag production and that the gag 5′ UTR does not contain IRES activity.
Evidence in Support of Internal Initiation on HIV-1 Unspliced RNA
Buck et al. also evaluated a bicistronic reporter plasmid in which CAT was placed in the 5′ cistron, HIV-1LAI gag was placed in the 3′ cistron, and a 77 bp fragment of the HIV-1LAI 5′ UTR was placed in the intergenic region [14]. Infection with poliovirus was used to suppress cap-dependent translation in transfected 293T cells over a period of six hours. Protein synthesis was evaluated by immunoprecipitation with antibodies against CAT and HIV-1. CAT protein synthesis was reduced by a factor of six, which is consistent with suppression of cap-dependent translation by poliovirus. By contrast, Gag protein synthesis was not inhibited, and this trend mimicked that of the EMCV IRES-cat control. Northern blot analysis eliminated the possibility that gag mRNA translation initiated from cap-dependent alternative transcripts generated by aberrant splicing or by cryptic promoter activity. The authors conclude that since synthesis is sustained of Gag Pr55 protein from bicistronic reporter RNA during shutdown of cap-dependent translation by poliovirus, IRES activity is conferred by sequences within the gag gene.
Brasey et al. used bicistronic renilla luciferase and firefly luciferase RNA to systematically evaluate sixteen segments of the HIVNL4-3 5′ UTR and gag open reading frame for IRES activity. In contrast to the results of Miele et al. and Buck et al., this analysis identified IRES activity in the HIV 5′ UTR in HeLa cells. Results of dual Luciferase assays indicated that reporters containing the HIVNL4-3 5′ UTR produced firefly Luciferase activity at a level similar in magnitude to the poliovirus IRES positive control. When the 5′ UTR was extended to include gag sequences, firefly Luciferase activity was reduced. The results in this bicistronic reporter system conflict with the conclusion by Buck et al. that gag coding sequences support IRES activity. A possible explanation is differences in the structure of the bicistronic reporter constructs.
Brasey et al. used nocodazole to induce cellular G2 arrest and investigate the possibility that HIV-1 IRES activity is active during cell cycle arrest and the consequential suppression of cap-dependent initiation [13]. HeLa cells were transduced with a bicistronic murine leukemia virus (MLV)-based retroviral vector that contained the HIVNL4-3 5′ UTR in the intergenic region between 5′ placental alkaline phosphatase (plap) and 3′ neomycin phosphotransferase (neo) reporter cistrons. Metabolic labeling showed that nocodazole treatment suppressed total cellular protein synthesis to 47% of the control. Immunoblot results determined that Neo protein synthesis was not reduced but rather was maintained at a level of 168% of the untreated control. The observation in the context of this bicistronic reporter construct that translation is sustained during nocodazole-induced G2 arrest supports the conclusion that sequences of the HIV-1 5′ UTR support cap-dependent translation initiation.
Indirect evidence supporting the presence of an IRES in the HIV-1 UTR is derived from the observation of interaction between La autoantigen and the HIV-1 5′ UTR [18]. La has been shown to be an ITAF that is necessary for EMCV IRES-mediated translation [44]. Results of mobility shift assay and detection of HIV-1 TAR RNA in immunoprecipitates from HIV-1 infected lymphocytes using anti-La serum showed that La binds the 5′ UTR by recognizing TAR secondary structure in vitro and in vivo [18]. Moreover, this interaction correlated with increased translation from HIV-1 TAR-containing CAT mRNA in rabbit reticulocyte lysates [80]. Genetic analysis will be an informative approach to further explore the possibility that La is an ITAF that modulates HIV-1 IRES activity.
