Abstract
Background
Although dopamine agonists (DAs) are useful in Parkinson′s disease (PD), they are not frequently used in elderly patients due to adverse effects. However, there is a lack of evidence because few elderly PD patients are enrolled in clinical trials.
Aims of the study
The aims of this study were to analyze the reasons of DA withdrawal (DAW) in a group of PD patients in clinical practice and to identify the related factors. Specifically, we studied the effect of age, comorbidity, and polypharmacy as potential risk factors for DAW.
Methods
A retrospective chart review of the follow‐up (from May, 2012 to March, 2015) of a subgroup of PD patients receiving a DA (n = 68; 60.3% males, 69.3 ± 9.2 years old) from a cohort (n = 150) previously studied in detail in 2012 was used to identify predictive factors of DAW.
Results
The DAW percentage was 18.2% (12/66; follow‐up of 690.2 ± 232.6 days). DAW causes were cognitive impairment (3), reduction therapy (3), hallucinations (2), dyskinesia (2), and excessive diurnal somnolence (2). Only a higher levodopa daily dose (HR 1.003; 95% CI 1.001–1.006; P = 0.044) was an independent predictor of DAW after adjustment for other explanatory variables.
Conclusions
The frequency of DAW was low. Advanced age alone is not a contraindication to the administration of DAs.
Keywords: Age, comorbidity, dopamine agonist, Parkinson′s disease, polypharmacy, tolerability
Introduction
Levodopa is the gold standard symptomatic treatment in Parkinson′s disease (PD), although its long‐term use is associated with the development of motor complications. Alternatively, dopamine agonists (DAs) have shown to be effective, both as monotherapy in the early stages of PD or in association with levodopa in advanced PD (Antonini et al. 2009). However, DAs are associated with a greater risk of developing dopaminergic side effects (somnolence, hallucinations, edema, impulse control disorders, etc.) compared with levodopa, and their use is avoided in the elderly for reasons including altered drug metabolism, an increased risk of adverse effects, increased comorbid conditions, increased risk of drug interactions, and a higher risk of cognitive problems and behavior disorders in PD patients over the age of 70 years (Kempster et al. 2007; Stowe et al. 2008). However, there is no compelling evidence for such concerns because few elderly PD patients have been enrolled in clinical trials (Mitchell et al. 1997). Moreover, a previous study demonstrated that trials with DAs are warranted in selected very elderly patients (Shulman et al. 2000).
The aims of this study were as follows: (1) to determine the reasons of DA withdrawal (DAW) in a group of PD patients in clinical practice; and (2) to identify the predictive factors of DAW. Specifically, we studied the effect of age, comorbidity, and polypharmacy as potential risk factors for DAW.
Materials and Methods
We conducted a retrospective chart review of the follow‐up of a subgroup of nondemented PD patients receiving a DA (n = 68; 60.3% males, 69.3 ± 9.2 years old) from a cohort (n = 150) previously studied in detail (Santos‐García and de la Fuente‐Fernández 2013, 2015). The patients from the cohort who were receiving a DA, such as pramipexole, ropinirole, and rotigotine at baseline (cross‐sectional study conducted in 2012) were included in this study. Figure 1 shows a flowchart of the selection and inclusion of patients. Baseline assessment included motor dysfunction (ON‐state Hoehn & Yahr [H&Y] / Unified Parkinson's Disease Rating Scale [UPDRS] part III and motor complications [UPDRS part IV]), mood (Beck Depression Inventory [BDI]), nonmotor symptoms (Nonmotor Symptoms Scale [NMSS]), disability (Schwab & England Activities of Daily Living Scale [ADLS]), socio‐demographic variables, and other disease‐related variables.
Figure 1.

Flowchart of inclusion in the clinical practice cohort. ADLS, Activities of Daily Living Score; BDI, Beck Depression Inventory; COMTI, catechol‐O‐methyl transferase inhibitor; DA, dopamine agonist; DAW, dopamine agonist withdrawal; MAOBI, monoamine oxidase B inhibitor; MSA, multiple system atrophy; NMSS, Nonmotor Symptoms Scale; UPDRS, Unified Parkinson's Disease Rating Scale (part III, motor examination; part IV, motor complications). *Data are from 149 patients because one case was diagnosed with MSA “a posteriori”.
