Abstract
Objective
The serious mental illnesses schizophrenia, schizoaffective disorder, and bipolar disorder are complex conditions affecting 1–4% of the population. Individuals with serious mental illnesses express interest in genetic counseling; an intervention showing promise for increasing patient knowledge and adaptation. This trial aimed to evaluate the effects of genetic counseling for people with serious mental illnesses as compared to an educational intervention or waitlist.
Methods
A pilot three-arm (each n=40; genetic counseling, a control intervention involving an educational booklet, or waitlist), parallel group, randomized clinical trial was conducted from September 2008–November 2011 in Vancouver, Canada. Participants with schizophrenia, bipolar disorder, or schizoaffective disorder (DSM-IV) completed outcome measures assessing knowledge, risk perception, internalized stigma, and perceived control over illness at baseline and one-month follow-up. The Brief Symptom Inventory was administered to control for current symptoms. Analyses included linear mixed effects models and chi-squared tests.
Results
Knowledge increased for genetic counseling/educational booklet compared to waitlist at follow-up (LRT=19.33, df=1, Holm-adjusted p=0.0003, R2LMM(m)=0.17). Risk perception accuracy increased at follow-up for genetic counseling compared to waitlist (Yates’ continuity corrected χ2=9.1, df=1, Bonferroni p=0.003) and educational booklet (Yates’ continuity corrected χ2=8.2, df=1, Bonferroni p=0.004). There were no significant differences between groups for stigma or perceived control scores.
Conclusions
Genetic counseling and the educational booklet improved knowledge; and genetic counseling, but not the educational booklet, improved risk perception accuracy for this population. The impact of genetic counseling on internalized stigma and perceived control is worth further investigation. Genetic counseling should be considered for patients with serious mental illnesses.
Trial registration
clinicaltrials.gov identifier: NCT00713804; http://clinicaltrials.gov/ct2/show/NCT00713804?term=genetic+counseling&rank=4
Keywords: mental illness, genetic counseling, psychiatric disorders, bipolar disorder, schizophrenia, schizoaffective disorder, internalized stigma, perceived control, knowledge, risk perception, randomized clinical trial
INTRODUCTION
The serious mental illnesses (SMIs) schizophrenia, schizoaffective disorder, and bipolar disorder cumulatively affect ~1–4% of the population worldwide1,2 and, like other common conditions (e.g. diabetes, cardiovascular disease), have a heterogeneous etiology typically involving both genetic variants and environmental factors3,4. Currently, no genetic tests are clinically useful in establishing, refining or excluding a psychiatric diagnosis.
Genetic counseling (GC) is “the process* of helping people understand and adapt to the medical, psychological and familial implications of genetic contributions to disease.”5. Individuals and families affected by SMIs express interest in receiving GC6,7 which has potential benefits, even without genetic testing8–10. Although GC for SMI is suggested in clinical practice guidelines of the American and Canadian Psychiatric Associations11,12, there is little empirical evidence regarding outcomes of GC in this context.
Two non-randomized pilot studies13,14 of GC for family members of individuals with psychotic disorders revealed positive effects, as did a similarly designed study in a group of people with schizophrenia or schizoaffective disorder15. In the latter, GC increased knowledge of causes of schizophrenia, and decreased recurrence risk (RR) estimates, concern regarding recurrence, stigma, and self-blame (albeit temporarily).
We conducted the first pilot randomized clinical trial (RCT) of the impact of GC for SMI, and also the first empirical investigation into the effect of GC for bipolar disorder.
Hypotheses
We hypothesized that 1) mean scores for knowledge, risk perception accuracy, and perceived control over illness would be higher, and scores for internalized stigma would be lower for the GC group compared to an intervention group provided with an educational booklet (EB), and 2) mean differences in scale scores between outcome (T3) and baseline (T1) for the two intervention groups (GC, EB) would be significantly different than waitlist, with GC/EB mean scores being higher for knowledge, risk perception accuracy, and perceived control over illness, and lower for internalized stigma.
METHODS
Participants & Ethics Statement
The Institutional Review Boards at the University of British Columbia and BC Children’s and Women’s Hospital approved this study (H07-02427) and it was registered on clinicaltrials.gov (NCT00713804). Participants were recruited from the community in Vancouver, Canada (September 2008–November 2011) via referrals from psychiatrists and self-referrals from study advertisements. Study appointments occurred in inpatient or outpatient settings. Each potential participant received a consent form and, if interested, an in-person baseline appointment (T1) to provide written consent and confirm eligibility. After an informed consent process, written consent was obtained. Individuals were enrolled if they reported a diagnosis of schizophrenia, bipolar disorder, or schizoaffective disorder, were fluent in English, and had the capacity to provide informed and autonomous consent (e.g. ≥19 years of age). Individuals were ineligible if their SMI diagnosis was substance-induced, or their ability to provide autonomous informed consent was compromised (e.g. intellectual disability (IQ<70), or currently floridly psychotic and/or intoxicated).
