Abstract
Long-distance calling is a common behaviour in animals that has various important social functions. At a physiological level, calling is often mediated by gonadal hormones such as testosterone (T), particularly when its function is linked to intra-sexual competition for mates or territory. T also plays an important role in the development of vocal characteristics associated with dominance in humans. However, the few available studies of T and vocal behaviour in non-human primates suggest that in primates T has less influence on call production than in other animals. We tested this hypothesis by studying the relationship between T concentrations and pant hooting in wild male chimpanzees (Pan troglodytes schweinfurthii) of the Kanyawara community in the Kibale National Park, Uganda. We found three kinds of correlation. Hourly T averages were positively associated with hourly rates of pant-hooting. Monthly T levels were likewise correlated with monthly rates of pant hooting after controlling for other influences such as fission-fusion rates. Finally, males with high T levels had higher peak frequency at the start of the call climax. These results suggest that T affects the production of pant-hoots in chimpanzees. This implies that the pant-hoot call plays a role in male-male competition. We propose that even in cognitively sophisticated species, endocrine mechanisms can contribute to regulating vocal production.
Keywords: chimpanzee, testosterone, vocal behaviour, pant hooting, acoustic structure
Introduction
Long distance calls have been identified in a variety of animal species, ranging from insects to mammals (Bailey 2003; Delgado 2006; Hall 2009). Multiple functions have been attributed to these calls, including attracting mates, repelling sexual rivals, alarm-calling, signalling the identity and location of the caller, advertising social bonds, and defending territorial boundaries (Waser 1977; McComb 1991; Geissmann 1999; Furmankiewicz et al. 2001; Zuberbühler 2001). On a physiological level, animal vocal behaviour is often influenced by hormones, and gonadal hormones such as testosterone (T) and its metabolites, are especially important in regulating vocal production (Floody 1981; Harding 1991; Emerson and Boyd 1999; Moore et al. 2005).
Since gonadal hormones play a crucial role in animal reproductive behaviour (Nelson 2000; Adkins-Regan 2005), these hormones are often involved in modulating the production of calls given in mating contexts. For example, in anuran species T stimulates the production of male advertisement calls (Townsend and Moger 1987; Marler and Ryan 1996; Solıs and Penna 1997; Wilczynski et al. 2005). In many birds T regulates the production of songs that function to attract mates or repel sexual rivals (Nowicki and Ball 1989; Harding 1991; Ketterson et al. 1992; McDonald et al. 2001; Boseret et al. 2006). T also affects the production of vocalisations used by rodents in sexual and agonistic interactions (Floody et al. 1979; Floody 1981; Kapusta and Pochroń 2011; Pasch et al. 2011).
In some species T can also influence the acoustic properties of a call. For instance, high levels of T lower the fundamental frequency (F0) of songs in zebra finches (Cynx et al. 2005), and the pitch of male crowing in Japanese quail (Beani et al. 2000). T implants decrease the minimum frequency, while increasing the duration and frequency range, of male calls in the grey partridge (Fusani et al. 1994; Beani et al. 1995). In singing mice, castrated males treated with T implants sing at lower frequencies than those receiving empty implants (Pasch et al. 2011). In human men, high T levels are associated with low voice pitch (due to lengthening and thickening of the vocal folds), which in turn correlates with perceived social dominance, and may reflect immuno-competence (Dabbs and Mallinger 1999; Archer 2006; Apicella and Feinberg 2009; Hodges-Simeon et al. 2014; Puts et al. 2014).
Several bird studies support the idea that T has acute, activational effects on singing motivation, but delayed, chronic effects on the acoustic structure of song (Ritschard et al. 2011). For example, Cynx et al. (2005) found that the F0 of arbitrarily chosen harmonic stacks in zebra finch songs was not significantly different following three weeks of T administration. After five weeks, however, the fundamental frequency was significantly lower, and remained so for at least one year. T’s effects on acoustic structure may involve long-term changes to anatomical structures of the syrinx (Ritschard et al. 2011) and the brain (Beani et al. 1995). It is unclear, however, whether a similar, long-term effect of T on acoustic characteristics of calls occurs in non-avian species.
Literature on non-human primates (hereafter: primates), however, provides mixed evidence for the claim that T influences calling behaviour. Whilst one recent study on white-handed gibbons showed that male androgen levels affects the song pitch (Barelli et al. 2013), most studies have failed to find a relationship between T and calling. For example, castration affected mouse lemur calling rates, but variation in natural levels of T did not (Zimmermann 1996). Likewise, the production of long-distance calls in male black howler monkeys was not correlated with T levels (Rangel-Negrín et al. 2011). Ontogenetic changes in T levels did not affect call production in Thomas langurs (Wich et al. 2003). These predominantly negative results have led some researchers to conclude that primate vocal behaviour is less tightly linked to hormones than that of many animals, and that experience and social factors play a more prominent role in highly intelligent species (Zimmermann 1996; Wich et al. 2003). However, such a conclusion is premature given how few primates have been studied, the small sample sizes in these studies, and the diversity of call functions in primates.
The purpose of this study was to investigate the relationship between T production and vocal behaviour in wild male chimpanzees. Chimpanzees live in multi-male, multi-female communities in which individuals form temporary subgroups (“parties”) that frequently change in size and composition (Chapman et al. 1995; Aureli et al. 2008). Chimpanzees produce long-distance “pant hoot” vocalizations that play a crucial role in coordinating grouping in their unstable society (Mitani and Nishida 1993; Fedurek et al. 2014). Because chimpanzees appear capable of recognizing the calls of specific community members (Mitani et al. 1996; Kojima et al. 2003), pant-hoots allow listeners to locate other individuals.
Although male chimpanzees often cooperate with each other, and form strong social bonds (Watts 2002; Mitani 2009), aggressive interactions are also important to the achievement and maintenance of male status (Muller and Mitani 2005). It has been suggested that chimpanzee pant hoots might be involved in male status competition. For example, high-ranking individuals pant hoot more often than low-ranking ones, suggesting that the call signals social status (Mitani and Nishida 1993; Clark and Wrangham 1994; Fedurek et al. 2014). Males often pant hoot in choruses, and such chorusing may facilitate coalition formation against other males (Fedurek et al. 2013a). In addition, pant-hooting rates are elevated on days when between-male competition is high, for example when valuable resources such as oestrous females or high-quality food are available (Fedurek et al 2014). Consequently, male pant hoots, apart from coordinating movements of community members, may play an important signalling role in male-male competition. Although this hypothesis has not been explored in detail, a positive relationship between pant-hooting and T levels would be consistent with this function, since in many animals, ranging from amphibians to mammals, the production of calls associated with mating or territorial behaviour is regulated by T (Floody 1981; Van Duyse et al. 2002; Wilczynski et al. 2005).
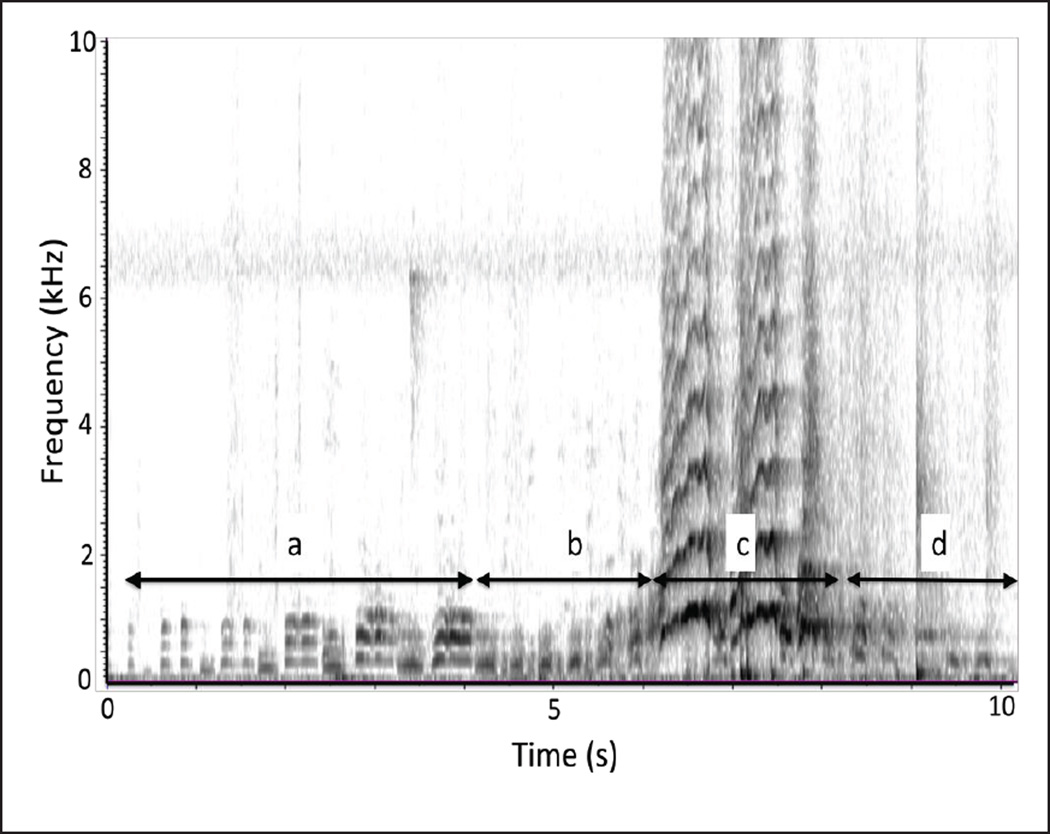
A pant hoot has a distinct and complex acoustic structure (Fig. 1; Marler and Hobbett 1975). The call starts with a short introduction phase consisting of low-frequency elements. The introduction is followed by the build-up phase, comprising short inhalations and exhalation. The build-up grades into the climax, which is the loudest part of the call, comprising one or several “screams” (climax elements) produced in succession. The call might be finalised by a short low-frequency let-down, which has similar acoustic features to the build-up. Although pant hoots may play an important role in male-male competition, it is unclear whether or how T may affect the acoustic features of this call. We sought to test the following three hypotheses of how T may affect the loudest part of the pant hoot call: the climax element (see Table 1). First, high levels of T may be correlated with low pitched vocalisations. As reviewed above, previous research has shown several species, including humans, to demonstrate this pattern and indicated that low pitched vocalisations are signals of good health or condition (Beani et al. 2000; Pasch et al. 2011; Puts et al. 2014). In addition, decreasing vocal tract resonant frequencies likely helps to exaggerate perceived body size, making an animal sound more formidable to competitors (Fitch and Reby 2001). In male chimpanzees, T levels appear to mediate aggressive behaviour towards other males (Muller and Wrangham 2004) and potentially reflect physical condition or health (Muehlenbein and Watts 2010), which in turn might be signalled by producing pant hoots with low F0. If this were the case, high T levels should be associated with pant hoot climaxes with low mean F0s.