Another study used rapamycin to interrogate the 5′ UTR of the related primate retrovirus, SIVmac for evidence of internal initiation [58]. These experiments utilized an MLV-based retroviral vector that expressed plap in a 5′ cistron and neo in the 3′ cistron with the intergenic region containing the SIV 5′ UTR. Rather than transient transfection assays, NIH-3T3 cells were transduced with the retroviral bicistronic reporter vectors. The transductants were subjected to long-term selection with G418 for exclusive selection of cells expressing the 3′ cistron of the bicistronic reporter RNA. Then G418-resistant clones were treated with rapamycin to disrupt cap-dependent translation by inactivation of the mTOR pathway. Rapamycin treatment for six hours decreased Plap activity by 43% relative to an untreated control. Under these conditions, Neo activity increased twofold. These results are consistent with internal initiation on the SIV 5′ UTR within these G418-selected transduced cells.
STUDIES OF IRES-LIKE ACTIVITY IN OTHER RETROVIRUSES
Capacity for internal initiation in other retroviruses is also being evaluated in studies with bicistronic reporter plasmids. Sequences from HIV-2, human T lymphotropic virus type 1 (HTLV-1), reticuloendotheliosis virus strain A (RevA), RSV, and MLV have been shown to be capable of directing IRES activity in reporter mRNA [4, 8, 13, 24, 25, 39, 49, 58, 83]. One example of these studies tested the 5′ UTRs of RSV and vSRC for IRES activity in the context of bicistronic neomycin-lacZ reporter plasmid [25]. Significant translation of lacZ was produced in response to the RSV and vSRC containing reporters even when a stable (−50 Kcal/mol) hairpin was inserted upstream of the 5′cistron to inhibit ribosome scanning and control for reinitiation. A separate study looked for MLV IRES activity in NIH3T3 cells permanently expressing bicistronic reporter RNA containing the 5′ leader of MLV envelope between placental alkaline phosphatase (plap) and neomycin, respectively. After treatment with rapamycin to inhibit cap-dependent translation, plap activity decreased by 26% while neomycin expression increased by 316% when compared to untreated cells [24]. A similar approach was used to identify an IRES in the 5′ UTR of RevA [49]. The RevA IRES was recently shown to facilitate translation initiation in mouse neural precursors and to be capable of maintaining activity after cell differentiation, indicating that ubiquitous ITAF are utilized. [26]. Overall, the studies of bicistronic reporter plasmids with sequences from several divergent retroviruses identify small, but positive stimulation of internal initiation. A bona fide role for internal initiation in translation of their respective natural viral transcripts remains to be determined.
Consideration of Unified Parameters to Define Positive IRES Activity
As enumerated by van Eden et al., bicistronic reporter gene assays performed in combination with structural and quantitative RNA analysis have been a productive approach to identify candidate viral and cellular IRESes [84]. For the retroviral literature, effective comparison of the magnitude of IRES activity identified in the various bicistronic reporter assays has been difficult. Two challenges to effective comparison of the results of studies have been differences in the definition of the baseline activity and differences in the definition of positive IRES activity.
RNA transfection of bicistronic reporter transcripts is an alternative approach to plasmid-based transfection assays. This RNA-based approach overcomes the potential pitfall that protein production is mediated by alternative transcripts generated by cryptic promoter activity or aberrant splicing, rather than internal ribosome entry on the bicistronic reporter RNA [84]. Experiments with viral or chemical treatment to inhibit cap-dependent translation have provided a complementary aspect to the bicistronic reporter assay [13, 14] and could be further utilized for analysis of natural mRNA templates expressed from authentic provirus. Furthermore, this approach is useful to validate authentic cap-independent translation, which is an expected characteristic of an mRNA that contains a typical IRES. This approach may also be useful to address the possibility of activity conferred by a cap-dependent ribosome shunt [71].