Study Design and Treatments
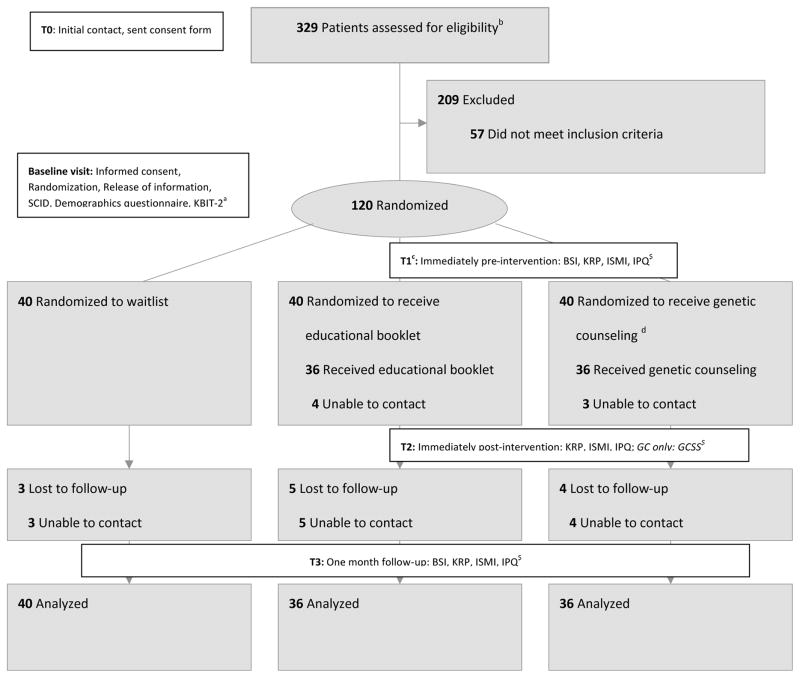
This was a pilot, prospective, three-arm, parallel RCT (N=120). Randomization was equal (1:1:1) and stratified (44% males, 56% females – a ratio balancing desire to have male representation against feasibility concerns regarding male recruitment). In the absence of more relevant data, we used information on the effect of GC on knowledge/anxiety in the context of cancer16 to set our sample size assuming an attrition rate of 25%, equal loss to follow-up between groups, and a power of >80% to detect a medium effect size (d=0.5) at p≤0.01 for each outcome variable (See Figure 1 Note). The three arms were: 1. receiving GC from a board certified/eligible genetic counselor (BC/EGC), 2. meeting with a research coordinator to read an educational booklet (EB), and 3. waitlist (WL). The EB intervention was designed as a rigorous control intervention; it was face-to-face and provided the same general information as GC, but without the ‘active ingredient’ of personalization of information/counseling by a BC/EGC. All participants who were not randomized to GC were offered GC following study completion. Questionnaires were administered at baseline (T1), immediately post-intervention (T2, GC/EB groups only), and at one-month follow-up (T3).
Figure 1.
Flow of participants through the trial
a Abbreviations: SCID = Structured Clinical Interview for Diagnosis; KBIT-2 = Kaufman Brief Intelligence Test version 2; BSI = Brief Symptom Inventory (current symptoms); KRP = Knowledge and Risk Perception Questionnaire; ISMI = Internalized Stigma of Mental Illness Scale; IPQ = Illness Perception Questionnaire (perceived control); GCSS = Genetic Counseling Satisfaction Scale. At the time of study initiation, there were no existing data about the impact of GC for individuals with SMI, and the effect sizes for our outcome variables were unknown. Therefore, we based our a priori power calculation on the effect of GC for hereditary cancer on increasing knowledge and diminishing anxiety16.
bWe used diverse recruitment strategies to reach potential participants, including: posters in psychiatrists’ offices/waiting areas/inpatient units, online advertisements, direct approach at community mental health organizations’ meetings/events, direct approach at low-income housing units catering to those with mental illness, emails to mental health professionals and clients’ listservs, and mental health practitioners providing information about the study (recruitment brochure) to their patients.
CFor the waitlist group, baseline and T1 occurred on the same day. Participants had the option of bringing a support person with them to appointments if they wished. In-person visits were arranged for some participants to complete the outcome measures at one month follow-up at their request. One of the participants in the waitlist group had received GC for SMI prior to the study. The trial was stopped once the pre-determined number of participants had been recruited and those who were not lost to follow up had completed the study. The full protocol can be obtained from the corresponding author.
dOne patient discontinued participation because of rapid exacerbation of illness.
While the nature of the study and interventions precluded blinding for participants or providers, an independent party blind to group status conducted data analyses.
Baseline
All participants completed a Structured Clinical Interview for DSM disorders17 and signed a release of information for their psychiatric history records. A psychiatrist (JC) reviewed these data to confirm diagnoses. To confirm IQ≥70, the Kaufman Brief Intelligence Test, Second Edition (KBIT-2)18 was administered. Participants completed a demographic questionnaire and were randomized. For the randomization procedure, equally-sized laminated cards were sorted into two opaque envelopes (one for males, containing 18 GC, and 17 of each EB and WL, and one for females, containing 22 GC, and 23 of each EB and WL). Participants were asked to choose a card from the appropriate (male/female) envelope without looking (under the supervision of AR or AI). Baseline appointments lasted ~1–3 hours, depending on informational detail shared by the participant. Questionnaires assessing outcome measures (Figure 1) were administered at T1 (~1 hour).
Interventions
For participants in EB/GC, outcome measures were assessed immediately pre- and post-intervention.
GC sessions (~1 hour) were provided by a psychiatric-specialist BC/EGC (JA, CH, AI). The GC session followed standard procedures19–21. Specifically, family histories and existing explanations for illness were elicited, then participants were provided with evidence-based information about illness etiology, in the context of a psychotherapeutically-oriented interaction designed to support and address emotional sequelae, as described elsewhere21. Participants received written information to take home (booklet described below) and information on RR, personalized to family history, if requested. No genetic testing was provided. AI and CH were trained by JA to ensure competency/consistency. Adherence to GC protocol was ensured by GC checklist completion, peer session observation and feedback, and regular peer-supervision meetings.
EB sessions (~30 minutes) were provided by the research coordinator (AR), who answered questions regarding literal interpretations of text, but responded to participants’ queries that aimed to make personal meaning of the material with responses such as: “I’m sorry, but I’m afraid I’m unable to answer that. If you’d like to meet with someone who can help you with questions like that, we can set up a GC appointment after you finish the study”. Thus, EB sessions did not evolve into GC, yet were a stringent control intervention. Through observation, the research coordinator confirmed participant adherence to the intervention.
The booklet (16 color pages, reading grade level 8) was designed in collaboration with individuals with SMI and included: a graphical depiction of the concepts of vulnerability (genetic and environmental) and resilience (the “mental illness jar”13), with specific examples and a table of general RRs for relatives of people with SMI.