Fig. 1.
A spectrogram showing the four phases of a pant hoot. a = introduction, b = build-up, c = climax, d = let-down (from Fedurek et al. 2013b)
Table 1.
An outline of the two hypotheses regarding the relationship between T levels and acoustic features of the pant hoot climax (the loudest element of this long distance call)
| Hypothesis | Prediction |
|---|---|
| High T levels are associated with pant hoot climaxes with low pitch |
T levels correlate negatively with mean pitch of the climax |
| High T levels are associated with pant hoot climaxes with high pitch |
T levels correlate positively with mean and maximum pitch of the climax or peak frequency at the start of the climax |
An alternative hypothesis, however, is that high levels of T may correlate with high frequency vocalisations. For instance, male gibbons with elevated androgen levels produce songs with higher pitch (Barelli et al. 2013). In several mammalian species, production of calls with high rather than low F0 has been shown to reflect good physical condition (Taylor and Reby 2010). For example, in baboons dominant individuals give calls with higher F0 than low-ranking ones (Fischer et al. 2004) and, in red deer, stags with higher F0 are preferred by oestrous females and have higher reproductive success (Reby and McComb 2003; Reby et al. 2010). It has been suggested that high frequency calling may be an effective signal of an individual’s condition (Garcia et al. 2013). For example, the production of high F0 requires a high sub-glottal pressure and large muscular effort that in turn may reflect an individual’s physical capacity or endurance (Titze 1989; Titze and Riede 2010). It is possible, therefore, that males producing pant hoots with high rather than low frequency climax elements are associated with high T levels. If this were the case, T levels should be positively associated with the mean and maximum pitch of the climax of the call. In a similar vein, Riede et al (2007) suggested that producing climaxes with high frequencies is physiologically costly (e.g., Zahavi 1975, 1977) since it requires the caller to immediately switch from the low-frequency build-up phase to the high-frequency climax phase, and that males in good physical condition are more likely to produce climaxes with high initial frequencies that contrast the low-frequency build-up elements. This “calling at the edge” model predicts that T level should be most closely associated with F0 at the beginning of the climax, which directly follows the low-pitched build-up. Consequently, we predicted that T levels should be associated with the peak frequency at the start of the first climax.
In this study, we examined the relationship between T levels and different aspects of male pant hooting behaviour in the Kanyawara community of chimpanzees, Kibale National Park, Uganda. In particular, we examined whether there was both within- and between-individual variation in terms of the relationship between T levels and pant hooting behaviour. T levels in male chimpanzees fluctuate over time, on both a daily and monthly basis, and diurnal variation in T levels is independent from seasonal or monthly variation (Muller and Lipson 2003; Muller and Wrangham 2004). Therefore, we investigated whether temporal changes in T production corresponded to temporal changes in both pant hoot rates and key acoustic parameters of the pant hoot climax, such as peak frequency and pitch. First, we examined whether annual T levels were correlated with annual rates of pant hooting or acoustic features of the call. Second, we examined whether mean monthly levels of T were associated with monthly rates of pant hooting or acoustic features of the call. Since factors such as male fission-fusion rates with other males, time spent travelling, the type of food consumed, the presence of a parous female in oestrus, and the rank of the caller are known to substantially influence male pant hoot rates in chimpanzees (Mitani and Nishida 1993; Fedurek et al. 2014), we examined whether T levels interacted with these factors to influence the rate of call production. It is important to note, however, that the purpose of this work was not to explore the function of pant hooting, but to provide insight on a possible regulating mechanism by examining its association with T. Third, considering that diurnal variation in T levels affects F0 of male voices (Evans et al. 2008) and that in chimpanzees T levels decline steadily over the course of the day (Muller and Lipson 2003), we investigated whether acoustic features of the pant hoot or rate of calling were associated with mean hourly levels of T. With regard to the potential association between T levels and the acoustic features of the call, we specifically examined whether high T levels were associated with high or low F0 (pitch) of the call climax so as to test the two hypotheses outlined above (summarised in Table 1).
Methods
Study subjects and study site
We collected the data between October 2010 and September 2011. The study was conducted on the Kanyawara community in Kibale National Park, located in south-western Uganda (0” 13’-0” 41’ N and 30” 19’-30” 32’ E). At the beginning of the study, in October 2010, the community included 54 individuals (including 10 adult males and 14 adult females). Their home range covered approximately 14 km2 of the park in 2006 (Wilson et al. 2012). Kibale is transitional between lowland rainforest and montane forest (Struhsaker 1975). The area occupied by the Kanyawara chimpanzees is a mosaic of semideciduous primary forest, regenerated forest, grasslands, and swamps (Chapman and Wrangham 1993). The community is well habituated, and has been studied continuously since 1987 by the Kibale Chimpanzee Project and also from 1983 to 1985 (Isabirye-Basuta 1987; Wrangham et al. 1992). The study subjects were eleven adult males (i.e., >15 years old; estimated mean age at the beginning of the study in October 2010=31.18 (SD=14.25 years; Table 2)).
Table 2.
Age (*estimated age of the male) and dominance rank (rank; 1–11) of the focal males
| Male ID | Age (years) | Rank |
|---|---|---|
| AJ | 36* | 3 |
| BB | 44* | 4 |
| ES | 16 | 6 |
| KK | 25 | 1 |
| LK | 28* | 2 |
| PB | 15 | 10 |
| PG | 22 | 7 |
| ST | 55* | 8 |
| TJ | 15 | 5 |
| TU | 50* | 9 |
| YB | 37* | 11 |
Data collection
a) Vocalisations
A randomly chosen male was followed by PF for a whole day (i.e., from nest to nest) and all vocal behaviour of the focal male was noted including the context in which vocalizations were produced (i.e., travelling, feeding and resting). Vocalisations were audio-recorded from both the focal male and males present in his party where possible, using a Marantz Professional PMD661 solid state recorder and a Sennheiser ME67 directional microphone. Instantaneous scan samples were also conducted at 5-min intervals to record (i) the behaviour of the focal male (i.e., travelling, feeding, and resting or grooming), (ii) the type of any food consumed by the focal animal (see below), and (iii) the composition of the focal male’s party. In line with established protocols at this field site, the focal individual’s party was defined as all individuals within 50 m of him (Clark and Wrangham 1994). An individual that had not been seen in the party within 15 min was excluded from the party (Clark and Wrangham 1994). Data on all pant grunts received and given by the focal male and all dyadic agonistic interactions (i.e., physical attack, chase, charge, displacements, etc. (Bygott 1979; Goodall 1986; Muller and Wrangham 2004)) involving the focal were also recorded. In total, 185 days of focal observations were collected. Only days of data collection in which the focal was followed for at least 6 h were incorporated in the analyses (N=168; mean=549.98 min of direct focal observation/day; see Supplementary Material for the number of days of data collection per male per month). Since our study involved focal animals in the field, it was not possible to record data blind.
b) Testosterone
Urinary T levels in chimpanzees show a clear diurnal pattern with the highest levels in the early morning (from 5:00–9:00), followed by a steady decline through the day (Muller and Lipson 2003). To control for this effect, in the analyses concerning annual and monthly relationships between pant hooting and T, we incorporated only samples collected before 9:00 am (N=141, Mean=12.82 samples per focal male, SD=7.13; see Supplementary Material for the number of T samples per male per month).
