Abstract
Among adults with nonalcoholic fatty liver disease (NAFLD), 25% of deaths are attributable to cardiovascular disease (CVD). CVD risk reduction in NAFLD requires not only modification of traditional CVD risk factors but identification of risk factors unique to NAFLD. In a NAFLD cohort, we sought to identify non-traditional risk factors associated with CVD. NAFLD was determined by a previously described algorithm and a multivariate logistic regression model determined predictors of CVD. Of the 8,409 individuals with NAFLD, 3,243 had CVD and 5,166 did not. On multivariable analysis, CVD among NAFLD patients was associated with traditional CVD risk factors including family history of CVD (OR 4.25, P=0.0007), hypertension (OR 2.54, P=0.0017), renal failure (OR 1.59, P=0.04), and age (OR 1.05, P<0.0001). Several non-traditional CVD risk factors including albumin, sodium, and Model for End-Stage Liver Disease (MELD) score were associated with CVD. On multivariable analysis, an increased MELD score (OR 1.10, P<0.0001) was associated with an increased risk of CVD. Albumin (OR 0.52, P<0.0001) and sodium (OR 0.96, P=0.037) were inversely associated with CVD. In addition, CVD was more common among those with a NAFLD fibrosis score >0.676 than those with a score ≤0.676 (39 vs. 20%, P<0.0001). CVD in NAFLD is associated with traditional CVD risk factors, as well as higher MELD scores and lower albumin and sodium levels. Individuals with evidence of advanced fibrosis were more likely to have CVD. These findings suggest that the drivers of NAFLD may also promote CVD development and progression.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease in the United States, affecting an estimated 80 million adults (1). Nonalcoholic steatohepatitis (NASH) is the progressive form of NAFLD and can lead to the development of cirrhosis and hepatocellular carcinoma (2–5). Although liver-related complications are frequent among those with NAFLD, cardiovascular disease (CVD) is the most common cause of mortality, accounting for 25% of deaths (6). NAFLD is associated with an increased prevalence of aortic and coronary atherosclerosis, high-risk coronary plaques, and increased coronary artery calcium scores. Further, NAFLD is associated with increased fatal and non-fatal CVD events including acute coronary syndromes (7–9).
The identification of CVD risk factors among the general population has been the focus of considerable investigation. Identifying which patient characteristics confer an increased risk of CVD has contributed to the understanding of CVD pathophysiology. Unlike in the general population, little attention has been focused on elucidating non-traditional CVD risk factors in NAFLD. A single study evaluated the Framingham Risk Score, a composite score of traditional risk factors including age, gender, cholesterol, high-density lipoprotein (HDL) level, smoking status, and hypertension, as a CVD predictor in NAFLD (10). Although the Framingham Risk Score accurately predicted a 10-year CVD risk, none of its individual components were found to be predictors of CVD, and no novel risk factors were evaluated. Thus, little is known about the value of non-traditional CVD risk factors in NAFLD.
CVD events are believed to be rare in individuals with chronic and end-stage liver disease (11). The systemic vasodilatation and decreased lipid synthesis that accompany liver disease are thought to decrease CVD risk (12,13). However, NAFLD is, in many ways, distinct from other causes of liver disease. Even in late stages, NAFLD is associated with dyslipidemia and hypertension, which confer increased CVD risk (14). We hypothesize that the same drivers of progressive NAFLD, systemic inflammation, lipid oxidation, and endothelial dysfunction, may also drive the development of CVD, making CVD increasingly prevalent as NAFLD progresses and associated with markers of liver disease progression. By using a large electronic medical record (EMR)-based cohort of 8,409 individuals with NAFLD, we evaluated those with and without CVD to identify unique CVD risk factors.
METHODS
Patients and data for the present study were drawn from a previously described cohort created from the Partners Health-Care EMR utilizing the Partners Research Patient Data Registry (RPDR). This centralized clinical data registry contains data from all institutions in the Partners HealthCare System and includes data on ~10 million patients with ~2.3 billion EMR facts. We utilized data from the Massachusetts General Hospital and Brigham and Women’s Hospital, both in Boston, that serve the greater Northeast United States.