Outcome Measures
The choice of outcome measures – knowledge, risk perception, internalized stigma, and perceived control over illness – was informed by the definition and goals of GC as well as psychiatric GC literature5,7,8,10,13–15,22–25 (Supplemental Material). One month post-intervention, outcome assessments were sent to participants, usually by mail, including a postage-paid return envelope. For GC only, participants completed the Genetic Counseling Satisfaction Scale26 (GCSS) immediately post-intervention.
Knowledge and risk perception
The 9-item Knowledge and Risk Perception Questionnaire (KRP) was designed for this study (Supplemental Material). The risk perception item was previously piloted extensively with the target population (N>400)24. Six knowledge items were adapted from ones used previously in studies of GC23. One item asked whether participants found the GC/EB useful (Likert-type item (0=not at all useful, 4=very useful)) and another whether they’d shared information from the GC/EB.
Internalized stigma
The Internalized Stigma of Mental Illness scale (ISMI) is a 29-item self-report scale designed to measure subjective experiences of stigma among people with SMI, with subscales measuring alienation, stereotype endorsement, perceived discrimination, social withdrawal, and stigma resistance27. Each item is rated on a Likert scale (1=strongly disagree, 4=strongly agree). The ISMI has strong internal consistency (α=.90), good test-retest reliability (r=.92), and robust construct validity.
Perceived control
We used a version of the Illness Perception Questionnaire that was revised and validated for individuals with SMI (IPQ-S)28. The 5 subscales used in this study consist of 34 items, rated on a Likert scale (1=strongly disagree, 5=strongly agree). All of these subscales have shown good internal consistency (α~.75) and test-retest reliability (r=.57–.95). There is no total score for the IPQ-S.
Current symptoms
We administered the Brief Symptom Inventory (BSI, a 53-item self-report measure that assesses psychological symptoms over the previous week29) to control for the potential confound of current mood. It has good internal consistency (α=.71–.85), test-retest reliability, and construct validity. It yields 3 global domains (a global severity index (GSI), a positive symptom distress index, and a total positive symptom score). Items are rated on a Likert scale (0=not at all, 4=extremely); higher scores indicate greater symptom severity. For all analyses we used the GSI.
Statistical Analysis
The primary analysis included all participants with complete demographic and baseline data. Two analyses were carried out in R30 to: 1) examine differences over the three time points for GC and EB groups (longitudinal; n=69), and 2) to assess effects of treatment (GC/EB) relative to WL between T3 and T1 (n=112). Analyses 1 and 2 both used linear mixed effects models with subject ID as the random nesting effect, and ln(GSI) at T1 and diagnosis type (BD, SZ, or SZA) as moderating covariates. The main effects were time (1, 2 and 3 for analysis 1; 1 and 3 for analysis 2), and group membership. Tests for group x time interaction terms were conducted. P-values for all tests of interactions and main effects were corrected using Holm’s correction31. Uncorrected p-values are also reported for comparison where relevant. R2LMM(m) (marginal R2 for linear mixed effects models = variance explained by the fixed effects) was calculated for all significant models using the method of Nakagawa and Schielzeth32.
Perceived RR estimates were transformed into dichotomous responses, accurate vs. not accurate (see Table 2 Note for definition of ‘accurate’), and compared among groups using chi-square tests at each time-point. P-values for these tests were included in the Holm adjustment for Knowledge, ISMI and IPQ analyses. Post-hoc pairwise chi-square comparisons were conducted and Bonferonni-corrected where required. We also calculated effect sizes (d) for ISMI, KRP, and IPQ scores and effect sizes (phi or Cramer’s V) for the RR comparisons. Data analysis was conducted using SPSS 17.0 (IBM) and R.
Table 2.
Mean scores (+SD) of the raw data for knowledge, internalized stigma, and perceived control, as well as numbers and percentages of participants reporting ‘accurate’ RRs, overestimated RRs, and underestimated RRs by group (GC, EB, WL) at each time point (T1, T2, T3).
| T1 | T2 | T3 | |
|---|---|---|---|
| Knowledgea | Mean Scores (SD) | ||
|
| |||
| GC | 2.8 (1.9) | 4.6 (2.1) | 4.8 (1.2) |
| EB | 3.2 (1.9) | 4.1 (2.0) | 4.4 (1.8) |
| WL | 3.3 (1.6) | n/a | 3.4 (1.8) |
|
| |||
| Risk Perception | Number (Percentage) | ||
|
| |||
| GC – Accurate | 12 (50.0) | 20 (80.0) | 17 (85.0) |
| EB – Accurate | 3 (8.6)b | 17 (48.6) | 8 (29.6) |
| WL – Accurate | 5 (21.7) | n/a | 7 (31.8) |
| GC – Overestimate | 11 (45.8) | 3 (12.0) | 1 (5.0) |
| EB – Overestimate | 30 (85.7) | 14 (40.0) | 14 (51.9) |
| WL – Overestimate | 16 (69.6) | n/a | 14 (63.6) |
| GC – Underestimate | 1 (4.2) | 2 (8.0) | 2 (10.0) |
| EB – Underestimate | 2 (5.7) | 4 (11.4) | 5 (18.5) |
| WL – Underestimate | 2 (8.7) | n/a | 1 (4.5) |
|
| |||
| Internalized Stigmac | Mean Scores (SD) | ||
|
| |||
| GC | 59.2 (14.8) | 57.6 (15.4) | 56.8 (14.3) |
| EB | 60.3 (15.2) | 58.7 (14.8) | 61.4 (15.9) |
| WL | 61.2 (17.5) | n/a | 62.7 (17.8) |
|
| |||
| Perceived Control | Mean Scores (SD) | ||
|
| |||
| Consequences subscaled | |||
| GC | 37.5 (6.9) | 34.6 (7.8) | 34.6 (7.8) |
| EB | 39.8 (7.9) | 39.7 (10.2) | 40.4 (5.9) |
| WL | 39.4 (7.9) | n/a | 39.1 (8.6) |
| Personal control subscalee | |||
| GC | 16.9 (3.4) | 16.3 (3.2) | 17.3 (2.9) |
| EB | 16.4 (2.5) | 17.1 (2.9) | 16.6 (3.3) |
| WL | 16.5 (2.9) | n/a | 17.2 (2.0) |
| Treatment control subscalee | |||
| GC | 19.0 (3.5) | 19.9 (3.5) | 19.6 (3.1) |
| EB | 18.6 (3.5) | 19.9 (3.5) | 18.2 (4.2) |
| WL | 19.0 (3.4) | n/a | 19.2 (3.5) |
| Illness coherence subscalee | |||
| GC | 10.3 (3.2) | 9.4 (3.0) | 9.3 (3.1) |
| EB | 10.1 (3.1) | 9.6 (3.2) | 9.5 (3.5) |
| WL | 11.3 (4.0) | n/a | 10.3 (3.9) |
| Emotional representation subscaled | |||
| GC | 29.1 (7.8) | 28.3 (8.2) | 27.5 (8.0) |
| EB | 29.2 (8.1) | 28.1 (8.0) | 31.4 (6.1) |
| WL | 29.0 (7.6) | n/a | 28.8 (8.4) |
High knowledge scores reflect a greater number of questions answered correctly.
