Abstract
Purpose
The purpose of this study was to evaluate the utility of postural sway variability as a potential assessment to detect altered postural sway in youth with symptoms related to a concussion.
Methods
Forty participants (20 who were healthy and 20 who were injured) aged 10 to 16 years were assessed using the Balance Error Scoring System (BESS) and postural sway variability analyses applied to center-of-pressure data captured using a force plate.
Results
Significant differences were observed between the 2 groups for postural sway variability metrics but not for the BESS. Specifically, path length was shorter and Sample and Renyi Entropies were more regular for the participants who were injured compared with the participants who were healthy (P < .05).
Conclusion
The results of this study indicate that postural sway variability may be a more valid measure than the BESS to detect postconcussion alterations in postural control in young athletes.
Keywords: adolescent, analysis of variance, brain concussion/diagnosis, brain concussion/physiopathology, child, female humans, male, mild traumatic brain injury, postural balance
INTRODUCTION
Once dismissed as a minor injury, mounting evidence indicates that mild traumatic brain injuries, or concussions, can lead to life-altering effects on a person’s physical, mental, and emotional health in the short term.1,2 Long-term complications include depression,3 chronic traumatic encephalopathy,4 and persistent motor control deficits.5,6 Concussion-related complications may be especially troubling for youth because of ongoing brain development that continues throughout adolescence.7–9 Therefore, concussions in youth are a significant public health concern. Nearly all states have passed legislation requiring medical clearance for youths with suspected concussions to allow them to return to sports and recreation-related activities.10 In addition, although most concussions resolve within a few weeks of the initial injury, an estimated 10% to 40% of patients experience lingering symptoms and impairments that last for months to years.11,12 As such, physical therapists have become an integral part of the health care concussion management team.1
Postconcussion Postural Control Assessments
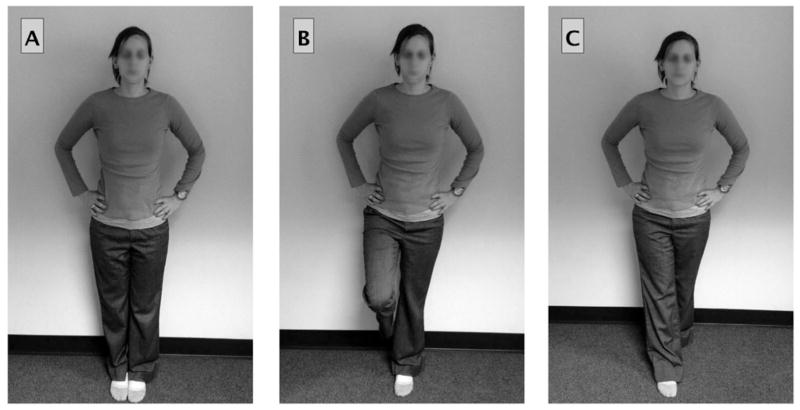
Brain injuries, including concussions, are known to disrupt the nervous system’s ability to process and integrate sensorimotor information, which can lead to difficulty with postural control. Thus, postural control is considered an important physiological parameter to assess following mild head injuries.1,13 Physical therapists can provide unique insight into the assessment and rehabilitation of impairments associated with head injuries. One of the most commonly used and cited postconcussion assessment tools is an observer-rated test that uses 3 different stances (double leg, single leg, and tandem) on 2 different surfaces (floor and foam) referred to as the Balance Error Scoring System (BESS; Figure 1).13 The BESS has been shown to distinguish between healthy and impaired states for 3 to 5 days following injury in college athletes.14 However, rater reliability issues15 and learning effects with serial administration can compromise the validity of the BESS.16
Fig. 1.
This figure demonstrates the various stances (double leg, single leg, and tandem), which are performed on 2 different surfaces (floor and foam), during administration of the Balance Error Scoring System (BESS). Participants stand with their eyes closed for 20-second trials while a trained observer counted the number of errors that are made.
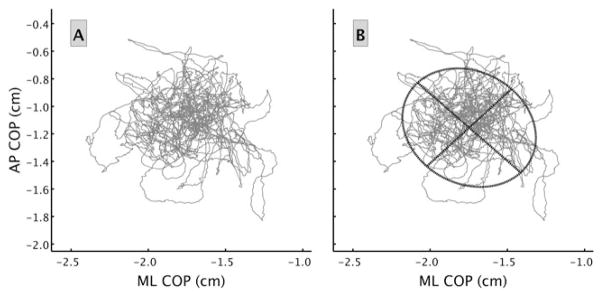
An alternative to the BESS is to evaluate postconcussion postural control using center-of-pressure (COP) trajectories measured with a force platform (Figure 2). The COP is the average spatial location of the ground reaction force vector and correlates highly with center of mass displacements during quiet stance.17 Trajectories of the COP are strings of data points that provide objective and precise records of how the body is swaying over a specified period of time.17 Metrics that summarize COP trajectories have become commonplace for quantitatively describing healthy or impaired states of postural control systems and may provide more reliable and sensitive assessments of postconcussion deficits.18–20 Nonetheless, the ability to detect postconcussion deficits using specific metrics related to COP trajectories may vary.18 To provide a better understanding of why this is so, describing some of the most common examples of such metrics is helpful. Perhaps, the simplest way to understand these metrics is to visualize a COP trajectory plot over the time course of an experimental trial (see Figure 3A). One way to summarize how much the participant sways is to compute the total distance the COP traveled during the trial. Imagine unraveling the plot in Figure 3A and measuring the resulting line with a tape measure. The number yielded from this process is called the COP path length (PL), which represents 1 metric that captures the amount of variability (Vamount) in the COP data (see Table 1). Another metric that captures Vamount is calculation of the 2-dimensional area the COP trajectory covered (COP area) as depicted in Figure 3B.
Fig. 2.
This figure demonstrates an example of the administration of a force platform protocol to measure center-of-pressure (COP) trajectories.
Fig. 3.
(A) This figure provides an example plot of a COP trajectory during a 2-minute trial. (B) This figure provides a visual image of the COP area determined by fitting an ellipse that contains 95% of the data (the oval) and computing the area contained within the ellipse. AP, anterior-posterior; COP, center of pressure; ML, medial-lateral.
TABLE 1.
A Summary of the Center-of-Pressure (COP) Trajectory Metrics Used in This Study
| Metric | Type | Basic Description |
|---|---|---|
| Path length (PL) | Vamount | Distance COP traveled during a given trial (Figure 3A) |
| Center-of-pressure area (COP area) | Vamount | Area of an ellipse fitted to 95% of the data (Figure 3B) |
| Standard deviation (SD) | Vamount | The dispersion of positions of the COP around the mean position of the COP in either the anterior-posterior or medial-lateral time series (the lighter lines in Figure 4A and B) |
| Sample entropy (SampEn) | Vstructure | Probability that a small segment of the COP trajectory will be reproduced at a later time in the time series (Figure 5) |
| Renyi entropy (RenyiEn) | Vstructure | Probability that a measured COP position will reside in a given location (Figure 6) |
Abbreviations: Vamount, amount of variability; Vstructure, structure of the variability.