The follow‐up information was collected from medical records by a neurology resident (E.S.C.). The follow‐up period was from the baseline evaluation (between 11 May and 24 December 2012 depending on the case) to 23 March 2015. The reason for DAW, age at DAW, follow‐up time, time until DAW (time from baseline to DA withdrawal), time taking DA therapy (time taking the DA before baseline) and DA dose (Tomlinson et al. 2010) changes were collected. The study (CADAW‐PD, Causes and fActors related to Dopamine Agonist Withdrawal in Parkinson′s Disease; DIE‐LEV‐2015‐01) was approved by the local ethics committee. All participants signed an informed consent form.
Data were processed using SPSS 21.0 for Windows. Proportions between groups were compared using the chi‐square test. Continuous variables are expressed as the mean ± SD or median and quartiles, depending on whether they were normally distributed and were compared using the Student′s t test or the Mann–Whitney test, as appropriate. We defined two groups relative to the primary endpoint (DA withdrawal): (1) patients who discontinued DA therapy during follow‐up (DAW group); and (2) patients who did not discontinue DA therapy (non‐DAW group). Univariate and multiple regression analyses were performed to evaluate predictors of DAW. Specifically, we studied the effect of age, comorbidity, and polypharmacy as potential risk factors for DAW. The number of drugs different of antiparkinsonian agents taken at baseline was considered a surrogate marker of comorbidity. Polypharmacy was assessed by counting the total number of pills at baseline. Values of p < 0.05 were considered significant.
Results
Sixty‐eight PD patients from the cohort (n = 150) (Santos‐García and de la Fuente‐Fernández 2013, 2015) were receiving DA therapy at baseline: 30 pramipexole, 20 rotigotine, 17 ropinirole, and one pramipexole + rotigotine. Seven patients died and two cases (taking ropinirole) were lost to follow‐up. Twelve of 66 patients (18.2%) discontinued DA therapy during follow‐up (690.2 ± 232.6 days; range, 128–1080). Figure 2 shows the frequency of DAW according to DA therapy and the patients' medications at baseline. The reasons for DAW are shown in Table 1.
Figure 2.

Percentage of patients taking DA therapy (pramipexole, rotigotine and/or ropinirole) at baseline (top bar) and at the end of follow‐up (bottom bar). Cases who discontinued DA therapy are indicated by a dark color in the bottom bar. The DAW percentage was 10% for pramipexole (3/30), 20% for rotigotine (4/20), 26.7% for ropinirole (4/15) and for the only case taking pramipexole + rotigotine. At the end of the follow‐up, 54 patients were receiving DA therapy: 50% pramipexole (27/54), 29.6% rotigotine (16/54) and 20.4% ropinirole (11/54). DA, dopamine agonist.
Table 1.
Reasons of DAW considering different type of DA therapy. “Time until DAW” refers to time from baseline to DA withdrawal. “Time taking DA therapy” refers to time taking the DA before baseline
| DA Therapy | Reasons of DAW | Time until DAW (days) | Age at DAW | Time taking DA therapy (days) |
|---|---|---|---|---|
| Pramipexole (n = 30) | 3 cases (10%) | 437.7 ± 476.2 | 71.3 ± 7 | 1250.3 ± 724.8 |
| Case 1 simplification of treatment (DBS) | 12 | 64 | 1987 | |
| Case 2 diurnal somnolence | 349 | 78 | 1226 | |
| Case 3 starting with levodopa enteral | 882 | 72 | 538 | |
| Rotigotine (n = 20) | 4 cases (20%) | 511.7 ± 333.9 | 68.8 ± 6.3 | 548.2 ± 563.3 |
| Case 1 cognitive impairment ‐> dementia | 112 | 66 | 48 | |
| Case 2 skin reaction and diurnal somnolence | 644 | 67 | 155 | |
| Case 3 cognitive impairment ‐> dementia | 719 | 78 | 723 | |
| Case 4 visual hallucinations | 846 | 64 | 1267 | |
| Ropinirole (n = 15) | 4 cases (26.7%) | 503 ± 118.2 | 60.3 ± 11.7 | 1295.5 ± 1052.2 |
| Case 1 visual hallucinations | 385 | 62 | 1232 | |
| Case 2 dyskinesia | 429 | 63 | 2689 | |
| Case 3 simplification of treatment | 554 | 72 | 1126 | |
| Case 4 dyskinesia/dystonia (ON) | 644 | 44 | 135 | |
| Pramipexol + rotigotine (n = 1) | 1 case (100%) | 551 | 70 | 1815 |
| Cognitive impairment ‐> dementia |
DA, dopamine agonist; DBS, deep brain stimulation; DAW, dopamine agonist withdrawal.