“Accurate” responses for each group were as follows: for the GC group, if they fell within the range provided in the GC session; for the EB group, if they fell within the range quoted in the booklet; for the WL group, if they fell within the range determined by consensus of the three BC/EGCs (CH, AI, JA) based on family history analysis. It was more difficult to achieve an ‘accurate’ rating at baseline for the EB group because the risk range (10–15%) was typically narrower than for those that were personalized to the family history (for the GC and WL groups).
High scores reflect high levels of internalized stigma.
High scores on the consequences and emotional representation subscales represent strongly held beliefs about the negative consequences of the illness, and a strong negative emotional response to the illness, respectively.
High scores on the personal control, treatment control and illness coherence subscales represent positive beliefs about the controllability of the illness and a personal understanding of the condition.
RESULTS
Patient Characteristics
Characteristics of participants are summarized in Table 1. Flow of participants through the trial is depicted in Figure 1.
Table 1.
Sociodemographic Characteristics of Participants (N=120)
| n (%) for all groups | n (%) for GC group | n (%) for EB group | n (%) for WL group | |
|---|---|---|---|---|
| Sex
| ||||
| Female | 68 (56.3) | 22 (55) | 23 (57.5) | 23 (57.5) |
| Male | 52 (43.7) | 18 (45) | 17 (42.5) | 17 (42.5) |
|
| ||||
| Age (mean, rangea) | 41.6 (17 – 73) | 40.1 (21 – 62) | 40.5 (17 – 68) | 44.1 (23 – 73) |
|
| ||||
| Diagnosis | ||||
|
| ||||
| Bipolar Disorder | 83 (69.2) | 29 (72.5) | 28 (70) | 26 (65) |
| Schizoaffective Disorder | 13 (10.8) | 4 (10) | 6 (15) | 3 (7.5) |
| Schizophrenia | 20 (16.7) | 7 (17.5) | 3 (7.5) | 10 (25) |
| Otherb | 4 (3.3) | 0 (0) | 3 (7.5) | 1 (2.5) |
|
| ||||
| Global Severity Index (GSI) at T1 (mean, SD) | 1.0 (0.8) | 1.0 (0.8) | 1.0 (0.8) | 1.1 (0.8) |
|
| ||||
| Marital Status | ||||
|
| ||||
| Single (including divorced, separated) | 79 (65.8) | 22 (55) | 25 (62.5) | 32 (80) |
| Partnered (including married, common law) | 41 (34.2) | 18 (45) | 15 (37.5) | 8 (20) |
|
| ||||
| Socioeconomic Status (Annual Household Income - CAD) | ||||
|
| ||||
| <20,000 | 61 (51.3) | 18 (45) | 18 (46.2) | 25 (62.5) |
| 20,000 – 40,000 | 17 (14.3) | 6 (15) | 6 (15.4) | 5 (12.5) |
| 41,000 – 60,000 | 13 (10.9) | 2 (5) | 4 (10.3) | 7 (17.5) |
| 61,000 – 80,000 | 11 (9.2) | 5 (12.5) | 6 (15.4) | 0 (0) |
| 81,000 – 100,000 | 10 (8.4) | 5 (12.5) | 3 (7.7) | 2 (5) |
| >100,000 | 7 (5.9) | 4 (10) | 2 (5.1) | 1 (2.5) |
|
| ||||
| Highest Level of Education | ||||
|
| ||||
| Some High School | 19 (16.0) | 5 (12.5) | 8 (20.5) | 6 (15) |
| Completed High School | 9 (7.6) | 1 (2.5) | 3 (7.7) | 5 (12.5) |
| Attended College or University | 91 (76.5) | 34 (85) | 28 (71.8) | 29 (72.5) |
Note. Percentages are based on non-missing data. The average number of years since diagnosis was 11.5 years (range: 0–42).
After consulting with the IRB we allowed one 17 year old to participate, due to their desire to do so, and their capability to provide fully informed consent.
Examples of other diagnoses included Major Depressive Disorder and Major Depressive Disorder with Psychosis.
Outcomes
Mean scores and effect sizes for outcome measures are in Table 2 and Table 3, respectively.
Table 3.