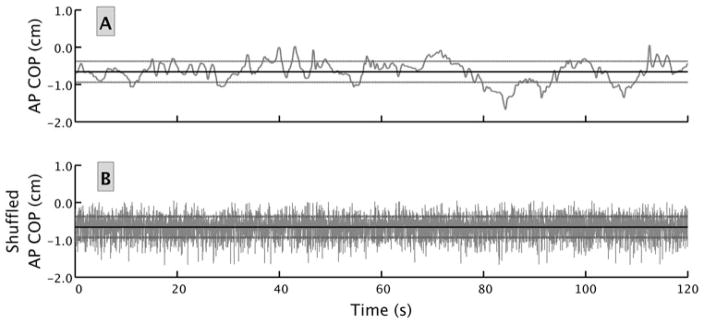
In addition to considering the COP as a whole, it is possible to break down the trajectory into the anterior-posterior (AP) and medial-lateral (ML) directional components to compute other metrics related to Vamount. For example, Figure 4A provides a visual representation of COP positional changes in the AP direction for an experimental trial. A classic calculation of the standard deviation (SD) of the AP positional changes (dashed lines in Figure 4A) around the mean AP COP position (solid line in the plot for Figure 4A) is another way to measure Vamount.
Fig. 4.
This figure provides a graphical representation of the COP positional changes over time in the anterior-posterior direction for an experimental trial. The time series in panel B was derived from the time series in panel A by randomly shuffling the location of each observation. Notice that the mean (identified by the dark line) of each time series is exactly the same, as is the SD (identified by the light lines). Yet, it is clear that the time series have different structural properties. The times series in panel A has more patterns or regularity in the signal and thus appears smoother in contrast to the time series in panel B, which is completely random and irregular. Typically, Vamount metrics will fail to capture the obvious structural differences in the 2 time series. AP, anterior-posterior; COP, center of pressure.
From a different perspective, another important consideration regarding postural sway is the structure (ie, patterns) of the variability (Vstructure) of the data in the time series.21–23 To visualize how Vstructure metrics can be a unique and distinguishing characteristic for a time series, compare Figure 4A with Figure 4B. Qualitatively, it is easy to see differences between the 2 time series. For example, Figure 4A has more regularity and predictability in its structure, whereas Figure 4B is characterized by more irregularity, randomness, and unpredictability. Structural variability metrics can help quantify these characteristics. Moreover, as Figure 4 demonstrates, 2 COP time series can have identical means and SDs and yet be qualitatively and quantitatively very different in terms of Vstructure. Evidence indicates that Vstructure can provide valuable theoretical and clinical information regarding the function of a person’s postural control system.22,24–26 Similar to the example provided in Figure 4, the differences between healthy and impaired states that Vstructure metrics may detect could be present even when Vamount metrics indicate a person’s postural control is not impaired.18
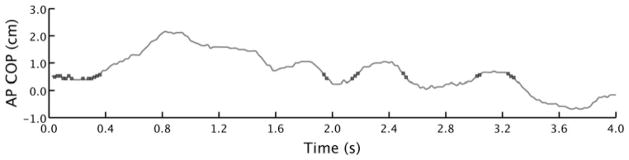
A common way to quantitatively summarize Vstructure is to compute entropy metrics. Entropy metrics provide a measure of the amount of randomness or disorder in a system.20,27 Time series that are more random or contain more disorder in the structure (eg, Figure 4B or the fluctuations of a volatile stock market) typically yield higher entropy scores, whereas time series that contain more order or regularity in the structure produce lower entropy scores (eg, Figure 4A or consider the back-and-forth motion of the pendulum of a clock). Entropy can be computed several ways. Common entropy metrics applied to COP data include approximate entropy (ApEn), Sample Entropy (SampEn), and Renyi Entropy (RenyiEn).20,27,28 All of these involve computing the likelihood that a small segment of the COP trajectory will be reproduced at a later time—an event more likely for a regular, predictable data sequence than an irregular one. Approximate entropy and SampEn are very similar. Approximate entropy was the preferred method, but an improved understanding of these methods has resulted in a general belief that ApEn is a biased statistic.27 Therefore, SampEn, which overcomes some of these biases, has become the preferred method.27 Figure 5 provides a visual representation of how SampEn is computed. Renyi entropy is calculated in a somewhat different way and is akin to the Vstructure for COP area described previously. Renyi entropy characterizes the likelihood a measured COP position will reside in a given location (Figure 6).28,29 Lower values of RenyiEn indicate a more predictable or regular structure, whereas higher RenyiEn indicate the Vstructure is more unpredictable or irregular.
Fig. 5.
This figure demonstrates the calculation of SampEn (see Kuznetsov et al27 for a tutorial on SampEn). Specifically, this figure shows the manner in which the repeatability of a time series is computed. In this case, data points that are recurrent with the first data point are marked by black dots. A data point is considered recurrent with another data point if their positions are separated by less than a specified tolerance (r). This procedure of determining recurrent data points is commonly performed with consecutive data points of a chosen length called a template (m), not just a single data point as illustrated above. Values of r = 0.10 and m = 2 were chosen for the present analysis on the basis of methods discussed in Ramdani et al.71 AP, anterior-posterior; COP, center of pressure; SampEn, sample entropy.
Fig. 6.
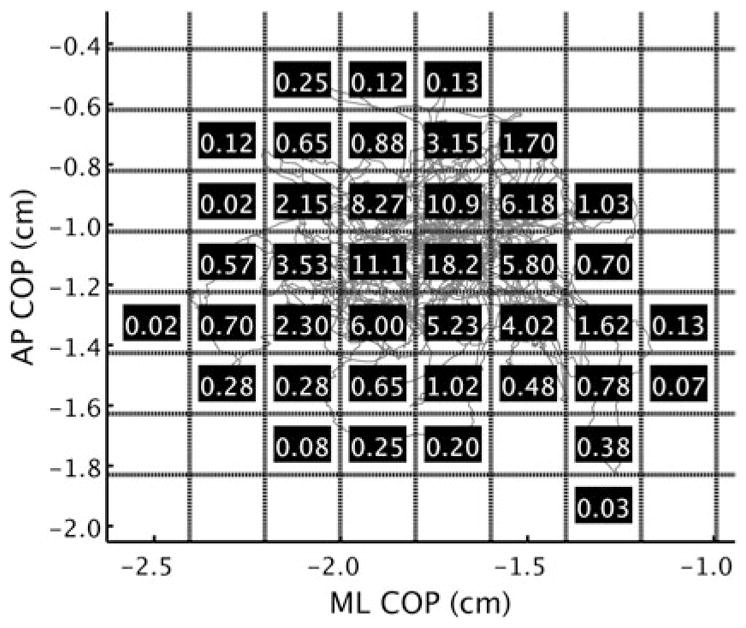
This figure demonstrates the calculation of RenyiEn. The first step in the calculation is to divide the 2-dimensional space containing the COP trajectory into several unit areas. The size of the units was determined individually for a trial by averaging the SD of the separate AP and ML time series—yielding a unit area the size of the mean SD.2 The dashed lines represent the unit areas’ boundaries schematically, with the number inside each unit area indicating the percentage of data points it contains—blank unit areas are not used in the calculation. An order of q = −1 was used for all Renyi calculations (see Gao et al28 for a more thorough description of q and RenyiEn). AP, anterior-posterior; COP, center of pressure; ML, medial-lateral; RenyiEn, Renyi entropy.