As shown in Table 2, an earlier age at PD onset, a longer disease duration, a higher daily dose of levodopa, a greater number of pills for PD, polypharmacy, and a higher number of nonmotor symptoms were associated with DAW. However, age was not related to DAW. In fact, only one patient discontinued DA therapy in the subgroup of patients over the age of 75 years old (Fig. 3). On the contrary, mortality was significantly higher in this group of patients.
Table 2.
Factors related (at baseline) to DAW (n = 12) versus non‐DAW (n = 54)
| Non‐DAW (n = 54) | DAW (n = 12) | P | |
|---|---|---|---|
| Age | 70.3 ± 9.1 | 65.3 ± 8.8 | 0.092 |
| Males (%) | 63 | 58.3 | 0.505 |
| Age of symptoms onset | 63.5 ± 7.9 | 54.8 ± 13.1 | 0.004 |
| Disease durarion (years) | 7.3 ± 3.9 | 10.6 ± 8.4 | 0.040 |
| Hoehn&Yahr (ON) | 2 [2, 2.5] | 2 [1.6, 2] | 0.171 |
| UPDRS‐III (ON) | 15.3 ± 7.6 | 15.4 ± 9.5 | 0.974 |
| UPDRS‐IV | 3.1 ± 2.7 | 4.3 ± 2.7 | 0.176 |
| Motor fluctuations (%) | 38.9 | 50 | 0.347 |
| Dyskinesia (%) | 40.7 | 58.3 | 0.215 |
| Levodopa (%) | 85.2 | 100 | 0.181 |
| COMT‐inhibitor (%) | 53.7 | 25 | 0.068 |
| MAO B inhibitor (%) | 33.3 | 25 | 0.425 |
| Time taking DA therapy (days) | 1288.3 ± 805.7 | 1078.4 ± 812.6 | 0.419 |
| Levodopa daily dose (mg) | 490.7 ± 321.1 | 820.8 ± 520.7 | 0.006 |
| DA equivalent daily dose (mg) | 246.6 ± 117.1 | 253.8 ± 179.2 | 0.864 |
| Levodopa equivalent daily dose (mg) | 795.2 ± 432 | 1089.2 ± 641.7 | 0.064 |
| Comorbidity | 4.1 ± 2.6 | 4 ± 2.6 | 0.896 |
| Total number of pills for PD | 5.6 ± 2.6 | 7.7 ± 3.6 | 0.019 |
| Polypharmacy | 9.4 ± 3.4 | 12 ± 4.4 | 0.028 |
| NMSS total score (0–360) | 65.5 ± 54.1 | 90.8 ± 58.3 | 0.154 |
| NMSS domains 2+3+4 | 26.2 ± 25.6 | 34.9 ± 26 | 0.293 |
| Number of nonmotor symptoms | 14.9 ± 5.7 | 19.3 ± 6.3 | 0.022 |
| BDI (0–63) | 11.9 ± 7.6 | 15.1 ± 9 | 0.214 |
| ADLS (0–100) | 80 [60, 90] | 65 [35, 80] | 0.112 |
ADLS, Activities of Daily Living Score; BDI, Beck Depression Inventory; COMT, catechol‐O‐methyl transferase; DA, dopamine agonist; DAW, dopamine agonist withdrawal; MAO, monoamine oxidase; NMSS, Nonmotor Symptoms Scale; UPDRS, Unified Parkinson's Disease Rating Scale (part III, motor examination; part IV, motor complications).
Bold values: Results are expressed as mean ± SD or %.