Urine samples were collected opportunistically from both focal individuals and others in the focal party by four Kibale Chimpanzee Project field assistants. We regularly collected first-morning samples, as chimpanzees predictably urinate upon waking. When a chimpanzee urinated from a tree, we trapped the urine in a disposable plastic bag attached to a two-meter pole. Urine samples were also collected opportunistically throughout the day. Whenever possible, we captured samples on plastic; if a bag could not be placed in time, then urine was pipetted from leaves in the ground layer of vegetation (Muller and Wrangham 2004). After collection, we recorded the identity of the chimpanzee, the date, and the time of urination. Specific gravity of the samples was assayed with a hand-held refractometer (Atago PAL-10S). Samples were stored in a propane-powered freezer and transported frozen to the Hominoid Reproductive Ecology Laboratory at the University of New Mexico, where they were assayed for T.
Prior to analysis, we deconjugated urine samples with beta-glucuronidase (Helix pomatia, Calbiochem, <2% aryl sulfatase activity) to recover the principal metabolite of testosterone and performed an ether extraction (as in Muller and Wrangham 2004). Samples were then assayed with a widely used enzyme immunoassay provided by the University of California at Davis Clinical Endocrinology Laboratory (R156/7). The antibody has strong cross-reactivity (57%) with 5alpha-dihydrotestosterone, which itself derives exclusively from testosterone. Cross-reactivities with the adrenal androgen androstenedione (0.3%) and other androgens/derivatives are minimal (< 0.04%). Inter-assay coefficients of variation (CVs) were 13.9% and 11.9%, respectively, for a low and high urine pool. Intra-assay CV, assessed from the mean CV of sample duplicate determinations, was 4.9%. Testosterone concentrations (in pg/ml) were standardized to specific gravity.
Data collected and definitions
Vocalisations
a) Pant hoot rates
We defined a call as a pant hoot only if it included the climax (Mitani and Gros-Louis 1998). We recorded, in total, 1320 pant hoots (see Supplementary Material for the number of pant hoots recorded per male per month). We calculated a focal male’s daily pant hoot rate by dividing the total number of pant hoots he produced on a given day by the duration of focal observations that day (Fedurek et al. 2014). We calculated monthly pant hoot rates by averaging a male’s daily pant hoot rates in a given month, which resulted in 12 data-points per focal male, corresponding to the 12 months of data collection. Similarly, a focal male’s hourly pant hoot rates were calculated by averaging daily values for each one-hour period (i.e., between 7am and 7:59, between 8am and 8:59, etc. up to 6pm).
b) Acoustic parameters
Only audio-recorded solo pant hoots were incorporated into the acoustic analyses (N=144 calls, Mean=13.09 calls per focal male, SD=8.93; see Supplementary Material for the number of acoustically analyzed pant hoots per focal male per month), and acoustic parameters were taken from the climax of the pant hoot. Pant hoots were recorded in travelling (N=67 calls) and feeding (N=77 calls) contexts. If more than one climax was present in a call (which occurred in 47.30% of the pant hoots recorded), we measured the first climax in the sequence. We took three acoustic measurements from the climax (Table 3).
Table 3.
Acoustic measures taken from a pant hoot climax
| Measure | Definition |
|---|---|
| Peak frequency start | The frequency at which most acoustic energy was present in the F0 at the start of the climax (Hz) |
| Mean pitch | Mean pitch of the climax (Hz) |
| Maximum pitch | Maximum pitch of the climax (Hz) |
All acoustic measurements were conducted using Praat software (version 5.2.19). Peak frequency was determined by taking a spectral slice (frequency vs amplitude) at the start of the climax call. Pitch range was set to 75 −2500 Hz and pitch measurements (see Table 3) for the selected climax call were calculated automatically by Praat. As Praat was designed for the analysis of human speech, rather than chimpanzee calls, the tracking of pitch, as depicted by the pitch line on the spectrogram, was also visually checked to ensure that measurements were accurate.
Each focal male’s mean monthly values for these three acoustic parameters were calculated by averaging these values from a given month. Similarly, each focal male’s hourly values of these parameters were calculated by averaging daily values, across the entire study period, for each one-hour period. Annual values of particular pant hoot characteristics were calculated by averaging values over the whole study period.
Testosterone levels
A focal’s hourly T levels were calculated by averaging values from each one-hour interval between 07:00 and 18:00, across the entire study period. For each focal, monthly T levels were calculated by averaging values from all samples collected prior to 9:00 am in a given month. A focal’s annual T levels were calculated by averaging values from all samples collected prior to 9:00 over the entire study period.
Fission-fusion rates with males
The focal animal’s fission–fusion rates with males were based on the number of changes in the presence of males in the focal’s party, as recorded during the instantaneous scan samples of party composition over one day of data collection (Fedurek et al. 2014). For example, if one or more males left or joined the party in one party composition scan, compared with the previous scan, this was recorded as a single change in the composition of males in the focal’s party. The total number of scans containing changes in the composition of males (mean=6.33 changes/day, SD=3.95) in the focal’s party on a given day was then divided by the number of hours of focal observation on that day. This represented the focal’s overall daily rate of fission–fusion with male community members. We then calculated each male’s monthly fission–fusion rates with males by averaging his daily fission–fusion rates with males in a given month.
Time spent travelling
We established the proportion of time spent travelling by the focal male during a given day of data collection by dividing the number of scans in which the focal was observed travelling by the total number of scans on that day (Fedurek et al. 2014). We then calculated monthly time spent travelling by averaging the focal male’s daily time spent traveling in a given month.
Social rank
Each male was assigned an ordinal linear rank (1–11, where 1 is the highest ranking). The linear hierarchy was based on the outcomes of win–lose interactions combining pant grunt and agonistic interactions recorded during the whole study period using focal animal sampling (Muller and Wrangham 2004). The analysis was carried out using Matman Software Package (version 1.1, Noldus Information Technology; de Vries, 1993). Male dominance hierarchies were significantly linear using a two-step randomization procedure with 10000 iterations (de Vries 1993, 1995).
Food type consumed
If, during the scan, the focal male was observed feeding, the species and plant part consumed were recorded. Chimpanzees tend to form larger groups during seasons of fruit abundance (Conklin-Brittain et al. 1998; Basabose 2002; see also Wrangham 2002), and the consumption of fruits, especially non-fig fruits, correlates positively with energetic status in the Kanyawara chimpanzees (Emery Thompson et al. 2009). We established the proportion of time spent feeding on non-fig fruits by the focal male during a given day of data collection by dividing the number of scans in which he was observed consuming non-fig fruits by the total number of scans during which he was observed feeding (Fedurek et al. 2014). We then calculated monthly proportion of time spent feeding on non-fig fruits by averaging his daily time spent feeding on this food type in a given month.
The presence of an oestrous female
Females were considered to be in oestrous when their genital swellings were maximally swollen. For each day of data collection, we recorded whether (N=61) or not (N=107) a parous female (N=12) in oestrous was present (i.e., during at least one party composition scan) in the focal male’s party. For each focal individual, we calculated the monthly proportion of days in which a parous oestrous female was present in his parties by dividing the number of focal days in which such females were present in his party in a given month by the total number of days in which that male was a focal during that month.
Statistical analyses
Linear mixed-effect models (LMM) were used in the analyses as the main statistical tools. LMM deals effectively with the problem of non-independence of data by incorporating into the model data on entities from which repeated measurements were taken as ‘random effects’. In all of our models we put the ID of the caller as a random effect. There was no colinearity between the examined independent variables (variance inflation factors (VIF) of the independent variables were below the value of 2 (Mean=1.34)), which allowed us to include all of the independent variables in the same model. Before running the analyses, the values of all quantitative variables were z-transformed to a mean of 0 and standard deviation of 1. We used Stata 12.0 (StataCorp LP) and SPSS 22 (IBM Corp) software for all statistical analyses.
Models created
To investigate the relationship between monthly pant hoot rates and monthly T levels, we created a LMM with monthly pant hoot rate as the dependent variable, and monthly T levels as the independent variable. In this model we also included the following independent variables: the rank of the caller, focal’s monthly rates of fission-fusion with other males, focal’s monthly proportion of time spent traveling, focal’s monthly proportion of time spent feeding on non-fig fruits, and focal’s monthly proportion of days in which there was an oestrous parous female present in his party. To investigate the relationship between pant hoot rates and the above independent variables within a specific period of the study, we included in the model only data-points from the months of interest. We then created five additional models with monthly pant hoot rates as the dependent variable. In these models we included interactions between monthly levels of T and the above independent variables, to investigate whether T level interacted with these variables in terms of its influence on pant hoot rates.