The inconsistencies between results of the bicistronic assays to investigate IRES activity in HIV-1 sequences may also be attributed to: 1) the caveat that the bicistronic reporter system evaluates HIV-1 sequences in an artificial context that may not recapitulate the natural HIV-1 RNA secondary or tertiary structure; 2) fibroblastic cell lines were used rather than CD4+ human T-lymphocytes, a natural target for HIV-1. These cell lines are expected to present a unique set of ITAFs, each of which may or may not be important for the activity of natural IRESes present in the HIV-1 genome. The inconsistencies exhibited between the results strongly warrant testing in CD4+ T-lymphocytes; 3) possible interplay between cap-dependent translation and IRES-mediated translation may affect internal entry in the natural HIV-1 RNA context, which is not recapitulated in the bicistronic reporter system. A challenge is to go beyond the artificial bicistronic approach and evaluate translation initiation in the context of the HIV-1 provirus. Evaluation of natural HIV-1 mRNAs expressed from provirus in primary lymphocytes is necessary to validate that internal initiation is a bona fide control mechanism of importance during HIV-1 replication.
Clues Supporting Coordinate Cap-Dependent and Cap-Independent Initiation on HIV-1 mRNA
Another challenge is to address the possibility that cap-dependent and cap-independent translation mechanisms in HIV-1 function in a coordinate manner. The existence of redundant initiation mechanisms is supported by the observation that HIV-1 mRNA is subject to cap-dependent ribosome scanning and that HIV-1 sequences exhibit IRES activity in at least some bicistronic reporter assays. What criteria are pertinent to determine which mechanism is dominant and what conditions favor use of the cap-dependent versus cap-independent mechanism? Cap-dependent translation initiation is used by most cellular RNAs and is expected to be a more efficient mechanism then internal initiation, therefore, yielding more protein product. On the other hand, constitutive high level synthesis of viral proteins may be detrimental to the growth and viability of the host cell and increase immunogenicity, which may be deleterious to the progression of HIV-1 replication. Internal initiation enabled by a regulatable IRES would be a versatile strategy to fine-tune viral protein production in response to progression of virus infection and/or changes in the cellular environment. Early in HIV-1 infection, a cap-dependent mechanism may be predominant and sustain a level of viral protein synthesis that is not deleterious to the cell. In later stages of infection, or in response to increased stress in the cellular environment, the cap-dependent mechanism may be replaced by the internal initiation mechanism. This alternation between initiation strategies would provide a mechanism for HIV-1 to sustain translation during the G2 arrest that is induced by Vpr. Identifying which translation initiation mechanism(s) is utilized by HIV-1 during sequential phases of host cell infection may reveal new targets for antiviral therapy. Identification of ITAFs that modulate internal initiation from various HIV-1 mRNAs will provide new insight into how this virus modulates viral protein under conditions of cellular distress and against the odds.
Acknowledgments
We are grateful to Tim Vogt for figure preparation and Dr. Kate Hayes for editing and Dr. Ihab Younis, Nicole Placek and Shuiming Qian for critical comments on the manuscript. This work was supported by National Institutes of Health National Cancer Institute P01 CA100730 and P30 CA16058.