Figure 3.
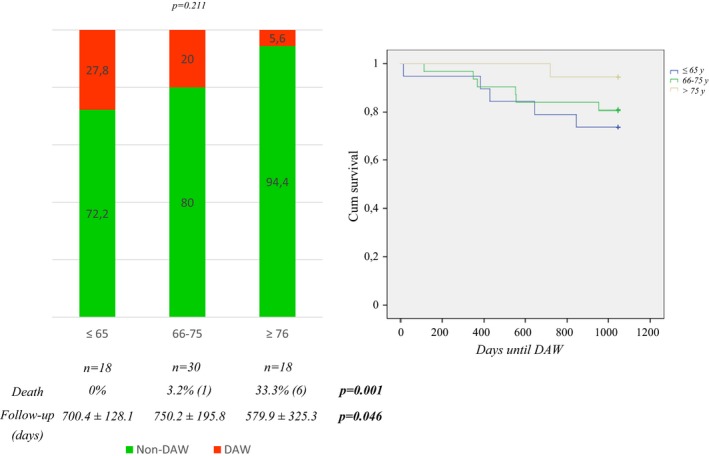
DAW, deaths and follow‐up time in the subgroups of PD patients in relation to age: 1) ≤65 years (5 DAW cases); 2) 66–75 years (6 DAW cases and 1 death); 3) >75 years (DAW in only 1 case and 6 deaths). DAW, dopamine agonist withdrawal.
Cox‐regression analysis showed that only the levodopa daily dose was a predictor of DAW. Table 3 shows different models in which DAW was the dependent variable and levodopa daily dose, comorbidity, polypharmacy, socio‐demographic patient‐related variables (age and gender), disease‐related variables (disease duration, age of symptoms onset, ON‐H&Y, ON‐UPDRS‐III, UPDRS‐IV, NMSS total score), and confounder variables (time taking DA therapy and follow‐up time) were independent variables. The nonadjusted hazard ratio (HR) of the levodopa daily dose for DAW was 1.002 (95% confidence interval [CI], 1.001–1.003; P = 0.006). The levodopa daily dose HR was 1.003 (95% CI, 1.001–1.006; P = 0.044) after adjustment for covariates. As expected, when PD patients were classified according to the levodopa daily dose, a significantly higher percentage of DAW was observed in those receiving more than 750 mg per day (42.8% vs. 11.5%; P = 0.014) (Table 4).
Table 3.
Analysis of the factors predicting dopamine agonist withdrawal
| Parameter | Univariate (HR; 95% CI; P‐value) | Multivariate Cox regression3 (HR; 95% CI; P‐value) | Multivariate Cox regression4 (HR; 95% CI; P‐value) | Multivariate Cox regression5 (HR; 95% CI; P‐value) |
|---|---|---|---|---|
| Levodopa daily dose1 | 1.002; 1.001–1.003; 0.006 | 1.002; 1.001–1.003; 0.001 | 1.002; 1.001–1.004; 0.019 | 1.003; 1.001–1.006; 0.044 |
| Age1 | 0.935; 0.883–0.991; 0.023 | 1.331; 0.541–3.278; 0.534 | 2.211; 0.561–8.707; 0.257 | |
| Gender | 0.830; 0.261–2.644; 0.275 | 0.831; 0.247–2.797; 0.765 | 0.241; 0.038–1.516; 0.129 | |
| Age at symptoms onset1 | 0.703; 0.282–1.755; 0.450 | 0.412; 0.102–1.667; 0.214 | ||
| Disease duration1 | 0.696; 0.274–1.765; 0.445 | 0.411; 0.102–1.654; 0.211 | ||
| Time taking DA therapy1 | 1.000; 0.999–1.001; 0.631 | 0.999; 0.998–1.001; 0.364 | ||
| Follow‐up time1 | 1.000; 0.997–1.003; 0.927 | 1.000; 0.995–1.004; 0.846 | ||
| Comorbidity1 | 1.040; 0.680–1.592; 0.856 | |||
| Polypharmacy1 | 1.243; 0.929–1.663; 0.143 |
1Hazard ratio is the risk multiplier per unit increase in the parameter.