NAFLD was defined using a previously validated algorithm for the identification of NAFLD in an EMR database (15). The algorithm calculates a NAFLD probability per patient based on the most recent triglycerides measurement, the total number of billing codes for NAFLD (ICD-9 571.8 or 571.9), and the total number of mentions of NAFLD in clinical narrative notes. The algorithm incorporates text processing to identify clinical narrative notes associated with NAFLD. As a first step, the algorithm was applied to the RPDP cohort to identify all individuals with NAFLD. As a second step, patients with either a diagnosis of cirrhosis or a non-viral hepatitis were excluded. In total, 8,409 adults aged ≥18 years of age exceeded the NAFLD probability threshold of 0.85 and were considered in our analysis.
CVD was considered present when an individual had ≥1 ICD-9 or CPT code for myocardial infarction, CVD, ischemic heart disease, angina, or peripheral vascular disease. Comorbidities were determined by ≥1 ICD-9 or CPT code for the comorbidity over their lifetime prior to the diagnosis of CVD. We extracted from the notes expressions to determine an individual’s most recent smoking status (past, present, never). In addition, to determine whether the patient had a family history of CVD, we identified in clinical narrative notes the indication of at least one family member being reported as having myocardial infarction, heart attack, angina, coronary artery bypass surgery, cardiovascular percutaneous intervention, or sudden death. For laboratory variables, when more than one value was present the average of all available values was used.
NAFLD fibrosis score (NFS) was calculated according to the published formula (16):
Model for End-Stage Liver Disease (MELD) score was calculated according to the published formula (17):
Statistical analysis
Categorical variables were compared using the χ2-test. Continuous variables were compared using the t-test or the Mann–Whitney’s test, as appropriate. To determine odds ratio (OR) for the variables associated with the presence of NAFLD, logistic regression was performed. The following variables, based on statistical significance and clinical relevance, were included: age, gender, ethnicity, diabetes, hypertension, dyslipidemia, obstructive sleep apnea, non-HDL cholesterol, renal failure, low-density lipoprotein level (LDL), alanine aminotransferase level, NFS, MELD score, and family history of CVD. Statistical analysis was performed on SAS 9.4 (SAS Institute, Cary, NC). We examined the collinearity among covariates in the multivariable model based on their variance inflation factor. The multivariable model consists of covariates that do not have overly high variance inflation factor (maximum VIF 3.3). This study was approved by the Partners Healthcare Human Research Committee that serves as the institutional review board for both Brigham and Women’s Hospital and Massachusetts General Hospital.
RESULTS
Baseline characteristics
Of the 8,409 individuals, 3,243 individuals had CVD, whereas 5,166 individuals had no evidence of CVD (Table 1). Individuals with NAFLD and CVD were older (61.9 years vs. 52.3 years, P<0.0001), more likely to be male (55.2 vs. 51.3%, P=0.0006), and Caucasian (92.6 vs. 87.1%, P<0.0001). There was no difference in the mean BMI or prevalence of obesity between groups (33.3 vs. 33.4 kg/m2, P=0.30). All variables considered were calculated based on the available values or measurements from date of birth of a patient to the last EMR fact that was available in the cohort—i.e., September 2010.
Table 1.
Baseline characteristics
| Variable | NAFLD −CVD | NAFLD +CVD | P value |
|---|---|---|---|
| Mean age±s.d. (years) | 52.3±14.1 | 61.9±13.1 | <0.0001 |
| Gender; no. (%) | |||
| Male | 2,652 (51.3%) | 1,789 (55.2%) | 0.0006 |
| Female | 2,514 (48.7%) | 1,454 (44.8%) | — |
| Ethnicity; no. (%) | |||
| White | 3,399 (87.1%) | 2,574 (92.6%) | <0.0001 |
| African American | 296 (7.6%) | 146 (5.25%) | — |
| Other | 206 (5.3%) | 60 (2.2%) | — |
| BMI±s.d (kg/m2) | 33.4±7.6 | 33.3±10.6 | 0.30 |
| Obesity; no. (%) | 1,747 (33.8%) | 1,074 (33.1%) | 0.51 |
| Diabetes mellitus; no. (%) | 3,340 (64.7%) | 2,651 (82.0%) | <0.0001 |
| Hypertension; no. (%) | 2,946 (57.0%) | 2,745 (84.6%) | <0.0001 |
| Family history of CVD; no. (%) | 3,014 (58.3%) | 2,172 (67.0%) | <0.0001 |
| Renal failure; no. (%) | 363 (7.0%) | 546 (16.8%) | <0.0001 |
| LDL±s.d. (mg/dl) | 112.5±38.2 | 106.3±38.8 | <0.0001 |
| Non-HDL-C±s.d. (mg/dl) | 189.7±63.6 | 182.3±58.8 | <0.0001 |
| Albumin±s.d. (g/dl) | 3.9±0.69 | 3.7±0.63 | <0.0001 |
| Sodium±s.d. (mmol/l) | 139.0±2.9 | 138.0±2.7 | <0.0001 |
| MELD score±s.d. | 10.2±5.0 | 12.3±5.7 | <0.0001 |
BMI, body mass index; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; MELD, Model for End-Stage Liver Disease; NAFLD, nonalcoholic fatty liver disease.
