Abstract
Background
Cyclooxygenase-2 (COX-2)-dependent signaling represents a potential mechanism of resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy. This is mediated in part through an EGFR-independent activation of MAPK/Erk by PGE2. PGE2 promotes downregulation of E-cadherin and epithelial to mesenchymal transition. This study investigated EGFR and COX-2 inhibition in patients with NSCLC and elevated baseline urinary metabolite of PGE2 (PGEM).
Methods
Patients with stage IIIB/IV NSCLC who progressed following at least one line of therapy or refused standard chemotherapy were randomized to erlotinib/celecoxib versus erlotinib/placebo. The primary endpoint was PFS with 80% power to detect a 50% improvement with a 0.2 one-sided significance level in the intent-to-treat (IIT) and elevated baseline PGEM populations. Secondary endpoints included response rate, OS and evaluation of molecular markers to assess targeting COX-2-related pathways and evaluate EGFR TKI-resistance.
Results
107 patients were enrolled with comparable baseline characteristics. Patients with EGFR wild type had an increased PFS in the celecoxib group (3.2 v 1.8 mos; p=0.03). PFS was numerically improved in the IIT group who received erlotinib/celecoxib compared to erlotinib/placebo (5.4 v 3.5 mos; p=0.33) and increased in patients in the celecoxib arm with elevated baseline PGEM (5.4 v 2.2mos; p=0.15). Adverse events (AEs) were similar in both arms.
Conclusions
Combined erlotinib/celecoxib did not improve outcomes in an unselected population, but selection by elevated baseline PGEM led to an increase in PFS with the celecoxib combination. Patients with EGFR wild type status may benefit from the combination.
Keywords: cyclooxygenase-2, erlotinib, celecoxib, non-small cell lung cancer, epidermal growth factor receptor, prostaglandin E2
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy provides significant benefit for patients with advanced non-small cell lung cancer (NSCLC) with activating EGFR mutations.1-2 Patients with wild type EGFR experience limited improvement with EGFR TKI therapy.3
Cyclooxygenase (COX) is the rate-limiting enzyme in the conversion of arachidonic acid to prostaglandins (PGs) and thromboxanes. COX-2 can be up regulated in response to growth factors, cytokines, tumor promoters, and other stimuli, and is constitutively overexpressed in a variety of malignancies, including NSCLC.4 Signaling through the cyclooxygenase-2 (COX-2) pathway presents a novel mechanism of resistance to EGFR TKI therapy in NSCLC. This resistance is mediated through an EGFR-independent activation of MAPK/Erk by the COX-2 metabolite PGE25, in addition to promotion of epithelial to mesenchymal transition (EMT) through the downregulation of E-cadherin expression.6
Urinary 11α-hydroxy-9,15-dioxo-2,3,4,5-tetranor-prostane-1,20-dioic acid is a metabolite of PGE2, and can be used as a surrogate for tumor levels of PGE2.7 Decreases in PGE-M in urine have been shown to correlate with improvement in time to tumor progression and overall survival.7-9
Combination EGFR and COX-2 inhibition may potentiate responses in NSCLC. In a phase I trial, the optimal dose of celecoxib combined with erlotinib was determined to be 600-mg twice daily with manageable toxicity and tumor responses in patients with both mutated and wild type EGFR status.10 We hypothesized that interfering with trans-activating and downstream pathways through the use of combination COX-2 and EGFR inhibition can enhance the efficacy of clinically tolerable doses of EGFR TKIs. The current study investigated the combination of high-dose celecoxib and erlotinib in a randomized, placebo-controlled phase II trial in patients with advanced NSCLC. A pre-planned analysis evaluating the association of baseline urine PGEM and clinical outcome based on treatment was performed.
Materials and Methods
Patients
Eligible patients had to be over the age of 18 with confirmed stage IIIB or IV NSCLC and tumor tissue available for mutation analysis; have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; have measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST); have progressive disease despite ≥1 prior chemotherapy regimens or subject's refusal or inability to receive standard chemotherapy; and have adequate hematologic, renal, and liver function. Key exclusion criteria included prior history of EGFR or COX-2 inhibitor therapy for the treatment of cancer; previous history of gastrointestinal ulceration, bleeding or perforation; concurrent use of COX-2 inhibitors or other NSAIDS (low dose aspirin was not allowed); active central nervous system metastasis; or any history of myocardial infarction or cerebral vascular accident or major medical condition that would interfere with participation. The study was approved by independent ethics review boards and in accord with an assurance filed with and approved by the Department of Health and Human Services by each site. The study was conducted according to the Declaration of Helsinki. All patients provided written informed consent prior to study participation.