References
- 1.Agy MB, Wambach M, Foy K, Katze MG. Expression of cellular genes in CD4 positive lymphoid cells infected by the human immunodeficiency virus, HIV-1: evidence for a host protein synthesis shut-off induced by cellular mRNA degradation. Virology. 1990;177:251–258. doi: 10.1016/0042-6822(90)90478-a. [DOI] [PubMed] [Google Scholar]
- 2.Ali IK, McKendrick L, Morley SJ, Jackson RJ. Truncated initiation factor eIF4G lacking an eIF4E binding site can support capped mRNA translation. EMBO Journal. 2001;20:4233–4242. doi: 10.1093/emboj/20.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen JL, Planelles V. The role of Vpr in HIV-1 pathogenesis. Current HIV Research. 2005;3:43–51. doi: 10.2174/1570162052772988. [DOI] [PubMed] [Google Scholar]
- 4.Attal J, Theron MC, Taboit F, Cajero-Juarez M, Kann G, Bolifraud P, Houdebine LM. The RU5 (‘R’) region from human leukaemia viruses (HTLV-1) contains an internal ribosome entry site (IRES)-like sequence. FEBS Letters. 1996;392:220–224. doi: 10.1016/0014-5793(96)00815-0. [DOI] [PubMed] [Google Scholar]
- 5.Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO Journal. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 6.Beretta L, Svitkin YV, Sonenberg N. Rapamycin stimulates viral protein synthesis and augments the shutoff of host protein synthesis upon picornavirus infection. Journal of Virology. 1996;70:8993–8996. doi: 10.1128/jvi.70.12.8993-8996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkhout B. Structure and function of the human immunodeficiency virus leader RNA. Progress in Nucleic Acid Research and Molecular Biology. 1996;54:1–34. doi: 10.1016/s0079-6603(08)60359-1. [DOI] [PubMed] [Google Scholar]
- 8.Berlioz C, Darlix JL. An internal ribosomal entry mechanism promotes translation of murine leukemia virus gag polyprotein precursors. Journal of Virology. 1995;69:2214–2222. doi: 10.1128/jvi.69.4.2214-2222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnal S, Pileur F, Orsini C, Parker F, Pujol F, Prats AC, Vagner S. Heterogeneous nuclear ribonucleoprotein A1 is a novel internal ribosome entry site trans-acting factor that modulates alternative initiation of translation of the fibroblast growth factor 2 mRNA. Journal of Biological Chemistry. 2005;280:4144–4153. doi: 10.1074/jbc.M411492200. [DOI] [PubMed] [Google Scholar]
- 10.Bonnal S, Schaeffer C, Creancier L, Clamens S, Moine H, Prats AC, Vagner S. A single internal ribosome entry site containing a G quartet RNA structure drives fibroblast growth factor 2 gene expression at four alternative translation initiation codons. Journal of Biological Chemistry. 2003;278:39330–39336. doi: 10.1074/jbc.M305580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonneau AM, Sonenberg N. Involvement of the 24-kDa cap-binding protein in regulation of protein synthesis in mitosis. Journal of Biological Chemistry. 1987;262:11134–11139. [PubMed] [Google Scholar]
- 12.Borovjagin A, Pestova T, Shatsky I. Pyrimidine tract binding protein strongly stimulates in vitro encephalomyocarditis virus RNA translation at the level of preinitiation complex formation. FEBS Letters. 1994;351:299–302. doi: 10.1016/0014-5793(94)00848-5. [DOI] [PubMed] [Google Scholar]
- 13.Brasey A, Lopez-Lastra M, Ohlmann T, Beerens N, Berkhout B, Darlix JL, Sonenberg N. The leader of human immunodeficiency virus type 1 genomic RNA harbors an internal ribosome entry segment that is active during the G2/M phase of the cell cycle. Journal of Virology. 2003;77:3939–3949. doi: 10.1128/JVI.77.7.3939-3949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buck CB, Shen X, Egan MA, Pierson TC, Walker CM, Siliciano RF. The human immunodeficiency virus type 1 gag gene encodes an internal ribosome entry site. Journal of Virology. 2001;75:181–191. doi: 10.1128/JVI.75.1.181-191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bushell M, McKendrick L, Janicke RU, Clemens MJ, Morley SJ. Caspase-3 is necessary and sufficient for cleavage of protein synthesis eukaryotic initiation factor 4G during apoptosis. FEBS Letters. 1999;451:332–336. doi: 10.1016/s0014-5793(99)00614-6. [DOI] [PubMed] [Google Scholar]