2Figures in bold are statistically significant (P < 0.005).
3Variables entered: levodopa daily dose, age and gender.
4Variables entered: levodopa daily dose, age, gender, age at diagnosis, disease duration, time taking DA therapy and follow‐up time.
5Variables entered: levodopa daily dose, age, gender, age at diagnosis, disease duration, time taking DA therapy, follow‐up time, comorbidity, polypharmacy, and other covariates (ON‐H&Y, ON‐UPRDS‐III, UPDRS‐IV, NMSS total score).
Table 4.
Percentage of patients who presented dopamine agonist withdrawal (DAW) according to levodopa daily dose (>750 mg vs. ≤750 mg): 42.9% (6/14) vs. 11.5% (6/52); P = 0.014
| Levodopa dose ≤ 750 mg/day | Levodopa dose > 750 mg/day | Number of patients | |
|---|---|---|---|
| Non DAW | 46 (88.5%) | 8 (57.1%) | 54 |
| DAW | 6 (11.5%) | 6 (42.9%) | 12 |
| 52 | 14 | 66 |
Bold values: Results are expressed as number of patients and %.
Discussion
There is considerable controversy regarding the use of DAs in the elderly because of the concern for a higher rate of side effects. However, there is no evidence for such concerns because few individuals older than 75 years have been enrolled in clinical trials for antiparkinsonian medications (Mitchell et al. 1997; Fitzsimmons et al. 2012). Our study demonstrates that an advanced age alone is not a contraindication for the administration of DA therapy. In PD, therapeutic decisions should be individualized, and we must keep in mind that the risk of DAW is higher when DA therapy is administered to PD patients with a longer disease duration, polypharmacy, and a higher daily dose of levodopa.
Clinical–pathological studies have demonstrated that cognitive disability, visual hallucinations, or residential care is more frequent in PD patients over the age of 70 years (Kempster et al. 2007). In addition, older patients metabolize drugs differently than younger patients and may be more susceptible to drug side effects, which are often complicated by comorbid conditions (Robertson et al. 1989). For these reasons, DAs historically have been avoided by some clinicians as therapy in elderly patients. However, Shulman et al. (2000) reported that DAs were well tolerated by very elderly (≥80 years old) and appropriately selected PD patients in clinical practice. In our study, about half of the PD patients were early elderly (from 65 to 74 years old) and a quarter were late elderly (over 75 years old). Furthermore, Oertel et al. (2013) observed that rotigotine was generally well tolerated regardless of age. Our results are in line with these studies.
Treatment considerations for elderly PD patients typically have centered on chronological age, and somewhat arbitrary cutoffs have been established for determining whether DA therapy should be used. However, patients with similar chronological ages may differ substantially with respect to functional or physiological age. Functional age can be assessed by measuring motor and nonmotor symptoms, including cognition, mood, behavior, disability, comorbidity, and other dimensions that contribute to functionality. When considering functional age in treatment planning, the clinician can appreciate variability among patients of the same age (Silver 2006). Our results support this idea. The risk of DAW seems to be higher in PD patients with longer disease duration, polypharmacy and more nonmotor symptoms. Indeed, levodopa dosage determines the probability of DAW, with a thirty percent higher risk for every increase in 1.000 mg of levodopa. In other words, almost half of the patients receiving more than 750 mg per day of levodopa discontinued with DA therapy versus one in ten of patients receiving a smaller dose. Related to this finding, compliance with once‐daily DA therapy has been reported to be suboptimal and to depend upon the total daily dose of levodopa and the total number of daily drugs (Santos‐García et al. 2012). Although comorbidity is a risk factor for the development of somnolence, edema, or hallucinations in PD patients (Biglan et al. 2007), these conditions were not related to DAW in our study. No statistical determinants for the discontinuation of DAs were detected in other studies (Arbouw et al. 2008).