Study Design and Treatment
This phase II, double-blind, placebo-controlled, randomized trial randomized patients in a 1:1 ratio using a random permuted block design with stratification by smoking status (non-smoker < 100 cigarettes smoked in lifetime vs. current/former smoker) and ECOG PS (0 vs. 1). Patients received erlotinib 150-mg p.o. daily plus placebo in the control arm (Arm A) or high-dose celecoxib at 600-mg p.o. twice daily plus erlotinib in the combination treatment arm (Arm B) continuously for a 28-day cycle. The combination of erlotinib and celecoxib or placebo was given for up to 12 months, after that time, the patient discontinued celecoxib/placebo and continued on erlotinib alone until disease progression or unacceptable toxicity. Dose reductions for erlotinib were allowed to 100 mg daily (no dose reduction was permitted for celecoxib), and dosing for either or both drugs could be interrupted up to 14 days.
Assessments
Radiographic assessments for tumor measurement were obtained every 8 weeks (2 cycles) to evaluate PFS, overall survival (OS), objective response rate (ORR) and disease control rate (DCR). Investigator assessed disease status was performed per RECIST v1.0. Adverse events (AEs) were assessed per the Common Terminology Criteria for Adverse Events (CTCAE version 3.0).
Biomarker Evaluation
Urine PGEM measurement
Urine samples were analyzed in a blinded manner by G.L.M. (Vanderbilt University, Nashville, TN). Twenty-four-hour urine samples were collected at baseline, at week 4, and at week 8 of the study. Each specimen was aliquoted and stored at -80°C. Urinary PGE-M levels were measured by mass spectrometry as previously described (normal levels were 10ng/mg Cr in men and 6ng/mg Cr in women).11-12
EGFR mutation analysis
EGFR mutational analysis was done at the CLIA certified laboratory at City of Hope through the pathology core. Formalin-fixed, paraffin embedded archival tumor specimens were submitted. Slides were reviewed by a board-certified pathologist who demarcated areas of tumor for dissection. Needle microdissection was done under a microscope, taking two representative areas from the region demarcated by the pathologist. These areas were digested overnight. Each dissected area was analyzed independently. Exons 18 to 21 of the EGFR gene were amplified from the digested products by PCR. Negative controls were included to rule out contamination. The amplified products were directly sequenced using ABI's automated fluorescent sequencing kit and sequencer. The chromatogram data were then reviewed for changes and reported.
Immunohistochemistry
Formalin-fixed, paraffin embedded archival tumor specimens were submitted to the University of California at Los Angeles (UCLA) pathology core. COX-2 and E-cadherin immunohistochemistry was performed as previously described.6 Each slide was assessed for the following: (a) % cells positive for each stain (E-cadherin, COX-2), (b) intensity of stain (0 to +3); (c) pattern of staining (membranous, cytoplasmic, and nuclear); and (d) in slides that had double staining, the percentage of cells that showed coexpression of both antigens (E-cadherin and COX-2). Staining intensity of +2 or greater was considered positive.
Statistical Analysis
The primary endpoint was progression-free survival (PFS) in the intent-to-treat population, and a second primary endpoint was added in May 2010 to evaluate PFS in patients with elevated baseline urine PGEM. Secondary endpoints included response rate; overall survival; correlation of PFS with urinary PGEM and EGFR mutation status; and measurement of COX-2, and E-cadherin expression. PFS was defined as time from randomization to disease progression by RECIST. The initial sample size was 86 with a total number of events required of 67, log rank test of equality of survival curves with a 0.2 one-sided significance level would have 80% power to detect the difference between the combination therapy associated exponential parameter of 0.22 (median progression-free survival of 3.2 months) and single agent erlotinib exponential parameter of 0.33 (median PFS 2.1 months), corresponding to a constant hazard ratio of 0.66.
The second primary endpoint increased the sample size by 20 patients (to 106) to account for the percentage of patients with low urinary PGEM in the analysis. This improved the power of the analysis, under the hypothesis that celecoxib will add benefit to erlotinib therapy when patients have elevated baseline urinary PGE-M levels (COX-2 pathway activation), to 82-85%.