With regard to concussion-related studies using force plate measures, the Sensory Organization Test on a NeuroCom Smart Balance Master System (NeuroCom International, Clackamas, Oregon) has been used to test for differences in COP trajectories between healthy and impaired states following a head injury.18,19,30 These studies indicate that although both Vamount and Vstructure metrics may be able to reveal differences between healthy and impaired states, the sensitivity of the Vamount and Vstructure measures may differ. For example, Vamount metrics were lower than baseline scores for some but not all participants with a head injury within the first 48 hours, whereas the Vstructure metrics for the injured cohort as a whole were decreased from baseline within the first 48 hours.19 By 96 hours, Vamount measures had mostly returned to baseline for the athletes who were injured, whereas the Vstructure measures remained lower.19 This suggests a greater sensitivity of Vstructure measures to detect concussion-induced postural impairments.
Extending that work, Sosnoff and colleagues.30 Demonstrated that postconcussion alterations in Vstructure may persist for months to years following a mild head injury. These alterations have also been found in conjunction with changes on functional magnetic resonance imaging (fMRI) in patients postconcussion when examined as many as 9 months after injury.6 Although the exact extent to which such alterations indicate a risk for future injury and long-term sequelae is currently unknown, the literature strongly suggests that such risks are possible.5,6,13,18,19,23,31 Moreover, alterations in Vstructure metrics may indicate a fundamental, pathological change in the underlying organization of the postural control system32 similar to findings related to other neurological conditions such as the Parkinson disease26 and developmental delay.33,34
It should be noted that the prior studies investigating Vamount and Vstructure in athletes with concussions all used test protocols that were only 20 seconds long.18,19,30 However, the Vstructure analysis methods have evolved since those studies were completed, and to optimize the reliability and validity of these methods, longer trials are needed.27,28 Specific to concussions and Vstructure, a study performed by Gao and colleagues indicated that trial lengths of at least 2 minutes should be used in this patient population to maximize the ability to detect postconcussion COP Vstructure changes.28 A further limitation of prior studies in this area is that they have used different COP measurement protocols and inconsistently applied different COP trajectory metrics, some of which have been shown to have less validity that originally though (ie, ApEn), making it difficult to compare results across studies.
Postconcussion Assessment of Postural Control for Children and Adolescents
A majority of studies on concussion, and particularly those regarding postural control evaluation techniques, have been performed with subjects that were young adults or older.2,35 Currently, little is known about which of these tools, if any, are appropriate for use with younger children. Moreover, many experts have raised concerns that younger individuals’ brains may respond differently to head trauma compared with older individuals.2,9,36,37 Therefore, it is possible that the effects of concussions on postural control may differ for younger individuals.
Some evidence suggests that the BESS may be less reliable and valid for the purposes of identifying deficits following a concussion in younger individuals38,39 compared with older individuals. From a developmental standpoint, numerous biomechanical studies indicate that maturational processes affect lower extremity and neuromuscular control.40–45 Good postural stability, particularly for single-leg stance, necessitates good neuromuscular control and strength of the lower extremity (hip, knee, and ankle) musculature.46–48 Because the single-leg test is typically the most effective stance to differentiate between individuals with and without concussions, the BESS may not be developmentally appropriate for younger individuals as the tool’s limitations may mask deficits that may be present.2,28
Results from studies focused on Vstructure characterizations of postural sway have shown great potential as a powerful method to detect changes in postconcussion postural control. However, all of the studies that involved patients with concussions were carried out with young adults. Although not well studied, the possibility exists that COP Vamount and Vstructure may change over the course of motor development, and therefore the effects of concussion on COP Vamount and Vstructure may be different for children. For example, the Vstructure for infants’ sway patterns tend to become more complex and adaptable as their skill level in movements improves.25,49 Similarly, 7-year-old children have demonstrated COP Vstructure that differs from 10-year-old children and adults during a performance of a goal-directed precision task.45 These developmental differences in COP Vamount and Vstructure may indicate transitions to more mature postural control strategies as children develop.45,50 Therefore, the utility and feasibility of Vamount and Vstructure approaches for use with children and adolescents also remain unclear.
The purpose of this study was to (1) to test the ability of COP trajectory metrics to detect differences between a cohort of children who were healthy (aged 8–18 years) and a cohort of children diagnosed with unresolved concussions, and (2) to directly compare the 2 cohorts with regard to their performance on the BESS and the different COP Vamount (PL, COP area, SD-AP, and SD-ML) and Vstructure metrics (SampEn and RenyiEn). We hypothesized that the cohorts would differ in Vstructure, similar to the findings with young adults, suggesting that concussions may cause a fundamental alteration in the underlying organization of the postural control system.
METHODS
Participants
Participants included 20 young athletes (13 males and 7 females) under the care of a physician for concussion-associated symptoms and 20 age, sex, and activity-matched participants serving as healthy controls. The mean age (± SD) for the participants was 13.23 ± 1.28 years with a range of 10.55 to 16.56 years. Participants for the injured cohort were recruited if they were referred to physical therapy for a postconcussion postural control assessment while under the care of a physician for postconcussion symptoms. None of the patients reported prior history of concussions nor had they received physical therapy for concussive symptoms prior to participating in the study.
The mean number of days from the concussive effect for the injured cohort was 48.70 ± 64.85 (range of 4 days to 250 days; 9 patients were within a month of the injury and 11 patients were a month or more postinjury). Participants for the healthy control group had a self-reported medical history free of any head injuries. Participants were excluded from both groups if they reported any health condition expected to affect sway or balance such as a developmental disorder, vestibular disorder, chronic ankle or knee instability, back pain, recent musculoskeletal injury (back, hips, knees, or ankles), or attention deficit disorder/attention deficit hyperactivity disorder. Signed participant assent/parental permission forms were obtained prior to the collection of the data and all study procedures abided by a protocol approved by the investigators’ Institutional Review Board.
Procedures
Postural control was assessed for both cohorts using the BESS and COP trajectory collected on a force platform. The assessments were performed in a randomized order for both cohorts. All assessments were performed by 1 of 4 physical therapists who had engaged in formal training to ensure convergence of techniques and decrease rater reliability issues.