- 16.Butsch M, Boris-Lawrie K. Translation is not required to generate virion precursor RNA in human immunodeficiency virus type 1-infected T cells. Journal of Virology. 2000;74:11531–11537. doi: 10.1128/jvi.74.24.11531-11537.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butsch M, Boris-Lawrie K. Destiny of unspliced retroviral RNA: ribosome and/or virion? Journal of Virology. 2002;76:3089–3094. doi: 10.1128/JVI.76.7.3089-3094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang YN, Kenan DJ, Keene JD, Gatignol A, Jeang KT. Direct interactions between autoantigen La and human immunodeficiency virus leader RNA. Journal of Virology. 1994;68:7008–7020. doi: 10.1128/jvi.68.11.7008-7020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- 20.Chiu SY, Lejeune F, Ranganathan AC, Maquat LE. The pioneer translation initiation complex is functionally distinct from but structurally overlaps with the steady-state translation initiation complex. Genes and Development. 2004;18:745–754. doi: 10.1101/gad.1170204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coldwell MJ, Mitchell SA, Stoneley M, MacFarlane M, Willis AE. Initiation of Apaf-1 translation by internal ribosome entry. Oncogene. 2000;19:899–905. doi: 10.1038/sj.onc.1203407. [DOI] [PubMed] [Google Scholar]
- 22.Creancier L, Mercier P, Prats AC, Morello D. c-myc internal ribosome entry site activity is developmentally controlled and subjected to a strong translational repression in adult transgenic mice. Molecular and Cellular Biology. 2001;21:1833–1840. doi: 10.1128/MCB.21.5.1833-1840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das AT, van Dam AP, Klaver B, Berkhout B. Improved envelope function selected by long-term cultivation of a translation-impaired HIV-1 mutant. Virology. 1998;244:552–562. doi: 10.1006/viro.1998.9124. [DOI] [PubMed] [Google Scholar]
- 24.Deffaud C, Darlix JL. Characterization of an internal ribosomal entry segment in the 5′ leader of murine leukemia virus env RNA. Journal of Virology. 2000;74:846–850. doi: 10.1128/jvi.74.2.846-850.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deffaud C, Darlix JL. Rous sarcoma virus translation revisited: characterization of an internal ribosome entry segment in the 5′ leader of the genomic RNA. Journal of Virology. 2000;74:11581–11588. doi: 10.1128/jvi.74.24.11581-11588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derrington EA, Lopez-Lastra M, Darlix JL. Dicistronic MLV-retroviral vectors transduce neural precursors in vivo and co-express two genes in their differentiated neuronal progeny. Retrovirology. 2005;2:60. doi: 10.1186/1742-4690-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehrenfeld E. Initiation of Translation by Picornavirus RNAs. In: Hershey JWB, Mathews M, Sonenberg N, editors. Translational Control. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 549–573. [Google Scholar]
- 28.Gatfield D, Le Hir H, Schmitt C, Braun IC, Kocher T, Wilm M, Izaurralde E. The DExH/D box protein HEL/UAP56 is essential for mRNA nuclear export in Drosophila. Current Biology. 2001;11:1716–1721. doi: 10.1016/s0960-9822(01)00532-2. [DOI] [PubMed] [Google Scholar]
- 29.Geballe AP, Gray MK. Variable inhibition of cell-free translation by HIV-1 transcript leader sequences. Nucleic Acids Research. 1992;20:4291–4297. doi: 10.1093/nar/20.16.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annual Review of Biochemistry. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 31.Gingras AC, Svitkin Y, Belsham GJ, Pause A, Sonenberg N. Activation of the translational suppressor 4E-BP1 following infection with encephalomyocarditis virus and poliovirus. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5578–5583. doi: 10.1073/pnas.93.11.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nature Medicine. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 33.Gudikote JP, Imam JS, Garcia RF, Wilkinson MF. RNA splicing promotes translation and RNA surveillance. Nature Structural and Molecular Biology. 2005;12:801–809. doi: 10.1038/nsmb980. [DOI] [PubMed] [Google Scholar]