Median PFS and OS were estimated from Kaplan-Meier curves. Comparison between treatment arms was made using log-rank test. Hazard ratios and 95% CI were estimated using a Cox regression model. Subgroup analyses for EGFR mutation, COX-2 and E-cadherin expression were performed, and a P value < 0.05 was considered significant, but was not adjusted for multiple testing.
Results
Patients
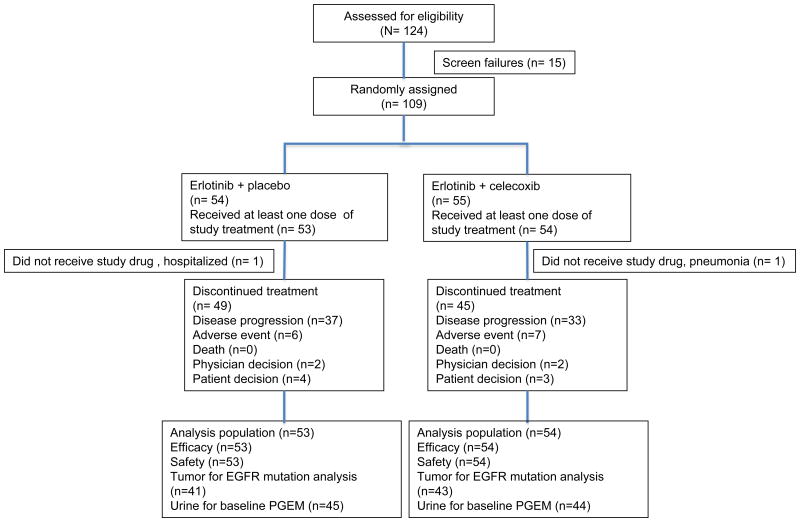
One hundred and nine patients were randomized to erlotinib and placebo (placebo) (n= 54) or erlotinib and celecoxib (n= 55) from November 2007 through May 2011 at City of Hope and University of Texas, Southwestern, and one hundred and seven patients received at least one dose of study drug (Figure 1). One patient in each arm did not receive study drug as planned and were excluded from analysis. Median follow-up was 12.1 months.
Figure 1.
CONSORT diagram. PGEM, urine prostaglandin E metabolite.
Baseline characteristics at baseline were well balanced between treatment arms (Table 1), including patients with EGFR mutation and baseline urine PGEM levels. Twenty-three patients (21%) had insufficient tissue for mutation analysis, 12 in the placebo group and 11 in the celecoxib group. Eighteen patients (17%) did not have adequate urine for baseline urine PGEM assessment 8 and 10 in the placebo and celecoxib groups, respectively.
Table 1. Patient Characteristics.
| Erlotinib + celecoxib (n= 54) |
Erlotinib + placebo (n= 53) |
||
|---|---|---|---|
| Age (range) | Median/yr | 63.5 (41-80) | 65 (30-80) |
| Male/Female | 26/28 | 24/29 | |
| Race (%) | White | 37 (69) | 35 (66) |
| Black of African American | 3 (6) | 2 (4) | |
| Asian | 12 (22) | 14 (26) | |
| American Indian | 0 | 1 (2) | |
| Not available | 2 (4) | 1 (2) | |
| ECOG PS (%) | 0 | 26 (48) | 26 (49) |
| 1 | 28 (52) | 27 (51) | |
| Histology (%) | Adenocarcinoma* | 32 (55) | 32 (60) |
| Squamous cell | 6 (11) | 5 (9) | |
| NSCLC NOS | 15 (28) | 16 (30) | |
| Not available | 1 (2) | 0 | |
| Smoking status (%) | < 100 cigarettes | 20 (37) | 20(38) |
| Former | 30 (56) | 31 (59) | |
| Current | 4 (7) | 2 (3) | |
| Stage (%) | IIIB | 6 (11) | 4 (8) |
| IV | 48 (89) | 49 (92) | |
| EGFR mutation (%) | Positive | 12 (22) | 14 (26) |
| Negative | 31 (57) | 27 (51) | |
| Not available | 11 (21) | 12 (23) | |
| Number of prior systemic therapies (%) | 0 | 6 (11) | 7 (13) |
| 1 | 27 (50) | 27 (51) | |
| 2 | 15 (28) | 11 (21) | |
| >2 | 6 (11) | 8 (15) | |
Includes 1 adenosquamous histology.