BESS Protocol
Administration of the BESS consisted of six 20-second balance trials (Figure 1). Each trial used a unique combination of stance (double-leg stance, single-leg stance, or tandem stance) and surface (floor or foam).51 Participants performed all trials with their eyes closed. Errors were defined as opening the eyes, lifting hands off hips, stepping, stumbling or falling out of the test position, lifting forefoot or heel, abducting the hip by more than 30°, or taking more than 5 seconds to return to the test position.51 The BESS yields 6 subtest scores (double-leg firm, single-leg firm, tandem firm, double-leg foam, single-leg foam, and tandem foam). A total BESS score was computed by totaling all errors made across all of these subtests with a minimum number of errors of 0 and a maximum number of 10 possible errors per trial. If a participant was never able to maintain a position for a minimum of 5 seconds across the trial, that trial was scored as an automatic 10.
Force Platform Protocol
An AccuSway+ force plate (AMTI, Boston, Massachusetts) and Balance Clinic software were used to collect COP trajectories with a data sampling rate of 50 Hz. Participants each completed 2 trials (1 with eyes open and 1 with eyes closed) presented in a counterbalanced order across subjects. Figure 2 demonstrates how participants were positioned for the study. The following script was read to participants: “Everyone naturally has a little postural sway. This is a test to look at your body’s natural sway. Don’t try to create any extra sway but don’t try to stop it either. We want you to be as natural as possible. We’re going to do 2 trials, 1 eyes open and the other eyes closed. You’re going to stand on the platform facing the ‘x’ with feet together so they’re touching with your hands naturally resting at your sides. You’ll be standing for 2 minutes, which feels like a really long time. Do your best to try not to fidget. Just try to remain as natural as possible throughout the 2 minute trial.” If a participant did not follow instructions (eg, talked, sneezed, or coughed), the trial was terminated and repeated.
The sway stances and conditions were chosen on the basis of the findings of Cavanaugh et al19 and Sosnoff et al30 in which differences were detected between healthy and injured states using bipedal eyes open and eyes closed stances. Moreover, Cavanaugh et al19 found that the greatest change scores from baseline to postinjury may actually be in the simple eyes open and eyes closed conditions rather than in the more challenging Sensory Organization Test conditions. The easier stances were desirable for 2 reasons—(1) to minimize the potential for confounding variables such as diminished hip strength or neuromuscular control needed for single-leg stance conditions, and (2) so the participants could perform longer trials, which are needed to optimally calculate accurate Vstructure metrics, with minimal risk for a fall or loss of balance. A trial length of 2 minutes was selected based on Gao et al’s28 recommendation to maximize the ability to detect post-concussion COP complexity changes. In a prior study, test-retest reliability for this protocol ranged from 0.77 to 0.90.52
Force Platform Data Processing
Custom MATLAB (The MathWorks, Inc, Natick, Massachusetts) code was used to compute the Vamount (PL, COP area, SD-AP, and SD-ML) and Vstructure metrics (SampEn-AP, SampEn-ML, and RenyiEn). Path length was calculated by summing the Euclidean distance between every consecutive point in the 2-dimensional (ie, AP and ML) COP trajectory. The area encompassed by the COP trajectory was calculated by determining the best fitting ellipse, using the least squares criterion, to 95% of the data and then calculating the area of the ellipse (Figure 3B). The COP SD provides an estimate of the trajectory variability (represented by the light lines in Figure 4A and B).
Two complementary Vstructure measures, SampEn and RenyiEn, were computed to capture the temporal and spatial structures, respectively, of the variability of the COP time series (see Figures 5 and 6 for more details). Sample entropy captures the Vstructure in terms of the repeatability of small subsets of data strings within the time series (Figure 5). A high degree of repeatability (lower SampEn) indicates a time series that is more structured (ie, more deterministic and less complex), whereas lower repeatability (higher SampEn) indicates a less structured time series. Renyi entropy is similar to SampEn in that it is a measure of repeatability in the time series. However, instead of examining the temporal structure of the time series, RenyiEn indexes the spatial variability of a 2-dimensional COP trajectory (Figure 6). Lower values of RenyiEn indicate a more predictable or regular trajectory (the trajectory is more likely to visit a certain location), and higher RenyiEn values indicate a more unpredictable or irregular COP trajectory. Sample entropy and RenyiEn were computed in MATLAB using software available from PhysioNet53,54 and software provided by Gao et al,28 respectively.
To test the validity of identified patterns of variability in the COP time series, which would indicate that Vstructure metrics could be useful, tests for differences between the acquired time series data and surrogate time series data were needed.55 Two surrogate analyses were performed—1 for SampEn and 1 for RenyiEn. For the SampEn analysis, a surrogate time series of shuffled data points was compared with the original (unshuffled) COP time series to verify that the identified structure of the variability of the COP signals was due to the temporal ordering of the recorded COP data. The random shuffling was expected to produce a less regular time series as the ordered patterns in the original time series are removed. To borrow an intuitive example from Webber and Zbilut,56 one can liken the shuffling of the COP time series to shuffling a string of Morse code. The random shuffling would not change the frequency of dots and dashes, but the intended meaning of the code is lost because the order, or temporal structure of the dots and dashes, conveys that meaning. Figure 4 illustrates the effect random shuffling has on a time series. The second surrogate analysis was designed to test the RenyiEn metric by randomly sampling hypothetical COP positions from a normal distribution of numbers having the same mean and SD of each original AP and ML time series. We expected that the surrogate data would be less regular than the original because the probability of a surrogate data point residing in a given unit area would be lower than the naturally structured original COP trajectory.
Analysis
To test for differences in the BESS between the injured and the healthy cohorts, independent sample t tests were used with a preset ∝ level of 0.05. Correlational analyses (the Pearson correlation coefficient) were also performed to determine the relationship between days out from injury and BESS scores. On the basis of a preset ∝ level of 0.05, a correlation coefficient (r) was considered significant if it was greater than 0.44. For postural sway variability analyses, a 2 (injury condition; children postconcussion vs children who were healthy) × 2 (vision condition; eyes open vs eyes closed) mixed-design analysis of variance was performed for each dependent postural sway variable. Injury condition was compared as a between-subjects factor, and the vision condition was treated as a within-subjects factor. An estimate of effect size (partial eta squared [ηp2]) is presented for each significant result. This measure provides a metric of the difference between 2 variables (eg, injured vs healthy state) without reference to the study’s sample size. Correlational analyses were performed, as described above, to determine the relationship between days since injury and each COP metric.
RESULTS
BESS
No statistically significant differences were found between the healthy and postconcussion cohorts for any of the BESS subtest scores or total BESS score (P > .05). Likewise, no significant correlations were observed between days since injury and BESS scores.
Amount of COP Variability (Vamount)
A summary of the results for each condition is provided in Table 2. No significant main effects or interactions involving the injury condition in the SD of the AP or ML COP were found (all P > .05). The SD of the AP COP was significantly greater, F1,38 = 34.56, P < .01, ηp2 = 0.48, with participants’ eyes closed (M = 0.29 ± 0.07 cm) than with their eyes open (M = 0.25 ± 0.06 cm). This result did not occur for the ML direction (P > .05). No significant main effect of injury condition for the COP area was found, nor did the injury condition interact with the vision condition (both P > .05). A significant effect of vision on COP area was found, F1,38 = 12.12, P < .01, ηp2 = 0.24, with the eyes open condition (M = 0.92 ± 0.60 cm2) having a smaller area than the eyes closed condition (M = 1.19 ± 0.71 cm2).