Traditional CVD risk factors
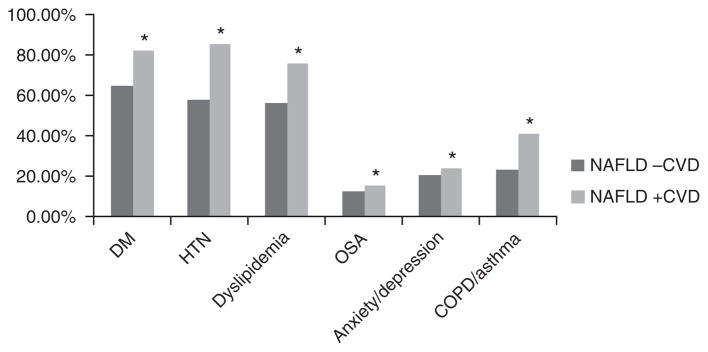
Traditional risk factors for CVD were more prevalent in individuals with NAFLD and CVD compared with those with NAFLD alone on univariate analysis (Figure 1). Type 2 diabetes (82.0 vs. 64.7%, P<0.0001) was more frequent and median HbA1C (7.3 vs. 7.08%, P=0.0009) was significantly higher in those with both CVD and NAFLD compared with those with NAFLD alone. Hypertension (84.6 vs. 57.0%, P<0.0001), family history of CVD (67.0 vs. 58.3%, P<0.0001), and current or past tobacco use (53.7 vs. 41.1%, P<0.0001) were associated with the presence of CVD in NAFLD patients. Other comorbidities including obstructive sleep apnea, anxiety and depression, chronic obstructive pulmonary disease, and asthma were more frequent in NAFLD and CVD when compared with NAFLD alone (Figure 1).
Figure 1.
Prevalence of comorbidities by CVD status in NAFLD. COPD, chronic obstructive pulrnonary disease; CVD, cardiovascular disease; DM, Diabetes; HTN, hypertension; NAFLD, nonalcoholic fatty liver disease; OSA, sleep apnea. * P<0.0001..
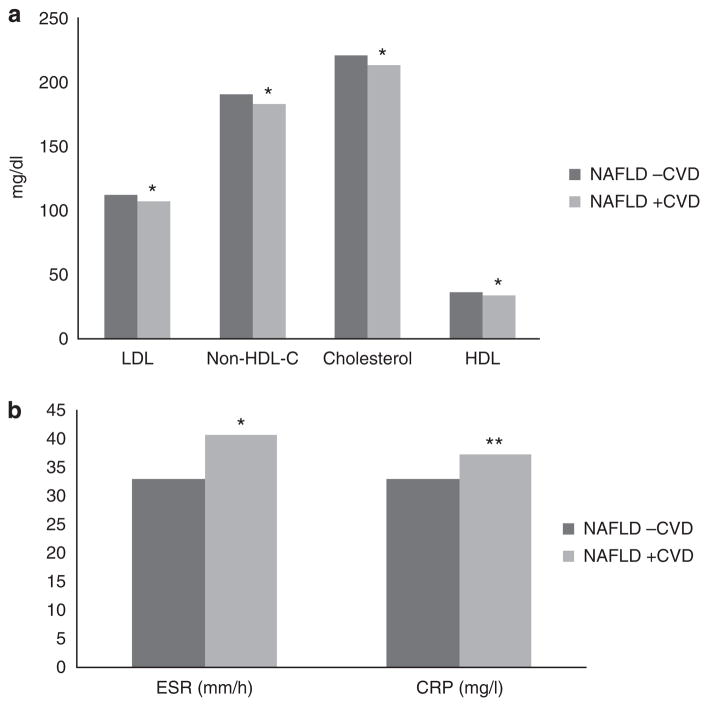
Dyslipidemia and statin use were more frequent in individuals with both NAFLD and CVD than those with NAFLD alone (75.4 vs. 55.5%, P<0.0001 and 49.0 vs. 26.1%, P<0.0001, respectively). Mean LDL (106.3 mg/dl vs. 112.5 mg/dl, P<0.0001), total cholesterol (214.5 mg/dl vs. 221.9 mg/dl, P<0.0001), and non-HDL cholesterol (182.3 mg/dl vs. 189.7 mg/dl, P<0.0001) were lower in those with NAFLD and CVD compared with those with NAFLD alone (Figure 2a). HDL levels were lower in those with CVD (35.9 mg/dl vs. 37.9 mg/dl, P<0.0001), although there was no difference in triglyceride levels. Other risk markers of CVD disease including ESR (40.7 mm/h vs. 33.2 mm/h, P<0.0001) and C-reactive protein (37.3 mg/l vs. 32.9 mg/l, P=0.007) were higher in those with CVD and NAFLD (Figure 2b and Figure 3).