ECOG PS, Eastern Cooperative Oncology Group performance status.
Efficacy
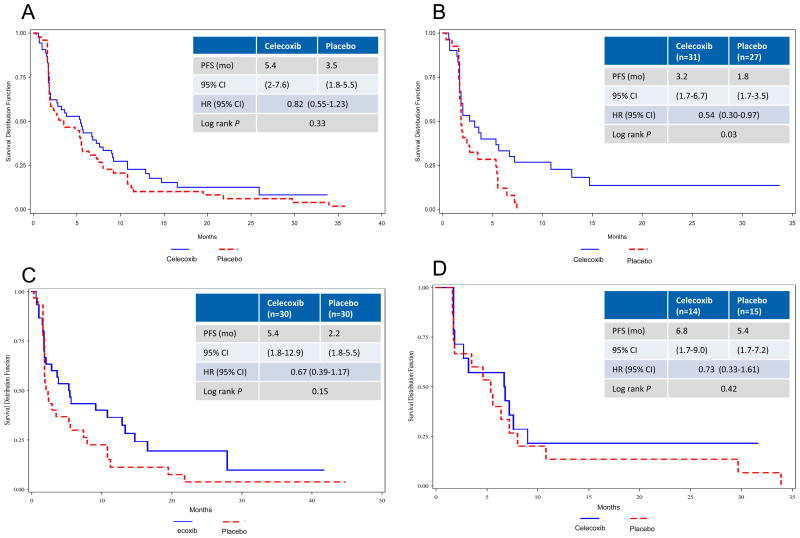
In the IIT population, PFS was numerically higher in the erlotinib plus celecoxib arm compared to the erlotinib plus placebo arm, although this did not reach statistical significance (median, 5.4 v 3.5 months; HR, 0.82; p = 0.33; Fig 2A). In patients selected with elevated baseline PGEM, an improvement in PFS was demonstrated (median, 5.4 v 2.2 months; HR 0.67; p = 0.15; Fig 2C). Those with low baseline PGEM did not benefit from combination treatment with celecoxib (Fig 2D). Analysis by EGFR mutation status revealed that patients with wild type tumors had an improvement in PFS (median, 3.2 v 1.8 months; HR 0.54; p = 0.03; Fig 2B). Those with EGFR mutation or unavailable results did not experience improved PFS (Table 2). In an exploratory analysis of patients with wild type EGFR and elevated PGEM at baseline, progression-free survival was longer for those who received celecoxib (median, 3.8 v 1.8 months; HR 0.42; p = 0.04) and overall survival was similar in each group (median, 11.1 v 12.3 months; HR 0.58; p = 0.22), data not shown.
Figure 2.
Kaplan-Meier estimates of progression free survival (PFS) in (A) intent-to-treat, (B) EGFR wild type, (C) elevated baseline PGEM, (D) low baseline PGEM. HR, hazard ratio; PGEM, urine prostaglandin E metabolite.
Table 2. Patient Response by intent to treat and EGFR mutation status.
| All patients | Erlotinib + celecoxib (n= 54) |
Erlotinib + placebo (n= 53) |
|---|---|---|
| CR/PR (%) | 12 (22.6) | 17 (32.7) |
| SD (%) | 22 (41.5) | 13 (25) |
| PD (%) | 19 (35.8) | 22 (42.3) |
| DCR (CR/PR/SD) (%) | 34 (64) | 30 (57.7) |
| EGFR wild type | Erlotinib + celecoxib (n= 30) |
Erlotinib + placebo (n= 27) |
| CR/PR (%) | 3 (10) | 0 (0) |
| SD (%) | 12 (40) | 10 (37) |
| PD (%) | 15 (50) | 17 (63) |
| DCR (CR/PR/SD) (%) | 15 (50) | 10 (37) |
| PFS (mo) | 3.2 (1.7-6.7) | 1.8 (1.7-3.5) |
| HR (95% CI) | 0.54 (0.30-0.97), p= 0.03 | |
| EGFR mutation positive | Erlotinib + celecoxib (n= 12) |
Erlotinib + placebo (n= 14) |
| CR/PR (%) | 5 (41.7) | 9 (64.3) |
| SD (%) | 6 (50) | 4 (29) |
| PD (%) | 1 (8) | 1 (7) |
| DCR (CR/PR/SD) (%) | 11 (91.7) | 13 (92.9) |
| PFS (mo) | 9.2 (5.4-16.5) | 9.2 (4.8-10.8) |
| HR (95% CI) | 0.98 (0.41-2.39), p= 0.97 | |
| EGFR not available | Erlotinib + celecoxib (n= 11) |
Erlotinib + placebo (n= 12) |
| CR/PR (%) | 1 (9) | 2 (16.7) |
| SD (%) | 5 (45.5) | 4 (33.3) |
| PD (%) | 5 (45.5) | 6 (50) |
| DCR (CR/PR/SD) (%) | 6 (54.6) | 6 (50) |
| PFS (mo) | 2.8 (1.0-8.0) | 2.9 (1.8-11.5) |
| HR (95% CI) | 1.48 (0.62-3.5), p= 0.36 | |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR (disease control rate); PFS, progression free survival; HR, hazard ratio.