TABLE 2.
Descriptive Statistics (Group Mean ± SD and Range) for Amount of Variability (Vamount) and Structure of Variability (Vstructure)
| Group and Condition | PL (cm)a | COP Area (cm)2 | SD (cm)
|
SampEn
|
RenyiEna | ||
|---|---|---|---|---|---|---|---|
| AP | ML | APa | MLa | ||||
| Healthy EO | |||||||
| Mean ± SD | 99.06 ± 22.46a | 0.86 ± 0.46 | 0.25 ± 0.05 | 0.25 ± 0.08 | 0.35 ± 0.09a | 0.37 ± 0.11a | 5.09 ± 0.16a |
| Range | 64.70–139.60 | 0.31–2.00 | 0.15–0.34 | 0.14–0.49 | 0.22–0.55 | 0.22–0.62 | 4.75–5.29 |
| Healthy EC | |||||||
| Mean ± SD | 126.17 ± 27.19a | 1.07 ± 0.50 | 0.28 ± 0.07 | 0.27 ± 0.08 | 0.40 ± 0.09a | 0.43 ± 0.13a | 5.10 ± 0.16a |
| Range | 79.85–176.50 | 0.45–2.00 | 0.17–0.44 | 0.15–0.47 | 0.22–0.52 | 0.23–0.74 | 4.83–5.47 |
| Injured EO | |||||||
| Mean ± SD | 82.93 ± 19.86a | 0.98 ± 0.72 | 0.25 ± 0.08 | 0.29 ± 0.12 | 0.30 ± 0.10a | 0.28 ± 0.10a | 4.96 ± 0.16a |
| Range | 61.53–143.20 | 0.30–2.71 | 0.12–0.43 | 0.14–0.53 | 0.15–0.51 | 0.10–0.48 | 4.69–5.15 |
| Injured EC | |||||||
| Mean ± SD | 106.99 ± 23.50a | 1.31 ± 0.87 | 0.30 ± 0.08 | 0.30 ± 0.11 | 0.33 ± 0.11a | 0.33 ± 0.10a | 5.01 ± 0.13a |
| Range | 71.22–160.40 | 0.42–4.11 | 0.16–0.47 | 0.15–0.47 | 0.16–0.58 | 0.18–0.53 | 4.75–5.30 |
Indicates a statistical difference between cohorts.
Abbreviations: AP, anterior-posterior; COP, center of pressure; EC, eyes closed; EO, eyes open; ML medial-lateral; PL, path length; RenyiEn, Renyi entropy; SampEn, sample entropy; SD, standard deviation.
For the COP PL, significant main effects of the injury and vision conditions were found, F1,38 = 6.29, P < .05, ηp2 = 0.13, and F1,38 = 125.57, P < .01, ηp2 = 0.77, respectively (Table 2). Participants who were injured had significantly shorter PL (M = 94.96 ± 20.94 cm) than participants who were healthy (M = 112.65 ± 23.49 cm). When participants’ eyes were open they had shorter PL (M = 91.00 ± 22.46 cm) than when their eyes were closed (M = 116.58 ± 26.90 cm). The injury and vision conditions did not interact (P > .05). No significant correlations between days since injury and any of these Vamount metrics were found (all P > .05).
Structure of COP Variability (Vstructure)
For SampEn, significant main effects of injury and vision in the AP direction were found, F1,38 = 4.41, P < .05, η2 = 0.10, and F1,38 = 13.41, P < .01, ηp2 = 0.26, respectively (Table 2). Participants who were injured (M = 0.32 ± 0.10) had more regular COP trajectories than participants who were healthy (M = 0.38 ± 0.08). For both cohorts, COP trajectories were more regular when participants had their eyes open (M = 0.33 ± 0.10) than eyes closed (M = 0.36 ± 0.11). Significant main effects for injury and vision were also found in SampEn for the ML direction, F1,38 = 10.34, P < .01, ηp2 = 0.21, and F1,38 = 11.72, P < .01, ηp2 = 0.24, respectively. Participants who were injured (M = 0.30 ± 0.09) had more regular COP trajectories than participants who were uninjured (M = 0.40 ± 0.11). Also, participants’ COP trajectories were more regular with eyes open (M = 0.32 ± 0.12) than eyes closed (M = 0.37 ± 0.13). The main effects of injury and vision did not interact for either direction (P > .05).
A significant main effect of injury condition was found for RenyiEn, F1,38 = 7.74, P < .05, ηp2 = 0.17. Participants who were injured (M = 4.99 ± 0.11) had more regular COP patterns than participants who were healthy (M = 5.09 ± 0.13). The main effect of vision was not significant, nor did it interact with the injury condition (both P > .05). Similar to the Vamount results, no significant correlations were found between days since injury and the Vstructure metrics (all P > .05).
Surrogate Data Analysis
Two paired-samples t tests revealed that the SampEn values of the surrogate data were significantly different from the original data for both the AP and ML COP trajectories, t79 = 211.88, P < .01, and t79 = 189.26, P < .01, respectively. For both directions, the shuffled time series (AP: M = 2.82 ± 0.04; ML: M = 2.81 ± 0.06) were more irregular than the original time series (AP: M = 0.35 ± 0.10; ML: M = 0.35 ± 0.12). Similar to the comparisons between SampEn values for the shuffled surrogate data and the original data, a paired samples t test, t79 = 26.43, P < .01, revealed that the original RenyiEn values (M = 5.04 ± 0.16) were significantly more regular than the surrogate data (M = 5.57 ± 0.09).
DISCUSSION
The purpose of this study was to compare performance scores on the BESS, the postural sway Vamount metrics, and the Vstructure metrics to test for differences between a cohort of children who were healthy and a cohort of children with unresolved concussion. On the basis of these data, the healthy and injured cohorts did not differ in their performances on the BESS but did demonstrate differences for 1 of the Vamount metrics (PL) and both of the Vstructure metrics (SampEn and RenyiEn). Thus, the results of this study indicate that, like the early studies performed with young adults, postural sway Vstructure may also be altered in children and adolescents with unresolved concussion symptoms, and that Vstructure metrics may be both feasible and useful for quantifying postconcussion postural deficits in children.
The lack of differences between the groups for the BESS were not surprising, as the mean time from injury was approximately 49 days. In adults, the BESS is only expected to track differences for about 3 to 5 days postinjury.15 Results from prior studies have also raised concerns about the measurement properties of the BESS for children and adolescents, which could decrease the sensitivity of the measure when used with youth.38,39 Therefore, that no differences were found was not unexpected.