Effect sizes for risk perception comparisons at all three time points and for treatment vs. WL (analysis 2; T3 minus T1) for knowledge, internalized stigma, and perceived control.
| Overall effect size [95%CI] | Effect size [95%CI] – Schizophrenia | Effect size [95%CI] – Schizoaffective disorder | Effect size [95%CI] – Bipolar disorder | |
|---|---|---|---|---|
| Knowledge | 0.87 [0.46 to 1.28] | 1.11 [−0.67 to 2.89] | 0.67 [−0.96 to 2.3] | 0.41 [−0.15 to 0.98] |
|
| ||||
| Risk Perception: T1 | Cramer’s V = 0.37 | - | - | - |
| Risk Perception: T2 | phi = 0.35 | - | - | - |
| Risk Perception: T3 | Cramer’s V = 0.44 | - | - | - |
|
| ||||
| Internalized Stigma | −0.12 [−0.52 to 0.27] | −1.13 [−2.92 to 0.65] | −0.41 [−2.01 to 1.2] | −0.44 [−1.01 to 0.13] |
|
| ||||
| Perceived Control: Consequences | −0.26 [−0.66 to 0.13] | −1.35 [−3.19 to 0.48] | −1.47 [−3.25 to 0.32] | −0.45 [−1.02 to 0.12] |
| Perceived Control: Personal Control | −0.16 [−0.56 to 0.23] | −1.35 [−3.19 to 0.48] | 0.24 [−1.35 to 1.83] | 0.37 [−1.32 to 2.05] |
| Perceived Control: Treatment Control | −0.05 [−0.44 to 0.34] | 1.13 [−0.66 to 2.91] | −1.16 [−2.88 to 0.55] | 0.28 [−0.29 to 0.84] |
| Perceived Control: Illness Coherence | 0.10 [−0.29 to 0.49] | −1.93 [−3.92 to 0.06] | 0.12 [−1.47 to 1.71] | 0.30 [−0.27 to 0.87] |
| Perceived Control: Emotional Representation | −0.02 [−0.41 to 0.37] | −0.81 [−2.54 to 0.92] | −1.46 [−3.25 to 0.32] | −0.41 [−0.97 to 0.16] |
Note. The effect sizes for schizophrenia, schizoaffective disorder, and bipolar disorder were calculated from the difference in scores (T3 minus T1) between groups (GC minus EB) for each diagnostic group separately.
Knowledge
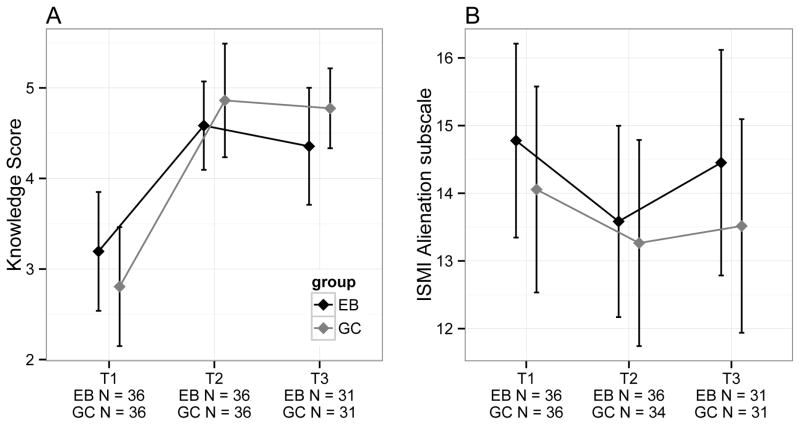
For analysis 1, there was no significant interaction between time and group (Likelihood ratio test statistic (LRT)=3.13, df=2, p=0.21), and no significant difference between GC and EB for knowledge score (LRT=0.14, df=1, p=0.71); however, there was a significant difference across time points (LRT=60.4, df=2, Holm-adjusted p<0.0001, R2LMM(m)=0.25) with T1 significantly lower than T2 (Tukey-adjusted p<0.001) and T3 (Tukey-adjusted p<0.001), but no significant difference between T2 and T3 (Tukey-adjusted p>0.05) (Figure 2a). For analysis 2, there was a significant group (GC/EB vs. WL) by time (T1 vs. T3) interaction term for knowledge scores (LRT=19.33, df=1, Holm-adjusted p=0.0003, R2LMM(m)=0.17) with treatment groups having knowledge scores on average 1.59 (95%CI=0.91–2.26) points higher than WL at T3.
Figure 2.
a) Mean knowledge scores by group and time, b) Mean ISMI alienation subscale scores by group and time. All error-bars represent 95% CI.
Risk Perception
There was a significant difference among groups in the proportion of accurate responses at T1, but not after p-value adjustment (unadjusted p=0.005, χ2=10.8, df=2, Holm-adjusted p=0.11), with EB having significantly fewer accurate responses than GC (Yates’ continuity corrected χ2=8.6, df=1, Bonferroni p=0.003). There were no significant differences between GC and WL, or EB and WL at T1. At T2, GC had a significantly greater proportion of accurate responses compared to EB, but not after p-value adjustment (unadjusted p=0.02, Yates’ continuity corrected χ2=5.9, df=1, Holm-adjusted p=0.32). There was a significant difference among groups at T3 (unadjusted p=0.001, χ2=13.8, df=2, Holm-adjusted p=0.03), with GC having more accurate responses than both EB (Yates’ continuity corrected χ2=8.2, df=1, Bonferroni p=0.004) and WL (Yates’ continuity corrected χ2=9.1, df=1, Bonferroni p=0.003).
Internalized Stigma
For analysis 1, there were no significant interaction terms, and no significant differences between GC and EB or over time for any ISMI subscale or total ISMI score (all unadjusted p>0.05), except Alienation, which showed a marginally non-significant difference across time points after p-value adjustment (unadjusted p=0.003, LRT=11.74, df=2, Holm-adjusted p=0.07). Specifically, scores at T1 were significantly higher than at T2 (Tukey-adjusted p=0.002), but there was no difference between T1 and T3, or T2 and T3 (all p>0.05) (Figure 2b). For analysis 2, there were no significant interaction terms, and no differences between groups (GC/EB vs. WL) for any ISMI subscale or ISMI total score (all unadjusted p>0.05).