- 34.Hann SR. Regulation and function of non-AUG-initiated proto-oncogenes. Biochimie. 1994;76:880–886. doi: 10.1016/0300-9084(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 35.Harris ME, Hope TJ. RNA export: insights from viral models. Essays in Biochemistry. 2000;36:115–127. doi: 10.1042/bse0360115. [DOI] [PubMed] [Google Scholar]
- 36.Hellen CU, Pestova TV, Litterst M, Wimmer E. The cellular polypeptide p57 (pyrimidine tract-binding protein) binds to multiple sites in the poliovirus 5′ nontranslated region. Journal of Virology. 1994;68:941–950. doi: 10.1128/jvi.68.2.941-950.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellen CU, Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes and Development. 2001;15:1593–1612. doi: 10.1101/gad.891101. [DOI] [PubMed] [Google Scholar]
- 38.Henis-Korenblit S, Strumpf NL, Goldstaub D, Kimchi A. A novel form of DAP5 protein accumulates in apoptotic cells as a result of caspase cleavage and internal ribosome entry site-mediated translation. Molecular and Cellular Biology. 2000;20:496–506. doi: 10.1128/mcb.20.2.496-506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbreteau CH, Weill L, Decimo D, Prevot D, Darlix JL, Sargueil B, Ohlmann T. HIV-2 genomic RNA contains a novel type of IRES located downstream of its initiation codon. Nature Structural and Molecular Biology. 2005;12:1001–1007. doi: 10.1038/nsmb1011. [DOI] [PubMed] [Google Scholar]
- 40.Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nature Cell Biology. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- 41.Jang SK, Krausslich HG, Nicklin MJ, Duke GM, Palmenberg AC, Wimmer E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. Journal of Virology. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johannes G, Sarnow P. Cap-independent polysomal association of natural mRNAs encoding c-myc, BiP, and eIF4G conferred by internal ribosome entry sites. RNA. 1998;4:1500–1513. doi: 10.1017/s1355838298981080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorman N, Lever AML. Comparison of viral genomic RNA sorting mechanisms in HIV-1, HIV-2, and MMLV. Journal of Virology. 2000;74:11413–11417. doi: 10.1128/jvi.74.23.11413-11417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YK, Jang SK. La protein is required for efficient translation driven by encephalomyocarditis virus internal ribosomal entry site. Journal of General Virology. 1999;80(Pt 12):3159–3166. doi: 10.1099/0022-1317-80-12-3159. [DOI] [PubMed] [Google Scholar]
- 45.Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiological Reviews. 1983;47:1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Research. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozak M. The scanning model for translation: an update. Journal of Cell Biology. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LeBlanc JJ, Beemon KL. Unspliced Rous sarcoma virus genomic RNAs are translated and subjected to nonsense-mediated mRNA decay before packaging. Journal of Virology. 2004;78:5139–5146. doi: 10.1128/JVI.78.10.5139-5146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Lastra M, Gabus C, Darlix JL. Characterization of an internal ribosomal entry segment within the 5′ leader of avian reticuloendotheliosis virus type A RNA and development of novel MLV-REV-based retroviral vectors. Human Gene Therapy. 1997;8:1855–1865. doi: 10.1089/hum.1997.8.16-1855. [DOI] [PubMed] [Google Scholar]
- 50.Luo ML, Zhou Z, Magni K, Christoforides C, Rappsilber J, Mann M, Reed R. Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature. 2001;413:644–647. doi: 10.1038/35098106. [DOI] [PubMed] [Google Scholar]
- 51.Mathews DH, Sabina J, Zuker M, Turner DH. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. Journal of Molecular Biology. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 52.Meerovitch K, Pelletier J, Sonenberg N. A cellular protein that binds to the 5′-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes and Development. 1989;3:1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- 53.Merrick WC, Hershey JWB. The Pathway and Mechanism of Eukaryotic Protein Synthesis. In: Hershey JWB, Mathews DH, Sonenberg N, editors. Translational Control. Plainview. Cold Spring Harbor Laboratory Press; 1996. pp. 31–69. [Google Scholar]