The acoustic structure of a pant hoot may depend on the context in which it is produced (Notman and Rendall 2005). To examine whether acoustic parameters differed between pant hoots given in travelling and feeding contexts, we created three LMMs. The dependent variables in these models were the average monthly values for the following acoustic measures: 1) peak frequency at the start of the climax or 2) mean pitch of the climax or 3) maximum pitch of the climax, and the independent variable was whether the pant hoot was produced in the travelling (0) or feeding (1) context.
To investigate the relationship between monthly values of particular acoustic parameters and T levels, we created three models. The dependent variables in these models were the average monthly values for the following acoustic measures: 1) peak frequency at the start of the climax or 2) mean pitch of the climax or 3) maximum pitch of the climax. Mean monthly T level was entered as the independent variable. Since peak frequency or pitch of the climax might be mediated by social rank and age (Riede 2007), we included these attributes as additional independent variables. We then created additional models with interactions between significant independent variables to investigate whether these factors exerted independent influence on pant hooting.
To investigate the relationships between hourly pant hoot rates or hourly values of particular pant hoot acoustics and hourly levels of T, we created four LMMs. The dependent variables in these models were the average monthly values for the following measures: 1) pant hoot rates or 2) peak frequency at the start of the call or 3) mean pitch of the climax or 4) maximum pitch of the climax. Independent variables were the hourly level of T, and the age and social rank of the individual.
To investigate the relationship between annual T levels and annual values of pant hoot rates or particular acoustic features of the call for each male, we used Spearman’s rank correlations (N=11 males).
In situations where multiple post hoc tests were conducted on the same dataset, the α-level for significance was corrected using Sidak's adjustment equation (Sidak 1967) to control for family-wise error.
Results
Pant hoot rates and testosterone levels
Annual testosterone levels and pant hoot rates
There was no significant correlation between yearly individual pant hoot rates and T levels (rs=0.236, P=0.484, N=11).
Monthly testosterone levels and pant hoot rates
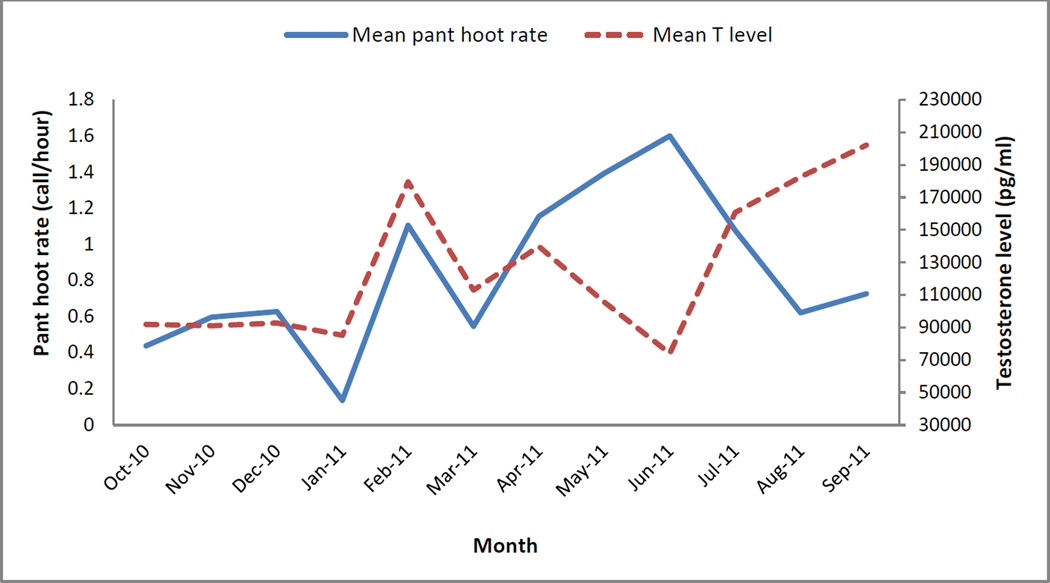
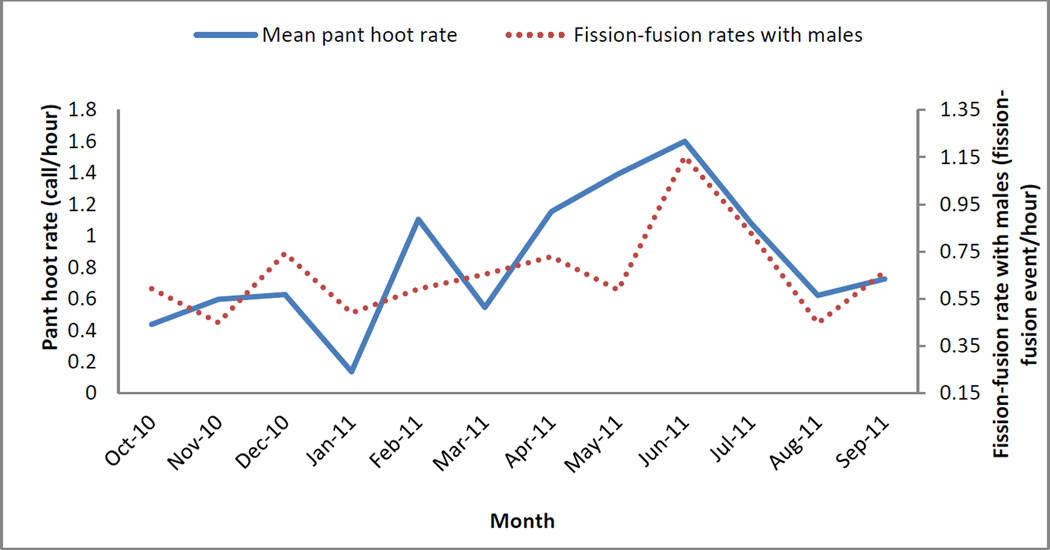
There was a positive relationship between a male’s monthly levels of T and his monthly pant hoot rates (Table 4, Fig. 2). Male monthly proportion of time spent travelling, fission-fusion rates with other males, proportion of time spent on feeding on non-fig fruits, and his social rank were also significantly associated with monthly pant hoot rates (Table 4). The variable “fission-fusion rates with other males” was most strongly associated with pant hoot rates (Table 4). There was no interaction between male monthly T levels and monthly proportion of days in which there was a parous oestrus female in his party (P=0.616), fission-fusion rate with other males (P=0.281), proportion of time spent travelling (P=0.828), proportion of non-fig fruits consumed (P=0.818), or male social rank (P=0.801), suggesting that these variables predicted pant hoot rates independently from T. The relationship between pant hoot rates and T levels was strongest between October 2010 and April 2011 (β±SE=0.32±0.10, z=3.22, P=0.001), and weakest between May and September 2011 (Fig. 2; β±SE=0.09±0.11, z=0.83, P=0.405). In contrast, there was a positive relationship between pant hoot rates and fission-fusion rates with males between May and September 2011 (β±SE=0.23±0.10, z=2.25, P=0.024), but not between October 2010 and April 2011 (Fig. 3; β±SE=0.14±0.10, z=1.39, P=0.163).
Table 4.
The relationship between monthly pant hoot rates and the investigated (independent) variables [LMM; Dependent variable: monthly pant hoot rates; Random effect: Focal ID]
| Independent variables | Coef. | Std. Err. | z | P | [95% Conf. Interval] | |
|---|---|---|---|---|---|---|
| Testosterone level | 0.19 | 0.09 | 2.08 | 0.037 | 0.01 | 0.36 |
| Time travel | 0.26 | 0.09 | 2.98 | 0.003 | 0.09 | 0.43 |
| Rank | −0.27 | 0.11 | −2.50 | 0.012 | −0.48 | −0.06 |
| Fission-fusion rates with males | 0.41 | 0.08 | 4.64 | ≤0.001 | 0.24 | 0.58 |
| Presence of oestrous female | 0.18 | 0.09 | 1.91 | 0.056 | −0.00 | 037 |
| Non-fig fruit consumption | 0.25 | 0.09 | 2.55 | 0.011 | 0.06 | 0.45 |
Fig. 2.
Mean monthly pant hoot rates and testosterone levels
Fig. 3.