Perceived Control
For analysis 1, there were no significant interaction terms, and no significant differences between GC and EB or time for all five IPQ subscales (all Holm-adjusted p>0.1). For analysis 2, there were no significant interaction terms, and no differences between groups (GC/EB vs. WL) or time for any IPQ subscale (all Holm-adjusted p>0.1).
Impact of GC/EB
Average scores for “usefulness” of GC were 3.31 (T2) and 2.93 (T3); for EB, scores were 3.03 (T2) and 2.68 (T3).
In GC, 23 participants reported sharing information from GC with family, friends, health care professionals, and teachers (mean=2.52; range:1–6). In EB, 15 participants reported sharing information from EB with family, friends, health care professionals, and fellow participants in a self-help group (mean=2.13; range:1–6).
GCSS data are reported elsewhere33.
DISCUSSION
This study represents the first pilot RCT of GC for individuals with SMI. Consistent with studies of GC in other areas34–37, we observed significant increases in knowledge scores post-intervention (GC/EB) as compared to WL and increases in risk accuracy for GC as compared to EB and WL. This is subtly, yet importantly, different from previous findings14,15, where mean risk estimates decreased from a baseline of overestimation, but remained overestimated. Increasing knowledge and risk perception accuracy may play a necessary, though not sufficient, role in empowering patients to make informed decisions about managing their mental health and, for some, whether to have children38.
Participants felt that EB and GC were useful, with GC having qualitatively higher mean scores on usefulness than EB (statistical testing was not conducted). Proportionally more participants who had GC, as compared to EB, reported knowledge sharing; and the mean score for the number of people with whom knowledge was shared was also higher for the GC group (although, again, no statistical testing was conducted). It is possible that participants who received GC had greater confidence in sharing their new knowledge, as compared to those who received EB. These promising results are worthy of further investigation.
While the effect of GC on ISMI scores was largely not significant, mean scores did decrease following GC, while for the EB group an initial drop in ISMI scores was followed by an increase one month later. Given the clinical importance of decreasing internalized stigma for those with SMI, and that our sample was underpowered, future studies with larger group sizes may be worthwhile, especially given other recent (uncontrolled) work which showed an effect of GC on ISMI scores15.
There was a decrease in IPQ consequences subscale mean scores post-intervention that persisted at one-month follow up. Changes in this subscale seem to indicate feelings of greater optimism for the future, and that SMI is perceived to be more manageable. Though this difference was not significant, we attribute the lack of statistical significance to small sample size. This is the first study to evaluate the impact of any intervention on perceived control in SMI. Previous studies evaluating GC in other contexts have shown increases in perceived control39,40.
It is possible that overall effects of the intervention on the outcomes of interest were influenced by difference in response between diagnostic groups (see Table 3). However, as the sample sizes for groups of individuals with schizophrenia and schizoaffective disorder were small, this requires further study.
Strengths and Weaknesses
Strengths of this study include a rigorous control group and recruitment of individuals from the general population rather than using individuals recruited for studies of the genetics of SMI (avoiding potential bias towards individuals with more strongly genetic causal attributions at baseline). Additionally, this study avoids a common confound of other GC studies; the impact of receiving genetic test results at the same time as GC. However, importantly, our sample size was underpowered to detect the observed effect sizes for internalized stigma and perceived control. It is possible that differing responses to GC between individuals with bipolar disorder and schizophrenia diluted our ability to detect differences. Additionally, blinding was not possible; due to the nature of the study, participants were aware of the group to which they had been randomized. Furthermore, the risk range used in the educational booklet was narrower than that typically provided on the basis of a family history evaluation, thus biasing towards less accurate results for the EB group. However, the ranges for the GC and WL groups were comparable. We excluded individuals not fluent in English; our findings, therefore, may not be generalizable to other cultural contexts.
Future Research
There are many avenues ripe for future psychiatric GC research: studies of GC efficacy for other psychiatric illnesses, the timing of GC in relation to time of diagnosis, and optimal number of GC sessions. Adequately powered RCTs of GC for individuals and family members of individuals with psychiatric illnesses, including recordings and manualization of the intervention(s) are an important next step. We would recommend that future RCTs focus on GC for only one psychiatric illness (rather than three, as in this pilot), especially given the potential difference in size of the effect of the intervention between diagnostic groups. Last, in future studies, the use of outcome measures related to those used here, but purpose-designed for exploring the outcomes of GC41 could be considered. Conducting research into psychiatric GC efficacy for populations with a variety of cultural backgrounds would be valuable.
Conclusions
These data support the value in referral to GC for individuals with SMI, the creation of psychiatric GC clinical practice guidelines, and the establishment of specialist psychiatric GC services. The potential for psychiatric GC to empower individuals with psychiatric illness makes it a very exciting addition to the range of services that are available to this disadvantaged population.
Supplementary Material
Clinical Points.
Patients with serious mental illnesses are interested in genetic counseling when it is made available to them, and genetic counseling is recommended by clinical practice guidelines, but systematic evaluation of its outcomes for these populations have been limited or unavailable, in the case of bipolar disorder.
Physicians seeing patients with serious mental illnesses could consider referring to a specialist psychiatric genetic counselor (who can be found using the “Find a counselor” tool at www.nsgc.org), particularly when patients have sufficient insight into their illness and would benefit from a greater understanding of risk and protective factors in managing their illness.
Acknowledgments
Funding/Support:
We would like to acknowledge the BC Medical Services Foundation (grant number: BCM07-0155), the Women’s Health Research Institute, and the Mood Disorders Association of BC, all of whom contributed financial assistance but had no role in the design or conduct of the study, data collection/interpretation, or manuscript development or approval. CH and AA are supported by the Women’s Health Research Institute. JCA was supported by the Canada Research Chairs program, the Canadian Institutes for Health Research, the Michael Smith Foundation for Health Research, and the BC Mental Health and Addictions Research Institute.