- 54.Messer LI, Levin JG, Chattopadhyay SK. Metabolism of viral RNA in murine leukemia virus-infected cells; evidence for differential stability of viral message and virion precursor RNA. Journal of Virology. 1981;40:683–690. doi: 10.1128/jvi.40.3.683-690.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miele G, Mouland A, Harrison GP, Cohen E, Lever AM. The human immunodeficiency virus type 1 5′ packaging signal structure affects translation but does not function as an internal ribosome entry site structure. Journal of Virology. 1996;70:944–951. doi: 10.1128/jvi.70.2.944-951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nevins TA, Harder ZM, Korneluk RG, Holcik M. Distinct regulation of internal ribosome entry site-mediated translation following cellular stress is mediated by apoptotic fragments of eIF4G translation initiation factor family members eIF4GI and p97/DAP5/NAT1. Journal of Biological Chemistry. 2003;278:3572–3579. doi: 10.1074/jbc.M206781200. [DOI] [PubMed] [Google Scholar]
- 57.Nott A, Le Hir H, Moore MJ. Splicing enhances translation in mammalian cells: an additional function of the exon junction complex. Genes and Development. 2004;18:210–222. doi: 10.1101/gad.1163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohlmann T, Lopez-Lastra M, Darlix JL. An internal ribosome entry segment promotes translation of the simian immunodeficiency virus genomic RNA. Journal of Biological Chemistry. 2000;275:11899–11906. doi: 10.1074/jbc.275.16.11899. [DOI] [PubMed] [Google Scholar]
- 59.Parkin NT, Cohen EA, Darveau A, Rosen C, Haseltine W, Sonenberg N. Mutational analysis of the 5′ non-coding region of human immunodeficiency virus type 1: effects of secondary structure on translation. EMBO Journal. 1988;7:2831–2837. doi: 10.1002/j.1460-2075.1988.tb03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- 61.Pelletier J, Kaplan G, Racaniello VR, Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5′ noncoding region. Molecular and Cellular Biology. 1988;8:1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pestova TV, Hellen CU, Shatsky IN. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Molecular and Cellular Biology. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pickering BM, Mitchell SA, Evans JR, Willis AE. Polypyrimidine tract binding protein and poly r(C) binding protein 1 interact with the BAG-1 IRES and stimulate its activity in vitro and in vivo. Nucleic Acids Research. 2003;31:639–646. doi: 10.1093/nar/gkg146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pickering BM, Mitchell SA, Spriggs KA, Stoneley M, Willis AE. Bag-1 internal ribosome entry segment activity is promoted by structural changes mediated by poly(rC) binding protein 1 and recruitment of polypyrimidine tract binding protein 1. Molecular and Cellular Biology. 2004;24:5595–5605. doi: 10.1128/MCB.24.12.5595-5605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pollard VW, Malim MH. The HIV-1 Rev protein. Annual Review of Microbiology. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 66.Purcell DF, Martin MA. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. Journal of Virology. 1993;67:6365–6378. doi: 10.1128/jvi.67.11.6365-6378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pyronnet S, Dostie J, Sonenberg N. Suppression of cap-dependent translation in mitosis. Genes and Development. 2001;15:2083–2093. doi: 10.1101/gad.889201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pyronnet S, Pradayrol L, Sonenberg N. A cell cycle-dependent internal ribosome entry site. Molecular Cell. 2000;5:607–616. doi: 10.1016/s1097-2765(00)80240-3. [DOI] [PubMed] [Google Scholar]
- 69.Pyronnet S, Pradayrol L, Sonenberg N. Alternative splicing facilitates internal ribosome entry on the ornithine decarboxylase mRNA. Cellular and Molecular Life Sciences. 2005;62:1267–1274. doi: 10.1007/s00018-005-5020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin X, Sarnow P. Preferential translation of internal ribosome entry site-containing mRNAs during the mitotic cycle in mammalian cells. Journal of Biological Chemistry. 2004;279:13721–13728. doi: 10.1074/jbc.M312854200. [DOI] [PubMed] [Google Scholar]