Mean monthly pant hoot rates and fission-fusion rates with males
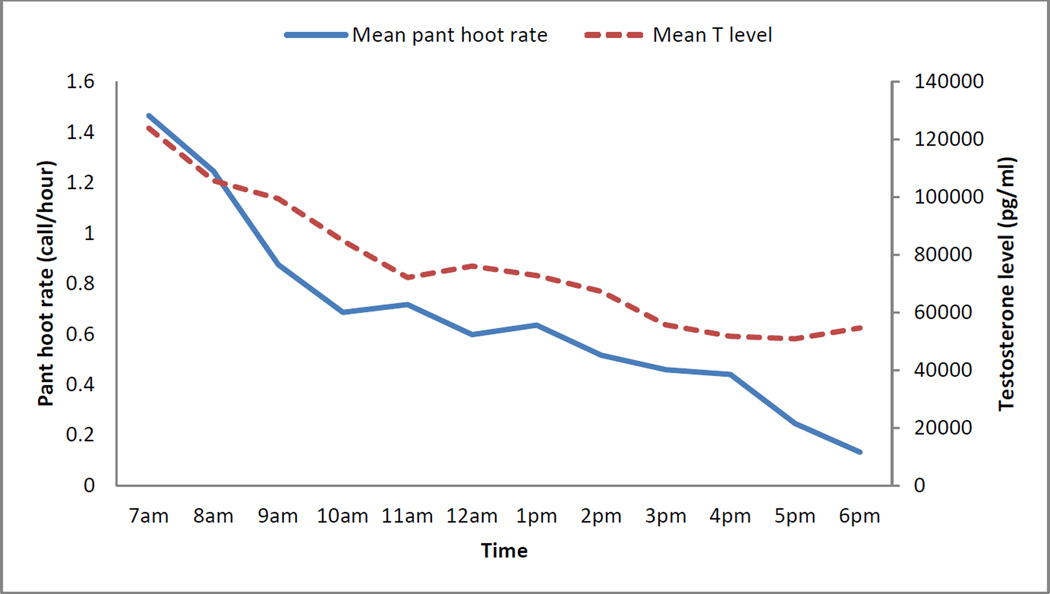
Hourly testosterone levels and pant hoot rates
There was a positive relationship between male hourly pant hoot rates and hourly T levels (Fig. 4; β±SE=0.47±0.09, z=5.35, P≤0.001). There was also a negative relationship between male hourly pant hoot rates and social rank, with high-ranking individuals calling more often than low-ranking ones (β±SE=-0.29±0.14, z=-2.10, P=0.036). There was a significant interaction between hourly T levels and male social rank in predicting pant hoot rates, with high-ranking males showing a stronger relationship between T levels and pant hoot rates (β±SE=-0.15±0.08, z=-1.98, P=0.047). There was no significant relationship between male hourly pant hoot rates and his age (β±SE=0.09±0.14, z=0.65, P=0.517).
Fig. 4.
Mean hourly levels of pant hoot rates and testosterone levels
Pant hoot acoustic features and testosterone levels
Acoustic features of pant hoots produced in travelling and feeding contexts
The acoustic features of pant hoots recorded in travelling and feeding contexts did not differ significantly in terms of the peak frequency at the start of the climax (P=0.313), and the mean (P=0.534) and maximum (P=0.231) pitch of the climax, which allowed us to include acoustic values from these two contexts in the same models.
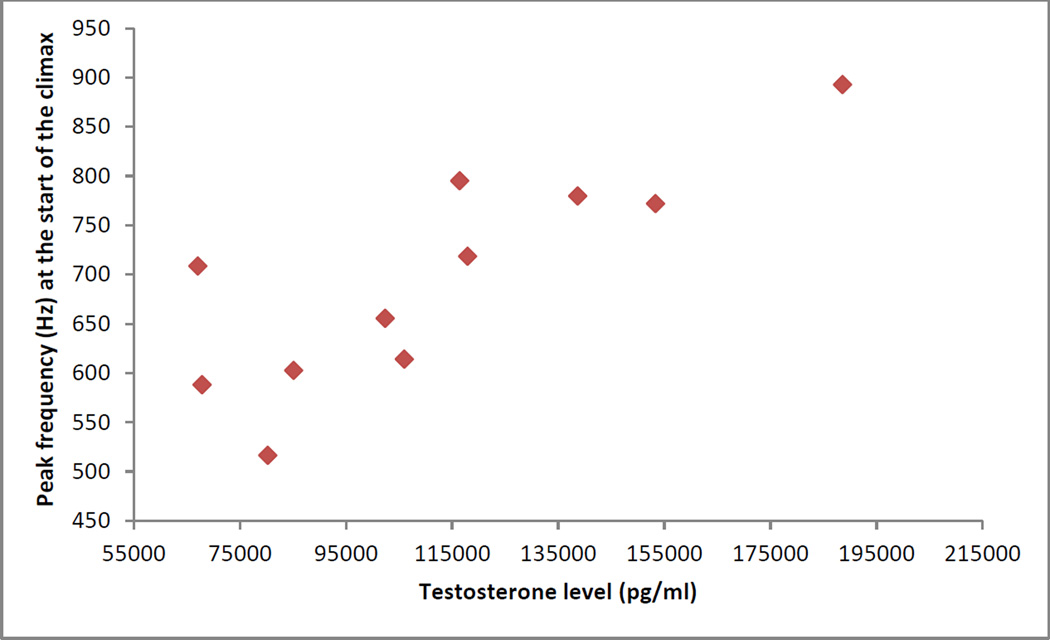
Annual testosterone levels and acoustic parameters of calls
Table 5 shows that there was both within- and between-individual variation in terms of the examined acoustic parameters. There was a significant positive correlation between mean annual T levels and mean peak frequencies at the start of the climax (rs=0.782, P=0.004, N=11, Sidak-corrected α level for significance = 0.006, Fig. 5). Age (rs=-0.469, P=0.145, N=11) and rank (rs=0.273, P=0.446, N=11) did not correlate significantly with peak frequency at the start of the climax. There was no significant correlation between annual T level and annual mean (rs=0.445, P=0.170, N=11) or maximum (rs=0.364, P=0.272, N=11) pitch of the climax.
Table 5.
Mean (±SD) values of the examined acoustic parameters of the pant hoot climax for each male: peak frequency start, mean pitch and maximum pitch
| Male ID | Peak frequency start (Hz) |
Mean pitch (Hz) | Maximum pitch (Hz) |
|---|---|---|---|
| AJ | 655.33 ± 131.56 | 1012.35 ± 151.28 | 1250.02 ± 113.25 |
| BB | 708.50 ± 122.80 | 1178.41 ± 119.65 | 1391.76 ± 160.77 |
| ES | 779.64 ± 174.76 | 1065.50 ± 176.04 | 1379.69 ± 135.81 |
| KK | 772.00 ± 145.69 | 1231.01 ± 257.53 | 1470.59 ± 243.31 |
| LK | 516.40 ± 81.49 | 662.44 ± 206.03 | 801.75 ± 252.59 |
| PB | 718.50 ± 207.18 | 1150.67 ± 103.21 | 1359.34 ± 12.86 |
| PG | 614.09 ± 164.96 | 1023.48 ± 167.09 | 1259.19 ± 170.32 |
| ST | 602.77 ± 120.40 | 739.36 ± 126.44 | 904.59 ± 70.80 |
| TJ | 794.97 ± 152.57 | 1161.92 ± 202.17 | 1354.48 ± 162.74 |
| TU | 588.00 ± 80.61 | 853.20 ± 203.54 | 986.03 ± 190.50 |
| YB | 892.71 ± 214.73 | 1095.85 ± 217.60 | 1249.21 ± 188.68 |
Fig. 5.
The relationship between mean annual levels of testosterone and mean peak frequencies at the start of the climax
Monthly testosterone levels and acoustic parameters of calls
There was a positive relationship between monthly values of peak frequency at the start of the climax and monthly T levels (β±SE=0.37±0.15, z=2.47, P=0.014). Age (β±SE=-0.47±0.15, z=-3.29, P=0.001), but not rank (β±SE=0.11±0.15, z=0.70, P=0.481) of the focal was also significantly associated with peak frequency at the start of the climax, with younger individuals having higher peak frequency. There was a non-significant negative interaction between T levels and age in predicting peak frequency (β±SE=-0.24±0.13, z=-1.82, P=0.069), with the relationship between T levels and peak frequency at the start of the climax being stronger for younger individuals.
There was no relationship between monthly T levels and mean (β±SE=0.21±0.13, z=1.56, P=0.119) or maximum (β±SE=0.22±0.14, z=1.58, P=0.113) pitch of the climax.
Hourly testosterone levels and acoustic parameters of calls
There was no relationship between hourly T levels and hourly values of peak frequency at the start of the climax (β±SE=-0.05±0.13, z=-0.37, P=0.712), mean (β±SE=-0.17±0.10, z=-1.73, P=0.085) or maximum (β±SE=0.21±0.13, z=1.56, P=0.119) pitch of the climax.