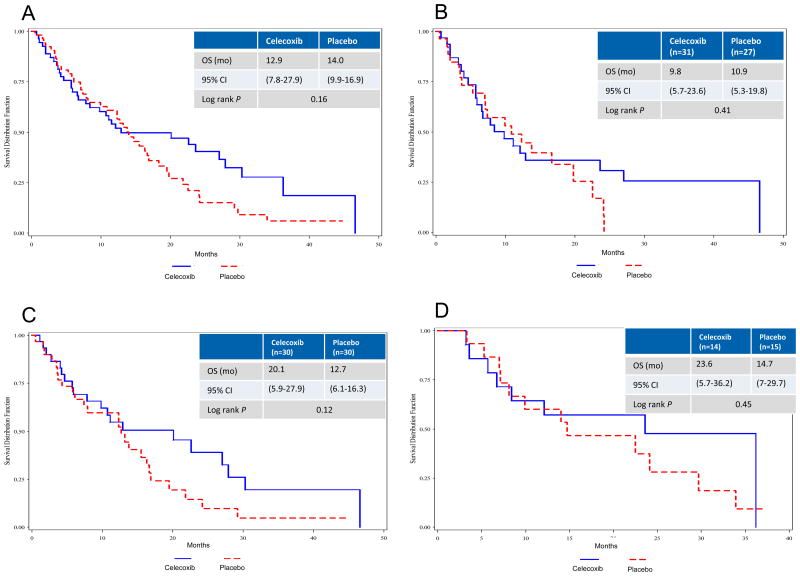
A trend toward improved OS was seen in the IIT population (Fig 3A) and in those with elevated baseline PGEM (Fig 3C). Patients with low baseline PGEM did not experience improved OS (Fig 3D). Survival was similar by treatment group regardless of EGFR mutation status (Fig 3B).
Figure 3.
Kaplan-Meier estimates of overall survival (OS) in (A) intent-to-treat, (B) EGFR wild type, (C) elevated baseline PGEM, (D) low baseline PGEM. HR, hazard ratio; PGEM, urine prostaglandin E metabolite.
Safety
All patients who received at least one dose of study drug were assessed for safety (n= 107). The rate of discontinuation due to AEs was similar in the erlotinib plus celecoxib and erlotinib plus placebo arm (13.0% v 11.3%). The most frequent cause for treatment discontinuation was disease progression in both groups.
The most common AEs (all grades) in both groups were rash, diarrhea, dry skin, fatigue and elevated AST and did not differ between groups (Table 3). Anemia (33% v 15%), elevated creatinine (32% v 15%), and paronychia (30% v 13%) occurred more often in the patients who received erlotinib plus celecoxib. Two patients who received erlotinib plus celecoxib experienced cerebrovascular ischemia (grade 2 and grade 3) and one patient on the erlotinib plus placebo arm had cardiac ischemia, grade 2. No deaths were attributable to treatment.