Although it might be expected that individuals who were injured would differ from individuals who were healthy in their PL, the direction of the difference observed was somewhat surprising. The injured cohort in this study exhibited significantly smaller PL than the healthy cohort. Under conventional views of postural stability, a smaller PL would likely be interpreted as more stable.18,19 Interpreted in this way, these results would indicate that the injured cohort demonstrated “better” postural control than the healthy cohort. Nonetheless, there are a couple of explanations for the smaller PL observed in the injured cohort. First, it may be specific to the younger population. As no studies have used a 2-minute protocol comparing PL for adults, it is difficult to know if this is a phenomenon specific to younger individuals or if it is something that might also be observed in adults. From a theoretical perspective, an exploratory component of postural sway may exist, through which the central nervous system uses sway to create sensory stimulation (visual, somatosensory, and vestibular) to gather input about the environment and components of the postural control system.57–59 This mechanism is hypothesized to facilitate perception of postural stability and thus could perhaps help prime the body for action when needed.60 From this perspective, the results of this study may indicate that head injury may degrade the natural exploratory component of sway, and consequently result in a suboptimal ability for the body to take action. Another possible explanation is that the postural control systems of individuals who were injured are attempting to exert more control to resist a sense of instability resulting in co-contractions of the muscles around the lower extremity joints. This would likely result in a reduced length of the COP trajectory.
Prior studies investigating Vamount and Vstructure in athletes with concussions all used a NeuroCom with trials that were only 20 seconds long.18,19,30 These studies also used slightly different forms of the Vamount metrics in comparison to what was used for this study. Although some-what analogous to standard deviation and PL, the Vamount metrics for these prior studies were based on NeuroCom-specific calculations of COP amplitude displacements. Although authors of these reports did indicate that Vstructure may be altered for days to months following a concussion, the Vamount metrics reported in these earlier studies either did not differ between healthy and injured states or only differed for about 2 to 3 days following the injury.18,30 Although this may seem somewhat inconsistent with the findings of this study, where differences were observed in the Vamount metric PL, it is important to note that as highlighted by Gao et al,28 differences in trial length and calculation of the Vamount metrics could potentially explain the contrasting results.28 Future work is needed to determine the robustness of this finding relative to trial length, recovery, and across age and sex.
In terms of the Vstructure metrics, the results of this study supported our hypothesis that, like older individuals, children and adolescents may experience altered postural sway Vstructure. The data for this study yielded lower SampEn and RenyiEn values for the injured cohort indicating greater regularity in their COP patterns. The most compelling interpretation of these findings stems from biomedical complexity theories, which view the human body and its physiological processes as complex, adaptive systems.19,21,61,62 This viewpoint invokes the premise that optimal, healthy regulation of body systems necessitates a good ability to respond to physical and environmental demands (ie, adaptability).63–65
Metrics of Vstructure applied to physiological signals (eg, COP trajectories) are theorized to serve as indicators of robust integration of sensorimotor information and the physiological adaptability of body systems.62,64 These metrics can be viewed on a continuum with 2 extremes. On 1 end of the continuum, the signal is highly regular and predictable like a sinusoidal wave. At the other end of the continuum, the signal is completely random with no recognizable patterns of regularity.22 “Healthy” physiological signals are composed of a combination of regular, rhythmic, predictable aspects (thought to represent control, integration, or regulation) and random components (thought to represent adaptability) and thus fall somewhere in the middle of this continuum. Signals that are higher in regularity or higher in randomness—2 different routes to a pathological loss of complexity—could be indicative of a system that is less able to accommodate neurophysiologic challenges/perturbations.22,23,61 For example, in the case of congestive heart failure, increased regularity in heart rate dynamics has been observed.62,64 Likewise, increased regularity in postural control has been observed in patients with Parkinson disease.26 In both of these examples, diminished ability is a characteristic aspect of the disease—diminished adaptability to cardiovascular demands and diminished adaptability to postural challenges, respectively.
Thus, an interpretation of the results of this study from the perspective of complexity science suggests that like adults, children and adolescents may also have diminished postural control integrity and adaptability following concussions. In this regard, the findings from this study are thus fairly consistent with other studies that have explored postural sway Vstructure metrics for adults with mild head injuries. However, the earlier studies were not always in agreement in terms of the direction in which the COP regularity was altered. The findings in this study were similar to Cavanaugh et al’s findings18,19 with the injured cohort demonstrating greater regularity in their COP patterns in both the AP and ML directions. In contrast, however, Sosnoff et al30 found increased irregularity in the injured group for the AP direction, whereas increased regularity was observed for the ML direction. One reason for this could be that Sosnoff et al’s sample consisted of young adults 6 months or more after injury, whereas Cavanaugh et al studied patients within a few days of their head injuries.18,19 This difference in direction of the altered Vstructure metrics may also be explained by the short time series and the use of the biased ApEn metric by the Cavanaugh18,19 and Sosnoff studies.30 Additional studies are needed to help clarify the extent to which the differences in observed alterations are based on the length of trials, course of recovery, or potentially the presence of heterogeneous responses to concussive injuries in terms of postural dynamics.
Currently, a paucity of research exits with regard to diagnosing and monitoring mild head injuries, particularly for children and adolescents.2,35 Studies using certain imaging modalities (eg, fMRI and diffusion tensor imaging) indicate that these measures may correlate with postconcussion symptom severity and recovery tracking.66,67 However, neuroimaging studies can be expensive and time consuming, rendering them particularly impractical for monitoring the 80% to 90% of patients with concussions who are expected to recover within a relatively short period of time (1–4 weeks).1 The findings from this study provide initial evidence to suggest that postural sway Vstructure metrics could provide a feasible and useful way to non-invasively track subtle underlying postconcussion deficits, which could offer a less expensive and time-consuming way to track concussion injuries for both those who recover quickly and those who struggle to recover within a month. However, at this time, it is difficult to provide explicit guidelines for how clinicians should implement and interpret Vstructure metrics in younger patients. The results for the means and ranges for the metrics reported here are not meant to serve as clinical ranges for diagnostic purposes as this was a relatively small sample size. Future studies should be conducted to determine normative estimates for specific age and maturational stages.
An important limitation of this study is that the time from injury was not controlled. Although recovery time is variable, especially for younger individuals,35,68–70 this study did not show any relationship between days since injury and the COP metrics. Regardless, the longitudinal tracking of how these metrics correspond with recovery in children and adolescents is an important next step to integrate these measures into clinical practice.
CONCLUSION
The results of this study indicate that Vstructure metrics may be both feasible and useful for quantifying postconcussion postural impairments in children and adolescents. Specifically, children and adolescents with a diagnosis of an unresolved concussion exhibited postural sway structural variability that was more regular, predictable, and less complex than their peers who were healthy. Further investigation is needed to establish age-appropriate normative values and the relationship between these metrics and recovery processes.
Acknowledgments
Sources of Funding: The Cincinnati Children’s Hospital Research In-Patient Services PS2 Mentored Career Grant, University of Cincinnati Research Council Post-Doctoral Fellow Grant, and NIH grant 1K23HD074683-01A1.
Footnotes
The authors declare no conflicts of interest.