Overall, our findings suggest that DA therapy is well tolerated in properly selected PD patients in clinical practice. The number of patients who discontinued DA therapy in our series was low. In fact, DAW in three cases was not due to adverse events. Moreover, adverse events in three other cases were in the context of cognitive impairment progressing to dementia. Previous observational studies have largely focused on discontinuation rates for DAs introduced as a supplement to levodopa (Hauser et al. 2007; Arbouw et al. 2008, 2009; Parkinson Study Group 2009; Valldeoriola et al. 2009; Nissen et al. 2012). A maintenance of pramipexole in approximately 80% at 6 years (Parkinson Study Group 2009) and 90% between 5 and 10 years for ropinirole (Hauser et al. 2007) has been reported in extended open‐label observational studies. Future studies are needed to know the extent to which PD patients tolerate different DAs according to age (Oertel et al. 2013), and interestingly, the AD type (Kim et al. 2015) and genetic determinants (Arbouw et al. 2007).
This study has several limitations. First, the sample size was small, and as result, some differences between groups might have been insignificant. Second, death is a confounding factor because there were more cases in older patients, and follow‐up time was significantly shorter in older patients. However, the follow‐up time was included as a covariate in the model. Third, we used a surrogate marker of comorbidity, but we think that, in any case, it provides proper information on the variable (a larger number of drugs different of antiparkinsonian agents indicates more frequent and/or severe comorbidity). Fourth, some information may have been missed due to the methodology used (chart review). However, the findings are novel and have practical applicability in clinical practice. Fifth, a cognition assessment using a specific scale at baseline was not conducted. Finally, follow‐up information on adverse events was not collected in patients who continued receiving DA therapy at the end of the follow‐up. Nevertheless, this study was not designed to evaluate tolerability and effectiveness. Thus, we can assume that patients continued taking the medication in clinical practice because it is useful and well tolerated.
In conclusion, our study shows that DA therapy is generally well tolerated by nondemented PD patients in clinical practice. Advanced age alone is not a contraindication to the administration of DA therapy. We must keep in mind that the probability of successful treatment when we use DA therapy is lower in PD patients with a longer disease duration, polypharmacy, and a higher daily dose of levodopa.
Conflict of Interest
None declared.
Acknowledgments
Parkinson′s disease patients. We also thank Adam D. and Sadye P. who provided English editing services on behalf of Wiley English Language Editing Services.
Castro E. S., Santos‐García D., de Deus Fonticoba T., Ruíz I. E., Gesto C. T., Arribí M. M., Causes and factors related to dopamine agonist withdrawal in Parkinson′s disease, Brain and Behavior, 2016; 6(7), e00453, doi: 10.1002/brb3.453
References
- Antonini, A. , Tolosa E., Mizuno Y., Yamamoto M., and Poewe W. H.. 2009. A reassessment of risk and benefits of dopamine agonists in Parkinson′s disease. Lancet Neurol. 8:929–937. [DOI] [PubMed] [Google Scholar]
- Arbouw, M. E. , van Vugt J. P., Egberts T. C., and Guchelaar H. J.. 2007. Pharmacogenetics of antiparkinsonian drug treatment: a systematic review. Pharmacogenomics 8:159–176. [DOI] [PubMed] [Google Scholar]
- Arbouw, M. E. , Movig K. L., Guchejaar H. J., Poels P. J., van Vugt J. P., Neef C., et al. 2008. Discontinuation of ropinirole and pramipexole in patients with Parkinson's disease in clinical practice versus clinical trials. Eur. J. Clin. Pharmacol. 64:1021–1026. [DOI] [PubMed] [Google Scholar]