Discussion
Our results are consistent with the hypothesis that T levels in male chimpanzees mediate pant hooting behaviour. Temporal changes in T levels corresponded with temporal changes in pant hoot rates, at both hourly and monthly scales. Monthly and annual mean T levels were correlated with the peak frequency at the start of the pant hoot climax. Therefore, on a proximate level, pant hoot production in male chimpanzees seems to be mediated by T. In this respect, our results are consistent with studies of vocal behaviour in many non-primates showing that T influences long-distance calling (Floody 1981; Nowicki and Ball 1989; Marler and Ryan 1996). The positive relationship between chimpanzee pant hooting and T levels therefore implies that, even though an important function of longdistance calls concerns contact maintenance, pant-hoots are also associated with male-male competition (Fedurek et al. 2014).
Although monthly pant hoot rates were associated with monthly T levels, this relationship was not as clear as in the hourly relationship between these two variables. Extrinsic factors such as a male’s fission-fusion rates with other males, time spent travelling and the type of food consumed also predicted monthly pant hoot rates independently from T (see also Fedurek et al. 2014). Indeed, the relative influence of monthly T levels and extrinsic factors such as fission-fusion rates with other males varied in different seasonal time periods. It is clear that multiple factors influence pant hooting rates and this may reflect the numerous social functions of this call (Mitani and Nishida 1993; Fedurek et al. 2014). It is likely, therefore, that the relationship between long calling and T in chimpanzees is not as strong as in animals that perform long calls solely as mating or territorial displays. Our study, however, is not consistent with the view that primate vocal behaviour is fully independent from the influence of gonadal hormones (Zimmermann 1996). Our results shows that the strength of the relationship between T levels and particular call parameters depends on the time scale investigated. It is thus possible that in the studies on primate species where no relationship between call production and testosterone was found (Zimmermann 1996; Wich et al. 2003; Rangel-Negrín et al. 2011), exploring this relationship on different time scales would produce different results.
Our study has several limitations. Most notably, this was an opportunistic study in which we utilised two datasets, one on vocal behaviour, and another on T production by the Kanyawara chimpanzees. Although collected over the same period, these data were associated with independent projects. Consequently, we were unable to investigate the association between pant hooting and T levels on shorter timescales, which arguably would be more informative regarding the cause-effect nature of this association. Having data on T levels of the caller during the time of calling would be especially informative about the cause-effect relationships between calling and T production. Considering the multiple functions of pant hooting, it is possible that the strength of the relationship between T and pant hooting depends on contextual details of emission. We also note that, even though we found a positive relationship between calling and T, with our data it is not possible to investigate whether T influences calling rates directly, or indirectly through some other behaviour or mechanism associated with T.
Our results provide some evidence that T levels are related to some acoustic features of the call. Individuals with the highest T levels over the study period produced, on average, pant hoots with the highest peak frequency at the beginning of the climax. Similarly, although there was considerable individual variation in terms of the peak frequency of the pant hoot climax, during months of elevated T production, males produced pant hoot climaxes with higher initial peak frequencies. Our results contrast with studies on humans and other animals where high T levels are associated with low F0 in males (e.g., Titze 1994; Cynx et al. 2005; Puts et al. 2014). Our study, however, lends some support to T levels being associated with higher frequency calls, and is consistent with a recent study on male gibbons showing a positive association between androgen levels and song pitch (Barelli et al. 2013). It is important to note, however, that we only found a positive relationship between peak frequency at the start of the climax and T levels, not the mean or max pitch. Thus, the elevated frequencies produced by individuals with high T levels were not sustained over the climax element of the call. None the less, our result provides some support for the ‘calling at the edge’ hypothesis (Riede et al. 2007), suggesting that T levels should correlate with F0 especially at the start of the climax.
The effect of T on peak frequency of the climax might be partially confounded by age. Age correlated negatively with F0 at the start of the call, suggesting that younger individuals are more capable of producing calls with high frequencies than older ones. Age is negatively correlated with F0 in many other mammals, mainly because thinner and shorter vocal folds in young individuals generate higher pitched sounds than thicker and longer vocal folds characterising older individuals (Taylor and Reby 2010). Our results suggest that monthly changes in T levels also accounted for the variance in peak F0 of the climax, even though this process was stronger for younger individuals. This suggests that monthly fluctuations in T levels indeed relate to the F0 of the climax, with age modulating rather than driving this process. We suggest that future research uses playback experiments to test whether chimpanzee listeners are sensitive to the subtle acoustic differences we have identified here. Moreover, it is crucial to test whether this acoustic parameter is an effective signal of condition or dominance. Whether chimpanzee males that produce calls containing higher frequencies are perceived as more dominant by other males remains to be empirically tested.
We found no evidence that hourly T levels affect the acoustic structure of the call. This finding seems to contrast with studies on male voice in humans, which show that F0 is sensitive to daily circulating levels of T (Evans et al. 2008). Pant hoot rates, by contrast, were associated with T levels at the hourly but not yearly scale. This is consistent with the suggestion that the motivation to call is mediated by T (e.g., Taylor and Reby 2010). Thus, in line with previous studies on birds (Beani et al. 1995; Cynx et al. 2005; Ritschard et al. 2011), overall vocal activity may be sensitive to short-term changes in T levels, whereas the acoustic structure of the call may be influenced by T in the long term. This interpretation is consistent with studies on birds showing that while short-term changes in T levels affect call production, the acoustic structure of calls is influenced by T on a long-term basis. This may be because, for example, long-lasting high levels of T induce anatomical changes in the structures associated with the vocal tract, thereby allowing greater sub-glottal pressure. It is possible, therefore, that the chronic effect of T on the acoustic structure of calling is not an avian-specific process, but also occurs in chimpanzees and other mammals. Alternatively, the delay between T production and urine excretion might have obscured the results concerning the hourly effect of T on the subtle acoustic features of the call. Although more research is needed to explore the influence of T on the acoustic structure of primate calls, our study is consistent with studies on anuran and avian species showing that some aspects of the acoustic structure of calling are related to T levels (Solis 1994; Cynx et al. 2005).
Pant hoots seem to play an important role in signalling the social status of the caller and facilitating coalitions against other males (Clark and Wrangham 1994; Fedurek et al. 2013a), and therefore likely contribute to male fitness. However, little is known about the features that would make pant hoots an effective signal in this respect. In addition to containing salient acoustic cues to individual identity (Mitani et al. 1996), our data suggest that the rate of calling and initial peak frequency of the climax may reflect social status and hormonal state, as we have shown these are related to T levels. It is possible that these two T-related properties of pant hooting signal the likelihood with which the caller will be involved in agonistic interactions or coalitions against other males when challenged (e.g., Fedurek et al. 2013a, b). Playback experiments examining the listener’s responses to manipulations of call rate and the initial peak frequency of the climax element are needed to test this hypothesis directly. Similarly, considering that the initial peak frequency of the climax element of the call is associated with T levels and that high-ranking males often have higher T levels than low-ranking ones (Muller and Wrangham 2004), it would be interesting to examine whether a change in the male hierarchy brings about changes in this feature of the call. There is anecdotal support for the hypothesis that in chimpanzees, descent in the status hierarchy corresponds with a drop in the dominant frequency of the climax of the call (Riede et al. 2007).
Although we did not incorporate females in our study, it would be interesting to examine the relationship between T levels and female vocal behaviour. Female chimpanzees pant-hoot less frequently than males (Notman 2003), which on a proximate level may be the result of lower T levels in females. In other apes, call inhibition of unflanged orang-utan males might be mediated by the low T levels recorded in these males (Knott 2009). Similarly, the elevated long-calling behaviour of high-ranking compared to low-ranking males observed in baboons and black-crested macaques (Fischer et al. 2004; Neumann et al. 2010) might also be partly explained by differences in T levels. More studies are needed to investigate short-term and long-term effects of T on vocal behaviour in apes and other primates.
In conclusion, our data show that temporal changes in T levels correlate with aspects of male chimpanzee pant hoot behaviour, including the rate at which the calls are produced and the initial frequency of the climax element of the call. Our study is consistent with the hypothesis that, on a proximate level, male chimpanzee vocal behaviour is mediated by T levels, and thus pant hoots likely play a role in male-male competition.
Supplementary Material
Significance Statement.
Many animals, ranging from amphibians to mammals, produce long-distance calls. The production of these calls is often modulated by gonadal hormones such as testosterone, especially if they are involved in competition between males for mates or territory. However, little is known about the role of testosterone in vocal behaviour of non-human primates, especially among great apes. In this study, we examined the relationship between testosterone and pant hooting in wild male chimpanzees. We found that testosterone levels were associated with pant hoot rates and one acoustic feature of the call. More specifically, males pant hooted more often and produced pant hoots with higher peak frequencies during periods of elevated testosterone levels. These results imply that gonadal hormones are involved in regulating vocal behaviour in chimpanzees, and support the view that pant hoots play a role in male-male competition.