Figure 2.
(a) Lipid levels by CVD status in NAFLD. (b) ESR and CRP by CVD status in NAFLD. CRP, c-reactive protein; CVD, cardiovascular disease; ESR, erythrocyte sedimentation rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; non-HDL-C, non-high-density lipoprotein cholesterol. * P<0.0001, ** P=0.007.
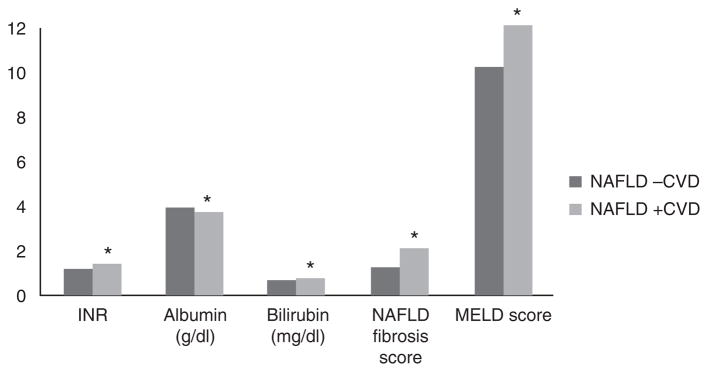
Figure 3.
Liver function by CVD status in NAFLD. CVD, cardiovascular disease; INR, international normalized ratio; MELD, model for End-stage liver disease; NAFLD, nonalcoholic fatty liver disease. * P<0.0001.
A diagnosis of renal failure was more common in CVD and NAFLD compared with those with only NAFLD (16.8 vs. 7.0%, P<0.0001). Individuals with CVD and NAFLD had higher serum creatinine levels (1.35 mg/dl vs. 1.16 mg/dl, P<0.0001) and lower estimated glomerular filtration rates (57.8 ml/min per 1.73 m2 vs. 62.9 ml/min per 1.73 m2, P<0.0001).
Non-traditional risk factors for CVD
Several non-traditional risk factors for CVD and NAFLD were identified on univariate analysis. CVD was associated with decreased albumin levels (3.7 g/dl vs. 3.9 g/dl, P<0.0001) and platelet counts (246.1 th/cumm vs. 259.6 th/cumm, P<0.0001) compared with those with NAFLD alone. In addition, patients with NAFLD and CVD had increased total bilirubin (0.8 mg/dl vs. 0.7 mg/dl, P<0.0001) and INR (1.4 vs. 1.2, P<0.0001). NFSs (2.1±1.52 vs. 1.3±1.45, P<0.0001) and mean MELD scores (12.3±5.7 vs. 10.2±5.0, P<0.0001) were also significantly higher in those with both CVD and NAFLD compared with those with NAFLD alone. No difference was seen in mean AST levels between groups, although those without CVD had slightly increased alanine aminotransferase level (45.8 U/l vs. 45.5 U/l, P<0.0001) when compared with those with CVD.