References
- 1.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 2.Guskiewicz KM, Valovich McLeod TC. Pediatric sports-related concussion. PM R. 2011;3(4):353–364. doi: 10.1016/j.pmrj.2010.12.006. quiz 364. [DOI] [PubMed] [Google Scholar]
- 3.Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 4.Baugh CM, Stamm JM, Riley DO, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6(2):244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 5.De Beaumont L, Lassonde M, Leclerc S, Theoret H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery. 2007;61(2):329–336. doi: 10.1227/01.NEU.0000280000.03578.B6. discussion 336–327. [DOI] [PubMed] [Google Scholar]
- 6.De Beaumont L, Mongeon D, Tremblay S, et al. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011;46(3):234–240. doi: 10.4085/1062-6050-46.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halstead ME, Walter KD American Academy of Pediatrics. Clinical report-sport-related concussion in children and adolescents. Pediatrics. 2010;126(3):597–615. doi: 10.1542/peds.2010-2005. [DOI] [PubMed] [Google Scholar]
- 9.Grady MF. Concussion in the adolescent athlete. Curr Probl Pediatr Adolesc Health Care. 2010;40(7):154–169. doi: 10.1016/j.cppeds.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Tomei KL, Doe C, Prestigiacomo CJ, Gandhi CD. Comparative analysis of state-level concussion legislation and review of current practices in concussion. Neurosurg Focus. 2012;33(6):E11, 1–9. doi: 10.3171/2012.9.FOCUS12280. [DOI] [PubMed] [Google Scholar]
- 11.Sigurdardottir S, Andelic N, Roe C, Jerstad T, Schanke AK. Post-concussion symptoms after traumatic brain injury at 3 and 12 months post-injury: a prospective study. Brain Inj. 2009;23(6):489–497. doi: 10.1080/02699050902926309. [DOI] [PubMed] [Google Scholar]
- 12.Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health. 2012;4(2):147–154. doi: 10.1177/1941738111433673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guskiewicz KM. Balance assessment in the management of sport-related concussion. Clin Sports Med. 2011;30(1):89–102. ix. doi: 10.1016/j.csm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Broglio SP, Puetz TW. The effect of sport concussion on neurocognitive function, self-report symptoms and postural control : a meta-analysis. Sports Med. 2008;38(1):53–67. doi: 10.2165/00007256-200838010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Bell DR, Guskiewicz KM, Clark MA, Padua DA. Systematic review of the balance error scoring system. Sports Health. 2011;3(3):287–295. doi: 10.1177/1941738111403122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valovich McLeod TC, Perrin DH, Guskiewicz KM, Shultz SJ, Diamond R, Gansneder BM. Serial administration of clinical concussion assessments and learning effects in healthy young athletes. Clin J Sport Med. 2004;14(5):287–295. doi: 10.1097/00042752-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Winter DA. Biomechanics and Motor Control of Human Movement. Hoboken, NJ: John Wiley and Sons; 2005. [Google Scholar]
- 18.Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer V, Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005;39(11):805–811. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer VS, Stergiou N. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41(3):305–313. [PMC free article] [PubMed] [Google Scholar]
- 20.Cavanaugh JT, Guskiewicz KM, Stergiou N. A nonlinear dynamic approach for evaluating postural control: new directions for the management of sport-related cerebral concussion. Sports Med. 2005;35(11):935–950. doi: 10.2165/00007256-200535110-00002. [DOI] [PubMed] [Google Scholar]
- 21.Riley MA, Turvey MT. Variability and determinism in motor behavior. J Mot Behav. 2002;34(2):99–125. doi: 10.1080/00222890209601934. [DOI] [PubMed] [Google Scholar]
- 22.Stergiou N, Decker LM. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Hum Mov Sci. 2011;30(5):869–888. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30(3):120–129. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- 24.Deffeyes JE, Harbourne RT, Kyvelidou A, Stuberg WA, Stergiou N. Nonlinear analysis of sitting postural sway indicates developmental delay in infants. Clin Biomech (Bristol, Avon) 2009;24(7):564–570. doi: 10.1016/j.clinbiomech.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Dev Psychobiol. 2003;42(4):368–377. doi: 10.1002/dev.10110. [DOI] [PubMed] [Google Scholar]
- 26.Schmit JM, Riley MA, Dalvi A, et al. Deterministic center of pressure patterns characterize postural instability in Parkinson’s disease. Exp Brain Res. 2006;168(3):357–367. doi: 10.1007/s00221-005-0094-y. [DOI] [PubMed] [Google Scholar]
- 27.Kuznetsov N, Bonnette S, Riley MA. Nonlinear time series methods for analyzing behavioral sequences. In: David K, Hristovski R, Araujo D, Sere NB, Button C, Passos P, editors. Complex Systems in Sport. New York, NY: Routledge; 2014. pp. 85–104. [Google Scholar]
- 28.Gao J, Hu J, Buckley T, White K, Hass C. Shannon and Renyi entropies to classify effects of Mild Traumatic Brain Injury on postural sway. PLoS One. 2011;6(9):e24446. doi: 10.1371/journal.pone.0024446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renyi A. On Measures of Entropy and Information. Paper presented at: Fourth Berkeley Symposium: Math, Statistics and Probability; Berkeley, CA. 1961. [Google Scholar]
- 30.Sosnoff JJ, Broglio SP, Shin S, Ferrara MS. Previous mild traumatic brain injury and postural-control dynamics. J Athl Train. 2011;46(1):85–91. doi: 10.4085/1062-6050-46.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Beaumont L, Theoret H, Mongeon D, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132(pt 3):695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- 32.Riley M, Kuznetsov N, Bonnette S. State-, parameter-, and graph-dynamics: constraints and the distillation of postural control systems. Sci Motricite. 2011;74:5–18. [Google Scholar]
- 33.Deffeyes JE, Harbourne RT, DeJong SL, Kyvelidou A, Stuberg WA, Stergiou N. Use of information entropy measures of sitting postural sway to quantify developmental delay in infants. J Neuroeng Rehabil. 2009;6:34. doi: 10.1186/1743-0003-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harbourne RT, Deffeyes JE, Kyvelidou A, Stergiou N. Complexity of postural control in infants: linear and nonlinear features revealed by principal component analysis. Nonlinear Dynamics Psychol Life Sci. 2009;13(1):123–144. [PubMed] [Google Scholar]
- 35.Makdissi M, Davis G, Jordan B, Patricios J, Purcell L, Putukian M. Revisiting the modifiers: how should the evaluation and management of acute concussions differ in specific groups? Br J Sports Med. 2013;47(5):314–320. doi: 10.1136/bjsports-2013-092256. [DOI] [PubMed] [Google Scholar]
- 36.Karlin AM. Concussion in the pediatric and adolescent population: “different population, different concerns”. PM R. 2011;3(10 suppl 2):S369–S379. doi: 10.1016/j.pmrj.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 37.McCrory P, Collie A, Anderson V, Davis G. Can we manage sport related concussion in children the same as in adults? Br J Sports Med. 2004;38(5):516–519. doi: 10.1136/bjsm.2004.014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheehan DP, Lafave MR, Katz L. Intra-rater and inter-rater reliability of the Balance Error Scoring System in pre-adolescent school children. Meas Phys Educ Exerc Sci. 2011;15(3):234–243. [Google Scholar]