- Arbouw, M. E. , Movig K. L., Egberts T. C., Poels P. J., van Vugt J. P., Wessels J. A., et al. 2009. Clinical and pharmacogenetic determinants for the discontinuation of non‐ergoline dopamine agonists in Parkinson's disease. Eur. J. Clin. Pharmacol. 65:1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglan, K. M. , Holloway R. G. Jr, McDermott M. P., Richard I. H., and Parkinson Study Group CALM‐PD Investigators . 2007. Risk factors for somnolence, edema, and hallucinations in early Parkinson disease. Neurology 69:187–195. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons, P. R. , Blayney S., Mina‐Corkill S., and Scott G. O.. 2012. Older participants are frequently excluded from Parkinson's disease research. Parkinsonism Relat. Disord. 18:585–589. [DOI] [PubMed] [Google Scholar]
- Hauser, R. A. , Rascol O., Korczyn A. D., Jon Soessl A., Watts R. L., Poewe W., et al. 2007. Ten‐year follow‐up of Parkinson's disease patients randomized to initial therapy with ropinirole or levodopa. Mov. Disord. 22:2409–2417. [DOI] [PubMed] [Google Scholar]
- Kempster, P. A. , Williams D. R., Selikhova M., Holton J., Revesz T., and Lees A. J.. 2007. Patterns of levodopa response in Parkinson's disease: a clinico‐pathological study. Brain 130:2123–2128. [DOI] [PubMed] [Google Scholar]
- Kim, J. M. , Chung S. J., Kim J. W., Jeon B. S., Singh P., Thierfelder S., et al. 2015. Rotigotine transdermal system as add‐on to oral dopamine agonist in advanced Parkinson's disease: an open‐label study. BMC Neurol. doi: 10.1186/s12883‐015‐0267‐7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, S. L. , Sullivan E. A., and Lipsitz L. A.. 1997. Exclusion of elderly subjects from clinical trials for Parkinson disease. Arch. Neurol. 54:1393–1398. [DOI] [PubMed] [Google Scholar]
- Nissen, T. , Newman E. J., Grosset K. A., Daghem M., Pal G., Stewart M., et al. 2012. Duration of L‐dopa and dopamine agonist monotherapy in Parkinson′s disease. Scott. Med. J. 57:217–220. [DOI] [PubMed] [Google Scholar]
- Oertel, W. , LeWitt P., Giladi N., Ghys L., Grieger F., and Boroojerdi B.. 2013. Treatment of patients with early and advanced Parkinson's disease with rotigotine transdermal system: age‐relationship to safety and tolerability. Parkinsonism Relat. Disord. 19:37–42. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group, CALM Cohort Investigators . 2009. Long‐term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch. Neurol. 66:563–570. [DOI] [PubMed] [Google Scholar]
- Robertson, D. R. , Wood N. D., Everest H., Monks K., Waller D. G., Renwick A. G., et al. 1989. The effect of age on the pharmacokinetics of levodopa administered alone and in the presence of carbidopa. Br. J. Clin. Pharmacol. 28:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐García, D. , and de la Fuente‐Fernández R.. 2013. Impact of non‐motor symptoms on health‐related and perceived quality of life in Parkinson's disease. J. Neurol. Sci. 332:136–140. [DOI] [PubMed] [Google Scholar]
- Santos‐García, D. , and de la Fuente‐Fernández R.. 2015. Factors contributing to caregivers' stress and burden in Parkinson's disease. Acta Neurol. Scand. 131:203–210. [DOI] [PubMed] [Google Scholar]
- Santos‐García, D. , Prieto‐Formoso M., and de la Fuente‐Fernández R.. 2012. Levodopa dosage determines adherence to long‐acting dopamine agonists in Parkinson's disease. J. Neurol. Sci. 318:90–93. [DOI] [PubMed] [Google Scholar]
- Shulman, L. M. , Minagar A., Rabinstein A., and Weiner W. J.. 2000. The use of dopamine agonists in very elderly patients with Parkinson's disease. Mov. Disord. 15:664–668. [DOI] [PubMed] [Google Scholar]
- Silver, D. 2006. Impact of functional age on the use of dopamine agonists in patients with Parkinson disease. Neurologist 12:214–223. [DOI] [PubMed] [Google Scholar]
- Stowe, R. L. , Ives N. J., Clarke C., van Hilten J., Ferreira J., Hawker R. J., et al. 2008. Dopamine agonist therapy in early Parkinson′s disease. Cochrane Database Syst. Rev.:CD006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson, C. L. , Stowe R., Patel S., Rick C., Gray R., and Clarke C. E.. 2010. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 25:2649–2653. [DOI] [PubMed] [Google Scholar]
- Valldeoriola, F. , Cobaledac S., and Lahuertac J.. 2009. A multicentre retrospective study of the clinical use of ropinirole in the treatment of Parkinson's disease: the ROPI‐PARK study. Clin. Neurol. Neurosurg. 111:742–747. [DOI] [PubMed] [Google Scholar]