- 71.Ryabova LA, Pooggin MM, Hohn T. Viral strategies of translation initiation: ribosomal shunt and reinitiation. Progress in Nucleic Acid Research and Molecular Biology. 2002;72:1–39. doi: 10.1016/S0079-6603(02)72066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schubert U, Bour S, Willey RL, Strebel K. Regulation of virus release by the macrophage-tropic human immunodeficiency virus type 1 AD8 isolate is redundant and can be controlled by either Vpu or Env. Journal of Virology. 1999;73:887–896. doi: 10.1128/jvi.73.2.887-896.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz S, Felber BK, Benko DM, Fenyo EM, Pavlakis GN. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. Journal of Virology. 1990;64:2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwartz S, Felber BK, Pavlakis GN. Mechanism of translation of monocistronic and multicistronic human immunodeficiency virus type 1 mRNAs. Molecular and Cellular Biology. 1992;12:207–219. doi: 10.1128/mcb.12.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sherrill KW, Byrd MP, Van Eden ME, Lloyd RE. BCL-2 translation is mediated via internal ribosome entry during cell stress. Journal of Biological Chemistry. 2004;279:29066–29074. doi: 10.1074/jbc.M402727200. [DOI] [PubMed] [Google Scholar]
- 76.Shi Y, Sharma A, Wu H, Lichtenstein A, Gera J. Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. Journal of Biological Chemistry. 2005;280:10964–10973. doi: 10.1074/jbc.M407874200. [DOI] [PubMed] [Google Scholar]
- 77.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Molecular and Cellular Biology. 1998;18:3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stoneley M, Chappell SA, Jopling CL, Dickens M, MacFarlane M, Willis AE. c-Myc protein synthesis is initiated from the internal ribosome entry segment during apoptosis. Molecular and Cellular Biology. 2000;20:1162–1169. doi: 10.1128/mcb.20.4.1162-1169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subkhankulova T, Mitchell SA, Willis AE. Internal ribosome entry segment-mediated initiation of c-Myc protein synthesis following genotoxic stress. Biochemical Journal. 2001;359:183–192. doi: 10.1042/0264-6021:3590183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Svitkin YV, Pause A, Sonenberg N. La autoantigen alleviates translational repression by the 5′ leader sequence of the human immunodeficiency virus type 1 mRNA. Journal of Virology. 1994;68:7001–7007. doi: 10.1128/jvi.68.11.7001-7007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Temin H. Origin and General Nature of Retroviruses. In: Levy JA, editor. The Retroviridae. New York: Plenum Press; 1992. pp. 1–18. [Google Scholar]
- 82.Vagner S, Touriol C, Galy B, Audigier S, Gensac MC, Amalric F, Bayard F, Prats H, Prats AC. Translation of CUG- but not AUG-initiated forms of human fibroblast growth factor 2 is activated in transformed and stressed cells. Journal of Cell Biology. 1996;135:1391–1402. doi: 10.1083/jcb.135.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vagner S, Waysbort A, Marenda M, Gensac MC, Amalric F, Prats AC. Alternative translation initiation of the Moloney murine leukemia virus mRNA controlled by internal ribosome entry involving the p57/PTB splicing factor. Journal of Biological Chemistry. 1995;270:20376–20383. doi: 10.1074/jbc.270.35.20376. [DOI] [PubMed] [Google Scholar]
- 84.Van Eden ME, Byrd MP, Sherrill KW, Lloyd RE. Demonstrating internal ribosome entry sites in eukaryotic mRNAs using stringent RNA test procedures. RNA. 2004;10:720–730. doi: 10.1261/rna.5225204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Eden ME, Byrd MP, Sherrill KW, Lloyd RE. Translation of cellular inhibitor of apoptosis protein 1 (c-IAP1) mRNA is IRES mediated and regulated during cell stress. RNA. 2004;10:469–481. doi: 10.1261/rna.5156804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wiegand HL, Lu S, Cullen BR. Exon junction complexes mediate the enhancing effect of splicing on mRNA expression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11327–11332. doi: 10.1073/pnas.1934877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]