- 39.Valovich McLeod TC, Barr WB, McCrea M, Guskiewicz KM. Psychometric and measurement properties of concussion assessment tools in youth sports. J Athl Train. 2006;41(4):399–408. [PMC free article] [PubMed] [Google Scholar]
- 40.Quatman-Yates CC, Myer GD, Ford KR, Hewett TE. A longitudinal evaluation of maturational effects on lower extremity strength in female adolescent athletes. Pediatr Phys Ther. 2013;25(3):271–276. doi: 10.1097/PEP.0b013e31828e1e9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quatman-Yates CC, Quatman CE, Meszaros AJ, Paterno MV, Hewett TE. A systematic review of sensorimotor function during adolescence: a developmental stage of increased motor awkwardness? Br J Sports Med. 2012;46(9):649–655. doi: 10.1136/bjsm.2010.079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford KR, Myer GD, Hewett TE. Longitudinal effects of maturation on lower extremity joint stiffness in adolescent athletes. Am J Sports Med. 2010;38:1829–1837. doi: 10.1177/0363546510367425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ford KR, Shapiro R, Myer GD, van den Bogert AJ, Hewett TE. Longitudinal sex differences during landing in knee abduction in young athletes. Med Sci Sports Exerc. 2010;42:1923–1931. doi: 10.1249/MSS.0b013e3181dc99b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004;86-A(8):1601–1608. doi: 10.2106/00004623-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Haddad JM, Van Emmerik RE, Wheat JS, Hamill J. Developmental changes in the dynamical structure of postural sway during a precision fitting task. Exp Brain Res. 2008;190(4):431–441. doi: 10.1007/s00221-008-1483-9. [DOI] [PubMed] [Google Scholar]
- 46.Takacs J, Hunt MA. The effect of contralateral pelvic drop and trunk lean on frontal plane knee biomechanics during single limb standing. J Biomech. 2012;45(16):2791–2796. doi: 10.1016/j.jbiomech.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 47.Lee SP, Powers CM. Individuals with diminished hip abductor muscle strength exhibit altered ankle biomechanics and neuromuscular activation during unipedal balance tasks. Gait Posture. 2013;39:933–938. doi: 10.1016/j.gaitpost.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Gribble PA, Hertel J. Effect of hip and ankle muscle fatigue on unipedal postural control. J Electromyogr Kinesiol. 2004;14(6):641–646. doi: 10.1016/j.jelekin.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Dusing SC, Harbourne RT. Variability in postural control during infancy: implications for development, assessment, and intervention. Phys Ther. 2010;90(12):1838–1849. doi: 10.2522/ptj.2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldfield EC. Emergent forms: Origins and Early Development of Human Action and Perception. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 51.Guskiewicz KM. Assessment of postural stability following sport-related concussion. Curr Sports Med Rep. 2003;2(1):24–30. doi: 10.1249/00149619-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Quatman-Yates CC, Lee A, Hugentobler J, Kurowski BG, Myer GD, Riley MA. Test-Retest consistency of a postural sway assessment protocol for adolescent athletes measured with a force plate. Int J Sports Phys Ther. 2013;8(6):741–748. [PMC free article] [PubMed] [Google Scholar]
- 53.Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2002;283(3):R789–R797. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- 54.Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–E220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 55.Theiler J, Eubank S, Longtin A, Galdrikian B, Farmer JD. Testing for nonlinearity in time series: the method of surrogate data. Physica D. 1992;58:77–94. [Google Scholar]
- 56.Webber CL, Zbilut JP. Recurrence quantification analysis of nonlinear dynamical systems. Riley MA, Van Orden GC, editors. [Accessed August 17, 2015];Tutorials in Contemporary Nonlinear Methods for the Behavioral Sciences. http://www.nsf.gov/sbe/bcs/pac/nmbs/nmbs.jsp. Published 2005.
- 57.Carpenter MG, Murnaghan CD, Inglis JT. Shifting the balance: evidence of an exploratory role for postural sway. Neuroscience. 2010;171(1):196–204. doi: 10.1016/j.neuroscience.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 58.Murnaghan CD, Horslen BC, Inglis JT, Carpenter MG. Exploratory behavior during stance persists with visual feedback. Neuroscience. 2011;195:54–59. doi: 10.1016/j.neuroscience.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 59.Murnaghan CD, Squair JW, Chua R, Inglis JT, Carpenter MG. Are increases in COP variability observed when participants are provided explicit verbal cues prior to COM stabilization? Gait Posture. 2013;38(4):734–738. doi: 10.1016/j.gaitpost.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Riccio G. Information in movement variability about the qualitative dynamics of posture and orientation. In: Newell KM, Corcos DM, editors. Variability and Motor Control. Champaign, IL: Human Kinetics Publishers; 1993. pp. 933–938. [Google Scholar]
- 61.Harbourne RT, Stergiou N. Movement variability and the use of nonlinear tools: principles to guide physical therapist practice. Phys Ther. 2009;89(3):267–282. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci USA. 2002;99(suppl 1):2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldberger AL. Heartbeats, hormones, and health: is variability the spice of life? Am J Respir Crit Care Med. 2001;163(6):1289–1290. doi: 10.1164/ajrccm.163.6.ed1801a. [DOI] [PubMed] [Google Scholar]
- 64.Goldberger AL. Fractal variability versus pathologic periodicity: complexity loss and stereotypy in disease. Perspect Biol Med. 1997;40(4):543–561. doi: 10.1353/pbm.1997.0063. [DOI] [PubMed] [Google Scholar]
- 65.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, III, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Ptito A, Chen JK, Johnston KM. Contributions of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. NeuroRehabilitation. 2007;22(3):217–227. [PubMed] [Google Scholar]
- 67.Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A. Focal cortical dysfunction and blood-brain barrier disruption in patients with postconcussion syndrome. J Clin Neurophysiol. 2005;22(1):1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- 68.Broglio SP, Sosnoff JJ, Ferrara MS. The relationship of athlete-reported concussion symptoms and objective measures of neurocognitive function and postural control. Clin J Sport Med. 2009;19(5):377–382. doi: 10.1097/JSM.0b013e3181b625fe. [DOI] [PubMed] [Google Scholar]
- 69.Makdissi M, Cantu RC, Johnston KM, McCrory P, Meeuwisse WH. The difficult concussion patient: what is the best approach to investigation and management of persistent (>10 days) postconcussive symptoms? Br J Sports Med. 2013;47(5):308–313. doi: 10.1136/bjsports-2013-092255. [DOI] [PubMed] [Google Scholar]
- 70.Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- 71.Ramdani S, Seigle B, Lagarde J, Bouchara F, Bernard PL. On the use of sample entropy to analyze human postural sway data. Med Eng Phys. 2009;31(8):1023–1031. doi: 10.1016/j.medengphy.2009.06.004. [DOI] [PubMed] [Google Scholar]