Footnotes
This process integrates the following: interpretation of family and medical histories to assess the chance of disease occurrence or recurrence; education about inheritance, testing, management, prevention, resources and research; and counseling to promote informed choices and adaptation to the risk or condition. Genetic and environmental contributors to illness are discussed in a holistic fashion.
Conflict of Interest Disclosures:
AR, AA, AI, JC, and RR report no competing interests. WGH has received consulting fees or sat on paid advisory boards for: MDH Consulting, In Silico, Lundbeck, Lilly and Roche; received honoraria from Rush University and the University of Ottawa, the Interior and Vancouver Coastal Health Authorities, the Canadian Psychiatric Association; and received grants from the Canadian Institutes of Health Research (CIHR). CH reported serving on the Canadian Association of Genetic Counselors Board of Directors from 2013–2016. JCA reported serving on the National Society of Genetic Counselors Board of Directors from 2011–2013 and has received grants from CIHR, the Michael Smith Foundation for Health Research, and Pfizer (investigator initiated grant).
Role of the sponsor:
Not applicable.
Disclaimer:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the BC Medical Services Foundation, the Canadian Association of Genetic Counsellors, the Mood Disorders Association of BC, the National Society of Genetic Counselors, the University of British Columbia, or the Women’s Health Research Institute.
Previous Presentations:
The 5th International Stigma Conference, Ottawa, Canada. June 4 – 6, 2012.
National Society of Genetic Counselors Annual Education Conference, Boston, U.S.A, Oct. 24 – 27, 2012
World Congress of Psychiatric Genetics, Boston, U.S.A., Oct 17 – 21, 2013.
Canadian Association of Genetic Counsellors Annual Education Conference, Toronto, Canada, Nov. 6 – 9, 2013
Additional Contributions:
We thank all those who participated in this study. We thank Dr. Allen Thornton, PhD, Simon Fraser University Department of Psychology, for his assistance in supervising and interpreting the KBIT-2. Dr. Thornton did not receive compensation for this contribution. We would like to thank Claudia Li for her graphic design work; the educational booklet wouldn’t have been the same without her. Claudia received compensation for this contribution (Mood Disorders Association of BC funding). We would like to thank Nick Alexander for his technical assistance. Finally, we thank all members of the Translational Psychiatric Genetics Group for their manifold support, insight, guidance, and commitment. None of these individuals have any conflicts of interest with this work.
References
- 1.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okkels N, Vernal DL, Jensen SO, McGrath JJ, Nielsen RE. Changes in the diagnosed incidence of early onset schizophrenia over four decades. Acta Psychiatr Scand. 2013;127(1):62–68. doi: 10.1111/j.1600-0447.2012.01913.x. [DOI] [PubMed] [Google Scholar]
- 3.Costain G, Bassett A. Clinical applications of schizophrenia genetics: genetic diagnosis, risk, and counseling in the molecular era. Appl Clin Genet. 2012;20(5):1–18. doi: 10.2147/TACG.S21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13(8):537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Society of Genetic Counselors’ Definition Task Force. Resta R, Biesecker BB, et al. A new definition of genetic counseling: national society of genetic counselors’ task force report. J Genet Couns. 2006;15(2):77–83. doi: 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- 6.DeLisi LE, Bertisch H. A preliminary comparison of the hopes of researchers, clinicians, and families for the future ethical use of genetic findings on schizophrenia. Am J Med Genet B. 2006;141B:110–115. doi: 10.1002/ajmg.b.30249. [DOI] [PubMed] [Google Scholar]
- 7.Lyus VL. The importance of genetic counseling for individuals with schizophrenia and their relatives: potential clients’ opinions and experiences. Am J Med Genet B. 2007;144B:1014–1021. doi: 10.1002/ajmg.b.30536. [DOI] [PubMed] [Google Scholar]
- 8.Austin JC, Honer WG. The genomic era and serious mental illness: a potential application for psychiatric genetic counseling. Psych Serv. 2007;58:254–261. doi: 10.1176/ps.2007.58.2.254. [DOI] [PubMed] [Google Scholar]
- 9.Finn CT, Smoller JW. Genetic counseling in psychiatry. Harv Rev Psychiatry. 2006;14(2):109–121. doi: 10.1080/10673220600655723. [DOI] [PubMed] [Google Scholar]
- 10.Hill MK, Sahhar M. Genetic counselling for psychiatric disorders. Med J Aust. 2006;185(9):507–510. doi: 10.5694/j.1326-5377.2006.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 11.Vergare MJ, Binder RL, Cook IA, Galanter M, Lu FG. Compendium 2006. Arlington, VA: American Psychiatric Association; Practice guidelines for the treatment of psychiatric disorders. [Google Scholar]
- 12.Canadian Psychiatric Association. Clinical practice guidelines: treatment of schizophrenia. Can J Psychiatry. 2005;50(13 Suppl 1):7S–57S. [PubMed] [Google Scholar]
- 13.Austin JC, Honer WG. Psychiatric genetic counselling for parents of individuals affected with psychotic disorders: a pilot study. Early Interv Psychiatry. 2008;2(2):80–89. doi: 10.1111/j.1751-7893.2008.00062.x. [DOI] [PubMed] [Google Scholar]
- 14.Costain G, Esplen MJ, Toner B, Hodgkinson KA, Bassett AS. Evaluating genetic counseling for family members of individuals with schizophrenia in the molecular age. Schizophr Bull. 2014;40(1):78–87. doi: 10.1093/schbul/sbs124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costain G, Esplen MJ, Toner B, Hodgkinson KA, Bassett AS. Evaluating genetic counseling for individuals with schizophrenia in the molecular age. Schizophr Bull. 2014;40(1):88–99. doi: 10.1093/schbul/sbs124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Castro M, Frydman M, Friedman I, et al. Genetic counseling in hereditary breast/ovarian cancer in Israel: psychosocial impact and retention of genetic information. Am J Med Genet. 2002;111:147–151. doi: 10.1002/ajmg.10550. [DOI] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW. User’s guide for the Structured Clinical Interview for DSM-IV-TR axis I disorders – research version. New York, NY: Biometric Research, New York State Psychiatric Institute; 2001. [Google Scholar]