Factors associated with CVD on regression analysis
On multivariable analysis after controlling for gender, ethnicity, diabetes, dyslipidemia, alanine aminotransferase, and obstructive sleep apnea, family history of CVD and hypertension were most strongly associated with the presence of CVD (Table 2). Age, non-HDL cholesterol, and renal failure remained directly associated with the presence of CVD. LDL was inversely associated with the presence of CVD. We assessed the correlation between LDL level and use of lipid lowering medication. Lipid lowering medication use was inversely correlated with the LDL level (r=−0.09, P<0.0001), indicating that statin use was likely associated with CVD.
Table 2.
Factors associated with CVD in NAFLD on multivariable analysisa
| Variable | OR (95% CI)a | P value |
|---|---|---|
| Hypertension | 2.54 (1.42–4.58) | 0.0017 |
| Renal failure | 1.59 (1.01–2.49) | 0.04 |
| MELD score | 1.10 (1.06–1.14) | <0.0001 |
| Age (years) | 1.05 (1.03–1.06) | <0.0001 |
| Non-HDL-C (mg/dl) | 1.01 (1.001–1.012) | 0.026 |
| LDL (mg/dl) | 0.99 (0.98–0.99) | 0.008 |
| Family history of CVD | 4.25 (1.84–9.83) | 0.0007 |
| Albumin | 0.52 (0.44–0.63) | <0.0001 |
| Sodium | 0.96 (0.93–0.99) | 0.037 |
CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein; MELD, Model for End-Stage Liver Disease; NAFLD, nonalcoholic fatty liver disease.
Adjusted for gender, ethnicity, diabetes, dyslipidemia, ALT, family history of CVD, and OSA.
In addition, the non-traditional CVD risk factors MELD score, albumin level, and sodium level were associated with increased risk of CVD. MELD score was associated with an increased risk of CVD after adjustment with an OR 1.10, 95% confidence interval (CI) 1.06–1.14. Albumin and sodium levels were inversely associated with risk of CVD, demonstrating that low albumin and sodium levels confer an increased risk of CVD (OR 0.52, 95% CI 0.44–0.63 and OR 0.96, 95% CI 0.93–0.99, respectively).
CVD by NFS
Histologic diagnosis of NASH was not available in the present cohort. To evaluate whether CVD prevalence differed in those with NASH and advanced fibrosis, we evaluated the CVD prevalence by the NFS. Individuals with NAFLD and a NFS>0.676 had a significantly higher prevalence of CVD compared with those with NFS≤0.676 (39 vs. 20%, P<0.0001). This finding further suggests an association between advanced fibrosis and CVD.
DISCUSSION
The present study demonstrates that among individuals with NAFLD, MELD score, albumin, and sodium are non-traditional predictors of CVD. Further, we confirm the validity of our model by demonstrating that several known risk factors for CVD in the general population are associated with CVD in NAFLD.
We found that MELD score, albumin, and sodium levels were associated with a diagnosis of CVD. Each of these factors is known to be independently associated with disease progression in chronic liver disease (18–26). Further, we demonstrated that those with advanced fibrosis as predicted by NFS had a higher prevalence of CVD, also suggesting that advanced liver disease is associated with increased risk of CVD. Our findings demonstrate that CVD may be associated with progressive liver disease among those with NAFLD and suggest that similar processes may drive the development of atherosclerosis, steatohepatitis, and liver fibrosis. This finding is counter to the widely held belief that CVD events are less frequent in end-stage liver disease in part due to systemic vasodilatation and impaired lipid synthesis (11). However, NAFLD may likely be an exception to this rule secondary to the associated systemic inflammatory response, endothelial dysfunction, and lipid peroxidation that accompanies advanced NAFLD histology and can impact the development of atherosclerotic disease (27–30). Furthermore, hypercoagulablity and impaired fibrinolysis found in NASH may also contribute to CVD progression (31,32). This finding has several important implications. First, a relationship between worsening liver disease and CVD suggests that individuals with advanced liver disease from NAFLD and those being evaluated for liver transplantation should undergo rigorous evaluation of CVD and CVD risk management. In addition, this finding further strengthens the link between NAFLD and CVD and suggests that treatment of one condition could positively impact the other.