Table 3. Adverse Events (All grades >10%).
| Adverse Events (all grades >10%) | # patients experienced (%) | ||
|---|---|---|---|
| Erlotinib + HD celecoxib | Erlotinib + placebo | ||
| Rash | 43 (80) | 42 (79) | |
| Diarrhea | 38 (70) | 40 (76) | |
| Dry skin | 36 (67) | 40 (76) | |
| Fatigue | 28 (52) | 31 (59) | |
| Elevated AST | 25 (46) | 27 (51) | |
| Anorexia | 17 (32) | 26 (49) | |
| Nausea | 14 (26) | 17 (32) | |
| Hypoalbuminemia | 16 (30) | 14 (26) | |
| Stomatitis | 15 (28) | 12 (23) | |
| Anemia | 18 (33) | 8 (15) | p = 0.028 |
| Elevated creatinine | 17 (32) | 8 (15) | p = 0.045 |
| Elevated AlkPhos | 12 (22) | 11 (21) | |
| Paronychia | 16 (30) | 7 (13) | P = 0.039 |
| Lymphopenia | 10 (19) | 4 (8) | |
| Weight loss | 5 (9) | 9 (17) | |
| Vomiting | 4 (7) | 7 (13) | |
| Elevated bilirubin | 4 (7) | 6 (11) | |
Biomarker Assessment
Thirty-six patients (44%) had tissue available for evaluation of COX-2 by IHC, 13 (68.4%) in the erlotinib plus celecoxib arm and 12 (70.6%) in the erlotinib plus placebo arm demonstrated high expression. Due to small numbers, this did not correlate with patient outcome. In addition, 35 had available tissue for E-cadherin assessment with elevated expression in 15 (83.3%) in the erlotinib plus celecoxib arm and 11 (64.7%) in the erlotinib plus placebo arm without association to response, PFS or OS.
Discussion
First described to be overexpressed in human NSCLC in 1998,13 COX-2 contributes to multiple aspects of the malignant phenotype, including angiogenesis,14 invasion,15 apoptosis resistance,16 and immune dysregulation.17-19 Information regarding selection of patients for COX-2 inhibitor therapy has matured, and it is clear that a method of selection will improve our ability to determine efficacy with the combination. Two methods for selection for COX-2 inhibitors are evaluation of COX-2 expression by IHC and PGEM. IHC was initially shown to potentially predict for those who could benefit from combination COX inhibition with chemotherapy,20 but the phase III trial did not confirm the results.21 Due to the variability in COX-2 expression by IHC and limited tissue availability, we chose to evaluate PGEM as a selective marker. PFS in patients with elevated baseline PGEM levels was higher in patients on the erlotinib plus celecoxib arm, and met our primary endpoint in this population. This suggests that baseline PGEM may identify a subgroup of patients with activation of the COX-2 pathway, and may be responsive to celecoxib therapy.
A number of studies have evaluated COX-2 and EGFR inhibition in small, unselected patient populations with limited benefit.22-25 Gadgeel et al.24 reported a Phase II study of gefitinib and celecoxib in patients with platinum refractory NSCLC. Patients received gefitinib 250 mg daily and celecoxib 400 mg twice daily. The response rate to the combination of celecoxib and gefitinib was similar to that observed with gefitinib alone. O'Byrne and colleagues25 reported combination therapy with gefitinib and rofecoxib in patients with platinum-pretreated relapsed NSCLC. Gefitinib combined with rofecoxib was found to provide disease control equivalent to that expected with single-agent gefitinib and was generally well tolerated. Fidler et al.23 evaluated erlotinib plus celecoxib in a phase II trial of patients with advanced NSCLC who had failed one prior chemotherapy regimen and found that 10 of 26 had disease control and elevated COX-2 expression by IHC was associated with improved PFS. The current study helps to improve our understanding of these combinations for the treatment of NSCLC. The prior studies did not dose COX-2 inhibition to adequately block COX-2 activity and induce antitumor effects, while our high-dose celecoxib was based on decline in PGEM levels in our phase I trial.10 Use of adequate COX-2 inhibition along with patient selection may optimize the use of this therapy.