- 18.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test manual. 2. Bloomington, MN: Pearson, Inc; 2004. [Google Scholar]
- 19.Austin JC, Palmer CG, Rosen-Sheidley B, et al. Psychiatric disorders in clinical genetics II: individualizing recurrence risks. J Genet Couns. 2008;17(1):18–29. doi: 10.1007/s10897-007-9121-4. [DOI] [PubMed] [Google Scholar]
- 20.Peay HL, McCarthy Veach P, Palmer CGS, Rosen-Sheidley B, Austin JC. Psychiatric disorders in clinical genetics I: addressing family histories of psychiatric illness. J Genet Couns. 2008;17(1):6–17. doi: 10.1007/s10897-007-9120-5. [DOI] [PubMed] [Google Scholar]
- 21.Inglis A, Koehn D, McGillivray B, Stewart SE, Austin J. Evaluating a unique, specialist psychiatric genetic counseling clinic: uptake and impact. Clin Genet. 2015;87(3):218–24. doi: 10.1111/cge.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasparian NA, Wakefield CE, Meiser B. Assessment of psychosocial outcomes in genetic counseling research: an overview of available measurement scales. J Genet Couns. 2007;16(6):693–712. doi: 10.1007/s10897-007-9111-6. [DOI] [PubMed] [Google Scholar]
- 23.Lerman C, Biesecker B, Benkendorf JL, et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Natl Cancer I. 1997;89:148–57. doi: 10.1093/jnci/89.2.148. [DOI] [PubMed] [Google Scholar]
- 24.Austin JC, Smith GN, Honer WG. The genomic era and perceptions of psychotic disorders: Genetic risk estimation, associations with reproductive decisions, and views about predictive testing. Am J Med Genet. 2006;141B:926–928. doi: 10.1002/ajmg.b.30372. [DOI] [PubMed] [Google Scholar]
- 25.Livingston JD, Boyd JE. Correlates and consequences of internalized stigma for 28414 people living with mental illness: a systematic review and meta-analysis. Soc Sci Med. 2010;71(12):2150–2161. doi: 10.1016/j.socscimed.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 26.DeMarco TA, Peshkin BN, Mars BD, Tercyak KP. Patient satisfaction with cancer genetic counseling: a psychometric analysis of the genetic counseling satisfaction scale. J Genet Couns. 2004;13(4):293–304. doi: 10.1023/b:jogc.0000035523.96133.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd Risher J, Otilingam PG, Grajales M. Internalized stigma of mental illness: psychometric properties of a new measure. Psychiatry Res. 2003;121:31–49. doi: 10.1016/j.psychres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Lobban F, Barrowclough C, Jones S. Assessing cognitive representations of mental health problems. I. The illness perception questionnaire for schizophrenia. Br J Psychiatry. 2005;44:147–162. doi: 10.1348/014466504X19497. [DOI] [PubMed] [Google Scholar]
- 29.Derogatis LR. The Brief Symptom Inventory: administration, scoring and procedures. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 30.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. URL http://www.R-project.org/ [Google Scholar]
- 31.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 32.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133–142. [Google Scholar]
- 33.Hippman CH, Lohn Z, Ringrose A, et al. “Nothing is absolute in life”: understanding uncertainty in the context of psychiatric genetic counseling from the perspective of those with serious mental illness. J Genet Couns. 2013;22(5):625–632. doi: 10.1007/s10897-013-9594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smerecnik CM, Mesters I, Verweij E, de Vries NK, de Vries H. A systematic review of the impact of genetic counseling on risk perception accuracy. J Genet Couns. 2009;18(3):217–228. doi: 10.1007/s10897-008-9210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. Fam Cancer. 2006;5(1):61–75. doi: 10.1007/s10689-005-2577-1. [DOI] [PubMed] [Google Scholar]
- 36.Hunter AG, Cappelli M, Humphreys L, et al. A randomized trial comparing alternative approaches to prenatal diagnosis counseling in advanced maternal age patients. Clin Genet. 2005;67(4):303–313. doi: 10.1111/j.1399-0004.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- 37.Kladny B, Williams A, Gupta A, Gettig EA, Krishnamurti L. Genetic counseling following the detection of hemoglobinopathy trait on the newborn screen is well received, improves knowledge, and relieves anxiety. Genet Med. 2011;13(7):658–661. doi: 10.1097/GIM.0b013e31821435f7. [DOI] [PubMed] [Google Scholar]
- 38.Elwyn G, Miron-Shatz T. Deliberation before determination: the definition and evaluation of good decision making. Health Expect. 2010;13(2):139–147. doi: 10.1111/j.1369-7625.2009.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davey A, Rostant K, Harrop K, Goldblatt J, O’Leary P. Evaluating genetic counseling: client expectations, psychological adjustment and satisfaction with service. J Genet Couns. 2005;14(3):197–206. doi: 10.1007/s10897-005-0519-6. [DOI] [PubMed] [Google Scholar]
- 40.Rothwell E, Kohlmann W, Jasperson K, Gammon A, Wong B, et al. Patient outcomes associated with group and individual genetic counseling formats. Fam Cancer. 2012;11:97–106. doi: 10.1007/s10689-011-9486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAllister M, Wood AM, Dunn G, Shiloh S, Todd C. The genetic counseling outcome scale: a new patient-reported outcome measure for clinical genetics services. Clin Genet. 2011;79(5):413–424. doi: 10.1111/j.1399-0004.2011.01636.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.