In the present study, we confirm that traditional CVD risk factors including age, family history, hypertension, and renal failure are risk factors for CVD in NAFLD. These findings demonstrate the ability of our algorithm (15) and the cohort that we created to accurately identify CVD and comorbidities. Family history of CVD was most strongly associated with CVD in individuals with NAFLD (OR 4.25, 95% CI 1.84–9.83), a previously unreported finding. Family history of CVD in the general population is a predictor of CVD-related death in men and women but has not been evaluated in a NAFLD population (33–35). This association suggests that a genetic component to CVD risk in NAFLD patients may exist, and further evaluation is needed in a prospective cohort. Traditional risk factors of age, renal failure, and hypertension were also associated with CVD in NAFLD (36).
The LDL level is a known risk factor for the development of CVD. However, in the present study, LDL was inversely associated with CVD (OR 0.99, 95% CI 0.98–0.99). Although the inverse relationship between LDL and CVD prevalence in NAFLD may seem to contradict data in non-NAFLD cohorts, we believe that this finding is due to the significantly higher frequency of lipid lowering medication use in those with CVD compared with those with NAFLD alone (49.0 vs. 26.1%, P<0.0001).
Our study has several important limitations. The cross-sectional design of our study allows for assessment of factors associated with the presence of CVD in NAFLD but does not allow us to comment on causality of those risk factors in the pathogenesis of CVD in NAFLD. However, it does allow for the identification of several novel CVD-associated variables that can be further assessed in prospective cohorts. Our study also uses a validated algorithm to identify NAFLD, but liver histology was not available to differentiate between steatosis and NASH or to determine fibrosis stage. To address this, NFSs, which serve as a proxy for the presence of NASH and advanced fibrosis, were calculated (16). Our study assessed MELD scores in a population that was not confined to those with cirrhosis. As a result, other causes of an elevated MELD score (e.g., anti-coagulation leading to an increased INR) are possible. However, the MELD has been demonstrated to have predictive value outside of a cirrhotic population and in our study was accompanied by positive correlations between CVD and other markers of liver disease including albumin and platelet count (19,37). In addition, comorbidities in our study were defined by the presence of one or more diagnostic codes of that condition. Further, patients with CVD may more frequently attend medical appointments, have more frequently laboratory testing, and may confound our results.
In conclusion, MELD score, sodium, and albumin levels are predictors of CVD in NAFLD. Further evaluation is needed to further elucidate the relationship between progressive CVD and NAFLD.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Nonalcoholic fatty liver disease (NAFLD) is an independent risk factor for cardiovascular disease (CVD).
Risk factors for CVD among those with NAFLD are not well-documented.
WHAT IS NEW HERE
CVD in those with NAFLD is associated with traditional CVD risk factors including age, hypertension, renal failure, and family history of CVD.
MELD score, albumin, and sodium are also associated with CVD in NAFLD.
Progressive NAFLD may be associated with worsening CVD.
Acknowledgments
We acknowledge Dr Mary Rinella for her critical review of this manuscript.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Kathleen E. Corey, MD, MPH, MMSc.
Specific author contributions: Study planning, conducting the study, data collection and interpretation, and drafting of the manuscript: Kathleen E. Corey and Uri Kartoun; data analysis and interpretation: Hui Zheng; study planning, data interpretation, and editing the manuscript: Raymond T. Chung and Stanley Y. Shaw. All the authors have approved the final submitted draft.
Financial Support: This study was funded in part by grants from the NIH K23 DK099422 (KEC), NIH U54 LM008748 (SYS), and NIH DK 078772 (RTC).