A randomized phase II trial with a novel COX-2 inhibitor, apricoxib, in combination with erlotinib used a run-in period to assess a decline in PGEM as a criteria for eligibility.26 This was based on dosing from the phase I trial that demonstrated at least a 50% reduction in PGEM.9 They randomized 120 patients and found a median time to progression of 1.8 in the apricoxib/erlotinib arm versus 2.1 months in the erlotinib/placebo arm. When subgroups were evaluated, patients aged 65 and less had a significant improvement in TTP with apricoxib/erlotinib. This was a unique design that attempted to identify those that could benefit from COX-2 blockage, although a higher cut-point for decline in PGEM may have been more beneficial as demonstrated by earlier studies.7, 10
Although clinical benefit has been demonstrated with erlotinib in patients with wild type EGFR27 responses are limited and patients have better outcomes with chemotherapy when compared to EGFR-directed treatment.3 Attempts to overcome resistance with pan-human epidermal growth factor receptor (HER) inhibitors have not resulted in better outcomes for patients with wild type EGFR.28-29 Patients with wild type EGFR experienced meaningful clinical benefit from the combination of erlotinib and high-dose celecoxib. This study enrolled a higher proportion of patients with EGFR mutation than would be expected in the general population, but both arms were balanced due to stratification by smoking status. The increased number with EGFR mutation may have led to a longer PFS in both arms for the IIT population. Ninety-six patient serum samples were retrospectively assessed by VeriStrat (VS) classification, which showed longer PFS and OS in the overall group for those with VS good classification.30 Analysis of the VS good group stratified by EGFR mutation status confirmed an improved PFS in the celecoxib group for those with EGFR wild type tumors.
Furthermore, we have learned from the use of COX-2 inhibition in colorectal carcinoma that has demonstrated improved outcomes associated with aspirin use following diagnosis related to tumor COX-2 expression by IHC.31 The linkage of PIK3CA mutations to aspirin use leading increased survival in these patients32 reaffirms the molecular basis and need for selection when evaluating COX inhibitor therapy. PIK3CA mutations are less common in NSCLC, and tissue was not sufficient to analyze in the current study. The heterogeneity of NSCLC is evident and understanding the molecular basis for response and resistance will enhance our therapies. Overexpression of neutrophil gelatinase associated lipocalin (NGAL) has also been shown to lead to EGFR TKI resistance, and may be modulated by COX-2 inhibition to improve sensitivity.33
The sample size in this study was too small to expand our knowledge of PIK3CA mutation status and outcome, but the phosphatidylinositol-3-kinase (PI3K) signaling is altered to promote cell proliferation, growth, and survival in many NSCLCs,34 and combined EGFR and COX-2 inhibition may effectively exploit this pathway. The results in the EGFR wild type and elevated baseline PGEM populations are encouraging, and provide supporting evidence to further evaluate these pathways in selected patients. Optimal use of biomarkers and genomic data may provide additional insights for improving eicosanoid modulatory agents in combination therapies for NSCLC.
Acknowledgments
Supported by NIH 1P50 CA90388, K12 CA01727, UCLA Claude Pepper Older Americans Independence Center funded by the National Institute of Aging 5P30AG028748 and Medical Research Funds from the Department of Veterans Affairs. Astellas Pharmaceuticals supplied erlotinib and patient care costs, and Pfizer provided celecoxib and placebo.
We acknowledge the support of the City of Hope Comprehensive Cancer Center Pathology Core in this work. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under grant number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We acknowledge Fran Rosen and Cecelia Arbayo for their contributions to this work.
Footnotes
Presented at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL June 1-5, 2012, and 15th World Conference on Lung Cancer, Sydney, Australia October 27-30, 2013.
Clinical trial information: NCT00499655
Disclosures: Trial funding from Astellas and Pfizer (KLR); all other authors report no relationships.