Acknowledgments
Permission to conduct the study was granted by the Uganda Wildlife Authority and the Uganda National Council for Science and Technology. We would like to thank the KCP field manager Emily Otali and KCP field assistants Francis Mugurusi, Solomon Musana, James Kyomuhendo, Wilberforce Tweheyo, Sunday John, and Christopher Irumba, who were extremely helpful during the fieldwork. We thank Hugh Notman for his insightful comments and suggestions that considerably improved the paper. This work was supported by a BBSRC studentship, an American Society of Primatologists General Small Grant, a NSF Graduate Research Fellowship, NSF grants #0849380 and #1355014, the Leakey Foundation and the Wenner-Gren Foundation. Research reported in this publication was also supported by the National Institute On Aging of the National Institutes of Health under Award Number R01AG049395. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ethical standards
The study complied with the current laws of Uganda. This study was approved by, and carried out in accordance with, the Department of Psychology Ethics Committee at the University of York.
References
- Adkins-Regan E. Hormones and animal behavior. Princeton University Press; 2005. [Google Scholar]
- Apicella CL, Feinberg DR. Voice pitch alters mate-choice-relevant perception in hunter-gatherers. Proc R Soc Lond B. 2009;276:1077–1082. doi: 10.1098/rspb.2008.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci Biobehav R. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Aureli F, Schaffner CM, Boesch C, Bearder SK, Call J, et al. Fission-fusion dynamics: new research frameworks. Curr Anthropol. 2008;49:627–654. [Google Scholar]
- Bailey WJ. Insect duets: underlying mechanisms and their evolution. Physiol Entomol. 2003;28:157–174. [Google Scholar]
- Barelli C, Mundry R, Heistermann M, Hammerschmidt K. Cues to androgen and quality in male gibbon songs. PLoS ONE. 2013;8:e82748. doi: 10.1371/journal.pone.0082748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basabose AK. Diet composition of chimpanzees inhabiting the montane forest of Kahuzi, Democratic Republic of Congo. Am J Primatol. 2002;58:1–21. doi: 10.1002/ajp.10049. [DOI] [PubMed] [Google Scholar]
- Beani L, Briganti F, Campanella G, Lupo C, Dessi-Fulgheri F. Effect of androgenes on structure and rate of crowing in the Japanise quail Coturnix japonica. Behaviour. 2000;137:417–435. [Google Scholar]
- Beani L, Panzica G, Briganti F, Persichella P, Dessì-Fulgheri F. Testosterone-induced changes of call structure, midbrain and syrinx anatomy in partridges. Physiol Behav. 1995;58:1149–1157. doi: 10.1016/0031-9384(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria) J Neurobiol. 2006;66:1044–1060. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- Bygott JD. Agonistic behavior, dominance, and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg D, McKown EA, editors. The Great Apes. Menlo Park, CA: Benjamin-Cummings; 1979. pp. 405–427. [Google Scholar]
- Chapman CA, Wrangham RW. Range use of the forest chimpanzees of Kibale: Implications of the understanding of chimpanzee social organization. Am J Primatol. 1993;31:263–273. doi: 10.1002/ajp.1350310403. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Wrangham RW, Chapman LJ. Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behav Ecol Sociobiol. 1995;36:59–70. [Google Scholar]
- Clark AP, Wrangham RW. Chimpanzee arrival pant-hoots: do they signify food or status? Int J Primatol. 1994;15:185–205. [Google Scholar]
- Conklin-Brittain NL, Wrangham RW, Hunt KD. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. II. Macronutrients. Int J Primatol. 1998;19:971–998. [Google Scholar]
- Cynx J, Bean NJ, Rossman I. Testosterone implants alter the frequency range of zebra finch songs. Horm Behav. 2005;47:446–451. doi: 10.1016/j.yhbeh.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Mallinger A. High testosterone levels predict low voice pitch among men. Pers Indiv Differ. 1999;27:801–804. [Google Scholar]
- de Vries H. Matman: a program for the analysis of sociometric matrices and behavioural transition matrices. Behaviour. 1993;125:157–175. [Google Scholar]
- de Vries H. An improved test of linearity in dominance hierarchies containing unknown or tied relationships. Anim Behav. 1995;50:1375–1389. [Google Scholar]
- Delgado RA. Sexual selection in the loud calls of male primates: signal content and function. Int J Primatol. 2006;27:5–25. [Google Scholar]
- Emerson SB, Boyd SK. Mating vocalizations of female frogs: control and evolutionary mechanisms. Brain Behav Evolut. 1999;53:187–197. doi: 10.1159/000006594. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW, Lwanga JS, Potts KB. Urinary C-peptide tracks seasonal and individual variation in energy balance in wild chimpanzees. Horm Behav. 2009;55:299–305. doi: 10.1016/j.yhbeh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Evans S, Neave N, Wakelin D, Hamilton C. The relationship between testosterone and vocal frequencies in human males. Physiol Behav. 2008;93:783–788. doi: 10.1016/j.physbeh.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Fedurek P, Donnellan E, Slocombe K. Social and ecological correlates of long-distance pant hoot calls in male chimpanzees. Behav Ecol Sociobiol. 2014;68:1345–1355. [Google Scholar]
- Fedurek P, Machanda Z, Schel AM, Slocombe KE. Pant hoot chorusing and social bonds in male chimpanzees. Anim Behav. 2013a;86:189–196. [Google Scholar]
- Fedurek P, Schel A, Slocombe KE. The acoustic structure of chimpanzee pant-hooting facilitates chorusing. Behav Ecol Sociobiol. 2013b;67:1781–1789. [Google Scholar]
- Fischer J, Kitchen DM, Seyfarth RM, Cheney DL. Baboon loud calls advertise male quality: acoustic features and their relation to rank, age, and exhaustion. Behav Ecol Sociobiol. 2004;56:140–148. [Google Scholar]
- Fitch WT, Reby D. The descended larynx is not uniquely human. Proc R Soc Lond B. 2001;268:1669–1675. doi: 10.1098/rspb.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floody OR. The hormonal control of ultrasonic communication in rodents. Am Zool. 1981;21:129–142. [Google Scholar]
- Floody OR, Walsh C, Flanagan MT. Testosterone stimulates ultrasound production by male hamsters. Horm Behav. 1979;12:164–171. doi: 10.1016/0018-506x(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Furmankiewicz J, Ruczynski I, Urban R, Jones G. Social calls provide tree-dwelling bats with information about the location of conspecifics at roosts. Ethology. 2001;117:480–489. [Google Scholar]
- Fusani L, Beani L, Dessi-Fulgheri F. Testosterone affects the acoustic structure of the male call in the grey partridge (Perdix perdix) Behaviour. 1994;128:301–310. [Google Scholar]
- Garcia M, Charlton BD, Wyman MT, Fitch WT, Reby D. Do red deer stags Cervus elaphus use roar fundamental frequency (F0) to assess rivals? PLoS ONE. 2013;8:e83946. doi: 10.1371/journal.pone.0083946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann T. Duet songs of the siamang, Hylobates syndactylus: II. Testing the pair-bonding hypothesis during a partner exchange. Behaviour. 1999;136:1005–1039. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: patterns of behavior. Cambridge: Harvard University Press; 1986. [Google Scholar]
- Hall ML. A review of vocal duetting in birds. Adv Stud Behav. 2009;40:67–121. [Google Scholar]
- Harding CF. Neuroendocrine integration of social behaviour in male songbirds. In: Archer T, Hansen S, editors. Behavioral Biology: Neuroendocrine Axis. New York: Hillsdale; 1991. pp. 53–66. [Google Scholar]
- Hodges-Simeon CR, Gurven M, Puts DA, Gaulin SJC. Vocal fundamental and formant frequencies are honest signals of threat potential in peripubertal males. Behav Ecol. 2014;25:984–988. doi: 10.1093/beheco/aru081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabirye-Basuta G. Feeding ecology of chimpanzees in the Kibale Forest. In: Heltne PG, Marquardt LA, editors. Understanding chimpanzees. Cambridge: Harvard University Press; 1987. 166-127. [Google Scholar]
- Kapusta J, Pochroń E. Effect of gonadal hormones and sexual experience on vocalizations and behavior of male bank voles (Myodes glareolus) Can J Zool. 2011;89:1117–1127. [Google Scholar]
- Ketterson EDVN, Jr, Wolf L, Ziegenfus C. Testosterone and avian life histories: effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis) Am Nat. 1992;140:980–999. [Google Scholar]
- Knott CD. Orangutans: sexual coercion without sexual violence. In: Muller MN, Wrangham RW, editors. Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Cambridge, Massachusetts: Harvard University Press; 2009. pp. 112–127. [Google Scholar]