Potential competing interests: None.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 3.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 4.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–16. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–8. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Puchner SB, Lu MT, Mayrhofer T, et al. High-risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: results from the ROMICAT II trial. Radiology. 2015;274:693–701. doi: 10.1148/radiol.14140933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–50. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–21. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 10.Treeprasertsuk S, Leverage S, Adams LA, et al. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int. 2012;32:945–50. doi: 10.1111/j.1478-3231.2011.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchesini G, Ronchi M, Forlani G, et al. Cardiovascular disease in cirrhosis—a point-prevalence study in relation to glucose tolerance. Am J Gastroenterol. 1999;94:655–62. doi: 10.1111/j.1572-0241.1999.00931.x. [DOI] [PubMed] [Google Scholar]
- 12.Albillos A, Rossi I, Cacho G, et al. Enhanced endothelium-dependent vasodilation in patients with cirrhosis. Am J Physiol. 1995;268:G459–G464. doi: 10.1152/ajpgi.1995.268.3.G459. [DOI] [PubMed] [Google Scholar]
- 13.Bosch J, Garcia-Pagan JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141–56. doi: 10.1016/s0168-8278(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 14.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007;24:1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 15.Corey K, Kartoun U, Zheng H, et al. Development and validation of an algorithm to identify nonalcoholic fatty liver disease in the electronic medical record. Dig Dis Sci. 2015:1–7. doi: 10.1007/s10620-015-3952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 17.Kamath PS, Kim WR Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 18.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 19.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–8. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 20.Telem DA, Schiano T, Goldstone R, et al. Factors that predict outcome of abdominal operations in patients with advanced cirrhosis. Clin Gastroenterol Hepatol. 2010;8:451–7. doi: 10.1016/j.cgh.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Berman K, Tandra S, Forssell K, et al. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–9. doi: 10.1016/j.cgh.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhangui P, Laurent A, Amathieu R, et al. Assessment of risk for non-hepatic surgery in cirrhotic patients. J Hepatol. 2012;57:874–84. doi: 10.1016/j.jhep.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Singal AG, Rahimi RS, Clark C, et al. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin Gastroenterol Hepatol. 2013;11:1335–41. doi: 10.1016/j.cgh.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younossi ZM, Henry L, Stepanova M. A new comorbidity model for predicting mortality in patients with cirrhosis: does it work? Gastroenterology. 2014;146:19–24. doi: 10.1053/j.gastro.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Schaubel DE, Goodrich NP, et al. Serum sodium and survival benefit of liver transplantation. Liver Transplant. 2015;21:308–13. doi: 10.1002/lt.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biggins SW, Rodriguez HJ, Bacchetti P, et al. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–9. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 27.De Vito R, Alisi A, Masotti A, et al. Markers of activated inflammatory cells correlate with severity of liver damage in children with nonalcoholic fatty liver disease. Int J Mol Med. 2012;30:49–56. doi: 10.3892/ijmm.2012.965. [DOI] [PubMed] [Google Scholar]
- 28.Sookoian S, Castano GO, Burgueno AL, et al. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis. 2010;209:585–91. doi: 10.1016/j.atherosclerosis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Thuy S, Ladurner R, Volynets V, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452–5. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 30.Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity. 2008;16:1394–9. doi: 10.1038/oby.2008.64. [DOI] [PubMed] [Google Scholar]
- 31.Tripodi A, Anstee QM, Sogaard KK, et al. Hypercoagulability in cirrhosis: causes and consequences. J Thromb Haemost. 2011;9:1713–23. doi: 10.1111/j.1538-7836.2011.04429.x. [DOI] [PubMed] [Google Scholar]
- 32.Targher G, Chonchol M, Miele L, et al. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009;35:277–87. doi: 10.1055/s-0029-1222606. [DOI] [PubMed] [Google Scholar]
- 33.Barrett-Connor E, Khaw K. Family history of heart attack as an independent predictor of death due to cardiovascular disease. Circulation. 1984;69:1065–9. doi: 10.1161/01.cir.69.6.1065. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and off spring. J Am Med Assoc. 2004;291:2204–11. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 35.Andresdottir MB, Sigurdsson G, Sigvaldason H, et al. Fifteen percent of myocardial infarctions and coronary revascularizations explained by family history unrelated to conventional risk factors. The Reykjavik Cohort Study. Eur Heart J. 2002;23:1655–63. doi: 10.1053/euhj.2002.3235. [DOI] [PubMed] [Google Scholar]
- 36.Jousilahti P, Vartiainen E, Tuomilehto J, et al. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99:1165–72. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 37.Wong VW, Chim AM, Wong GL, et al. Performance of the new MELD-Na score in predicting 3-month and 1-year mortality in Chinese patients with chronic hepatitis B. Liver Transplant. 2007;13:1228–35. doi: 10.1002/lt.21222. [DOI] [PubMed] [Google Scholar]