References
- 1.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14:981–988. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 4.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 5.Krysan K, Reckamp KL, Dalwadi H, et al. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–6281. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 6.Dohadwala M, Yang SC, Luo J, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338–5345. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- 7.Csiki I, Morrow JD, Sandler A, et al. Targeting cyclooxygenase-2 in recurrent non-small cell lung cancer: a phase II trial of celecoxib and docetaxel. Clin Cancer Res. 2005;11:6634–6640. doi: 10.1158/1078-0432.CCR-05-0436. [DOI] [PubMed] [Google Scholar]
- 8.Mutter R, Lu B, Carbone DP, et al. A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res. 2009;15:2158–2165. doi: 10.1158/1078-0432.CCR-08-0629. [DOI] [PubMed] [Google Scholar]
- 9.Reckamp K, Gitlitz B, Chen LC, et al. Biomarker-based phase I dose-escalation, pharmacokinetic, and pharmacodynamic study of oral apricoxib in combination with erlotinib in advanced nonsmall cell lung cancer. Cancer. 2011;117:809–818. doi: 10.1002/cncr.25473. [DOI] [PubMed] [Google Scholar]
- 10.Reckamp KL, Krysan K, Morrow JD, et al. A phase I trial to determine the optimal biological dose of celecoxib when combined with erlotinib in advanced non-small cell lung cancer. Clin Cancer Res. 2006;12:3381–3388. doi: 10.1158/1078-0432.CCR-06-0112. [DOI] [PubMed] [Google Scholar]
- 11.Murphey LJ, Williams MK, Sanchez SC, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Gross ND, Boyle JO, Morrow JD, et al. Levels of prostaglandin E metabolite, the major urinary metabolite of prostaglandin E2, are increased in smokers. Clin Cancer Res. 2005;11:6087–6093. doi: 10.1158/1078-0432.CCR-05-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang M, Stolina M, Sharma S, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–1216. [PubMed] [Google Scholar]
- 14.Pold M, Zhu LX, Sharma S, et al. Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res. 2004;64:1853–1860. doi: 10.1158/0008-5472.can-03-3262. [DOI] [PubMed] [Google Scholar]
- 15.Dohadwala M, Batra RK, Luo J, et al. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krysan K, Merchant FH, Zhu L, et al. COX-2-dependent stabilization of survivin in non-small cell lung cancer. FASEB J. 2004;18:206–208. doi: 10.1096/fj.03-0369fje. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Stolina M, Yang SC, et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961–968. [PubMed] [Google Scholar]
- 18.Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 19.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 20.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy--Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 21.Edelman MJ, Wang X, Hodgson L, et al. Phase III randomized, placebo controlled trial of COX-2 inhibition in addition to standard chemotherapy for advanced non-small cell lung cancer (NSCLC):CALGB 30801 (Alliance) Proc Am Assoc Cancer Res. 2014 abstr CT238. [Google Scholar]
- 22.Agarwala A, Fisher W, Bruetman D, et al. Gefitinib plus celecoxib in chemotherapy-naive patients with stage IIIB/IV non-small cell lung cancer: a phase II study from the Hoosier Oncology Group. J Thorac Oncol. 2008;3:374–379. doi: 10.1097/JTO.0b013e3181693869. [DOI] [PubMed] [Google Scholar]
- 23.Fidler MJ, Argiris A, Patel JD, et al. The potential predictive value of cyclooxygenase-2 expression and increased risk of gastrointestinal hemorrhage in advanced non-small cell lung cancer patients treated with erlotinib and celecoxib. Clin Cancer Res. 2008;14:2088–2094. doi: 10.1158/1078-0432.CCR-07-4013. [DOI] [PubMed] [Google Scholar]
- 24.Gadgeel SM, Ruckdeschel JC, Heath EI, et al. Phase II study of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), and celecoxib, a cyclooxygenase-2 (COX-2) inhibitor, in patients with platinum refractory non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007;2:299–305. doi: 10.1097/01.JTO.0000263712.61697.69. [DOI] [PubMed] [Google Scholar]
- 25.O'Byrne KJ, Danson S, Dunlop D, et al. Combination therapy with gefitinib and rofecoxib in patients with platinum-pretreated relapsed non small-cell lung cancer. J Clin Oncol. 2007;25:3266–3273. doi: 10.1200/JCO.2006.09.2791. [DOI] [PubMed] [Google Scholar]
- 26.Gitlitz BJ, Bernstein E, Santos ES, et al. A randomized, placebo-controlled, multicenter, biomarker-selected, phase 2 study of apricoxib in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9:577–582. doi: 10.1097/JTO.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 28.Ramalingam SS, Blackhall F, Krzakowski M, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2012;30:3337–44. doi: 10.1200/JCO.2011.40.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reckamp KL, Giaccone G, Camidge DR, et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer. 2014;120:1145–1154. doi: 10.1002/cncr.28561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reckamp KL, Koczywas M, Cristea M, Dowell JE, Figlin RA, Dubinett SM, Grigorieva J, Roder H. Evaluation of VeriStrat in the Randomized, Placebo-Controlled, Phase II Trial of Erlotinib and High-Dose Celecoxib in Advanced Non-small Cell Lung Cancer. J Thorac Oncol. 2012;7:S337. Abstract LBOA1. [Google Scholar]
- 31.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krysan K, Cui X, Gardner BK, et al. Elevated neutrophil gelatinase-associated lipocalin contributes to erlotinib resistance in non-small cell lung cancer. Am J Transl Res. 2013;5:481–496. [PMC free article] [PubMed] [Google Scholar]
- 34.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]