- Kojima S, Izumi A, Ceugniet M. Identification of vocalizers by pant hoots, pant grunts and screams in a chimpanzee. Primates. 2003;44:225–230. doi: 10.1007/s10329-002-0014-8. [DOI] [PubMed] [Google Scholar]
- Marler CA, Ryan MJ. Energetic constraints and steroid hormone correlates of male calling behaviour in the túngara frog. J Zool. 1996;240:397–409. [Google Scholar]
- Marler P, Hobbett L. Individuality in a long-range vocalization of wild chimpanzee. Z Tierpsychol. 1975;38:97–109. [PubMed] [Google Scholar]
- McComb KE. Female choice for high roaring rates in red deer, Cervus elaphus. Anim Behav. 1991;41:79–88. [Google Scholar]
- McDonald PG, Buttemer WA, Astheimer LB. The influence of testosterone on territorial defence and parental behavior in male free-living rufous whistlers, Pachycephala rufiventris. Horm Behav. 2001;39:185–194. doi: 10.1006/hbeh.2001.1644. [DOI] [PubMed] [Google Scholar]
- Mitani JC. Male chimpanzees form enduring and equitable social bonds. Anim Behav. 2009;77:633–640. [Google Scholar]
- Mitani JC, Gros-Louis J. Chorusing and call convergence in chimpanzees: tests of three hypotheses. Behaviour. 1998;135:1041–1064. [Google Scholar]
- Mitani JC, GrosLouis J, Macedonia JM. Selection for acoustic individuality within the vocal repertoire of wild chimpanzees. Int J Primatol. 1996;17:569–583. [Google Scholar]
- Mitani JC, Nishida T. Contexts and social correlates of long-distance calling by male chimpanzees. Anim Behav. 1993;45:735–746. [Google Scholar]
- Moore FL, Boyd SK, Kelley DB. Historical perspective: hormonal regulation of behaviors in amphibians. Horm Behav. 2005;48:373–383. doi: 10.1016/j.yhbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Muehlenbein M, Watts D. The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. Biopsychosoc Med. 2010;4:1–12. doi: 10.1186/1751-0759-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Lipson SF. Diurnal patterns of urinary steroid excretion in wild chimpanzees. Am J Primatol. 2003;60:161–166. doi: 10.1002/ajp.10103. [DOI] [PubMed] [Google Scholar]
- Muller MN, Mitani JC. Conflict and cooperation in wild chimpanzees. Adv Stud Behav. 2005;35:275–331. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, aggression and testosterone in wild chimpanzees: a test of the 'challenge hypothesis' . Anim Behav. 2004;67:113–123. [Google Scholar]
- Nelson RJ. An introduction to behavioural endocrinology. Sunderland: Sinauer; 2000. [Google Scholar]
- Neumann C, Assahad G, Hammerschmidt K, Perwitasari-Farajallah D, Engelhardt A. Loud calls in male crested macaques, Macaca nigra: a signal of dominance in a tolerant species. Anim Behav. 2010;79:187–193. [Google Scholar]
- Notman H. The meaning, structure and function of chimpanzee pant hoots from the Budongo Forest, Uganda. PhD thesis. Calgary: University of Calgary; 2003. [Google Scholar]
- Notman H, Rendall D. Contextual variation in chimpanzee pant hoots and its implications for referential communication. Anim Behav. 2005;70:177–190. [Google Scholar]
- Nowicki S, Ball GF. Testosterone induction of song in photosensitive and photorefractory male sparrows. Horm Behav. 1989;23:514–525. doi: 10.1016/0018-506x(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Pasch B, George AS, Hamlin HJ, Guillette LJ, Jr, Phelps SM. Androgens modulate song effort and aggression in Neotropical singing mice. Horm Behav. 2011;59:90–97. doi: 10.1016/j.yhbeh.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Puts DA, Doll LM, Hill AK. Sexual selection on human voices. In: Weekes-Shackelford V, Shackelford TK, editors. Evolutionary perspectives on human sexual psychology and behavior. New York: Springer; 2014. pp. 69–86. [Google Scholar]
- Rangel-Negrín A, Dias PAD, Chavira R, Canales-Espinosa D. Social modulation of testosterone levels in male black howlers (Alouatta pigra) Horm Behav. 2011;59:159–166. doi: 10.1016/j.yhbeh.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Reby D, Charlton BD, Locatelli Y, McComb K. Oestrous red deer hinds prefer male roars with higher fundamental frequencies. Proc R Soc Lond B. 2010;277:2747–2753. doi: 10.1098/rspb.2010.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reby D, McComb K. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav. 2003;65:519–530. [Google Scholar]
- Riede T, Arcadi AC, Owren MJ. Nonlinear acoustics in the pant hoots of common chimpanzees (Pan troglodytes): vocalizing at the edge. J Acoust Soc Am. 2007;121:1758–1767. doi: 10.1121/1.2427115. [DOI] [PubMed] [Google Scholar]
- Ritschard M, Laucht S, Dale J, Brumm H. Enhanced testosterone levels affect singing motivation but not song structure and amplitude in Bengalese finches. Physiol Behav. 2011;102:30–35. doi: 10.1016/j.physbeh.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc. 1967;62:626–633. [Google Scholar]
- Solis R. Factores moduladores de las interacciones sociales acusticasde Pleurodema thaul. PhD thesis. Universidad de Chile; 1994. [Google Scholar]
- Solıs R, Penna M. Testosterone levels and evoked vocal responses in a natural population of the frog Batrachyla taeniata. Horm Behav. 1997;31:101–109. doi: 10.1006/hbeh.1997.1366. [DOI] [PubMed] [Google Scholar]
- Struhsaker TT. The red colobus monkey. Chicago: University of Chicago Press; 1975. [Google Scholar]
- Taylor AM, Reby D. The contribution of source-filter theory to mammal vocal communication research. J Zool. 2010;280:221–236. [Google Scholar]
- Titze IR. Principles of voice production. Englewood Cliffs: Prentice Hall; 1994. [Google Scholar]
- Titze IR. On the relation between subglottal pressure and fundamental frequency in phonation. J Acoust Soc Am. 1989;85:901–906. doi: 10.1121/1.397562. [DOI] [PubMed] [Google Scholar]
- Titze IR, Riede T. A cervid vocal fold model suggests greater glottal efficiency in calling at high frequencies. PLoS Comput Biol. 2010;6:e1000897. doi: 10.1371/journal.pcbi.1000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DS, Moger WH. Plasma androgen levels during male parental care in a tropical frog (Eleutherodactylus) Horm Behav. 1987;21:93–99. doi: 10.1016/0018-506x(87)90034-1. [DOI] [PubMed] [Google Scholar]
- Van Duyse E, Pinxten R, Eens M. Effects of testosterone on song, aggression, and nestling feeding behavior in male great tits, Parus major. Horm Behav. 2002;41:178–186. doi: 10.1006/hbeh.2001.1747. [DOI] [PubMed] [Google Scholar]
- Waser MS. Individual recognition, intragroup cohesion, and intergroup spacing: evidence from sound playback to forest monkeys. Behaviour. 1977;60:28–74. [Google Scholar]
- Watts DP. Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour. 2002;139:343–370. [Google Scholar]
- Wich SA, van der Post DJ, Heistermann M, Möhle U, van Hooff JARAM, Sterck EHM. Life-phase related changes in male loud call characteristics and testosterone levels in wild Thomas langurs. Int J Primatol. 2003;24:1251–1265. [Google Scholar]
- Wilczynski W, Lynch KS, O'Bryant EL. Current research in amphibians: studies integrating endocrinology, behavior, and neurobiology. Horm Behav. 2005;48:440–450. doi: 10.1016/j.yhbeh.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Kahlenberg SM, Wells M, Wrangham RW. Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Anim Behav. 2012;83:277–291. [Google Scholar]
- Wrangham RW. The cost of sexual attraction: is there a trade-off in female Pan between sex appeal and received coercion? In: Boesch C, Hohmann G, Marchant L, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge: Cambridge University Press; 2002. pp. 204–215. [Google Scholar]
- Wrangham RW, Clark AP, Isabirye-Basuta G. Female social relationships and social organization of Kibale Forest chimpanzees. In: Nishida T, McGrew WC, PMarler P, Pickford M, de Waal FBM, editors. Human origins. Tokyo: The University of Tokyo Press; 1992. pp. 81–98. [Google Scholar]
- Zahavi A. Mate selection- selection for a handicap. J Theor Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Zahavi A. The cost of honesty (further remarks on handicap principle) J Theor Biol. 1977;67:603–605. doi: 10.1016/0022-5193(77)90061-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann E. Castration affects the emission of an ultrasonic vocalization in a nocturnal primate, the grey mouse lemur (Microcebus murinus) Physiol Behav. 1996;60:693–697. doi: 10.1016/0031-9384(96)81674-x. [DOI] [PubMed] [Google Scholar]
- Zuberbühler K. Predator-specific alarm calls in Campbell’s guenons. Behav Ecol Sociobiol. 2001;50:414–422. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.